Abstract
A largely unanswered question in the study of arboviruses is the extent to which virus can overwinter in adult vectors during the cold winter months and resume the transmission cycle in summer. Buggy Creek virus (BCRV; Togaviridae, Alphavirus) is an unusual arbovirus that is vectored primarily by the swallow bug (Hemiptera: Cimicidae: Oeciacus vicarius) and amplified by the ectoparasitic bug's main avian hosts, the migratory cliff swallow (Petrochelidon pyrrhonota) and resident house sparrow (Passer domesticus). Bugs are sedentary and overwinter in the swallows' mud nests. We evaluated the prevalence of BCRV and extent of infection in swallow bugs collected at different times in winter (October–early April) in Nebraska and explored other ecological aspects of this virus's overwintering. BCRV was detected in 17% of bug pools sampled in winter. Virus prevalence in bugs in winter at a site was significantly correlated with virus prevalence at that site the previous summer, but winter prevalence did not predict BCRV prevalence there the following summer. Prevalence was higher in bugs taken from house sparrow nests in winter and (in April) at colony sites where sparrows had been present all winter. Virus detected by reverse transcription (RT)-polymerase chain reaction in winter was less cytopathic than in summer, but viral RNA concentrations of samples in winter were not significantly different from those in summer. Both of the BCRV lineages (A, B) overwintered successfully, with lineage A more common at sites with house sparrows and (in contrast to summer) generally more prevalent in winter than lineage B. BCRV's ability to overwinter in its adult vector probably reflects its adaptation to the sedentary, long-lived bug and the ecology of the cliff swallow and swallow bug host–parasite system. Its overwintering mechanisms may provide insight into those of other alphaviruses of public health significance for which such mechanisms are poorly known.
Key Words: Arbovirus, Buggy Creek virus, Oeciacus vicarius, Overwintering, Petrochelidon pyrrhonota, Virus ecology
Introduction
Transmission cycles of arthropod-borne viruses (“arboviruses”) in temperate latitudes are interrupted by cold weather during the winter months when the insect vectors die off or become dormant. Some North American arboviruses are vertically transmitted (Watts et al. 1973, Turell et al. 1982, Tesh 1984, Turell 1988, Goddard et al. 2003, Phillips and Christensen 2006), and overwintering of infected eggs may provide a mechanism for virus persistence at a given locale from year to year. Less common, however, is overwintering of arboviruses in adult insects (Reeves et al. 1958, Bellamy and Reeves 1963, Reeves 1974, 1990, Reisen et al. 1986, 2006a, Rosen 1987, Reisen 1990). Only a few virus isolations from adult mosquitoes in the winter have been reported in central and northern North America (Blackmore and Winn 1956, Bailey et al. 1978, Nasci et al. 2001, Farajollahi et al. 2005), and it is unclear whether overwintering in adult vectors is an important component of the transmission cycles for most of the well-studied arboviruses (Mitchell 1988). The difficulty in finding large numbers of adult vectors, particularly mosquitoes (e.g., Mitchell 1979), in the cold winter months has generally precluded detailed investigations into the frequency or success of virus overwintering, what ecological conditions promote virus survival in winter, and the extent to which winter might serve as a selective bottleneck for different arbovirus strains.
Buggy Creek virus (BCRV; Togaviridae, Alphavirus) is an unusual arbovirus within the western equine encephalomyelitis virus sero-complex of alphaviruses (Hayes et al. 1977, Calisher et al. 1980, 1988, Hopla et al. 1993, Pfeffer et al. 2006, Padhi et al. 2008). There are two BCRV lineages that are sympatric in portions of Nebraska and Colorado (Padhi et al. 2008). BCRV is vectored primarily by the hematophagous swallow bug (Hemiptera: Cimicidae: Oeciacus vicarius) and amplified by the ectoparasitic bug's main avian hosts, the cliff swallow (Petrochelidon pyrrhonota), and the introduced house sparrow (Passer domesticus; Hayes et al. 1977, Rush et al. 1980, 1981, Scott et al. 1984, Brown et al. 2001, 2007, 2008). The sedentary swallow bugs are wingless and remain in or near the cliff swallows' mud nests at the birds' nesting colonies throughout the year. Bugs can be easily collected in large numbers at predictable spatial foci (the colony sites) and typically have relatively high levels of BCRV infection (e.g., Brown et al. 2001, 2007, 2008, Moore et al. 2007); thus, these insects provide an unusual opportunity to study the winter ecology and lineage dynamics of BCRV.
Because BCRV vectors commonly survive the winter in the field and are known to maintain infectious BCRV in the laboratory for up to 310 days (Rush et al. 1980, Brown et al. 2009b), we hypothesized that this virus successfully overwinters in swallow bugs. Using a well-studied population of virus, vectors, and hosts in southwestern Nebraska, we investigated (i) the prevalence of BCRV in bug vectors during the winter months, (ii) ecological correlates associated with BCRV occurrence at sites in the winter, and (iii) potential differences in overwintering ecology between the two BCRV lineages. The lineages show a number of ecological differences in the summer season that may reflect a divergence in their transmission strategies (Brown et al. 2009), and in this paper we examine whether they also differ ecologically in winter. No other arbovirus in temperate latitudes of North America has been studied as systematically in winter, and the results offer insight into the potential ability of alphaviruses to overwinter in natural environments.
Materials and Methods
Study organisms
BCRV was first isolated in 1980 from swallow bugs collected at a cliff swallow colony along Buggy Creek in Grady County, west central Oklahoma (Hopla et al. 1993). Fort Morgan virus, found in Colorado and also associated with cliff swallows and swallow bugs (Hayes et al. 1977, Calisher et al. 1980, Scott et al. 1984), is a strain of BCRV (Pfeffer et al. 2006, Padhi et al. 2008). The two lineages of BCRV (lineages A and B) differ from each other by >6% at the nucleotide level (Pfeffer et al. 2006); A is the more southerly lineage and B the more northerly, and both occur at swallow nesting colonies in Nebraska (Padhi et al. 2008, Brown et al. 2009).
Cliff swallows are highly colonial passerines that breed throughout most of western North America (Brown and Brown 1995). They build gourd-shaped mud nests and attach them to the vertical faces of cliff walls, rock outcrops, or artificial sites such as the eaves of buildings, bridges, and highway culverts. Cliff swallows are migratory, wintering in southern South America, and have a relatively short breeding season in North America from late April to late July. Most pairs raise only one brood.
House sparrows were introduced into North America from Europe in the 1800s and are found in all parts of the United States (Lowther and Cink 1992). Sparrows usurp cliff swallow nests and will occupy them until the nests fall from the substrate. Numbers of sparrows vary among colony sites, with some sites having none and others having only sparrows. House sparrows are nonmigratory and resident around the swallow colonies throughout the year. Many individuals raise up to three broods per summer at our study site.
