Abstract
The lymphatic vasculature plays a key role in tissue homeostasis and immune surveillance. There is mounting evidence of a role for the lymphatic circulation and for newly formed lymphatic vessels in the pathogenesis of lung disease. Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, debilitating lung disease. In IPF, the lung parenchyma undergoes extensive remodeling. This review focuses on the current knowledge and understanding of the pathogenesis of IPF, and recent evidence of the involvement of lymphangiogenesis in lung injury and repair and the molecular and cellular pathways leading to the development of lymphatic vasculature.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease of unknown etiology, with an insidious onset leading to ventilatory restriction and respiratory failure. The disease, which is the most common form of interstitial lung disease (ILD), characteristically progresses to death, as existing conventional therapies are of limited benefit. There is currently no known cause or cure for IPF. In the United States (US), the most recent data show a prevalence ranging from 14 to 42.7 per 100,000 and an incidence ranging from 6.8 to 16.3 per 100,000.1 The prognosis is poor, with a median survival of 3–5 years from the time of diagnosis,2 similar to stage 1B lung cancer.3 Recent evidence suggests that from 1992 to 2003, mortality from IPF in the US increased by 28% and 41% for men and women, respectively.4 There has been little progress in the development of therapeutic strategies for the disease. Conventional therapy with immunosuppressive medications is disappointing, and the only currently effective therapy is lung transplantation.5 It is hoped that elucidating the molecular pathways involved in causing fibrosis may identify potential therapies and lead to improved clinical outcomes.
Pathogenesis of IPF
The pathogenesis of pulmonary fibrosis is poorly understood. While the mechanism of injury is unclear, recent evidence suggests the importance of genetic background in the pathogenesis of disease.6,7 The long-prevailing hypothesis is that IPF represents a disease of chronic inflammation that results in a persistent antigenic response, leading to fibrosis. Recently, the role of inflammation has been challenged. Selman et al.8 have proposed that IPF is an epithelial-fibroblastic disease, with alveolar epithelial cell (AEC) injury and apoptosis, provoking the migration and proliferation of mesenchymal cells and formation of active fibroblastic/myofibroblastic foci, resulting in an exaggerated deposition of extracellular matrix (ECM).8 The accumulation of fibroblasts and myofibroblasts in IPF may be due to decreased apoptosis, increased proliferation, or both.9
Recent evidence suggests that fibroblastic foci are sites of an organized reticulum surrounded by an extensive capillary network that runs through the lung.10 IPF may thus be similar to other chronic inflammatory processes that are associated with angiogenesis. Moreover, inhibition of vascular remodeling in an animal model of lung injury resulted in less fibrosis,11 lending support to the importance of vascular remodeling in sustaining the fibrotic process. In animal models and in lung tissue from IPF patients, an imbalance towards production of more angiogenic than angiostatic factors has been described.12–15 In IPF, fibroblasts were the predominant producers of angiogenic stimuli (CXCL8).12 These data were supported by finding that in conditioned medium from cultured IPF fibroblasts, a significant imbalance was observed towards the production of angiogenic factors (CXCL8) relative to medium from normal fibroblasts, which contained predominantly anti-angiogenic factors (CXCL10).12
The role of vascular endothelial growth factor (VEGF)-A in the angiogenic milieu of IPF remains controversial. Whereas capillary endothelial cells and type II cells in alveolar septae were reactive with anti-VEGF-A antibodies, fibroblasts in fibrotic areas showed less reactivity.16 Thus, VEGF-A may play a key role in the vascular heterogeneity pattern seen in IPF. In support of this model, Cosgrove et al.17 found increased vascularity in areas of severe destruction of lung architecture, but no vascularity was seen in the fibroblastic foci. Ebina et al.16 found increased vascularity in areas of low-grade fibrosis, with decreased vascularity in regions of extensive fibrosis. These studies confirmed the heterogeneous pattern of vascular distribution in IPF.
Lymphatics in the Normal Lung
Lymphatic vessels exist in close proximity of the airways and major blood vessels. However, the existence of lymphatic vessels in the alveolar spaces remains subject to much debate. Marchetti et al.,18 in their study of pig lung, found lymphatic vessels in the peribronchovascular interstitium but lymphatic vessels were not observed in the parenchyma between alveoli. Schraufnagel et al.19 used hyperoxia to induce alveolar damage in the rat lung and found perialveolar lymphatic vessels. These lymphatic vessels resolved over time, and at 14 days, the hyperoxia-exposed lungs resembled normal lung, with no lymphatic vessels in the perialveolar space. Casting of lymphatics in mouse lung20 showed that the peribronchiolar region is rich in lymphatic vessels, but that those lymphatic vessels do not enter the alveolar spaces. Conversely, Leak 21 described juxta-alveolar lymphatics, which were separated from alveolar spaces by the alveolar wall and a thin layer of connective tissue.
