Abstract
Development of vaccines is essential for the prevention of future recurrences of severe acute respiratory syndrome (SARS), caused by the SARS coronavirus (SARS-CoV). The spike (S) protein, especially receptor-binding domain (RBD) of SARS-CoV, plays important roles in the prevention of SARS infection, and is thus an important component in SARS vaccine development. In this study, we expressed a 219-mer (residues 318–536) RBD protein in Chinese hamster ovary (CHO)-K1 cells (RBD219-CHO), and tested its immune responses and protective immunity in a mouse model. The results showed that this recombinant protein was correctly folded, being able to maintain intact conformation and authentic antigenicity. It could induce strong humoral and cellular immune responses and high titers of neutralizing antibodies in the vaccinated mice. RBD219-CHO protein elicited potent protective immunity that protected all vaccinated mice from SARS-CoV challenge. These results suggest that the recombinant RBD219-CHO protein has great potential for the development of an effective and safe SARS subunit vaccine.
Introduction
Anewly emerging infectious disease, severe acute respiratory syndrome (SARS), is caused by SARS coronavirus (SARS-CoV) (27,46,58), a zoonotic virus that most likely originated in its natural reservoir bats, through intermediate transmission such as via palm civets and raccoon dogs, and was finally transmitted to humans (21,37,38). Transmission of SARS-CoV from humans to humans led to the global outbreak of SARS in 2003 (39,44,45,52). Though SARS is currently under control, it is necessary to develop effective and safe vaccines for the prevention of future SARS outbreaks that may arise from animal reservoirs or accidentally due to laboratory virus escape.
Recently developed SARS vaccines are of various categories (18), including inactivated virus vaccines (49,51,59), subunit vaccines (2,26), DNA vaccines (28,42,55), virus-like particles (40,41), viral vector-based vaccines (4,19,34), and different vaccine combinations (16,30,53). A variety of SARS vaccines have been tested in animals, including monkeys, ferrets, mice, and hamsters (1,7,9,20,33,48,49), and some of them have been evaluated in humans (42). These vaccines may target different antigens of the virus, but most of them are based on the spike (S) protein. It has been reported that an adenovirus-based vaccine expressing S protein prevented pneumonia in ferrets after SARS-CoV challenge, and stimulated potent immune responses in macaques (36). A recombinant SARS S-protein elicits neutralizing antibodies and protection in mice (29). Specific humoral and cellular immune responses and/or protection could be induced by SARS S DNA vaccines via different vaccination routes (28,55). A SARS-CoV-like particle carrying the S protein protected mice from virus challenge (40). These reports suggest that the S protein plays an important role in the prevention of SARS infection (3).
Our previous studies demonstrated that a recombinant fusion protein consisting of a 193-mer (residues 318–510) receptor-binding domain (RBD) of SARS-CoV S protein tagged with the Fc fragment of human IgG (RBD193-Fc) could induce highly potent neutralizing antibody responses and protective immunity (14,25). However, one potential disadvantage of this vaccine candidate is that the Fc tag, which was added to the C-terminus of RBD193 in the hope of increasing immunogenicity by binding Fc-tagged immunogen to the Fc receptor on antigen-presenting cells (5,47,57), may cause adverse effects when used as a vaccine component in humans. When we expressed a recombinant 193-mer RBD (residues 318–510) without fusing Fc (RBD193-CHO) in Chinese hamster ovary (CHO)-K1 cells, it induced RBD-specific immune responses and neutralizing antibodies, but could not fully protect vaccinated mice from SARS-CoV challenge, with virus replication detected in two of five vaccinated mice (15).
In the present study we expressed a 219-mer RBD protein covering residues 318–539 in CHO-K1 cells (RBD219-CHO). Like RBD193-Fc, this newly designed RBD without the Fc tag (RBD219-CHO) can also induce strong humoral and cellular immune responses, high titers of neutralizing antibodies, and induces potent protective immunity that protected all vaccinated mice from SARS-CoV challenge. These results suggest that RBD219-CHO has great potential for development into an effective and safe SARS subunit vaccine.
Materials and Methods
Gene construction, protein expression, and purification of RBD219-CHO
The gene construction and expression of RBD219-CHO protein was done as previously described (15). The genes encoding the fragment containing 219 aa (318–536) of the SARS-CoV S protein RBD region, plus a 6 × His tag at the C terminus, were amplified by PCR using a full-length S plasmid (Tor2 strain) as the template (12, 24). It was then inserted into the GS Gene Expression Vector PEE14.1. The constructed recombinant plasmid (RBD219) was confirmed by sequencing analysis. Briefly, the recombinant RBD219 plasmid was transiently transfected using FuGENE 6 transfection reagents (Roche Applied Science, Indianapolis, IN) into CHO-K1 cells precultured in F-12K medium (American Type Culture Collection, Manassas, VA). The culture medium was replaced by fresh OPTI-MEM I Reduced-Serum Medium (Invitrogen, Carlsbad, CA) 10 h later, and the supernatant was collected 72 h post-transfection. Culture supernatant containing expressed protein was added to protease inhibitor cocktails (Roche Applied Science) and purified using His columns (Promega, Madison, WI). The protein yield using this transient transfection method was about 10.6 mg/L supernatant.