The swallow bug is a hematophagous ectoparasite primarily of cliff swallows. Swallow bugs are nest-based parasites that overwinter in their hosts' nests or in the cracks and crevices of the nesting substrate near the nests. Infestations can reach 2600 bugs per nest. Swallow bugs are relatively long-lived and begin to reproduce as soon as they feed in the spring. Cliff swallows do not use all of the colony sites in a given year (Brown and Brown 1996), and the bugs can survive long periods of host absence (Smith and Eads 1978, Hopla et al. 1993, Rannala 1995). The bugs also parasitize house sparrows that nest in cliff swallow colonies (V. O'Brien and C. Brown, unpublished data). Swallow bugs disperse between nests within a colony by crawling on the substrate and disperse between colony sites by clinging to the feet and legs of cliff swallows that move from one site to another (Brown and Brown 2004, 2005).
Study site
Our study site is centered at the Cedar Point Biological Station (41°3′ N, 101°39′ W) near Ogallala, in Keith County, along the North and South Platte Rivers, southwestern Nebraska, United States, and also includes portions of Lincoln, Deuel, Garden, and Morrill counties. Cliff swallows have been studied there since 1982. Approximately 170 cliff swallow colony sites are in the 200 × 60 km study area; about a third of these are not occupied by swallows in a given year. In our study area, cliff swallow colony size ranges from 2 to 6000 nests, with some birds nesting solitarily. Over a 25-year period, mean (±SE) colony size (n = 1812) was 393 (±15) nests. The number of house sparrow nests at colony sites has been less well monitored but typically varies between 1 and 20. Each colony site tends to be separated from the next nearest by 1–10 km but in a few cases by ≥20 km. The study area is described in detail by Brown and Brown (1996) and Brown et al. (2008). Cliff swallow colonies in this study were situated either on large highway bridges that spanned the North or South Platte rivers, or in box-shaped culverts underneath roads or railroads.
Field collections of bugs
Winter collections of swallow bugs for virus isolation took place on six occasions during the birds' nonbreeding season: 27–28 December 2004, 6–8 October 2005, 6–8 January 2006, 8–10 April 2006, 9–12 February 2007, and 11 April 2007. The April dates were just before the spring return of any cliff swallows; none was observed by us on those dates, and the earliest the species has ever been recorded in the study area is 13 April (Brown 1998).
We removed inactive swallow nests to expose bugs on the substrate behind the nests, brushing these bugs off the wall into a collecting jar. We harvested additional bugs by picking them out individually from the mud nest fragments. Bugs were sorted into pools of 100 individuals while alive and frozen immediately at −70°C. We sampled bugs from throughout a colony site and only at sites that had been occupied by cliff swallows the previous summer; colony sites unused by swallows in the past summer yielded few bugs in winter. For each colony site, we recorded if house sparrows were present (known by our seeing them or by the presence of sparrow nesting material in the swallow nests). In the 2005–2006 winter season, we also collected some nests that had been or were currently occupied by sparrows (for winter roosting), indicated by the large quantities of grass and feathers that house sparrows use for bedding. Bugs collected in winter did not show evidence of any recent blood-feeding (i.e., none had a dark blood spot visible in the abdomen).
Comparative analyses in this paper used data from bug pools collected in the summers (May–July) of 2004–2007. The summer data were collected and analyzed as described in Moore et al. (2007) and Brown et al. (2007, 2008, 2009). All data here come from bug pools that were tested for BCRV; no vertebrate hosts were screened for virus in winter.
Virus screening and isolation
Bug pools were macerated by mortar and pestle or with a Mixer Mill (MM 31) from Qiagen (Valencia, CA). Homogenates were clarified by centrifugation at 11,000 g for 1 min and subsequently stored at −70°C. For RNA extraction, a 100 μL aliquot of the supernatant of each homogenate was added to 400 μL of a guanidine thiocyanate–based lysis buffer. After the addition of 400 μL of 100% ethanol, RNA was extracted using the QIAmp Viral RNA Mini Kit (Qiagen) following the manufacturer's protocol. A negative control was placed between every five samples during extraction and maintained in the same position for reverse transcription (RT)-polymerase chain reaction (RT-PCR). A positive BCRV control (isolated from swallow bugs) was also included in each extraction.
RT-PCR was performed using the OneStep RT-PCR Kit (Qiagen) following the manufacturer's protocol. Primers and themocycler conditions are given in Moore et al. (2007). A portion (6.5 μL) of each amplification product was electrophoresed on a 4% Nusieve/agarose gel, together with a BCRV amplicon and a 100-bp cDNA ladder, to identify BCRV-positive pools.
Bug pools that were positive by RT-PCR were subjected to plaque assay on Vero cells. We added 100 μL of each supernatant in duplicate to a confluent monolayer of Vero cells in a six-well cell culture plate, incubated it for 1 h at 37°C in 5% CO2, overlaid each monolayer with 3 mL 0.5% agarose in yeast extract–lactalbumin overlay medium supplemented with 2240 mg/L sodium bicarbonate, 292 mg/L L-glutamine, and antibiotics, and returned the plate to the incubator. A second 3 mL overlay, prepared as before but supplemented with 0.004% neutral red dye, was added after 2 days' incubation for plaque observation. Plaques were scored on the third and fourth days of incubation. For RT-PCR-positive samples that showed no plaques on Vero cells, we reextracted RNA from the remaining swallow bug homogenate and performed another RT-PCR assay to verify the presence of noncytopathic (noninfectious) viral RNA (Moore et al. 2007).
Determining viral RNA concentrations
To determine the relative viral RNA concentration of each isolate, we designed a one-step multiplex real-time RT-PCR assay (Gentle et al. 2001, Pfaffl 2001, Marino et al. 2003). Quantitative RT-PCR requires that the data be normalized to account for the variability involved in the reverse transcription of RNA and thus to ensure assay reproducibility (Bustin and Nolan 2004, Hugget et al. 2005). We used an externally added control RNA for this purpose (Gilsbach et al. 2006), as the pools of bugs had no inherent internal control suitable for quantitative RT-PCR. Samples were prepared for real-time RT-PCR by mixing 3 μL of each isolate with an equal volume of 20 ng/μL total human RNA (diluted from a 50 ng/μL stock of TaqMan® Control Total RNA, which was stored frozen in aliquots; Applied Biosystems, Foster City, CA). One-step quantitative RT-PCR was then performed using a QuantiTect Probe RT-PCR kit (Qiagen). The manufacturer's recommended protocol for custom assays was used except that a concentration of 0.2 μM was used for each of the four sequence-specific primers in the multiplex reaction. For each reaction, 2.5 μL of the combined RNA sample was used as a template in 25-μL reactions and cycled in a Cepheid SmartCycler® (Cepheid, Sunnyvale, CA). LUX™ Primers 418RU (5′-CGTGCAATGGTGGAAATTGATA-3′) and the FAM-labeled 406FL (5′-CAACAAGGTGCAGCGTAAGTTTG[FAM]TG-3′) were derived from BCRV E2 nucleotide positions 418–439 and 387–406, respectively. JOE-labeled Certified LUX Primers that target the human beta-2-microglobulin gene were obtained from Invitrogen (Carlsbad, CA). Melting curve analysis was performed on each sample at the end of cycling to verify the purity of amplicons.