Studies of lymphatics in the normal human lung are rare. Pusztaszeri et al.22 found that D2-40 reactivity (an epitope of podoplanin; a marker of lymphatic endothelial cells) followed the bronchovascular distribution, and was nonexistent or rare in the alveoli. Yamashita et al.23 showed that normal lymphatic vessels are in close proximity of the large bronchovascular trees and not seen in the alveolar spaces. More recently, Kambouchner et al.24, looking at the normal areas of lung in the vicinity of excised tumors, showed that about 3.6%–19% of alveolar spaces had an associated D2-40-reactive lymphatic vessel.
Lymphatics in IPF
There is a paucity of data regarding tissue fibrosis and lymphangiogenesis. The fibrotic process in a rat remnant kidney model was associated with newly formed lymphatic vessels.25 In human kidney disease, VEGF-C-mediated lymphangiogenesis is an intrinsic part of tubulo-interstitial fibrosis.26 There is scant information regarding the role of lymphatics and lymphangiogenesis in the pathogenesis of lung disease.27 In human studies, de novo lymphangiogenesis has been described in diffuse alveolar damage,23,28 where the outcome correlated with the size of the lymphatic vessels.28 In patients who died of severe asthma, a lack of lymphatic vessels was observed in their airways.29
Until recently,30,31 in humans, there were no studies showing de novo lymphangiogenesis in IPF. However, there are reports32,33 indicating a higher prevalence (40%–60%) of mediastinal lymphadenopathy. One study indicated a relationship between adenopathy and IPF progression, in the absence of infectious or neoplastic complications.32 Conversely, Souza et al.33 showed no relationship between the extent of disease on high resolution computed tomography (HRCT) scans and the presence of mediastinal lymph nodes.
In a rat bleomycin lung injury model,34 and based on types of collagen deposition, a temporal process was described involving angiogenesis followed by lymphangiogenesis. The bleomycin lung injury model is the most commonly used animal model of pulmonary fibrosis,35 however it has limitations.36
Histopathological Study of Lymphatics in IPF
In our study30 of lymphatics and lymphangiogenesis in IPF, lymphatic vessels were reactive with anti-D2-40 antibodies, and capillaries with anti-CD34 antibodies. In normal lung tissue, lymphatic vessels were found in proximity of large blood vessels. Normal alveolar spaces were devoid of lymphatic vessels but capillaries were found. In the IPF lung, in contrast to the normal lung, lymphatic vessels were observed in close proximity of alveolar spaces, even in those areas with a relatively well-preserved architecture (Fig. 1A). In addition, lymphatic vessels were present throughout the fibrotic tissue and in close proximity to the main bronchovascular tree (Figs. 1B and 1C). Fibroblastic foci were devoid of lymphatic vessels at their center, while lymphatics could be seen at their periphery.30 The lack of lymphatic vessels in the fibroblastic foci was also observed by Yamashita et al.31 Similar to the findings of other investigators,16 blood vessels were seen in fibrotic alveoli, throughout the fibrotic tissue, and at the edge of fibroblastic foci.
FIG. 1.
Lymphatic vessels area increases in parallel with disease severity in IPF tissue sections. In mild (A), moderate (B), and severe (C) IPF tissue sections, there is a marked increase of both lymphatic (in brown, arrows) and blood capillary vessels (in red, arrowheads). In mild IPF (A), lymphatic vessels (in brown, arrows) appear in close proximity of relatively normal alveolar spaces, with further increase in areas of fibrosis (*). (D) IPF lung tissue sections from mild (n = 3), moderate (n = 5), and severe IPF (n = 4) were stained with anti-CD34 (red) and anti-D2-40 (brown) antibodies. Image J® software (National Institutes of Health, Bethesda, MD) was used to analyze images (n = 15 per tissue) and measure the area of blood vessels (CD34-positive) and lymphatic vessels (D2-40-positive). There is a significant increase in lymphatic area with worsening disease (p < 0.05, severe compared to mild and moderate).
