Abstract
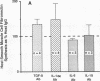
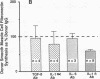
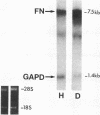
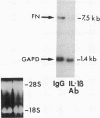
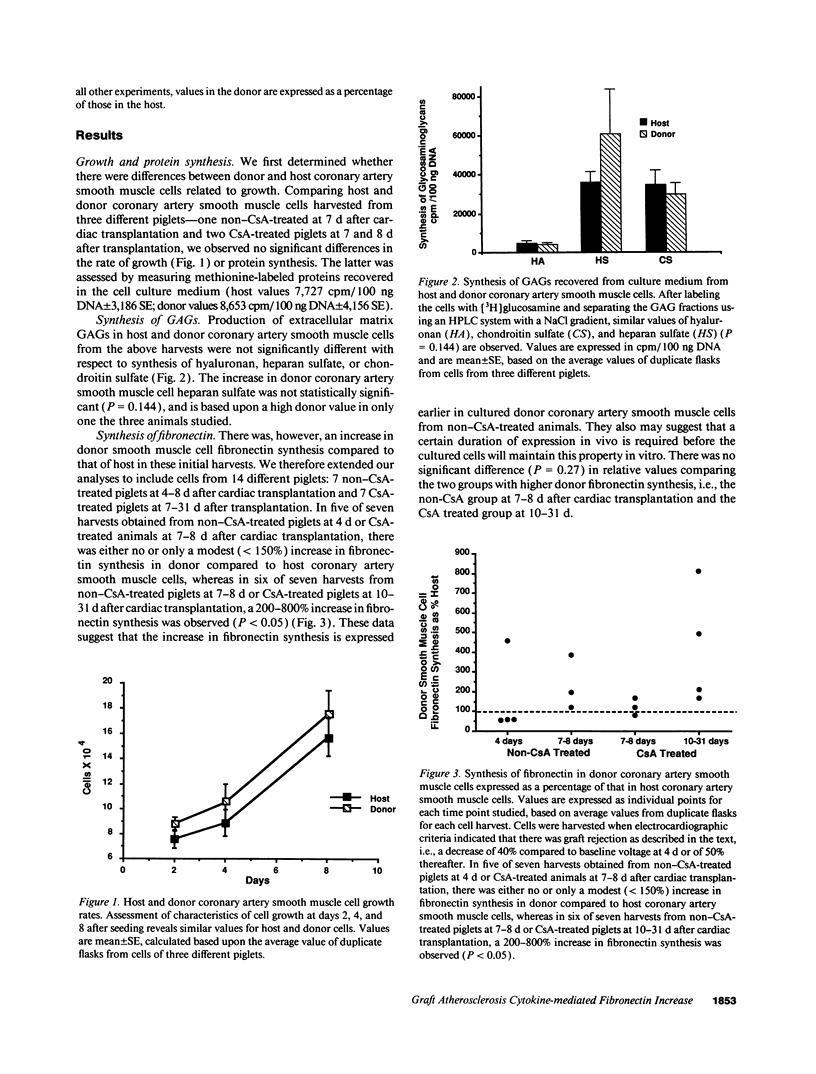
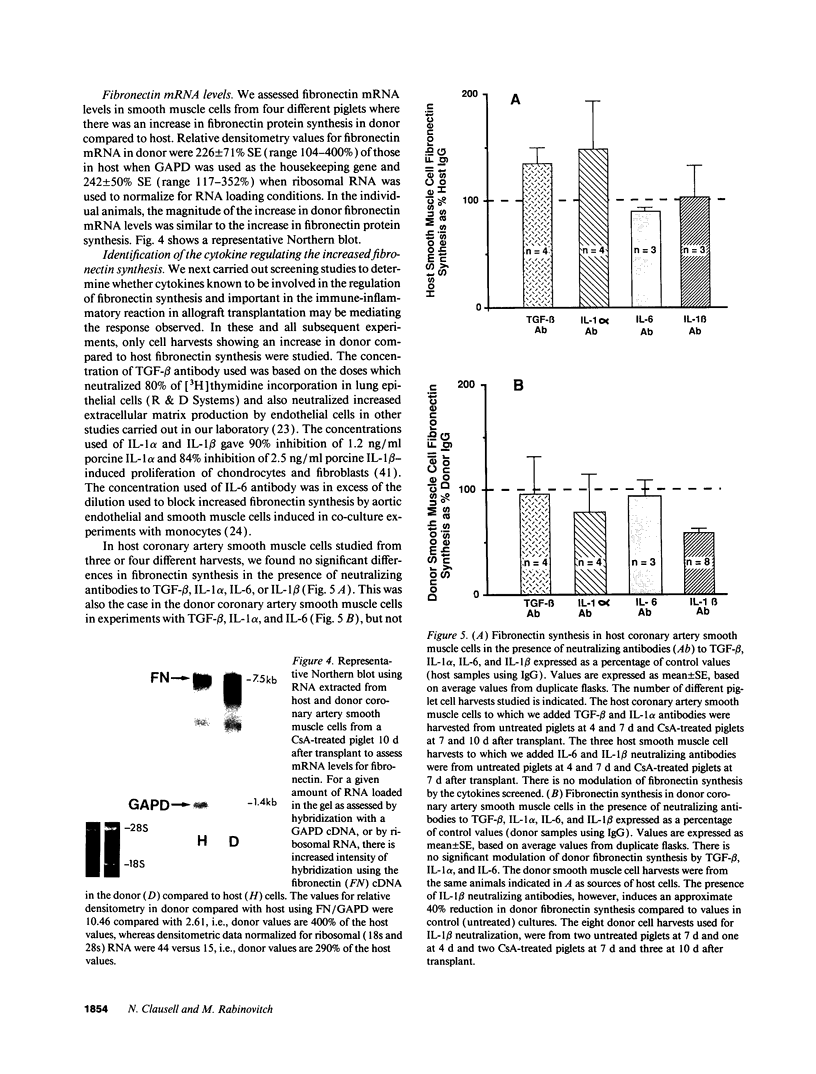
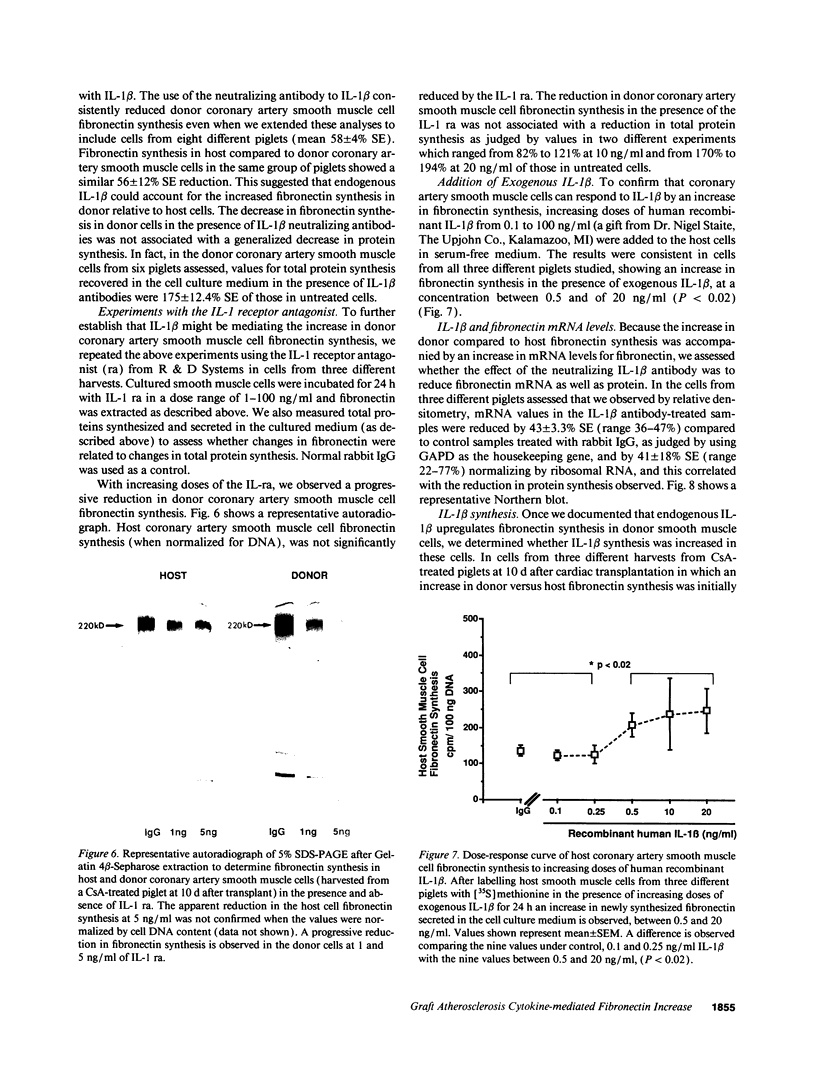
We previously described in piglets after heterotopic cardiac transplantation the early development of a coronary arteriopathy characterized by increased immunostaining for fibronectin and interleukin-1 beta (IL-1 beta) in the vessel wall. The objective of this study was to culture smooth muscle cells from donor and host coronary arteries in these piglets to determine whether donor cells produce more fibronectin than host cells as judged by increased protein and mRNA levels, and whether IL-1 beta may be regulating this increase by an autocrine mechanism involving increased production of the cytokine. We documented increased donor coronary artery smooth muscle cell fibronectin protein synthesis and mRNA compared to host. By using neutralizing antibodies to