To correct for variations in the efficiency of each RT reaction, the Ct value (cycle number at which the fluorescence exceeded a predefined threshold) obtained for the JOE-labeled beta-2-microglobulin amplicon was subtracted from the Ct value obtained for the FAM-labeled BCRV amplicon for each reaction, thus giving a normalized Ct (ΔCt) for each BCRV isolate measured (Gilsbach et al. 2006). As the ΔCt value increased for a sample, the RNA concentration of that sample relative to others decreased.
Determining virus lineages
RNA from each isolate was used in an alphavirus RT-PCR to amplify the entire 1269 bp of the E2 gene. See Brown et al. (2008) for details on how sequences were aligned. The lineage of each isolate was determined from maximum likelihood and Bayesian inference phylogenies constructed using PAUP* 4.0b10 (Swofford 2002) and MrBayes Version 3.1 (Huelsenbeck and Ronquist 2001). We used the E2 gene for determining lineages: this gene in alphaviruses codes for a glycoprotein that is responsible for cell receptor binding (Navaratnarajah and Kuhn 2007) and is the region of the genome most sensitive to selection brought about by the immune systems of different hosts (Strauss and Strauss 1994, Powers et al. 2001, Pfeffer et al. 2006). The E2 region also determines infection of invertebrate vectors (Brault et al. 2002). If there are functional differences among virus isolates or lineages that reflect varying levels of adaptation to cell receptors of different hosts or vectors, they may be expressed in the E2 gene. Not all samples determined to be positive for BCRV were assigned to lineage because some failed to amplify well enough to yield clean sequences. GenBank accession numbers of the sequences used in this paper are EU483798, EU483807, EU483814, EU483844, EU483856–EU483865, EU483879, EU483928, EU483935, EU483938, EU483939, EU483952, EU483955, EU483960, EU483963, EU483975–EU483978, EU483984, EU483989, EU483990, EU483992, EU483995, EU484002, EU484004, EU484009, EU484018, EU484033, EU708159–EU708171, and FJ754359–FJ754428.
Results
Seasonal BCRV prevalence
Across all colony sites, BCRV prevalence (by RT-PCR) was 17.2% (n = 239 pools) in October 2005, 16.0% (n = 169) in December 2004, 12.9% (n = 248) in January 2006, 16.3% (n = 356) in February 2007, 21.9% (n = 128) in April 2006, and 21.0% (n = 205) in April 2007. BCRV prevalence did not differ significantly between the three mid-winter sampling periods (December–February; 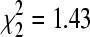 , p = 0.49) or when October was compared to the mid-winter periods collectively (
, p = 0.49) or when October was compared to the mid-winter periods collectively (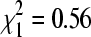 , p = 0.45). However, BCRV prevalence in the two April sampling periods was significantly greater than in the other sampling periods combined (
, p = 0.45). However, BCRV prevalence in the two April sampling periods was significantly greater than in the other sampling periods combined (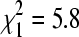 , p = 0.016). The overall BCRV prevalence across all sites in the three winter seasons (17.0%, n = 1345 pools) was significantly lower than that found for all sites sampled in the summers of 2004–2007 in the same study area (27.9%, n = 3769 pools;
, p = 0.016). The overall BCRV prevalence across all sites in the three winter seasons (17.0%, n = 1345 pools) was significantly lower than that found for all sites sampled in the summers of 2004–2007 in the same study area (27.9%, n = 3769 pools; 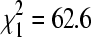 , p < 0.0001).
, p < 0.0001).
Temporal and ecological correlates of BCRV prevalence
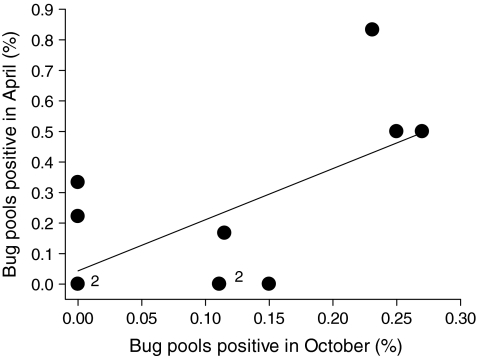
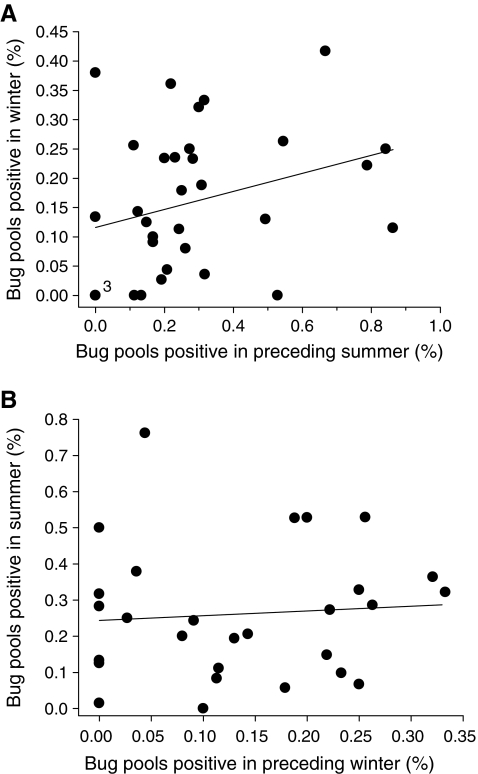
For colony sites sampled in October and again the following April, we found that BCRV prevalence in bug pools was significantly positively correlated between the two time periods (Fig. 1). Sites with higher prevalence at the start of the winter season maintained that higher prevalence until early spring. BCRV prevalence at a colony site in winter was significantly positively related to prevalence there the previous summer (Fig. 2A) but not significantly related to prevalence the following summer (Fig. 2B).
FIG. 1.
Percentage of swallow bug pools positive for BCRV by RT-PCR in early April (end of the winter) at a cliff swallow colony site in relation to the percentage positive there the previous October (start of the winter). Circles denoted with “2” represent two sites with the same values. Percentage positive in April was significantly correlated with percentage positive in October (r = 0.63, p = 0.039, n = 13 sites). BCRV, Buggy Creek virus; RT-PCR, reverse transcription polymerase chain reaction.
FIG. 2.
(A) Percentage of swallow bug pools positive for BCRV by RT-PCR in winter at a cliff swallow colony site in relation to the percentage positive there the previous summer. Circle denoted with “3” represents three sites with the same values. Percentage positive in winter was significantly correlated with percentage positive the previous summer (rs=0.35, p < 0.04, n = 33 sites). (B) Percentage of swallow bug pools positive for BCRV in summer at a cliff swallow colony site in relation to the percentage positive there the previous winter. Percentage positive in summer was not significantly correlated with percentage positive the previous winter (rs = 0.17, p = 0.40, n = 28 sites). For these analyses and that of Fig. 3, we used only mid-winter and April samples in calculating overall winter BCRV prevalence, because fall (October) sampling was done in only 1 year.