Detection of RBD219-CHO protein reactivity with RBD-specific mAbs
The reactivity of purified RBD219-CHO protein was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by Western blot, according to our previously described protocols (10). Briefly, purified protein (2 μg/well) was separated by non-reducing SDS-PAGE (no boiling and no reducing agent). The gel was transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA), which were then blocked overnight at 4°C with blocking buffer containing 5% non-fat milk dissolved in 0.1% Tween 20-PBS (PBST). The blots were incubated for 1 h at room temperature with serial RBD-specific monoclonal antibodies (mAbs) generated in our laboratory (25), at a final concentration of 0.5 μg/mL. After washing three times, the blots were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:5000; Zymed, Carlsbad, CA) for 1 h at room temperature. Signals were visualized with ECL Western blot Substrate Reagents and Amersham Hyperfilm (GE Healthcare, Piscataway, NJ).
Mouse vaccination and sample collection
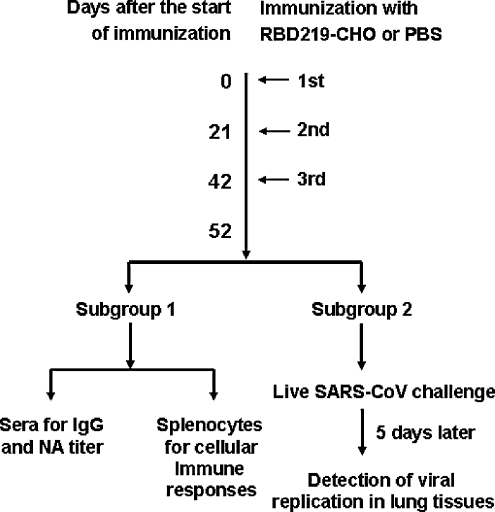
A group of five female BALB/c mice 4–6 wk of age were subcutaneously vaccinated with purified RBD219-CHO protein (20 μg/mouse) in the presence of Freund's complete adjuvant (FCA; Sigma, St. Louis, MO), and boosted twice with the same immunogen (10 μg/mouse) containing Freund's incomplete adjuvant (FIA; Sigma) at 21-day intervals. Mice injected with the same amount of PBS were used as negative controls. Sera were collected pre-immunization and 10 d after each vaccination, and analyzed for IgG and neutralizing antibody responses. Spleen cells collected 10 d after the last vaccination were used for the detection of cellular responses. For parallel experiments, mice were challenged with SARS-CoV 10 days after the last vaccination, and analyzed for viral load and RNA copies in challenged lung tissues 5 d post-challenge. Vaccination protocols are described in detail in Fig. 1.
FIG. 1.
Vaccination protocol for the experiments.
Enzyme-linked immunosorbent assay (ELISA)
Mouse sera were analyzed using ELISA for IgG antibody specific for RBD of SARS-CoV S protein using a previously described protocol with some modifications (14). Briefly, 96-well plates were pre-coated with 100 μL/well of RBD219-CHO protein (1 μg/mL) and sat overnight at 4°C, then blocked with 2% non-fat milk at 37°C for 2 h. Serially diluted mouse sera were added to the plates at 100 μL/well and incubated at 37°C for 1 h, followed by three washes with PBST. Bound antibodies were then reacted with HRP-conjugated goat anti-mouse IgG (1:2000) at 37°C for 1 h. The substrate 3,3′,5,5′-tetramethylbenzidine (TMB; Zymed) was added to the plates after three washes, followed by adding 1 N H2SO4 to stop the reaction. The absorbance at 450 nm (A450) was measured by an ELISA plate reader (Tecan Systems, San Jose, CA).
Neutralization assay for SARS pseudovirus infection
The neutralization assay against SARS-CoV pseudovirus infection was done according to our previously established method (25). Pseudotyped virus bearing SARS-CoV S protein, and defective HIV-1 genome expressing luciferase as reporter was prepared as before. Briefly, 293T cells were co-transfected with a plasmid encoding codon-optimized SARS-CoV S protein, and a plasmid encoding Env-defective, luciferase-expressing HIV-1 genome (pNL4-3.luc.RE), using FuGENE 6 transfection reagents (Roche Applied Science). Supernatants containing SARS pseudovirus were harvested 72 h post-transfection and used for single-cycle infection of 293T cells expressing ACE2 (ACE2/293T). The cells were plated at 104 cells/well in 96-well tissue culture plates 24 h pre-infection. The pseudovirus-containing supernatants were pre-incubated with serially diluted mouse sera at 37°C for 1 h before they were added to the cells. After 24 h, the cells were re-fed with fresh medium, which was followed by lysing the cells 72 h later using cell lysis buffer (Promega), and the lysates were transferred into 96-well luminometer plates. Luciferase substrate (Promega) was added to the plates, and relative luciferase activity was determined with an Ultra 384 luminometer (Tecan Systems). The neutralization of SARS pseudovirus was calculated (8), and presented as 50% neutralizing antibody titer (NT50).
Neutralization assay for live SARS-CoV infection
Neutralization assay against the live SARS-CoV (GZ50 strain; GenBank accession no. AY304495) was done as previously described (16,17), and neutralizing antibody titers of sera from mice immunized with RBD219-CHO protein were detected in Vero E6 cells. Briefly, the cells were seeded (104/well) on 96-well tissue culture plates 24 h before infection. Serial twofold dilutions of serum samples were separately mixed with 100 50% tissue-culture infectious dose (TCID50) of the SARS-CoV GZ50 strain, incubated at 37°C for 1 h, and added to the cells in triplicate. Cytopathic effect (CPE) was observed daily and recorded on day 3 post-infection. The neutralizing titer was determined as the highest dilution of sera that could completely prevent CPE in at least 50% of the wells (NT50).