Morphometric analysis of these newly formed lymphatic vessels showed that the mean perimeter and area and the total area of the lymphatic vessels per tissue section increased with worsening stages of IPF, while their number did not change significantly. In marked contrast, the number of newly formed blood capillaries increased significantly with increasing disease severity, whereas the area, perimeter, and total area of blood capillaries did not change with disease progression.
Similarly, in their study of the evolution of fibrosis in diffuse alveolar damage (DAD), Yamashita et al.23 showed that intra-alveolar lymphangiogenesis was a key element of the fibrotic process. Whereas newly formed capillaries appeared earlier than lymphatic vessels, the angiogenic process seemed to be reversible as evidenced by the decline in capillary density in end-stage fibrosis. The newly formed lymphatic vessels did not regress, and lymphatic density did not decline in the final stages of fibrosis.
Molecular and Cellular Pathways of Lymphangiogenesis in IPF
The presence of newly formed lymphatic vessels in the alveolar spaces of IPF lung suggests a role for soluble lymphangiogenic factors in the alveolar milieu.30 Bronchoalveolar lavage fluid (BALF) from subjects with IPF triggered significantly more migration of lymphatic endothelial cells (LEC) than did healthy volunteer BALF.
Surprisingly, VEGF-C and VEGF-D, known to play key roles in lymphangiogenesis in disease,37 did not seem to be driving lymphangiogenesis in IPF. VEGF-C levels were lower in IPF BALF than in that from healthy volunteers; levels of VEGF-D were similar.30 VEGF-A, an angiogenic growth factor, is known to induce lymphangiogenesis in inflammatory conditions. In mice overexpressing VEGF-A in the epidermis, triggering a delayed hypersensitivity reaction resulted in increased lymphangiogenesis, which can be significantly attenuated with function-blocking antibodies against VEGFR-1 and VEGFR-2.38 Moreover, in inflammation, VEGF-A can trigger lymphangiogenesis indirectly, via recruitment of macrophages, which in turn secrete VEGF-C and VEGF-D.39 In line with other studies,17,40,41 we found the levels of VEGF-A in IPF BALF to be markedly decreased, strongly suggesting the absence of a significant role for VEGF-A in the lymphangiogenic process.
Levels of fibroblast growth factor, which is known to induce lymphangiogenesis,42 and TIMP-2, which stabilizes vascular tubes,43 were not elevated in BALF of IPF subjects. Conversely, the levels of hepatocyte growth factor, which induces lymphangiogenesis during tissue repair and inflammation44 were higher in IPF BALF.30,45 In addition, levels of TIMP-1 and CCL-246,47 were higher in IPF BALF. Addition of function-blocking antibodies against HGF, TIMP-1, and CCL2 to IPF BALF did not change migration of LEC.30
Hyaluronan (HA) is known to play a key role in lung injury and repair,48 and to induce angiogenesis in a bleomycin-induced lung injury model.49 More recently, the role of HA in tumor lymphangiogenesis has been examined.50,51 In a study of tumor lymphangiogenesis, exogenous HA induced intratumoral lymphangiogenesis, but not angiogenesis.50 Mammary tumor cells overexpressing hyaluronan synthase 2 significantly increased intratumoral lymphangiogenesis in parallel with the development of stromal tissue.51 Stromal cells, particularly tumor-associated fibroblasts, seem to play a key role in the lymphangiogenic process by upregulation of VEGF-C and -D production. A direct role for HA in lymphangiogenesis could not be excluded.51 In our study,30 short-fragment HA in IPF BALF increased LEC migration and proliferation. The addition of short-fragment HA to healthy volunteer BALF increased LEC migration. These data suggest a direct role for hyaluronan in lymphangiogenesis in IPF. There are multiple known receptors for HA on the surface of blood endothelial cells.52,53 Savani et al.53 have shown that migration and proliferation are induced by HA receptors CD44 and CD168, respectively. These receptors seem to be redundant,54 since in CD44 null-mice, CD168 more efficiently sustained collagen-induced inflammation.