BCRV prevalence in winter bug pools did not vary significantly between colony sites on bridges (14.6% pools positive, n = 437) and culverts (18.2% positive, n = 908; 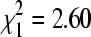 , p = 0.11), the two principal cliff swallow nesting substrates in the study area. BCRV prevalence in winter was significantly lower than in summer at both bridge sites (27.0% positive in summer, n = 1464;
, p = 0.11), the two principal cliff swallow nesting substrates in the study area. BCRV prevalence in winter was significantly lower than in summer at both bridge sites (27.0% positive in summer, n = 1464; 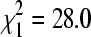 , p < 0.0001) and culvert sites (28.5% positive in summer, n = 2305;
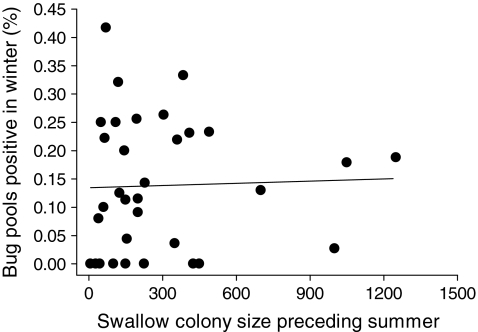
, p < 0.0001) and culvert sites (28.5% positive in summer, n = 2305; 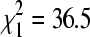 , p < 0.0001). For both substrates combined, there was no significant correlation between winter BCRV prevalence in bugs at a site and cliff swallow colony size (number of active nests) at that site the previous summer (Fig. 3).
, p < 0.0001). For both substrates combined, there was no significant correlation between winter BCRV prevalence in bugs at a site and cliff swallow colony size (number of active nests) at that site the previous summer (Fig. 3).
FIG. 3.
Percentage of swallow bug pools positive for BCRV by RT-PCR in winter at a cliff swallow colony site in relation to swallow colony size (number of active nests) the previous summer. Percentage positive in winter was not significantly correlated with colony size (r = 0.03, p = 0.85, n = 33 sites).
Bug pools taken directly from house sparrow nests in winter had significantly higher BCRV prevalence (37.5%, n = 24) than did those taken from cliff swallow nests during the same sampling periods (15.6%, n = 591; 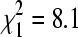 , p = 0.004). When we classified each colony site as having sparrows either present or not in winter, we found no overall difference in BCRV prevalence between sites with sparrows (19.0% pools positive, n = 594) and sites without sparrows (15.4%, n = 751;
, p = 0.004). When we classified each colony site as having sparrows either present or not in winter, we found no overall difference in BCRV prevalence between sites with sparrows (19.0% pools positive, n = 594) and sites without sparrows (15.4%, n = 751; 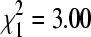 , p = 0.08) sampled simultaneously. However, if a single outlier site without sparrows sampled in December 2004 that had an unusually high BCRV prevalence (21.9%) was excluded, sites with sparrows had significantly higher overall virus prevalence than sites without (
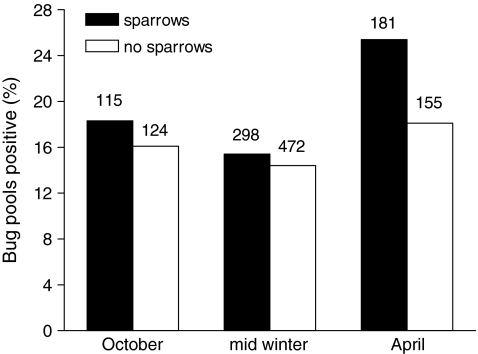
, p = 0.08) sampled simultaneously. However, if a single outlier site without sparrows sampled in December 2004 that had an unusually high BCRV prevalence (21.9%) was excluded, sites with sparrows had significantly higher overall virus prevalence than sites without (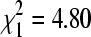 , p = 0.029). The effect of sparrows' presence varied across the winter season (Fig. 4). At sites with sparrows, BCRV prevalence in October did not differ from that in mid-winter, but prevalence in April at those sites increased significantly when compared to the other time periods (Fig. 4). In contrast, at sites without sparrows, there was no difference in BCRV prevalence between the three main sampling times (Fig. 4). For all April samples combined, BCRV prevalence was significantly lower than in summer (
, p = 0.029). The effect of sparrows' presence varied across the winter season (Fig. 4). At sites with sparrows, BCRV prevalence in October did not differ from that in mid-winter, but prevalence in April at those sites increased significantly when compared to the other time periods (Fig. 4). In contrast, at sites without sparrows, there was no difference in BCRV prevalence between the three main sampling times (Fig. 4). For all April samples combined, BCRV prevalence was significantly lower than in summer (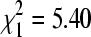 , p = 0.02), but when restricting the analysis to only sites with sparrows, BCRV prevalence in April did not differ significantly from that in summer (
, p = 0.02), but when restricting the analysis to only sites with sparrows, BCRV prevalence in April did not differ significantly from that in summer (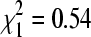 , p = 0.46).
, p = 0.46).
FIG. 4.
Percentage of swallow bug pools positive for BCRV by RT-PCR in the October, mid-winter (December–February), and early April sampling periods at colony sites with house sparrows (dark bars) and at sites without sparrows (clear bars). At sites with sparrows, percentage positive in the October and mid-winter periods did not differ significantly (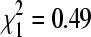 , p = 0.48), but early April differed significantly from the other two periods combined (
, p = 0.48), but early April differed significantly from the other two periods combined (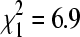 , p = 0.009). At sites without sparrows, percentage positive did not differ among the three sampling periods (
, p = 0.009). At sites without sparrows, percentage positive did not differ among the three sampling periods (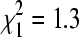 , p = 0.53). Numbers above bars indicate total numbers of bug pools tested.
, p = 0.53). Numbers above bars indicate total numbers of bug pools tested.
Winter cytopathicity
For all winter bug samples positive for BCRV by RT-PCR, 12.2% (n = 222) were cytopathic on Vero cells. This compares to 70.5% of RT-PCR positives in summer that were cytopathic (n = 1075). The highest concentration of virus found in a winter sample (collected in February) was 65 PFU/mL, in contrast to many summer samples that were >2000 PFU/mL. Across the winter season, 17.9% of positives were cytopathic in October (n = 28), 20.0% were cytopathic in mid-winter (n = 120; this percentage dropped to 7.0% when the anomalous site from December 2004 was excluded), and 1.4% were cytopathic in April (n = 71). The single cytopathic April bug pool was from a house sparrow nest.
Viral RNA concentrations
The mean (±SE) viral RNA concentration of BCRV in positive samples, as measured by ΔCt values, was 11.18 (±0.67) in October (n = 29), 10.77 (±0.50) in mid-winter (n = 98), and 13.41 (±0.47) in April (n = 59). The mean concentrations differed significantly among the three sampling periods (Wilcoxon test, p = 0.002), with April virus concentrations differing significantly from both the October (p = 0.009) and mid-winter samples (p < 0.001); the October and mid-winter samples were not significantly different (p = 0.86). The mean (±SE) for all winter samples collectively, 11.67 (±0.033), was not significantly different from that for all 2004–2006 positive summer samples, 11.04 (±0.18, n = 795 summer samples; Wilcoxon test, p = 0.15).