ELISPOT assay
ELISPOT assay was done using a mouse kit (Mabtech Inc., Mariemont, OH), according to the manufacturer's protocol and our previously described method (16,17). Briefly, 96-well ELISPOT plates were coated with anti-IFN-γ, anti-IL-2, and anti-IL-4 mAbs overnight at 4°C, and blocked with RPMI-1640 containing 10% FBS for 2 h at room temperature. Single-cell suspensions (2 × 105 cells/well) isolated from vaccinated mouse spleens were added to the wells, which was followed by incubation at 37°C for 24 h in the presence of an MHC-H-2d-restricted SARS-CoV RBD-specific cytotoxic T lymphocyte (CTL) peptide (N50: S365–374, KCYGVSATKL), or Th peptide (N60: S435–444, NYNYKYRYLR), at a final concentration of 1 μg/mL (30). The plates were washed with PBS, followed by incubation with biotinylated labeled anti-mouse IFN-γ, IL-2, and IL-4 mAbs at 1:1000 for 2 h at room temperature. After additional washes, the wells were incubated with streptavidin-conjugated HRP for 1 h at room temperature, and developed with TMB substrate. Spots of cytokine-producing T cells were counted by using an ELISPOT reader and ImmunoSpot 3 software (Cellular Technology Ltd., Cleveland, OH). Results were expressed as the number of spot-forming cells per 106 input cells.
Challenge of vaccinated mice with live SARS-CoV
Vaccinated mice were intranasally inoculated with SARS-CoV strain GZ50 (5 × 105 TCID50) as previously described (16,17). The mice were sacrificed 5 d after virus challenge, and the lungs were removed and stored at −80°C for further virological detection.
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
The viral RNA copies in the lung tissues were determined by qRT-PCR according to a previously described protocol (14). Briefly, total RNA was extracted from 20 mg of the lung tissues using an RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. SARS-CoV RNA was quantified in a 30-μL mixture containing 10 μL RNA, 15 μL 2 × TaqMan® One-Step RT-PCR Master Mix (Applied Biosystems, Foster City, CA), 0.75 μL 40 × multiscribe, 0.25 μM each forward primer Af (Taq-772F 5′-AAG CCT CGC CAA AAA CGT AC-3′), reverse primer Ar (Taq-1000R 5′-AAG TCA GCC ATG TTC CCG AA-3′), and probe (Taq-955T 5′-FAM-TCA CGC ATT GGC ATG GAA GTC ACA CT-TAMRA) (TIB Molbiol, Berlin, Germany), using a fluorometric PCR instrument (ABI 7300; Applied Biosystems).
Viral titer and total virus detection
The virus replication was detected by titration of the inoculated virus in lung tissues collected from sacrificed mice according to a previously described protocol (14). Briefly, the lung tissues were homogenized to a final concentration of 10% (w/v) suspension in DMEM. Tissue homogenates were centrifuged, filtered, and inoculated into the monolayer of Vero E6 cells seeded on 96-well tissue-culture plates. The results were evaluated after 3 d of culture under phase-contrast microscopy, and viral titers using a CPE-based TCID50 test were calculated by the Reed-Muench method. Viral titers were expressed as log10TCID50/g of tissue, with a lowest detection limit of 1.5 log10TCID50/g. The total amount of virus was calculated by multiplying the weight of the lung tissues and the viral titers measured in 10% of tissue homogenates.
Results
The expressed RBD219-CHO protein was correctly folded and maintained intact conformation, and was recognized by a majority of conformational epitope-specific mAbs against RBD
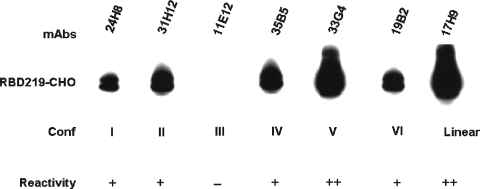
The expressed RBD protein was analyzed for conformation by using mAbs targeting the conformational epitopes in RBD. As shown in Fig. 2, the expressed protein reacted with 5 of 6 selected mAbs recognizing conformational epitopes, having the strongest reaction with the conformational V mAb 33G4. It also reacted with mAb 17H9, that recognizes the linear epitope in RBD. These results demonstrate that the expressed RBD219-CHO protein is correctly folded and maintains intact conformation.
FIG. 2.
Western blot for detecting reactivity of RBD219-CHO protein with a panel of mAbs specific for distinct conformational epitopes in RBD of SARS-CoV S protein. One mAb recognizing a linear epitope in RBD was also selected for detection.
RBD219-CHO induced high titers of RBD-specific antibodies in vaccinated mice
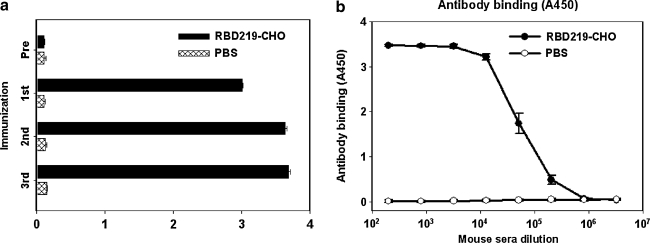
In order to detect humoral immune responses induced by RBD219-CHO protein, sera were collected from vaccinated mice and analyzed with ELISA for RBD-specific antibodies. As shown in Fig. 3A, all sera from 10 d after the first, second, and third vaccinations showed strong antibody responses, with those from the last vaccination demonstrating the highest reactivity. The sera from pre-immunization (Pre), and those from PBS controls had only background levels of antibody responses. To determine the titer of RBD-specific antibodies induced by RBD219-CHO protein, sera collected 10 d after the last vaccination were titrated at a series of fourfold dilutions and A450 was measured. Fig. 3B indicates that RBD219-CHO elicited high levels of RBD-specific antibodies, with the end-point titer of 1:3.3 × 106. In contrast, only background levels of antibody were detected in the PBS control group (Fig. 3B).