In inflammation and cancer, new lymphatic vessels arise from existing ones. Our data and those of others23,30 showed that newly formed alveolar lymphatic vessels were not connected to existing lymphatics, consistent with a role for a lymphatic progenitor endothelial cell.55 There is, however, conflicting evidence about the contribution of progenitor cells to lymphangiogenesis. Salven et al.56 described a CD34+/CD133+/VEGFR-3+ circulating progenitor endothelial cell, with the potential to differentiate into mature blood vascular and lymphatic endothelial cells in vitro. In cancer and metastasis, preexisting lymphatic endothelium appears to play a critical role in lymphangiogenesis, with little or no role for a bone marrow-derived endothelial progenitor cell.57 In humans, circulating progenitor lymphatic endothelial cells were first described in sex-mismatched kidney transplant recipients. After transplant rejection, lymphatic endothelial cells from the recipient were found in the donor kidney.55 A recent report58 showed that human mesenchymal stem cells grown in conditioned medium of human microvascular endothelial cells are capable of differentiating to LEC in vitro, and in vivo can restore lymphatic function in a mouse model of lymphedema.
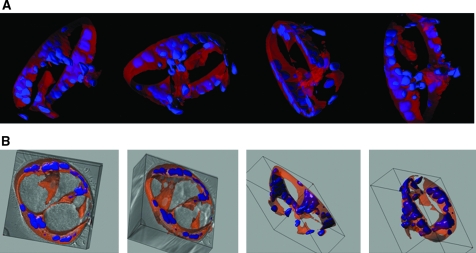
Maruyama et al.59 were the first to show that activated CD11b+ macrophages were able to differentiate into LEC both in vitro and in vivo in a corneal inflammation model. Earlier findings demonstrated that CD11b+ macrophages are increased in IPF BALF.60 In our study, CD45+/CD14+/CD11b+ macrophages were isolated from BALF of subjects with IPF and healthy volunteers, and subsequently grown in Matrigel®. No quantitative difference in CD11b+ macrophages was detected, but functional differences between CD11b+ macrophages isolated from IPF BALF and healthy volunteers were observed. CD11b+ macrophages, isolated from IPF BALF and grown in Matrigel, formed tube-like structures (Fig. 2), whereas CD11b+ macrophages isolated from healthy volunteers did not. These tube-like structures expressed LEC markers LYVE-1 and podoplanin.
FIG. 2.
CD11b+ alveolar macrophages in IPF develop tube-like structures in vitro. CD11b+ alveolar macrophages were cultured in Matrigel for up to 31 days and inspected under white light, or after fluorescent labeling of cytoplasm (CellTracker Orange CMTMR) and nuclei (Hoechst, Invitrogen, Carlsbad, CA, USA). Large tube-like structures (150 μm in diameter) were observed when cells from subjects with IPF were cultured. A series of confocal images were reconstructed in 3-D (three dimensional) renderings and representative snapshots of tubular structures are presented as overlayed fluorescence (A) or DIC and overlayed fluorescence (B).
The role(s) of these newly formed lymphatic vessels in IPF remains to be investigated. We hypothesize (Fig. 3) that they may contribute to the fibrotic process and the maintenance of the injury. Several mechanisms may play a role in this process: first, LEC produced CCL21 a chemokine,61 which was elevated in IPF BALF.30 CCL21 induced proliferation of IPF-derived fibroblasts via stimulation of its receptor CCR-7.62 Moreover, in IPF, CCL21 stimulated recruitment of dendritic cells,63 which might contribute to the persistence of the injury. Finally, in a different model of tissue fibrosis,64 inhibition of transforming growth factor (TGF)-ß1 resulted in acceleration of lymphatic regeneration, raising the possibility that the fibrotic process itself negatively regulates lymphangiogenesis.
FIG. 3.
Lymphangiogenesis in IPF: mechanisms and potential role. The deposition of hyaluronan after lung injury leads to lymphatic endothelial cell (LEC) migration and proliferation. Lung injury leads to the activation of CD11b+ macrophages, which can transdifferentiate to LEC. CCL21, a chemokine derived from LEC, attracts CCR7-expressing dendritic cells (DC) towards the newly formed lymphatic vessels, which contributes to the recirculation of injury in IPF. In addition, CCL21 induces fibroblasts proliferation. Fibrosis, in turn, negatively regulates lymphangiogenesis via transforming growth factor (TGF)-ß.
In summary, lymphangiogenesis is part of the disease process in IPF. Hyaluronan and CD11b+ macrophages seem to drive, at least partially, lymphangiogenesis in IPF. The role(s) of these lymphatic vessels and the interplay between fibrosis and lymphangiogenesis remain to be investigated. Understanding pathways leading to lymphangiogenesis may offer novel therapeutic targets in IPF.