Distribution of lineages
Of 121 winter BCRV isolates that were sequenced and identified to lineage, 58.7% were lineage A, with the remainder lineage B. This differed significantly from the summer BCRV isolates, in which 42.3% were lineage A (n = 385; 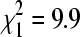 , p = 0.002). Some of this difference may have reflected disproportionate sampling for the lineages on bridges and culverts in winter versus summer. Lineage B was more common at bridge colony sites in summer, with only 9.8% of summer isolates on bridges belonging to lineage A (n = 143). Our winter samples that were sequenced came mostly from culverts (81%, n = 121), so we would expect more lineage A overall in the winter dataset. However, in comparing just bridges, we found 30.4% to be lineage A in winter (n = 23) versus 9.8% lineage A in summer, a significant difference (
, p = 0.002). Some of this difference may have reflected disproportionate sampling for the lineages on bridges and culverts in winter versus summer. Lineage B was more common at bridge colony sites in summer, with only 9.8% of summer isolates on bridges belonging to lineage A (n = 143). Our winter samples that were sequenced came mostly from culverts (81%, n = 121), so we would expect more lineage A overall in the winter dataset. However, in comparing just bridges, we found 30.4% to be lineage A in winter (n = 23) versus 9.8% lineage A in summer, a significant difference (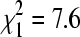 , p = 0.006). At culvert sites, lineage A represented 65.3% of isolates in winter (n = 98), compared to 61.5% in summer (n = 213), a nonsignificant difference (
, p = 0.006). At culvert sites, lineage A represented 65.3% of isolates in winter (n = 98), compared to 61.5% in summer (n = 213), a nonsignificant difference (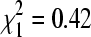 , p = 0.52).
, p = 0.52).
Lineage A was significantly more likely to be found in winter at colony sites where house sparrows were present (68.1% of 69 isolates were lineage A) than at sites without sparrows (36.5% of 52 isolates were lineage A; 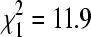 , p = 0.001). Of 8 isolates taken directly from bugs in house sparrow nests, 7 (87.5%) were lineage A, a significant preponderance of lineage A isolates (binomial test, p = 0.035).
, p = 0.001). Of 8 isolates taken directly from bugs in house sparrow nests, 7 (87.5%) were lineage A, a significant preponderance of lineage A isolates (binomial test, p = 0.035).
Across the winter season, lineage A represented 66.7% of isolates in October (n = 9), 58.7% of isolates in mid-winter (December–February, n = 75), and 56.8% of isolates in April (n = 37). The distributions of lineages A and B did not differ significantly across these three time periods (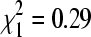 , p = 0.86).
, p = 0.86).
Combining all isolates (n = 506) across the summer and winter seasons, lineage A represented 46.2% of isolates and lineage B 53.8%. This did not differ significantly from a 50:50 ratio (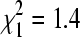 , p = 0.23).
, p = 0.23).
Discussion
These analyses show that BCRV successfully and routinely overwinters in swallow bugs in the central Great Plains of North America. The fact that we found this virus in unfed bugs in mid-April at sites without resident house sparrows and before cliff swallows had returned to nest demonstrates that the virus can persist at a given locale for an entire winter. Prevalence of the virus in bugs decreases during the winter months (October–February) relative to summer, but by April before the cliff swallows' arrival, prevalence starts to rise, and at sites with house sparrows, bugs are as likely to be infected then as during the summer months. Winter BCRV prevalence at a colony site seems to reflect the relative degree of bug infection there the preceding summer but does not predict relative prevalence the following summer at a site. We found evidence that both BCRV lineages overwinter successfully in bugs, but the lineage most common in summer (B) was not the one most commonly found in winter (A).
Seasonal changes in prevalence and infectivity
Our data suggest that most of the seasonal decline in BCRV prevalence in bugs was between the end of the summer season (i.e., July in our study area, when most cliff swallows depart) and October (when we took our first winter samples). The reason for this reduction in virus infection in the vectors is unclear but could mean that BCRV reduces survival of the bugs it infects, as documented in mosquitoes for other alphaviruses (Scott and Lorenz 1998, Mahmood et al. 2004).
Alternatively, BCRV could become harder to detect given the changes in the bugs' behavior and physiology in late summer and early fall (and with the onset of colder ambient temperatures in winter). By late July, many bugs in the Nebraska study area have stopped feeding and begun to aggregate in masses on the substrate behind the nests, where they remain all winter. They likely undergo physiological changes at that time as well, as they enter winter dormancy/diapause with associated hypometabolism. These changes in most insects involve loss of water from the tissues and hypertrophy of body fat (Chapman 1969, Mitchell 1988), and may be partly responsible for the greater difficulty in detecting viruses such as western equine encephalomyelitis virus, West Nile, and St. Louis encephalitis viruses in mosquitoes during winter or when held at relatively cool temperatures (Reeves et al. 1958, Bailey et al. 1978, Nasci et al. 2001, Dohm et al. 2002, Reisen et al. 2006b). Laboratory experiments would be useful in determining whether infection is costly to swallow bugs and the effects of winter temperature and/or bug winter physiology on the efficiency of detecting BCRV infection.
The significant decline in BCRV prevalence in bugs during the winter in Nebraska is in contrast to bugs collected in North Dakota (a colder winter environment) and in Oklahoma (a warmer winter environment); in both cases, BCRV prevalence did not differ between summer and winter seasons (Brown et al. 2009b, C.R. Brown et al. unpublished data). We cannot explain this geographic difference, but it might be related to the co-occurrence of both lineages in Nebraska, where different lineages might be favored in the different seasons (see below). Only one lineage has been detected at the other locations (Padhi et al. 2008). More work clearly is needed to understand the observed geographic variation in virus prevalence.
Does enough viable virus remain in bugs by early spring to infect returning birds and thus reinitiate local transmission? The facts that cytopathicity of BCRV in bugs declined strongly from summer to winter and that April samples had almost no cytopathic virus might suggest that RT-PCR positives in winter reflect only noninfectious viral RNA (e.g., White et al. 2005) that cannot infect other vectors or hosts at the beginning of the summer season. If so, this would imply that infectious BCRV present at a site in summer results mostly or exclusively from reintroduction by birds. We think this possibility is unlikely (Hayes et al. 1977, O'Brien et al. 2008) and that infectious virus is maintained by bugs through the winter season for the following reasons. (i) As we did with summer samples, we have obtained full 2076 bp sequences (Padhi et al. 2008) of winter isolates that were noncytopathic, not an expected result if we were detecting only viral RNA fragments. (ii) Various other arboviruses in overwintering insects are known to require that the vector terminate its winter diapause and take a blood meal before infectious virus can be isolated (Reeves et al. 1958, Bailey et al. 1978, Reisen et al. 2002). (iii) We see a similar result in summer in which bugs at sites without bird activity (i.e., unfed bugs) maintain largely noncytopathic virus, but upon arrival of the first cliff swallows at a site, the recently fed bugs immediately exhibit cytopathic virus (C. Brown and A. Moore, unpublished data). This suggests that blood-feeding per se (not temperature) activates the virus. (iv) Cytopathic virus was isolated from 45% of overwintering positive samples in North Dakota, indicating that BCRV can remain infectious all winter (Brown et al. 2009b). (v) Rush et al. (1980) found that swallow bugs in the laboratory could transmit infectious BCRV to nestling house sparrows as long as 310 days after initial infection and after being held for some of that time in conditions simulating winter.
