Abstract
Lassa virus pathogenesis is believed to involve dysregulation of cytokines. We have previously shown nuclear factor-κB (NF-κB) inhibition using a BSL-2 model for Lassa fever. Here we further define the potential mechanism for NF-κB inhibition as involving increased levels of repressive p50/p50 homodimers, and suggest a novel therapeutic strategy that acts via modulation of host signaling.
Introduction
The arenaviruses include several important emerging human pathogens, including Lassa virus and Junin virus. While these viruses remain significant causes of morbidity and mortality, little is known about the molecular basis of disease, and this has hindered the design of novel therapeutics. Hemorrhagic arenavirus pathogenesis is believed to involve the dysregulation of cytokines, which leads to impaired development of a protective immune response. Pichindé virus (PICV) causes a similar disease in guinea pigs to Lassa fever in humans (18). By using two variants of PICV-P2, which causes a mild infection, and P18, which causes a lethal disease (1), we can begin to delineate the cellular responses that lead to a protective immune response, and those that lead to a severe hemorrhagic disease (3–5).
In some guinea pig models of arenavirus hemorrhagic fever, differential expression of tumor necrosis factor-α (TNF-α) has been associated with pathogenicity (2), with high levels of TNF-α appearing at late stages of lethal infection, at a time when levels are declining following infection with the attenuated variant. There is also evidence suggesting that early elaboration of pro-inflammatory cytokines is actually associated with milder disease. Differential production of TNF-α has been shown to be involved in the pathogenesis of African swine fever virus (16), with TNF-α production elevated at early times following infection with a low-virulence isolate compared to infection with a more virulent isolate (15). These data suggest that an early inflammatory response is important in activating the required immune effectors that lead to survival, but that high levels of these mediators late in infection may lead to severe disease. Infection of macrophages with Lassa virus does not activate TNF-α expression, and inhibits production of this cytokine following treatment with lipopolysaccharide; this effect is not seen following infection with the apathogenic Mopeia virus (20). A similar effect is seen following infection with virulent, but not attenuated, PICV (11), suggesting that virulent arenaviruses are able to actively suppress the signaling events that lead to the development of an effective innate response, and may contribute to pathogenesis by facilitating increased viral replication and dissemination.
The transcription factor nuclear factor-κB (NF-κB) controls the expression of a number of genes including TNF-α (17). We have previously identified the NF-κB pathway as being highly interconnected in signaling networks induced during PICV infection using high-throughput methods and pathway analysis (3,4). NF-κB refers to a protein family that functions as homo- and heterodimers. We have shown that infection with the attenuated variant of PICV increases DNA binding of the transcription-activating p65/p50 heterodimer, whereas lethal infection increases binding of the transcription-repressing p50/p50 homodimer (11). We sought to increase our understanding of the mechanisms behind this finding, and further characterize the activation status of NF-κB during infection.
Materials and Methods
We infected triplicate cultures of P388D1 murine monocyte-like cells with P2 or P18 PICV at a multiplicity of infection of 5. Virus was purified by PEG precipitation to remove contaminating soluble factors that may activate cellular signaling pathways. Control cultures were mock-infected with PEG purification medium alone. Cells were harvested at various times post-infection (15 min to 16 h), and cytoplasmic and nuclear extracts were prepared as described previously (10). Protein levels were quantified using the BCA protein assay (Pierce, Rockford, IL). Equal amounts of protein were used for all experiments.