Footnotes
This study was supported by the Intramural Research Program NIH/NHLBI. Yoshihiko Ikeda was partially supported by a Senior Fellowship from the Oak Ridge Institute for Science and Education (ORISE).
Disclosure Statement
Drs. Souheil El-Chemaly, Gustavo Pacheco-Rodriguez, Yoshihiko Ikeda, Daniela Malide, and Joel Moss have no conflicts of interest or financial ties to report.
References
- 1.Raghu G. Weycker D. Edelsberg J. Bradford WZ. Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society. Idiopathic pulmonary fibrosis: Diagnosis and treatment. International consensus statement. American Thoracic Society (ATS) and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 3.Allen JT. Spiteri MA. Growth factors in idiopathic pulmonary fibrosis: Relative roles. Respir Res. 2002;3:13. doi: 10.1186/rr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson AL. Swigris JJ. Lezotte DC. Norris JM. Wilson CG. Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176:277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- 5.Walter N. Collard HR. King TE., Jr. Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:330–338. doi: 10.1513/pats.200602-016TK. [DOI] [PubMed] [Google Scholar]
- 6.Alder JK. Chen JJ. Lancaster L. Danoff S. Su SC. Cogan JD. Vulto I. Xie M. Qi X. Tuder RM. Phillips JA., 3rd Lansdorp PM. Loyd JE. Armanios MY. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosas IO. Ren P. Avila NA. Chow CK. Franks TJ. Travis WD. McCoy JP., Jr May RM. Wu HP. Nguyen DM. Arcos-Burgos M. MacDonald SD. Gochuico BR. Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:698–705. doi: 10.1164/rccm.200702-254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selman M. Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: From innocent targets to serial killers. Proc Am Thorac Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz JC. Thannickal VJ. Epithelial-mesenchymal interactions in pulmonary fibrosis. Semin Respir Crit Care Med. 2006;27:600–612. doi: 10.1055/s-2006-957332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cool CD. Groshong SD. Rai PR. Henson PM. Stewart JS. Brown KK. Fibroblast foci are not discrete sites of lung injury or repair: The fibroblast reticulum. Am J Respir Crit Care Med. 2006;174:654–658. doi: 10.1164/rccm.200602-205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burdick MD. Murray LA. Keane MP. Xue YY. Zisman DA. Belperio JA. Strieter RM. CXCL11 attenuates bleomycin-induced pulmonary fibrosis via inhibition of vascular remodeling. Am J Respir Crit Care Med. 2005;171:261–268. doi: 10.1164/rccm.200409-1164OC. [DOI] [PubMed] [Google Scholar]
- 12.Keane MP. Arenberg DA. Lynch JP., 3rd Whyte RI. MIannettoni MD. Burdick MD. Wilke CA. Morris SB. Glass MC. DiGiovine B. Kunkel SL. Strieter RM. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol. 1997;159:1437–1443. [PubMed] [Google Scholar]
- 13.Keane MP. Belperio JA. Arenberg DA. Burdick MD. Xu ZJ. Xue YY. Strieter RM. IFN-gamma-inducible protein-10 attenuates bleomycin-induced pulmonary fibrosis via inhibition of angiogenesis. J Immunol. 1999;163:5686–5692. [PubMed] [Google Scholar]
- 14.Keane MP. Belperio JA. Burdick MD. Lynch JP. Fishbein MC. Strieter RM. ENA-78 is an important angiogenic factor in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164:2239–2242. doi: 10.1164/ajrccm.164.12.2104106. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama S. Mukae H. Ishii H. Kakugawa T. Sugiyama K. Sakamoto N. Fujii T. Kadota J. Kohno S. Comparison of BALF concentrations of ENA-78 and IP10 in patients with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Respir Med. 2005;99:1145–1151. doi: 10.1016/j.rmed.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Ebina M. Shimizukawa M. Shibata N. Kimura Y. Suzuki T. Endo M. Sasano H. Kondo T. Nukiwa T. Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2004;169:1203–1208. doi: 10.1164/rccm.200308-1111OC. [DOI] [PubMed] [Google Scholar]