Viral RNA concentrations (as reflected in higher ΔCt values) were lower for bug samples taken in April than earlier in the winter, suggesting some degradation of virus in bugs with time (or continuing mortality of those infected). Surprisingly, however, we found no evidence that, overall, there was less virus (as measured by RNA concentration) in bug samples in winter than during the summer. Thus, the much lower cytopathicity of virus in winter (compared to summer) cannot be explained by lower virus titers in bugs in winter. This leads back to the suggestion that cytopathicity may be related mostly to whether bugs have recently blood-fed. Cytopathicity on Vero cells may not accurately reflect potential virus infectivity for winter samples.
House sparrows influence the winter prevalence of BCRV in bugs. We found more infected bugs in house sparrow nests than in cliff swallow nests in winter. By April the mere presence of house sparrows at a colony site increased virus prevalence in bugs from all nests to a level statistically indistinguishable from average summer prevalence. This result might be expected if bugs take blood meals from sparrows either occasionally during the winter months or in early spring before the cliff swallows return. Occasional blood-feeding may enhance virus persistence in bug tissues over winter (or make it more likely to be detected), perhaps by shortening the period of the bugs' winter dormancy. House sparrows roost in swallow nests throughout the winter (C. Brown et al., personnel observation) and are probably important in maintaining at least one of the BCRV lineages at some sites.
If we assume that BCRV truly declines in prevalence during the winter in Nebraska and does not simply become harder to detect, this suggests that amplification by vertebrate hosts in summer (and/or vertical transmission when bugs begin reproducing; Brown et al. 2009a) is critical to sustaining it over the long-term at a given colony site. BCRV thus may be similar to arboviruses that overwinter in eggs via vertical transmission and that need periodic boosts from summer amplification in hosts to avoid local extinction (Reisen 1990). The fact that BCRV prevalence in winter was not correlated with virus prevalence the following summer likely reflects variation in the number of avian hosts (cliff swallows and/or house sparrows) occupying a colony site from year to year, variation that also affects the annual population size of swallow bugs (Brown et al. 2001).
Differences among lineages
Both lineages successfully overwintered in bugs, with no changes in the relative proportion of lineages A and B across the winter season. However, the majority of winter isolates were lineage A, in contrast to summer when the majority were lineage B. The seasonal reduction in lineage B must happen in early fall, as the predominance of lineage A among our isolates was apparent as early as October. The overall reduction in BCRV prevalence between summer and winter (∼27% to ∼17%) can be accounted for mostly by the disappearance (for unknown reasons) of lineage B at bridge colony sites in particular.
Lineage A's close association with sparrows in winter supports the hypothesis that it is more closely associated with birds and depends on them more for transmission than does lineage B (Brown et al. 2009). This hypothesis is also consistent with the seasonal change in the proportions of the two lineages. During summer, lineage B is perhaps favored because it replicates mostly in bugs, and with a massive increase in bug populations over the summer, lineage B can be transmitted to instars through either vertical (Brown et al. 2009a) or mechanical (nonviremic) transmission in addition to transmission per os from bugs' feeding on viremic birds. With the die-off of early stage instars in early fall and the cessation of bug reproduction (and potential vertical transmission), disproportionately more of lineage B is removed from the population at that time of year. In contrast, lineage A's ability to exploit avian hosts (i.e., sedentary house sparrows) favors it in the winter, potentially allowing it to initiate its transmission season earlier in the spring and continue later into the fall than lineage B. If summer conditions favor lineage B and winter favors lineage A, the existence of the two lineages may represent a balanced polymorphism in transmission strategies in which neither lineage has an advantage over the other. This is supported by the percentages of lineages A and B not differing significantly from a stable 50:50 ratio when summer and winter are considered together. Additional data from both field and laboratory are needed to better test this hypothesis.
Unique aspects of BCRV
The central Great Plains represents a relatively inhospitable environment for overwintering of any arbovirus (and their arthropod vectors). During the three coldest months of the winter (December–February), the average daily low temperature in our study area (as measured at North Platte over 30 years) is −11.5°C, −13°C, and −9.8°C, with an all-time record low of −37°C recorded in January (NWS 2008). Most other arboviruses found in adult arthropods in winter (Blackmore and Winn 1956, Bailey et al. 1978, Nasci et al. 2001, Farajollahi et al. 2005) were in mines or buildings where indoor temperatures were warmer than outside ambient (Mitchell 1979). Both swallow bugs and BCRV seem able to survive in very cold winter environments, with this virus isolated from bugs in winter as far north as western North Dakota, where temperatures are even colder in mid-winter than in Nebraska (Brown et al. 2009b).
BCRV's ability to regularly overwinter in adult arthropods may reflect primarily its vector's ability to survive the winter as adults or late-stage instars in relatively large numbers. Overwintering is part of a suite of adaptations that the bugs and virus exhibit in response to the erratic colony-site use by cliff swallows and to the bugs' own dependence on cliff swallows (and more recently, introduced house sparrows) as their sole avian hosts. Because BCRV does not seem to be introduced to colony sites in the spring by returning cliff swallows (Hayes et al. 1977, O'Brien et al. 2008), overwintering in the bugs is key to the virus's long-term persistence. With about 60% of the virus present in summer (27.9% average prevalence) persisting into and detectable throughout the winter (17.0% average prevalence), overwintering would seem to be an important life-history component for BCRV that helps maintain its relatively stable prevalence in summer from year to year. Quantitative modeling is needed to study more precisely the relative importance of overwintering in BCRV's annual cycle. This virus may also provide a useful model for studying cold tolerance in arboviruses and the potential for other alphaviruses to overwinter in insect vectors.
Acknowledgments
Portions of this work represented an M.S. thesis by S.A.S. at the University of Tulsa. For laboratory and technical assistance, we thank Eric Edwards, Kenton Miller, Martin Pfeffer, and Karen Winans. The School of Biological Sciences at the University of Nebraska–Lincoln allowed us to use the Cedar Point Biological Station, and the Union Pacific Railroad and the Robert Clary, Dave Knight, and Loren Soper families granted us permission to access land. This work was supported by the National Institutes of Health (AI057569) and the National Science Foundation (DEB-0075199 and DEB-0514824).
Disclosure Statement
No competing financial interests exist.