FIG. 3.
Detection of RBD-specific antibodies in sera of mice immunized with RBD219-CHO protein and PBS controls. The data are presented as mean A450 ± standard error (SE) from five mice per group. (a) IgG antibody detected in sera (at 1:3000 dilution) collected before immunization (Pre) and 10 d after each vaccination are shown here. (b) RBD-specific antibody titer detected in sera collected 10 d after the last vaccination are shown here.
RBD219-CHO induced highly potent neutralizing antibodies against in-vitro infection by SARS pseudovirus and live SARS-CoV
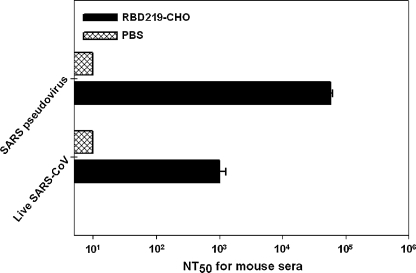
Sera collected 10 d after the last vaccination were tested for neutralizing activity against infection by SARS pseudovirus in ACE2/293T cells, and live SARS-CoV in Vero E6 cells. As shown in Fig. 4, 1:5.8 × 104 ± 4.9 × 103 of NT50 were reached in RBD219-CHO-vaccinated mouse sera to neutralize SARS pseudovirus infection, levels higher than those of PBS controls. High titers of RBD-specific IgG antibodies were also confirmed to neutralize live SARS-CoV in infected Vero E6 cells, with 1:1.0 × 103 ± 2.4 × 102 NT50 of neutralizing antibodies maintained in the vaccinated mice (Fig. 4). These results suggest that highly potent neutralizing antibodies may be induced by RBD219-CHO protein against both SARS pseudovirus and live SARS-CoV infection in cell cultures in vitro.
FIG. 4.
Detection of neutralizing antibodies in sera of mice vaccinated with RBD219-CHO or PBS controls. Sera collected 10 d after the last immunization were tested for neutralizing activity against infection by SARS pseudovirus in ACE2/293T cells, and live SARS-CoV in Vero E6 cells. The data are presented as mean NT50 ± SE of five mice per group.
RBD219-CHO was able to induce a relatively high level of CTL and Th1-/Th-2 responses in vaccinated mice
For detection of the cellular immune responses potentially induced by RBD219-CHO protein, mouse splenocytes were collected 10 d after the last vaccination, and T-cell responses were detected by ELISPOT. Splenocytes were stimulated with N50 and N60 peptides specific to CD8+ T (CTL) cells and CD4+ T (Th) cells, respectively, in the RBD of SARS-CoV (30). For further detection of the cellular immune response, purified RBD protein was also used for stimulation with a final concentration of 1 μg/mL. As shown in Table 1, RBD219-CHO induced RBD-specific CTL responses, with a particularly high frequency of IL-2-producing CD8+ (CTL) cells under the stimulation of the CTL peptide N50. Evaluation of Th-cell responses upon stimulation with the N60 peptide indicated that RBD219-CHO protein may elicit relatively higher levels of Th-1 response, as represented by the production of high numbers of IL-2-presenting T cells, in addition to the induction of Th-2 response, which was indicated by the presence of IL-4-secreting T cells in the spleens of vaccinated mice. The results in Table 1 also show that the cellular immune responses induced by RBD219-CHO protein stimulation were generally higher than those elicited by the CTL peptide N50 or Th peptide N60 stimulation alone, probably because the recombinant protein covering the RBD region contains much more CTL and/or Th epitopes. In contrast, only a background level of cellular response was detected in the splenocytes of mice from the PBS controls (Table 1), or the cells stimulated with the supernatants of CHO culture only (data not shown). These data suggest that the expressed RBD219-CHO protein was able to induce a relatively high level of cellular immune response in the vaccinated mice.
Table 1.
ELISPOT Detection of Cellular Immune Responses in RBD219-CHO-Vaccinated Micea
| |
RBD219-CHO (SFC/106 cells) |
PBS (SFC/106 cells) |
||||
|---|---|---|---|---|---|---|
| Stimulus | IFN-γ | IL-2 | IL-4 | IFN-γ | IL-2 | IL-4 |
| CTL peptide N50 | 63 ± 21 | 298 ± 100 | 163 ± 89 | 7 ± 3 | 4 ± 1 | 4 ± 0 |
| Th peptide N60 | 143 ± 66 | 645 ± 232 | 905 ± 196 | 8 ± 1 | 3 ± 0 | 3 ± 0 |
| RBD219-CHO protein | 465 ± 66 | 1044 ± 278 | 833 ± 273 | 5 ± 0 | 5 ± 1 | 5 ± 1 |
Cytokine-producing cells were detected by ELISPOT under the stimulation of N50 and N60 peptides containing SARS-CoV RBD-specific CD8+ T-cell and CD4+ T-cell epitopes, respectively, and RBD219-CHO protein. PBS was used as the control. The data are expressed as mean ± SE of cytokine spot-forming cells (SFC)/106 cells of five mice per group.