- 17.Cosgrove GP. Brown KK. Schiemann WP. Serls AE. Parr JE. Geraci MW. Schwarz MI. Cool CD. Worthen GS. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: A role in aberrant angiogenesis. Am J Respir Crit Care Med. 2004;170:242–251. doi: 10.1164/rccm.200308-1151OC. [DOI] [PubMed] [Google Scholar]
- 18.Marchetti C. Poggi P. Clement MG. Aguggini G. Piacentini C. Icaro-Cornaglia A. Lymphatic capillaries of the pig lung: TEM and SEM observations. Anat Rec. 1994;238:368–373. doi: 10.1002/ar.1092380311. [DOI] [PubMed] [Google Scholar]
- 19.Hainis KD. Sznajder JI. Schraufnagel DE. Lung lymphatics cast from the airspace. Am J Physiol. 1994;267:L199–L205. doi: 10.1152/ajplung.1994.267.2.L199. [DOI] [PubMed] [Google Scholar]
- 20.Peao MN. Aguas AP. de Sa CM. Pereira AS. Grande NR. Scanning electron microscopy of the deep lymphatic network of the murine lung as viewed in corrosion casts. Lymphology. 1993;26:42–48. [PubMed] [Google Scholar]
- 21.Leak LV. Lymphatic removal of fluids and particles in the mammalian lung. Environ Health Perspect. 1980;35:55–76. doi: 10.1289/ehp.803555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pusztaszeri MP. Seelentag W. Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54:385–395. doi: 10.1369/jhc.4A6514.2005. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita M. Iwama N. Date F. Chiba R. Ebina Miki H. Yamauchi K. Sawai T. Nose M. Sato S. Takahashi T. Ono M. Characterization of lymphangiogenesis in various stages of idiopathic diffuse alveolar damage. Hum Pathol. 2009;40:542–551. doi: 10.1016/j.humpath.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 24.Kambouchner M. Bernaudin JF. Intralobular pulmonary lymphatic distribution in normal human lung using D2-40 antipodoplanin immunostaining. J Histochem Cytochem. 2009;57:643–648. doi: 10.1369/jhc.2009.953067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui K. Nagy-Bojarsky K. Laakkonen P. Krieger S. Mechtler K. Uchida S. Geleff S. Kang DH. Johnson RJ. Kerjaschki D. Lymphatic microvessels in the rat remnant kidney model of renal fibrosis: Aminopeptidase p and podoplanin are discriminatory markers for endothelial cells of blood and lymphatic vessels. J Am Soc Nephrol. 2003;14:1981–1989. doi: 10.1097/01.asn.0000076078.50889.43. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto I. Ito Y. Mizuno M. Suzuki Y. Sawai A. Tanaka A. Maruyama S. Takei Y. Yuzawa Y. Matsuo S. Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int. 2009;75:828–838. doi: 10.1038/ki.2008.661. [DOI] [PubMed] [Google Scholar]
- 27.El-Chemaly S. Levine SJ. Moss J. Lymphatics in lung disease. Ann NY Acad Sci. 2008;1131:195–202. doi: 10.1196/annals.1413.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandal RV. Mark EJ. Kradin RL. Organizing pneumonia and pulmonary lymphatic architecture in diffuse alveolar damage. Hum Pathol. 2008;39:1234–1238. doi: 10.1016/j.humpath.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Ebina M. Remodeling of airway walls in fatal asthmatics decreases lymphatic distribution; Beyond thickening of airway smooth muscle layers. Allergol Int. 2008;57:165–174. doi: 10.2332/allergolint.O-07-497. [DOI] [PubMed] [Google Scholar]
- 30.El-Chemaly S. Malide D. Zudaire E. Ikeda Y. Weinberg BA. Pacheco-Rodriguez G. Rosas IO. Aparicio M. Ren P. MacDonald SD. Wu HP. Nathan SD. Cuttitta F. McCoy JP. Gochuico BR. Moss J. Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc Natl Acad Sci USA. 2009;106:3958–3963. doi: 10.1073/pnas.0813368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamashita M. Yamauchi K. Chiba R. Iwama N. Date F. Shibata N. Kumagai H. Risteli J. Sato S. Takahashi T. Ono M. The definition of fibrogenic processes in fibroblastic foci of idiopathic pulmonary fibrosis based on morphometric quantification of extracellular matrices. Hum Pathol. 2009;40:1278–1287. doi: 10.1016/j.humpath.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Attili AK. Kazerooni EA. Gross BH. Flaherty KR. Martinez FJ. Thoracic lymph node enlargement in usual interstitial pneumonitis and nonspecific-interstitial pneumonitis: Prevalence, correlation with disease activity and temporal evolution. J Thorac Imaging. 2006;21:288–292. doi: 10.1097/01.rti.0000213562.55914.9a. [DOI] [PubMed] [Google Scholar]