References
- Bailey CL. Eldridge BF. Hayes DE. Watts DM, et al. Isolation of St. Louis encephalitis virus from overwintering Culex pipiens mosquitoes. Science. 1978;199:1346–1349. doi: 10.1126/science.628843. [DOI] [PubMed] [Google Scholar]
- Bellamy RE. Reeves WC. The winter biology of Culex tarsalis (Diptera: Culicidae) in Kern County, California. Ann Entomol Soc Am. 1963;56:314–323. [Google Scholar]
- Blackmore JS. Winn FJ. A winter isolation of western equine encephalitis virus from hibernating Culex tarsalis Coquillett. Proc Soc Exp Biol Med. 1956;91:146–148. doi: 10.3181/00379727-91-22193. [DOI] [PubMed] [Google Scholar]
- Brault AC. Powers AM. Weaver SC. Vector infection determinants of Venezuelan equine encephalitis virus reside within the E2 envelope glycoprotein. J Virol. 2002;76:6387–6392. doi: 10.1128/JVI.76.12.6387-6392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CR. Swallow Summer. Lincoln, NE: University of Nebraska Press; 1998. [Google Scholar]
- Brown CR. Brown MB. Cliff swallow (Hirundo pyrrhonota) In: Poole A, editor; Gill F, editor. Birds of North America. Philadelphia, PA: Academy of Natural Sciences; Washington, DC: American Ornithologists' Union; 1995. no. 149. [Google Scholar]
- Brown CR. Brown MB. Coloniality in the Cliff Swallow: the Effect of Group Size on Social Behavior. Chicago, IL: University of Chicago Press; 1996. [Google Scholar]
- Brown CR. Brown MB. Empirical measurement of parasite transmission between groups in a colonial bird. Ecology. 2004;85:1619–1626. [Google Scholar]
- Brown CR. Brown MB. Between-group transmission dynamics of the swallow bug, Oeciacus vicarius. J Vector Ecol. 2005;30:137–143. [PubMed] [Google Scholar]
- Brown CR. Brown MB. Moore AT. Komar N. Bird movement predicts Buggy Creek virus infection in insect vectors. Vector Borne Zoonot Dis. 2007;7:304–314. doi: 10.1089/vbz.2006.0646. [DOI] [PubMed] [Google Scholar]
- Brown CR. Brown MB. Padhi A. Foster JE, et al. Host and vector movement affects genetic diversity and spatial structure of Buggy Creek virus (Togaviridae) Mol Ecol. 2008;17:2164–2173. doi: 10.1111/j.1365-294X.2008.03747.x. [DOI] [PubMed] [Google Scholar]
- Brown CR. Komar N. Quick SB. Sethi RA, et al. Arbovirus infection increases with group size. Proc R Soc Lond B. 2001;268:1833–1840. doi: 10.1098/rspb.2001.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CR. Moore AT. Knutie SA. Komar N. Overwintering of infectious Buggy Creek virus (Togaviridae: Alphavirus) in Oeciacus vicarius (Cimicidae) in North Dakota. J Med Entomol. 2009b;46:391–394. doi: 10.1603/033.046.0227. [DOI] [PubMed] [Google Scholar]
- Brown CR. Moore AT. Young GR. Padhi A. Komar N. Isolation of Buggy Creek virus (Togaviridae: Alphavirus) from field-collected eggs of Oeciacus vicarius (Hemiptera: Cimicidae) J Med Entomol. 2009a;46:375–379. doi: 10.1603/033.046.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CR. Padhi A. Moore AT. Brown MB, et al. Ecological divergence of two sympatric lineages of Buggy Creek virus, an arbovirus associated with birds. Ecology. 90 doi: 10.1890/08-1731.1. (In press). [DOI] [PubMed] [Google Scholar]
- Bustin SA. Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15:155–166. [PMC free article] [PubMed] [Google Scholar]
- Calisher CH. Karabatsos N. Lazuick JS. Monath TP. Wolff KL. Reevaluation of the western equine encephalitis antigenic complex of alphaviruses (family Togaviridae) as determined by neutralization tests. Am J Trop Med Hyg. 1988;38:447–452. doi: 10.4269/ajtmh.1988.38.447. [DOI] [PubMed] [Google Scholar]
- Calisher CH. Monath TP. Muth DJ. Lazuick JS, et al. Characterization of Fort Morgan virus, an alphavirus of the western equine encephalitis virus complex in an unusual ecosystem. Am J Trop Med Hyg. 1980;29:1428–1440. doi: 10.4269/ajtmh.1980.29.1428. [DOI] [PubMed] [Google Scholar]
- Chapman RF. The Insects: Structure and Function. New York, NY: Elsevier; 1969. [Google Scholar]
- Dohm DJ. O'Guinn ML. Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2002;39:221–225. doi: 10.1603/0022-2585-39.1.221. [DOI] [PubMed] [Google Scholar]
- Farajollahi A. Crans WJ. Bryant P. Wolf B, et al. Detection of West Nile viral RNA from an overwintering pool of Culex pipiens pipiens (Diptera: Culicidae) in New Jersey, 2003. J Med Entomol. 2005;42:490–494. doi: 10.1093/jmedent/42.3.490. [DOI] [PubMed] [Google Scholar]
- Gentle A. Anastasopoulos F. McBrien NA. High-resolution semi-quantitative real-time PCR without the use of a standard curve. Biotechniques. 2001;31:502–508. doi: 10.2144/01313st03. [DOI] [PubMed] [Google Scholar]
- Gilsbach R. Kouta M. Bönisch H. Brüss M. Comparison of in vitro and in vivo reference genes for internal standardization of real-time PCR data. Biotechniques. 2006;40:173–177. doi: 10.2144/000112052. [DOI] [PubMed] [Google Scholar]
- Goddard LB. Roth AE. Reisen WK. Scott TW. Vertical transmission of West Nile virus by three California Culex (Diptera: Culicidae) species. J Med Entomol. 2003;40:743–746. doi: 10.1603/0022-2585-40.6.743. [DOI] [PubMed] [Google Scholar]
- Hayes RO. Francy DB. Lazuick JS. Smith GC. Gibbs EPJ. Role of the cliff swallow bug (Oeciacus vicarius) in the natural cycle of a western equine encephalitis-related alphavirus. J Med Entomol. 1977;14:257–262. [Google Scholar]
- Hopla CE. Francy DB. Calisher CH. Lazuick JS. Relationship of cliff swallows, ectoparasites, and an alphavirus in west-central Oklahoma. J Med Entomol. 1993;30:267–272. doi: 10.1093/jmedent/30.1.267. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J. Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Huggett J. Dheda K. Bustin S. Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- Lowther PE. Cink CL. House sparrow (Passer domesticus) In: Poole A, editor; Stettenheim P, editor; Gill F, editor. Birds of North America. Philadelphia, PA: Academy of Natural Sciences; Washington, DC: American Ornithologists' Union; 1992. no. 12. [Google Scholar]
- Mahmood F. Reisen WK. Chiles RE. Fang Y. Western equine encephalomyelitis virus infection affects the life table characteristics of Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2004;41:982–986. doi: 10.1603/0022-2585-41.5.982. [DOI] [PubMed] [Google Scholar]
- Marino JH. Cook P. Miller KS. Accurate and statistically verified quantification of relative mRNA abundances using SYBR Green I and real-time RT-PCR. J Immunol Methods. 2003;283:291–306. doi: 10.1016/s0022-1759(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Mitchell CJ. Winter survival of Culex tarsalis (Diptera: Culicidae) hibernating in mine tunnels in Boulder County, Colorado, USA. J Med Entomol. 1979;6:482–487. [Google Scholar]