Results
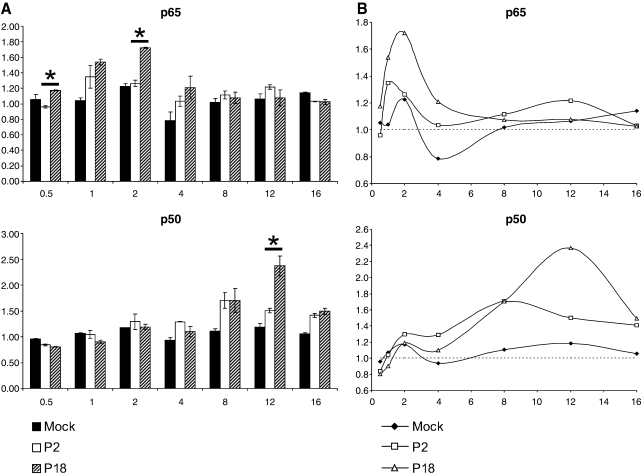
We performed high-throughput DNA-binding assays using the TransAM assay system (Active Motif, Carlsbad, CA). Nuclear extracts from 30 min to 16 h post-infection were assayed for levels of NF-κB p65 and p50 (Fig. 1), and c-Rel and p52 (data not shown) to bind to the consensus κB site (GGGACTTTCC) in vitro. Infection with the virulent P18 variant of the virus induced approximately a 1.7-fold increase in p65 DNA binding by 2 h post-infection; binding following P18 infection was significantly higher than that induced by P2 at 0.5 and 2 h post-infection (p = 0.004 and p = 0.008, respectively, by Student's t-test). No significant differences were observed at late time points post-infection. At 8 h post-infection clear differences in p50 binding were seen between virus- and mock-infected cells; at 12 h post-infection, P18 infection induced 1.6-fold more p50 binding than infection with P2 (p = 0.048). Analysis of nuclear p50 and p65 levels by immunoblotting showed significantly higher levels of p50 in the nucleus following infection with the virulent virus from 8 h post-infection (data not shown). Infection with p18 showed consistently higher levels of c-Rel binding from 4 h onward, and increased and sustained p52 binding from 8 h post-infection. The role of this pathway in pathogenesis has yet to be determined.
FIG. 1.
DNA-binding activity of NF-κB family members. We used 5 μg of nuclear extracts from mock, P2, and P18 PICV-infected cells to assay DNA binding of NF-κB p65 and p50 to the κB consensus site in vitro. (A) The bar graphs show the results from three independent infections. Error bars show the standard error of the mean. Data are normalized to the DNA-binding activity of a triplicate set of nuclear extracts from time-point zero. Asterisks indicate significant differences (p <0.05 by Students T-test). (B) Line graph representations are used to illustrate the oscillatory nature of the responses. The y axis shows absorbance, and the x axis is hours post-infection.
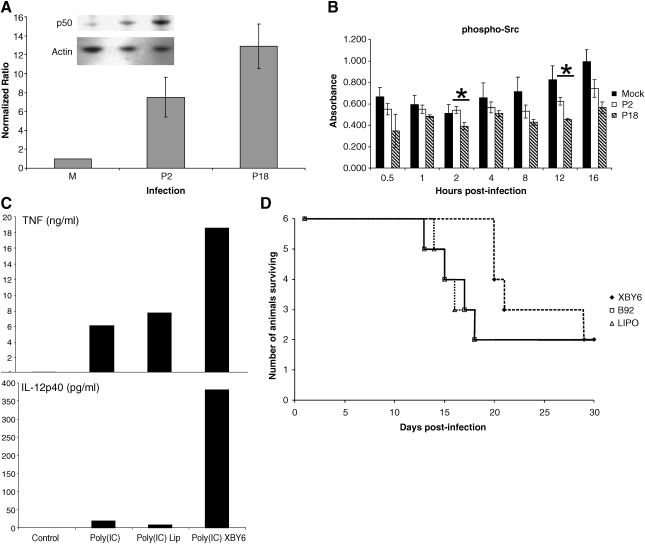
As NF-κB p50 showed increased nuclear translocation and DNA binding in P18-infected cells, we investigated the total levels of this protein by immunoblotting whole-cell extracts at 24 h post-infection. Bands were quantified by densitometry using the GelPro Analyzer (Media Cybernetics, Silver Spring, MD) (Fig. 2A). We observed an increase in levels of total p50 following infection with both P2 and P18. This finding is consistent with our previous report (3). The increased level of p50 may account for our previously reported increased binding of p50/p50 homodimers to the κB site (11). However, the increased nuclear levels are markedly higher in P18-infection, as shown by the DNA-binding assay in Fig. 1, and immunoblots of nuclear extracts following the time-course of infection (data not shown), whereas total cell levels were not statistically significantly greater in P18 infection compared to P2. This suggests that while both viruses may activate the signaling pathways that lead to p50 expression, P18 infection may activate signaling events that preferentially trigger p50 homodimer translocation. While specific signaling mechanisms leading to preferential p50/p50 activation remain to be identified, it has been shown that p50/p50 homodimers are preferentially activated by ethanol in monocytes, and that this may be the mechanism for the downregulation of pro-inflammatory cytokines in acute ethanol exposure (21). Pathway analysis using the Ingenuity Pathways Analysis software and knowledge base (Ingenuity Systems, Redwood City, CA), revealed one protein, c-terminal Src kinase (Csk), to be selectively involved in p50 localization, and that Src mediates an important alternative pathway