- 33.Souza CA. Muller NL. Lee KS. Johkoh T. Mitsuhiro H. Chong S. Idiopathic interstitial pneumonias: Prevalence of mediastinal lymph node enlargement in 206 patients. AJR Am J Roentgenol. 2006;186:995–999. doi: 10.2214/AJR.04.1663. [DOI] [PubMed] [Google Scholar]
- 34.Teles-Grilo ML. Leite-Almeida H. Martins dos Santos J. Oliveira C. Boaventura P. Grande NR. Differential expression of collagens type I and type IV in lymphangiogenesis during the angiogenic process associated with bleomycin-induced pulmonary fibrosis in rat. Lymphology. 2005;38:130–135. [PubMed] [Google Scholar]
- 35.Chua F. Gauldie J. Laurent GJ. Pulmonary fibrosis: Searching for model answers. Am J Respir Cell Mol Biol. 2005;33:9–13. doi: 10.1165/rcmb.2005-0062TR. [DOI] [PubMed] [Google Scholar]
- 36.Gauldie J. Kolb M. Animal models of pulmonary fibrosis: How far from effective reality? Am J Physiol Lung Cell Mol Physiol. 2008;294:L151. doi: 10.1152/ajplung.00520.2007. [DOI] [PubMed] [Google Scholar]
- 37.Cueni LN. Detmar M. The lymphatic system in health and disease. Lymphat Res Biol. 2008;6:109–122. doi: 10.1089/lrb.2008.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunstfeld R. Hirakawa S. Hong YK. Schacht V. Lange-Asschenfeldt B. Velasco P. Lin C. Fiebiger E. Wei X. Wu Y. Hicklin D. Bohlen P. Detmar M. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–1057. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- 39.Cursiefen C. Chen L. Borges LP. Jackson D. Cao J. Radziejewski C. D'Amore PA. Dana MR. Wiegand SJ. Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koyama S. Sato E. Haniuda M. Numanami H. Nagai S. Izumi T. Decreased level of vascular endothelial growth factor in bronchoalveolar lavage fluid of normal smokers and patients with pulmonary fibrosis. Am J Respir Crit Care Med. 2002;166:382–385. doi: 10.1164/rccm.2103112. [DOI] [PubMed] [Google Scholar]
- 41.Meyer KC. Cardoni A. Xiang ZZ. Vascular endothelial growth factor in bronchoalveolar lavage from normal subjects and patients with diffuse parenchymal lung disease. J Lab Clin Med. 2000;135:332–338. doi: 10.1067/mlc.2000.105618. [DOI] [PubMed] [Google Scholar]
- 42.Chang LK. Garcia-Cardena G. Farnebo F. Fannon M. Chen EJ. Butterfield C. Moses MA. Mulligan RC. Folkman J. Kaipainen A. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci USA. 2004;101:11658–11663. doi: 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saunders WB. Bohnsack BL. Faske JB. Anthis NJ. Bayless KJ. Hirschi KK. Davis GE. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175:179–191. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kajiya K. Hirakawa S. Ma B. Drinnenberg I. Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakai T. Satoh K. Matsushima K. Shindo S. Abe S. Abe T. Motomiya M. Kawamoto T. Kawabata Y. Nakamura T. Nukiwa T. Hepatocyte growth factor in bronchoalveolar lavage fluids and cells in patients with inflammatory chest diseases of the lower respiratory tract: Detection by RIA and in situ hybridization. Am J Respir Cell Mol Biol. 1997;16:388–397. doi: 10.1165/ajrcmb.16.4.9115749. [DOI] [PubMed] [Google Scholar]
- 46.Antoniades HN. Neville-Golden J. TGalanopoulos T. Kradin RL. Valente AJ. Graves DT. Expression of monocyte chemoattractant protein 1 mRNA in human idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA. 1992;89:5371–5375. doi: 10.1073/pnas.89.12.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Car BD. Meloni F. Luisetti M. Semenzato G. Gialdroni-Grassi G. Walz A. Elevated IL-8 and MCP-1 in the bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Crit Care Med. 1994;149:655–659. doi: 10.1164/ajrccm.149.3.8118632. [DOI] [PubMed] [Google Scholar]