Complete suppression of SARS-CoV replication in lung tissues of virus-challenged mice, possibly mediated by RBD219-CHO-induced neutralizing antibody responses
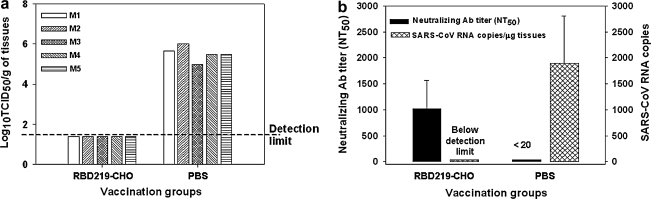
To detect the protective immunity induced by RBD219-CHO protein, vaccinated mice were challenged with SARS-CoV GZ50, and mouse lung tissues were collected 5 d post-virus challenge to measure virus replication. The mice did not develop obvious weight loss or clinical signs of disease in both the control and vaccinated groups. No detectable replication was seen in all five mice vaccinated with RBD219-CHO protein. In contrast, high levels of virus replication were found in the lung tissues of PBS control mice (Fig. 5A). The above results confirm that RBD219-CHO protein was able to completely protect vaccinated mice from subsequent SARS-CoV challenge.
FIG. 5.
Detection of protective immunity in mice immunized with RBD219-CHO protein or PBS controls. Mice vaccinated with RBD219-CHO protein were challenged with live SARS-CoV (GZ50). (a) SARS-CoV replication in the lung tissues of challenged mice was detected and expressed as log10TCID50/g of tissue. The detection limit was 1.5 log10TCID50/g of lung tissue. M1–M5 indicate the five mice used per group. (b) The number of SARS-CoV RNA copies in the lung tissues of mice challenged with live SARS-CoV GZ50 were measured by qRT-PCR, and expressed as RNA copies/μg of lung tissue. The neutralizing antibody titers (NT50) in the sera of the mice are also shown. The data are presented as mean ± SE of five mice per group. The experiment was performed three times and similar results were obtained.
To investigate the relationship between neutralizing antibody and virus protection, we compared the serum neutralizing antibody titer and the viral RNA copies in the lung tissues after the mice were challenged with live SARS-CoV (GZ50). As shown in Fig. 5B, no detectable virus replication was accompanied by high titers of neutralizing antibodies (1:1.0 × 103 ± 2.4 × 102) in the RBD219-CHO-vaccinated mice, while high levels of virus replication (1:1.9 × 103 ± 4.1 × 102 RNA copies/μg of lung tissue) and undetectable levels of serum neutralizing antibodies (<1:20) were found in the PBS control mice. These results suggest that neutralizing antibody responses may play a critical role in the inhibition of SARS-CoV replication.
Discussion
Vaccines based on the recombinant S protein of SARS-CoV have been shown to induce humoral immune responses, especially neutralizing antibodies against SARS-CoV (23,33,35,60). High titers of cross-neutralizing antibodies against pseudovirus in vitro were detected in rRBD-vaccinated mice and rabbits (24). Protective humoral responses were detected in protein-vaccinated rabbits, with antisera against S containing neutralizing antibodies for SARS-CoV infection in vivo (43). These reports demonstrated that S protein-induced neu-tralizing antibodies play important roles in fighting SARS-CoV infection.
We previously reported that a recombinant protein containing a 193-aa RBD with Fc tag (RBD193-Fc) induced highly potent neutralizing antibody responses and protective immunity in vaccinated animals (14). In order to eliminate any potential adverse effects that might be induced by RBD193-Fc when used as a vaccine component in humans, we have expressed several RBD proteins with the Fc tag removed in mammalian cells 293T (RBD-293T) and CHO-K1 (RBD193-CHO), insect cells Sf9 (RBD-Sf9),. and E. coli (RBD-Ec). Unlike RBD proteins expressed in 293T, Sf9, and E. coli, that may induce complete protection against live SARS-CoV challenge in vaccinated mice, the 193-aa RBD protein expressed in CHO-K1 cells (RBD193-CHO) protected a majority of the mice from virus challenge, with virus replication detected in two of five mice (13,15). When we expressed a 219-mer RBD protein in the same cell line of CHO-K1 (RBD219-CHO), the newly-designed protein elicited high titers of RBD-specific antibodies with potent neutralizing activity against both SARS pseudovirus and live SARS-CoV infection in cell cultures in vitro. Like rRBD proteins expressed in 293T, Sf9, and E. coli cells, RBD219-CHO was able to protect all vaccinated mice against SARS-CoV challenge in vivo (Fig. 5). Similarly to other rRBD proteins expressed above, this mammalian cell CHO-K1-expressed RBD protein without the Fc fusion tag reacted with the majority of the mAbs specific for the conformational and linear epitopes in RBD, suggesting that it maintains intact conformation and proper antigenicity (Fig. 2). Similarly to RBD193-CHO protein, a stable cell line could be established in the CHO-K1 cell lines for long-term expression of the RBD219-CHO protein (data not shown). The RBD219-CHO protein (residues 318–536) was expressed with an extension of 26 residues at the C terminus of the RBD193-CHO protein (residues 318–510). This extended fragment may assist RBD to refold into a more stable or more immunogenic conformation to induce more potent protective immunity in the vaccinated animals. Future comparison of the structure and immune responses and long-term protection of these two proteins is warranted.