for NF-κB activation. We investigated the phosphorylation of Src over time following mock, P2, or P18 infection by cell-based phospho-specific ELISA (Active Motif FACE assay performed per the manufacturer's instructions, with cell number controlled by crystal violet staining), and found that p-Src levels were consistently lower in P18- than P2-infected cells (Fig. 2B). However, p-Src levels following P18 infection were only significantly lower at two time-points (2 and 12 h post-infection), and levels of p-Src following P2 infection showed a trend toward being lower than in mock-infection, although the differences were not statistically significant at any individual time-point. Although the observed differences in phosphorylation are small, the fact that phosphorylation events can dramatically affect protein activity, these small-fold changes in phosphorylation may result in large functional changes.
FIG. 2.
PICV infection increases expression of NF-κB p50. Quadruplicate cultures of mock, P2, and P18 PICV-infected cells were harvested in SDS-PAGE loading buffer at 24 h post-infection, and total levels of p50 were detected by immunoblotting. (A) Levels of p50 in infected cells were calculated relative to levels in mock-infected cells by densitometry (n = 4). (B) A cell-based phospho-specific ELISA was used to investigate Src phosphorylation in vitro over the time-course of infection. Error bars show the standard error of the mean (n = 3). Statistically significant differences between P2 and P18 are shown by an asterisk (Student's t-test, p < 0.05). (C) Murine macrophage-like P388D1 cells were stimulated with poly(I:C) following treatment with XBY-6 or liposomes alone. Supernatant levels of TNF-α and IL-12p40 were assayed by ELISA. (D) Guinea pigs (n = 6 per group) were treated with thioaptamer XBY-6 in liposomes as described in (12), with either XBY-6 thioaptamer targeting NF-κB p50/p50 (XBY), a control (scrambled) thioaptamer B92 (B92), liposomes alone (LIPO), or no treatment (omitted here for clarity, but described in the text).
Discussion
The p50/p50 homodimers of NF-κB are likely to suppress the pro-inflammatory response (9,14). Several studies suggest that virulent hemorrhagic fever infection is associated with early innate immune dysregulation (6,15,28), and potentially inhibition of pro-inflammatory signaling events. We used a double-stranded DNA thioaptamer to NF-κB p50/p50 (19,29,30) to attempt to counteract the inhibitory effects mediated by transcriptionally repressive p50/p50 dimers. The thioaptamer XBY-6 was shown to be specific for the p50 homodimer by gel shift and supershift assay (31). In vitro, XBY-6 treatment of P388D1 cells led to increased TNF-α and IL-12 production following stimulation with poly(I:C) (Fig. 2C). XBY-6 was used to treat guinea pigs infected with PICV P18 following appropriate guidelines for institutional animal care and use. Total numbers of surviving guinea pigs were not increased, but XBY-6 thioaptamer treatment did appear to prolong survival compared to guinea pigs treated with liposomes alone or a scrambled control thioaptamer (Fig. 2D), although these results were not statistically significant by Kaplan-Meier survival curve analysis. Animals treated with the thioaptamers died earlier than infected-untreated animals. We hypothesize that this is due to the immunosuppressive effects of the liposome preparation we have characterized previously (12), although histopathologic evaluation of tissues harvested at time of death revealed slightly less severe pathologic lesions in the splenic marginal zone and red pulp of XBY-6-treated animals compared to untreated, infected control animals (data not shown). There was no difference between groups in mean viral titers or antibody titers at death.