- 48.Jiang D. JLiang J. Fan J. Yu S. Chen S. Luo Y. Prestwich GD. Mascarenhas MM. Garg HG. Quinn DA. Homer RJ. Goldstein DR. Bucala R. Lee RJ. Medzhitov R. Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 49.Garantziotis S. Zudaire E. Trempus CS. Hollingsworth JW. Jiang D. Lancaster LH. Richardson E. Zhuo L. Cuttitta F. Brown KK. Noble PW. Kimata K. Schwartz DA. Serum inter-alpha-trypsin inhibitor and matrix hyaluronan promote angiogenesis in fibrotic lung injury. Am J Respir Crit Care Med. 2008;178:939–947. doi: 10.1164/rccm.200803-386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo LX. Zou K. Ju JH. Xie H. Hyaluronan promotes tumor lymphangiogenesis and intralymphantic tumor growth in xenografts. Acta Biochim Biophys Sin (Shanghai) 2005;37:601–606. doi: 10.1111/j.1745-7270.2005.00083.x. [DOI] [PubMed] [Google Scholar]
- 51.Itano N. Zhuo L. Kimata K. Impact of the hyaluronan-rich tumor microenvironment on cancer initiation and progression. Cancer Sci. 2008;99:1720–1725. doi: 10.1111/j.1349-7006.2008.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banerji S. Ni J. Wang SX. Clasper S. Su J. Tammi R. Jones M. Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Savani RC. Cao G. Pooler PM. Zaman A. Zhou Z. DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem. 2001;276:36770–36778. doi: 10.1074/jbc.M102273200. [DOI] [PubMed] [Google Scholar]
- 54.Nedvetzki S. Gonen E. Assayag N. Reich R. Williams RO. Thurmond RL. Huang JF. Neudecker BA. Wang FS. Turley EA. Naor D. RHAMM, a receptor for hyaluronan-mediated motility, compensates for CD44 in inflamed CD44-knockout mice: A different interpretation of redundancy. Proc Natl Acad Sci USA. 2004;101:18081–18086. doi: 10.1073/pnas.0407378102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kerjaschki D. Huttary N. IRaab I. Regele H. Bojarski-Nagy K. Bartel G. Krober SM. Greinix H. Rosenmaier A. Karlhofer F. Wick N. Maza PR. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 56.Salven P. Mustjoki S. Alitalo R. Alitalo K. Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- 57.He Y. Rajantie I. Ilmonen M. Makinen T. Karkkainen MJ. Haiko P. Salven P. Alitalo K. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64:3737–3740. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- 58.Conrad C. Niess H. Huss R. Huber S. von Luettichau I. Nelson PJ. Ott HC. Jauch KW. Bruns CJ. Multipotent mesenchymal stem cells acquire a lymphendothelial phenotype and enhance lymphatic regeneration in vivo. Circulation. 2009;119:281–289. doi: 10.1161/CIRCULATIONAHA.108.793208. [DOI] [PubMed] [Google Scholar]
- 59.Maruyama K. Ii M. Cursiefen C. Jackson DG. Keino H. Tomita M. Van Rooijen N. Takenaka H. D'Amore PA. Stein-Streilein J. Losordo DW. Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaberg T. Rau M. Stephan H. Lode H. Increased number of alveolar macrophages expressing surface molecules of the CD11/CD18 family in sarcoidosis and idiopathic pulmonary fibrosis is related to the production of superoxide anions by these cells. Am Rev Respir Dis. 1993;147:1507–1513. doi: 10.1164/ajrccm/147.6_Pt_1.1507. [DOI] [PubMed] [Google Scholar]
- 61.Bromley SK. Thomas SY. Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 62.Pierce EM. Carpenter K. Jakubzick C. Kunkel SL. Evanoff H. Flaherty KR. Martinez FJ. Toews GB. Hogaboam CM. Idiopathic pulmonary fibrosis fibroblasts migrate and proliferate to CC chemokine ligand 21. Eur Respir J. 2007;29:1082–1093. doi: 10.1183/09031936.00122806. [DOI] [PubMed] [Google Scholar]
- 63.Marchal-Somme J. Uzunhan Y. Marchand-Adam S. Valeyre D. Soumelis V. Crestani B. Soler P. Cutting edge: Nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis. J Immunol. 2006;176:5735–5739. doi: 10.4049/jimmunol.176.10.5735. [DOI] [PubMed] [Google Scholar]
- 64.Clavin NW. Avraham T. Fernandez J. Daluvoy SV. Soares MA. Chaudhry A. Mehrara BJ. TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol. 2008;295:H2113–H2127. doi: 10.1152/ajpheart.00879.2008. [DOI] [PubMed] [Google Scholar]