In addition to humoral immune responses, cellular immune responses may play a role in the clearance of SARS-CoV infection, in which both CD4+ and CD8+ T cells are involved in the immune response against virus infection. Memory T-cell responses against S protein were shown to continue for more than 1 y after SARS-CoV infection, as indicated by the production of IFN-γ (54). An increased specific CTL response was induced by an S2 fragment encoding residues 681–980 (22). Immunization of mice with a DNA fragment encoding the S protein was demonstrated to induce both CD4+ and CD8+ T-cell responses (31). In a Phase I human study, an S protein-expressing DNA vaccine elicited SARS-CoV-specific CD4+ T-cell responses in all tested healthy adults, and CD8+ T-cell responses in 20% of individuals (42). Both CTL and Th-1/Th-2 responses were detected in this study in RBD219-CHO-vaccinated mouse splenocytes, more evidence of the high immunogenicity and antigenicity of the expressed RBD protein when used as a vaccine candidate (Table 1).
Although both humoral and cellular immune responses are important for suppression of SARS-CoV infection, neutralizing antibodies may be the key factor for protecting mice from subsequent virus challenge (13,14,50). Long-term protec-tion mediated by neutralizing antibodies could be achieved by immunization of mice with a single dose of an attenuated vesicular stomatitis virus (VSV) recombinant bearing S protein (VSV-S) (34). A recombinant secreted protein containing 14–762 residues of the S protein could provide sufficient neutralizing antibodies to protect against SARS-CoV infection (2). The protective immunity seen in the mice vaccinated with an S protein-expressing DNA vaccine was mediated by humoral immunity rather than by a T-cell-dependent immune mechanism (55). In agreement with the above reports, our results show that neutralizing antibodies induced by RBD219-CHO protein play a significant role in the suppression of SARS-CoV infection (Fig. 5).
We and others have demonstrated that RBD of SARS-CoV S protein is the basis for the development of vaccines against SARS-CoV (6,11,16,17,26,32). High titers of neutralizing antibodies and protective immunity could be induced by subunit vaccines based on the RBD protein with or without fusion segments (14,56). However, RBD-based vaccines without the fusion tag would eliminate any potential adverse effects induced by fusion proteins when used in humans (13). In agreement with our previous reports, the results of this study further confirm that the recombinant RBD protein without the Fc fusion fragment is able to induce potent neutralizing antibody responses and protection against infection by SARS-CoV, suggesting the possibility of the development of RBD subunit-based vaccines for SARS prevention.
Acknowledgments
This study was supported by the National Institutes of Health (NIH) of the United States (RO1 AI68002), by the Research Fund for the Control of Infectious Diseases, the Health, Welfare and Food Bureau of the Hong Kong SAR Government, and by the National 973 Basic Research Program of China (2005CB523001).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bai B. Lu X. Meng J, et al. Vaccination of mice with recombinant baculovirus expressing spike or nucleocapsid protein of SARS-like coronavirus generates humoral and cellular immune responses. Mol Immunol. 2008;45:868–875. doi: 10.1016/j.molimm.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisht H. Roberts A. Vogel L. Subbarao K. Moss B. Neutralizing antibody and protective immunity to SARS coronavirus infection of mice induced by a soluble recombinant polypeptide containing an N-terminal segment of the spike glycoprotein. Virology. 2005;334:160–165. doi: 10.1016/j.virol.2005.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchholz UJ. Bukreyev A. Yang L. Lamirande EW. Murphy BR. Subbarao K. Collins PL. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci USA. 2004;101:9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukreyev A. Lamirande EW. Buchholz UJ, et al. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363:2122–2127. doi: 10.1016/S0140-6736(04)16501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H. Xu X. Jones IM. Immunogenicity of the outer domain of a HIV-1 clade C gp120. Retrovirology. 2007;4:33. doi: 10.1186/1742-4690-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J. Miao L. Li JM. Li YY. Zhu QY. Zhou CL. Fang HQ. Chen HP. Receptor-binding domain of SARS-CoV spike protein: soluble expression in E. coli, purification and functional characterization. World J Gastroenterol. 2005;11:6159–6164. doi: 10.3748/wjg.v11.i39.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z. Zhang L. Qin C, et al. Recombinant modified vaccinia virus Ankara expressing the spike glycoprotein of severe acute respiratory syndrome coronavirus induces protective neutralizing antibodies primarily targeting the receptor binding region. J Virol. 2005;79:2678–2688. doi: 10.1128/JVI.79.5.2678-2688.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 9.Czub M. Weingartl H. Czub S. He R. Cao J. Evaluation of modified vaccinia virus Ankara based recombinant SARS vaccine in ferrets. Vaccine. 2005;23:2273–2279. doi: 10.1016/j.vaccine.2005.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du L. He Y. Wang Y, et al. Recombinant adeno-associated virus expressing the receptor-binding domain of severe acute respiratory syndrome coronavirus S protein elicits neutralizing antibodies: Implication for developing SARS vaccines. Virology. 2006;353:6–16. doi: 10.1016/j.virol.2006.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du L. He Y. Zhou Y. Liu S. Zheng BJ. Jiang S. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du L. Kao RY. Zhou Y, et al. Cleavage of spike protein of SARS coronavirus by protease factor Xa is associated with viral infectivity. Biochem Biophys Res Commun. 