- Mitchell CJ. Occurrence, biology, and physiology of diapause in overwintering mosquitoes. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology. Vol. 1. Boca Raton, FL: CRC Press; 1988. pp. 191–243. [Google Scholar]
- Moore AT. Edwards EA. Brown MB. Komar N. Brown CR. Ecological correlates of Buggy Creek virus infection in Oeciacus vicarius, southwestern Nebraska, 2004. J Med Entomol. 2007;44:42–49. doi: 10.1603/0022-2585(2007)44[42:ecobcv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Nasci RS. Savage HM. White DJ. Miller JR, et al. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg Infect Dis. 2001;7:742–744. doi: 10.3201/eid0704.010426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratnarajah CK. Kuhn RJ. Functional characterization of the Sindbis virus E2 glycoprotein by transposon linker-insertion mutagenesis. Virology. 2007;363:134–147. doi: 10.1016/j.virol.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NWS (National Weather Service) North Platte, Nebraska, 2008. www.crh.noaa.gov/lbf. [Jan 30;2009 ]. www.crh.noaa.gov/lbf
- O'Brien VA. Moore AT. Huyvaert KP. Brown CR. No evidence for spring re-introduction of an arbovirus by cliff swallows. Wilson J Ornithol. 2008;120:910–913. [Google Scholar]
- Padhi A. Moore AT. Brown MB. Foster JE, et al. Phylogeographical structure and evolutionary history of two Buggy Creek virus lineages in the western Great Plains of North America. J Gen Virol. 2008;89:2122–2131. doi: 10.1099/vir.0.2008/001719-0. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer M. Foster JE. Edwards EA. Brown MB. Komar N, et al. Phylogenetic analysis of Buggy Creek virus: evidence for multiple clades in the western Great Plains, United States of America. Appl Environ Microbiol. 2006;72:6886–6893. doi: 10.1128/AEM.00868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RA. Christensen K. Field-caught Culex erythrothorax larvae found naturally infected with West Nile virus in Grand County, Utah. J Am Mosq Control Assoc. 2006;22:562–562. doi: 10.2987/8756-971X(2006)22[561:FCELFN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Powers AM. Brault AC. Shirako Y. Strauss EG, et al. Evolutionary relationships and systematics of the alphaviruses. J Virol. 2001;75:10118–10131. doi: 10.1128/JVI.75.21.10118-10131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannala BH. Demography and Genetic Structure in Island Populations, Ph.D. dissertation. New Haven, CT: Yale University; 1995. [Google Scholar]
- Reeves WC. Overwintering of arboviruses. Prog Med Virol. 1974;17:193–220. [PubMed] [Google Scholar]
- Reeves WC. Epidemiology and Control of Mosquito-borne Arboviruses in California, 1943–1987. Sacramento, CA: California Mosquito Vector Control Association; 1990. [Google Scholar]
- Reeves WC. Bellamy RE. Scrivani RP. 1958. Relationships of mosquito vectors to winter survival of encephalitis viruses. I. Under natural conditions. Am J Hyg. 1958;67:78–89. doi: 10.1093/oxfordjournals.aje.a119922. [DOI] [PubMed] [Google Scholar]
- Reisen WK. North American mosquito-borne arboviruses: questions of persistence and amplification. Bull Soc Vector Ecol. 1990;15:11–21. [Google Scholar]
- Reisen WK. Fang Y. Lothrop HD. Martinez VM, et al. Overwintering of West Nile virus in southern California. J Med Entomol. 2006a;43:344–355. doi: 10.1603/0022-2585(2006)043[0344:oownvi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Fang Y. Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2006b;43:309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Kramer LD. Chiles RE. Wolfe TM. Green EGN. Simulated overwintering of encephalitis viruses in diapausing female Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2002;39:226–233. doi: 10.1603/0022-2585-39.1.226. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Meyer RP. Milby MM. Overwintering studies on Culex tarsalis (Diptera: Culicidae) in Kern County, California: temporal changes in abundance and reproductive status with comparative observations on C. quinquefasciatus (Diptera: Culicidae) Ann Entomol Soc Am. 1986;79:677–685. [Google Scholar]
- Rosen L. Overwintering mechanisms of mosquito-borne arboviruses in temperate climates. Am J Trop Med Hyg. 1987;37(Suppl):69S–76S. doi: 10.4269/ajtmh.1987.37.69s. [DOI] [PubMed] [Google Scholar]
- Rush WA. Francy DB. Bailey RE. Seasonal changes in susceptibility of a population of swallow bugs (Hemiptera: Cimicidae) to Fort Morgan virus. J Med Entomol. 1981;5:425–428. [Google Scholar]
- Rush WA. Francy DB. Smith GC. Cropp CB. Transmission of an arbovirus by a member of the family Cimicidae. Ann Entomol Soc Am. 1980;73:315–318. [Google Scholar]
- Scott TW. Bowen GS. Monath TP. A field study on the effects of Fort Morgan virus, an arbovirus transmitted by swallow bugs, on the reproductive success of cliff swallows and symbiotic house sparrows in Morgan County, Colorado, 1976. Am J Trop Med Hyg. 1984;33:981–91. doi: 10.4269/ajtmh.1984.33.981. [DOI] [PubMed] [Google Scholar]
- Scott TW. Lorenz LH. Reduction of Culiseta melanura fitness by eastern equine encephalomyelitis virus. Am J Trop Med Hyg. 1998;59:341–346. doi: 10.4269/ajtmh.1998.59.341. [DOI] [PubMed] [Google Scholar]
- Smith GC. Eads RB. Field observations on the cliff swallow, Petrochelidon pyrrhonota (Vieillot), and the swallow bug, Oeciacus vicarius Horvath. J Wash Acad Sci. 1978;68:23–26. [Google Scholar]
- Strauss JH. Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. PAUP*: Phylogenetic Analyses Using Parsimony (*and Other Methods) 4.0 β. Sunderland, MA: Sinauer; 2002. [Google Scholar]
- Tesh RB. Transovarial transmission of arboviruses in their invertebrate vectors. In: Harris KF, editor. Current Topics in Vector Research. Vol. 2. New York: Praeger; 1984. pp. 57–76. [Google Scholar]
- Turell MJ. Horizontal and vertical transmission of viruses by insect and tick vectors. In: Monath TP, editor. The Arboviruses: Epidemiology and Ecology. Vol. 1. Boca Raton, FL: CRC Press; 1988. pp. 127–152. [Google Scholar]
- Turell MJ. Hardy JL. Reeves WC. Stabilized infection of California encephalitis virus in Aedes dorsalis, and its implications for viral maintenance in nature. Am J Trop Med Hyg. 1982;31:1252–1259. doi: 10.4269/ajtmh.1982.31.1252. [DOI] [PubMed] [Google Scholar]
- Watts DM. Pantuwatana S. DeFoliart GR. Yuill TM. Thompson WH. Transovarial transmission of LaCrosse virus (California encephalitis group) in the mosquito, Aedes triseriatus. Science. 1973;182:1140–1141. doi: 10.1126/science.182.4117.1140. [DOI] [PubMed] [Google Scholar]
- White DM. Wilson WC. Blair CD. Beaty BJ. Studies on overwintering of bluetongue viruses in insects. J Gen Virol. 2005;86:453–462. doi: 10.1099/vir.0.80290-0. [DOI] [PubMed] [Google Scholar]