We have previously reported that PICV P18 is able to inhibit the DNA binding of NF-κB p65 to its DNA target sequence following stimulation with LPS (11). This result shows that virulent PICV is able to actively suppress the NF-κB pathway. As NF-κB transactivates its own expression, and expression of IκB, this is consistent with gene expression data from LCMV-infected macaques, in which the virulent strain downregulated p65 and IκB transcription, consistent with NF-κB inhibition, whereas the attenuated virus upregulated their transcription (8). In this study we have shown that P18 induces expression and nuclear translocation of high levels of p50. This suggests that the increased levels of p50 protein and its selective nuclear translocation may be the mechanism responsible for arenavirus-induced NF-κB inhibition. The pathways that lead to upregulation of p50 remain to be elucidated, and may involve a viral protein acting on upstream signaling pathways to activate p50 gene transactivating factors. It is known that NF-κB transactivates its own promoter (13); the early activation of p65-containing dimers in infected cells may be partly responsible for the observed p50 upregulation. However, the mechanisms that lead to preferential translocation of p50/p50 may be more significant in pathogenesis. Despite only encoding four proteins, arenaviruses have evolved the ability to directly interfere with host signaling. For example, the NP protein of arenaviruses, except Tacaribe virus, have been shown to inhibit the type-I interferon response by inhibiting IRF-3 translocation (22,23).
Activation of the pathways that lead to p50 expression and p50/p50 activation could be another mechanism used by arenaviruses to evade the immune response. Both NF-κB p65/p50 and p50/p50 dimers are held in the cytoplasm by IκB, which is degraded following phosphorylation by the IκB kinase complex. However, nuclear translocation and transactivation of NF-κB molecules require other regulatory mechanisms, many of which are due to phosphorylation events (24,25). While activation of a common pathway may lead to IκB degradation in both P2- and P18-infected cells, infection with attenuated virus could lead to differential activation of pathways that lead to the correct activating modifications of the p65 subunit of NF-κB, while virulent virus may fail to activate these pathways, or actively inhibit them. However, detectable levels of p65 in the nucleus (G.C.B. and N.K.H, unpublished results) during late-stage P18 infection suggest that other inhibitory mechanisms may be present, such as increased levels of nuclear IκBs, or incomplete post-translational activation of p65. Interestingly, a recent genomic study of LCMV infection suggested that increased levels of HSP60 may downregulate the inflammatory response, potentially through downregulation of NF-κB expression (7). The repeated identification of NF-κB and its various regulatory pathways in the host responses to arenavirus infection illustrate the central role of this transcription factor in arenavirus disease. The finding that increased p50/p50 binding in P18 infection does not occur until later time points suggests that this could be a secondary effect, such as following differential activation of a pathway responsible for p50/p50 activation, or may be associated with a certain stage in the virus replication cycle.
We have previously shown that modulation of host signaling can protect guinea pigs from lethal PICV infection (12). A more complete understanding of the role of NF-κB in arenavirus hemorrhagic fever may allow focused targeting of therapeutics, that by modulating this pathway, result in the development of an effective immune response. Modulation of NF-κB activity and of other transcription factors is likely to provide a useful basis for the development of novel antivirals. The fact that early TNF-α expression appears to correlate with infection with lower-virulence viruses and recovery suggests that an early pro-inflammatory response is critical in viral clearance. Inhibiting events that act to suppress this response, such as NF-κB p50/p50 and transcriptionally repressive forms of AP-1, may allow the correct development of these responses. In contrast, as pathogenesis late in infection correlates with high levels of pro-inflammatory cytokines, using a different set of inhibitors at later stages of disease to inhibit NF-κB p65/p50 may provide protection. In addition to targeting immune pathways, inhibition of other cellular pathways may be effective in controlling viral replication directly (26,27). We believe that this type of approach, modulating specific “arms” of the host-response at different stages of infection, in combination with supportive care and drugs that directly target pathogen replication, may provide an important therapeutic strategy for the control of a number of infectious diseases.
Acknowledgments
We wish to thank Barry Elsom for technical assistance. This work was supported by the National Institutes of Health (UO1 AI054827), and a grant to N.K.H. from the Western Regional Center for Excellence for Biodefense and Emerging Infectious Disease Research, NIH grant no. U54 AI057156.