2007;359:174–179. doi: 10.1016/j.bbrc.2007.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du L. Zhao G. Chan CC, et al. Recombinant receptor-binding domain of SARS-CoV spike protein expressed in mammalian, insect and E. coli cells elicits potent neutralizing antibody and protective immunity. Virology. 2009;393:144–150. doi: 10.1016/j.virol.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du L. Zhao G. He Y. Guo Y. Zheng BJ. Jiang S. Zhou Y. Receptor-binding domain of SARS-CoV spike protein induces long-term protective immunity in an animal model. Vaccine. 2007;25:2832–2838. doi: 10.1016/j.vaccine.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du L. Zhao G. Li L. He Y. Zhou Y. Zheng BJ. Jiang S. Antigenicity and immunogenicity of SARS-CoV S protein receptor-binding domain stably expressed in CHO cells. Biochem Biophys Res Commun. 2009;384:486–490. doi: 10.1016/j.bbrc.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du L. Zhao G. Lin Y, et al. Priming with rAAV encoding RBD of SARS-CoV S protein and boosting with RBD-specific peptides for T cell epitopes elevated humoral and cellular immune responses against SARS-CoV infection. Vaccine. 2008;26:1644–1651. doi: 10.1016/j.vaccine.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du L. Zhao G. Lin Y, et al. Intranasal vaccination of recombinant adeno-associated virus encoding receptor-binding domain of severe acute respiratory syndrome coronavirus (SARS-CoV) spike protein induces strong mucosal immune responses and provides long-term protection against SARS-CoV infection. J Immunol. 2008;180:948–956. doi: 10.4049/jimmunol.180.2.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enjuanes L. Dediego ML. Alvarez E. Deming D. Sheahan T. Baric R. Vaccines to prevent severe acute respiratory syndrome coronavirus-induced disease. Virus Res. 2008;133:45–62. doi: 10.1016/j.virusres.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faber M. Lamirande EW. Roberts A. Rice AB. Koprowski H. Dietzschold B. Schnell MJ. A single immunization with a rhabdovirus-based vector expressing severe acute respiratory syndrome coronavirus (SARS-CoV) S protein results in the production of high levels of SARS-CoV-neutralizing antibodies. J Gen Virol. 2005;86:1435–1440. doi: 10.1099/vir.0.80844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao W. Tamin A. Soloff A, et al. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362:1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan Y. Zheng BJ. He YQ, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y. Sun S. Wang K. Zhang S. Zhu W. Chen Z. Elicitation of immunity in mice after immunization with the S2 subunit of the severe acute respiratory syndrome coronavirus. DNA Cell Biol. 2005;24:510–515. doi: 10.1089/dna.2005.24.510. [DOI] [PubMed] [Google Scholar]
- 23.He Y. Li J. Heck S. Lustigman S. Jiang S. Antigenic and immunogenic characterization of recombinant baculovirus-expressed severe acute respiratory syndrome coronavirus spike protein: implication for vaccine design. J Virol. 2006;80:5757–5767. doi: 10.1128/JVI.00083-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Y. Li J. Li W. Lustigman S. Farzan M. Jiang S. Cross-neutralization of human and palm civet severe acute respiratory syndrome coronaviruses by antibodies targeting the receptor-binding domain of spike protein. J Immunol. 2006;176:6085–6092. doi: 10.4049/jimmunol.176.10.6085. [DOI] [PubMed] [Google Scholar]
- 25.He Y. Lu H. Siddiqui P. Zhou Y. Jiang S. Receptor-binding domain of severe acute respiratory syndrome coronavirus spike protein contains multiple conformation-dependent epitopes that induce highly potent neutralizing antibodies. J Immunol. 2005;174:4908–4915. doi: 10.4049/jimmunol.174.8.4908. [DOI] [PubMed] [Google Scholar]
- 26.He Y. Zhou Y. Liu S. Kou Z. Li W. Farzan M. Jiang S. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun. 2004;324:773–781. doi: 10.1016/j.bbrc.2004.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes KV. SARS-associated coronavirus. N Engl J Med. 2003;348:1948–1951. doi: 10.1056/NEJMp030078. [DOI] [PubMed] [Google Scholar]
- 28.Hu H. Lu X. Tao L, et al. Induction of specific immune responses by severe acute respiratory syndrome coronavirus spike DNA vaccine with or without interleukin-2 immunization using different vaccination routes in mice. Clin Vaccine Immunol. 2007;14:894–901. doi: 10.1128/CVI.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu MC. Jones T. Kenney RT. Barnard DL. Burt DS. Lowell GH. Intranasal Protollin-formulated recombinant SARS S-protein elicits respiratory and serum neutralizing antibodies and protection in mice. Vaccine. 2007;25:6334–6340. doi: 10.1016/j.vaccine.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J. Cao Y. Du J. Bu X. Ma R. Wu C. Priming with SARS CoV S DNA and boosting with SARS CoV S epitopes specific for CD4+ and CD8+ T cells promote cellular immune responses. Vaccine. 2007;25:6981–6891. doi: 10.1016/j.vaccine.2007.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J. Ma R. Wu CY. Immunization with SARS-CoV S DNA vaccine generates memory CD4+ and CD8+ T cell immune responses. Vaccine. 2006;24:4905–4913. doi: 10.1016/j.vaccine.2006.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang S. He Y. Liu S. SARS vaccine development. Emerg Infect Dis. 2005;11:1016–1020. doi: 10.3201/eid1107.050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kam YW. Kien F. Roberts A, et al. Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcgammaRII-dependent entry into B cells in vitro. Vaccine. 2007;25:729–740. doi: 10.1016/j.vaccine.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapadia SU. Rose JK. Lamirande E. Vogel L. Subbarao K. Roberts A. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. 2005;340:174–182. doi: 10.1016/j.virol.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keng CT. Zhang A. Shen S, et al. Amino acids 1055 to 1192 in the S2 region of severe acute respiratory syndrome coronavirus S protein induce neutralizing antibodies: implications for the development of vaccines and antiviral agents. J Virol. 2005;79:3289–3296. doi: 10.1128/JVI.79.6.3289-3296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobinger GP. Figueredo JM. Rowe T, et al. Adenovirus-based vaccine prevents pneumonia in ferrets challenged with the SARS coronavirus and stimulates robust immune responses in macaques. Vaccine. 2007;25:5220–5231. doi: 10.1016/j.vaccine.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau SK. Woo PC. Li KS, et al. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li W. Shi Z. Yu M, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 39.Liang G. Chen Q. Xu J, et al. Laboratory diagnosis of four recent sporadic cases of community-acquired SARS, Guangdong Province, China. Emerg Infect Dis. 2004;10:1774–1781. doi: 10.3201/eid1010.040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lokugamage KG. Yoshikawa-Iwata N. Ito N, et al. Chimeric coronavirus-like particles carrying severe acute respiratory syndrome coronavirus (SCoV) S protein protect mice against challenge with SCoV. Vaccine. 2008;26:797–808. doi: 10.1016/j.vaccine.2007.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu X. Chen Y. Bai B, et al. Immune responses against severe acute respiratory syndrome coronavirus induced by virus-like particles in mice. Immunology. 2007;122:496–502. doi: 10.1111/j.1365-2567.2007.02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin JE. Louder MK. Holman LA, et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26:6338–6343. doi: 10.1016/j.vaccine.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pang H. Liu Y. Han X, et al. Protective humoral responses to severe acute respiratory syndrome-associated coronavirus: implications for the design of an effective protein-based vaccine. J Gen Virol. 2004;85:3109–3113. doi: 10.1099/vir.0.80111-0. [DOI] [PubMed] [Google Scholar]
- 44.Peiris JS. Chu CM. Cheng VC, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peiris JS. Guan Y. Confronting SARS: a view from Hong Kong. Philos Trans R Soc Lond B Biol Sci. 2004;359:1075–1079. doi: 10.1098/rstb.2004.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peiris JS. Guan Y. Yuen KY. Severe acute respiratory syndrome. Nat Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regnault A. Lankar D. Lacabanne V, et al. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts A. Thomas WD. Guarner J, et al. Therapy with a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody reduces disease severity and viral burden in golden Syrian hamsters. J Infect Dis. 2006;193:685–692. doi: 10.1086/500143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.See RH. Petric M. Lawrence DJ, et al. Severe acute respiratory syndrome vaccine efficacy in ferrets: whole killed virus and adenovirus-vectored vaccines. J Gen Virol. 2008;89:2136–2146. doi: 10.1099/vir.0.2008/001891-0. [DOI] [PubMed] [Google Scholar]
- 50.Subbarao K. McAuliffe J. Vogel L, et al. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J Virol. 2004;78:3572–3577. doi: 10.1128/JVI.78.7.3572-3577.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang L. Zhu Q. Qin E, et al. Inactivated SARS-CoV vaccine prepared from whole virus induces a high level of neutralizing antibodies in BALB/c mice. DNA Cell Biol. 2004;23:391–394. doi: 10.1089/104454904323145272. [DOI] [PubMed] [Google Scholar]
- 52.Wenzel RP. Bearman G. Edmond MB. Lessons from severe acute respiratory syndrome (SARS): implications for infection control. Arch Med Res. 2005;36:610–616. doi: 10.1016/j.arcmed.2005.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woo PC. Lau SK. Tsoi HW, et al. SARS coronavirus spike polypeptide DNA vaccine priming with recombinant spike polypeptide from Escherichia coli as booster induces high titer of neutralizing antibody against SARS coronavirus. Vaccine. 2005;23:4959–4968. doi: 10.1016/j.vaccine.2005.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang LT. Peng H. Zhu ZL, et al. Long-lived effector/central memory T-cell responses to severe acute respiratory syndrome coronavirus (SARS-CoV) S antigen in recovered SARS patients. Clin Immunol. 2006;120:171–178. doi: 10.1016/j.clim.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang ZY. Kong WP. Huang Y. Roberts A. Murphy BR. Subbarao K. Nabel GJ. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zakhartchouk AN. Sharon C. Satkunarajah M, et al. Immunogenicity of a receptor-binding domain of SARS coronavirus spike protein in mice: implications for a subunit vaccine. Vaccine. 2007;25:136–143. doi: 10.1016/j.vaccine.2006.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang MY. Wang Y. Mankowski MK. Ptak RG. Dimitrov DS. Cross-reactive HIV-1-neutralizing activity of serum IgG from a rabbit immunized with gp41 fused to IgG1 Fc: possible role of the prolonged half-life of the immunogen. Vaccine. 2009;27:857–863. doi: 10.1016/j.vaccine.2008.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhong NS. Zheng BJ. Li YM, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou J. Wang W. Zhong Q, et al. Immunogenicity, safety, and protective efficacy of an inactivated SARS-associated coronavirus vaccine in rhesus monkeys. Vaccine. 2005;23:3202–3209. doi: 10.1016/j.vaccine.2004.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Z. Post P. Chubet R. Holtz K. McPherson C. Petric M. Cox M. A recombinant baculovirus-expressed S glycoprotein vaccine elicits high titers of SARS-associated coronavirus (SARS-CoV) neutralizing antibodies in mice. Vaccine. 2006;24:3624–3631. doi: 10.1016/j.vaccine.2006.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]