D.G.G and B.A.L. declare financial interests in AM Biotechnologies and AptaMed, University of Texas Medical Branch spin-off companies involved in thioaptamer technologies, including thioaptamer XBY-6. No other authors declare any potential competing financial interests.
References
- 1.Aronson JF. Herzog NK. Jerrells TR. Pathological and virological features of arenavirus disease in guinea-pigs—comparison of 2 Pichinde virus-strains. Am J Pathol. 1994;145:228–235. [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson JF. Herzog NK. Jerrells TR. Tumor-necrosis-factor and the pathogenesis of Pichinde virus-infection in guinea-pigs. Am J Trop Med Hyg. 1995;52:262–269. doi: 10.4269/ajtmh.52-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowick GC. Fennewald SM. Elsom BL. Aronson JF. Luxon BA. Gorenstein DG. Herzog NK. Differential signaling networks induced by mild and lethal hemorrhagic fever virus infections. J Virol. 2006;80:10248–10252. doi: 10.1128/JVI.01384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowick GC. Fennewald SM. Scott EP, et al. Identification of differentially activated cell-signaling networks associated with Pichinde virus pathogenesis by using systems kinomics. J Virol. 2007;81:1923–1933. doi: 10.1128/JVI.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowick GC. Spratt HM. Hogg AE, et al. Analysis of the differential host cell nuclear proteome induced by attenuated and virulent hemorrhagic arenavirus infection. J Virol. 2009;83:687–700. doi: 10.1128/JVI.01281-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dejean CB. Ayerra BL. Teyssie AR. Interferon response in the guinea pig infected with Junin virus. J Med Virol. 1987;23:83–91. doi: 10.1002/jmv.1890230110. [DOI] [PubMed] [Google Scholar]
- 7.Djavani M. Crasta OR. Zhang Y, et al. Gene expression in primate liver during viral hemorrhagic fever. J Virol. 2009;6:20. doi: 10.1186/1743-422X-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djavani MM. Crasta OR. Zapata JC, et al. Early blood profiles of virus infection in a monkey model for Lassa fever. J Virol. 2007;81:7960–7973. doi: 10.1128/JVI.00536-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driessler F. Venstrom K. Sabat R. Asadullah K. Schottelius AJ. Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: a role for p50. Clin Exp Immunol. 2004;135:64–73. doi: 10.1111/j.1365-2249.2004.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyer RB. Herzog NK. Isolation of intact nuclei for nuclear extract preparation from a fragile B-lymphocyte cell-line. Biotechniques. 1995;19:192–195. [PubMed] [Google Scholar]
- 11.Fennewald SM. Aronson JF. Zhang L. Herzog NK. Alterations in NF-kappa b and RBP-J kappa by arenavirus infection of macrophages in vitro and in vivo. J Virol. 2002;76:1154–1162. doi: 10.1128/JVI.76.3.1154-1162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fennewald SM. Scott EP. Zhang LH, et al. Thioaptamer decoy targeting of ap-1 proteins influences cytokine gene expression and the outcome of arenavirus infections. J Gen Virol. 2007;88:981–990. doi: 10.1099/vir.0.82499-0. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira V. Tarantino N. Korner M. Discrimination between rela and relb transcriptional regulation by a dominant negative mutant of IkappaBalpha. J Biol Chem. 1998;273:592–599. doi: 10.1074/jbc.273.1.592. [DOI] [PubMed] [Google Scholar]
- 14.Franzoso G. Bours V. Park S. Tomita-Yamaguchi M. Kelly K. Siebenlist U. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-kappa B-mediated inhibition. Nature. 1992;359:339–342. doi: 10.1038/359339a0. [DOI] [PubMed] [Google Scholar]
- 15.Gil S. Spagnuolo-Weaver M. Canals A, et al. Expression at mRNA level of cytokines and a238l gene in porcine blood-derived macrophages infected in vitro with African swine fever virus (ASFV) isolates of different virulence. Arch Virol. 2003;148:2077–2097. doi: 10.1007/s00705-003-0182-x. [DOI] [PubMed] [Google Scholar]
- 16.Gomez del Moral M. Ortuno E. Fernandez-Zapatero P. Alonso F. Alonso C. Ezquerra A. Dominguez J. African swine fever virus infection induces tumor necrosis factor alpha production: implications in pathogenesis. J Virol. 1999;73:2173–2180. doi: 10.1128/jvi.73.3.2173-2180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinata K. Gervin AM. Jennifer ZY. Khavari PA. Divergent gene regulation and growth effects by NF-kappa B in epithelial and mesenchymal cells of human skin. Oncogene. 2003;22:1955–1964. doi: 10.1038/sj.onc.1206198. [DOI] [PubMed] [Google Scholar]
- 18.Jahrling PB. Hesse RA. Rhoderick JB. Elwell MA. Moe JB. Pathogenesis of a Pichinde virus-strain adapted to produce lethal infections in guinea-pigs. Infect Immun. 1981;32:872–880. doi: 10.1128/iai.32.2.872-880.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King DJ. Bassett SE. Li X. Fennewald SA. Herzog NK. Luxon BA. Shope R. Gorenstein DG. Combinatorial selection and binding of phosphorothioate aptamers targeting human NF-kappa B rela(p65) and p50. Biochemistry. 2002;41:9696–9706. doi: 10.1021/bi020220k. [DOI] [PubMed] [Google Scholar]
- 20.Lukashevich IS. Maryankova R. Vladyko AS, et al. Lassa and Mopeia virus replication in human monocytes/macrophages and in endothelial cells: different effects on IL-8 and TNF-alpha gene expression. J Med Virol. 1999;59:552–560. [PMC free article] [PubMed] [Google Scholar]
- 21.Mandrekar P. Catalano D. Szabo G. Alcohol-induced regulation of nuclear regulatory factor-kappa beta in human monocytes. Alcohol Clin Exp Res. 1997;21:988–994. [PubMed] [Google Scholar]
- 22.Martinez-Sobrido L. Giannakas P. Cubitt B. Garcia-Sastre A. de la Torre JC. Differential inhibition of type I interferon induction by arenavirus nucleoproteins. J Virol. 2007;81:12696–12703. doi: 10.1128/JVI.00882-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Sobrido L. Zuniga EI. Rosario D. Garcia-Sastre A. de la Torre JC. Inhibition of the type I interferon response by the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J Virol. 2006;80:9192–9199. doi: 10.1128/JVI.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann M. Naumann M. Beyond IkappaBs: alternative regulation of NF-kappaB activity. FASEB J. 2007;21:2642–2654. doi: 10.1096/fj.06-7615rev. [DOI] [PubMed] [Google Scholar]
- 25.Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene. 2006;25:6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 26.Vela EM. Bowick GC. Herzog NK. Aronson JF. Exploring kinase inhibitors as therapies for human arenavirus infections. Future Virol. 2008;3:243–251. doi: 10.2217/17460794.3.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vela EM. Bowick GC. Herzog NK. Aronson JF. Genistein treatment of cells inhibits arenavirus infection. Antiviral Res. 2008;77:153–156. doi: 10.1016/j.antiviral.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villinger F. Rollin PE. Brar SS, et al. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J Infect Dis. 1999;179(Suppl 1):S188–S191. doi: 10.1086/514283. [DOI] [PubMed] [Google Scholar]
- 29.Volk DE. Yang X. Fennewald SM, et al. Solution structure and design of dithiophosphate backbone aptamers targeting transcription factor NF-kappaB. Bioorg Chem. 2002;30:396–419. doi: 10.1016/s0045-2068(02)00510-2. [DOI] [PubMed] [Google Scholar]
- 30.Wang H. Yang X. Bowick GC. Herzog NK. Luxon BA. Lomas LO. Gorenstein DG. Identification of proteins bound to a thioaptamer probe on a proteomics array. Biochem Biophys Res Commun. 2006;347:586–593. doi: 10.1016/j.bbrc.2006.06.132. [DOI] [PubMed] [Google Scholar]
- 31.Yang X. Fennewald S. Luxon BA. Aronson J. Herzog NK. Gorenstein DG. Aptamers containing thymidine 3′-o-phosphorodithioates: synthesis and binding to nuclear factor-kappaB. Bioorg Med Chem Lett. 1999;9:3357–3362. doi: 10.1016/s0960-894x(99)00600-9. [DOI] [PubMed] [Google Scholar]