IL-1 beta, fibronectin protein synthesis and mRNA levels were reduced in donor cells to the levels observed in the host cells and a similar reduction in synthesis was observed with the IL-1 receptor antagonist. Immunoprecipitation of newly synthesized IL-1 beta revealed increased endogenous levels in donor compared to host cells. We therefore suggest in the coronary arteriopathy a pathophysiologic mechanism whereby IL-1 beta-mediated increased fibronectin synthesis may promote lymphocyte trapping and migration of medial smooth muscle cells leading to progressive intimal thickening associated with the post-cardiac transplant coronary arteriopathy.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Humphries M. J. Use of synthetic peptides to probe lymphocyte--high endothelial cell interactions. Lymphocytes recognize a ligand on the endothelial surface which contains the CS1 adhesion motif. Int Immunol. 1990;2(10):921–928. doi: 10.1093/intimm/2.10.921. [DOI] [PubMed] [Google Scholar]
- Arend W. P. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J Clin Invest. 1991 Nov;88(5):1445–1451. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber C. P., Hunt S. A., Schwinn D. A., Jamieson S. A., Reitz B. A., Oyer P. E., Shumway N. E., Stinson E. B. Complications in long-term survivors of cardiac transplantation. Transplant Proc. 1981 Mar;13(1 Pt 1):207–211. [PubMed] [Google Scholar]
- Boudreau N., Clausell N., Boyle J., Rabinovitch M. Transforming growth factor-beta regulates increased ductus arteriosus endothelial glycosaminoglycan synthesis and a post-transcriptional mechanism controls increased smooth muscle fibronectin, features associated with intimal proliferation. Lab Invest. 1992 Sep;67(3):350–359. [PubMed] [Google Scholar]
- Boudreau N., Rabinovitch M. Developmentally regulated changes in extracellular matrix in endothelial and smooth muscle cells in the ductus arteriosus may be related to intimal proliferation. Lab Invest. 1991 Feb;64(2):187–199. [PubMed] [Google Scholar]
- Boudreau N., Turley E., Rabinovitch M. Fibronectin, hyaluronan, and a hyaluronan binding protein contribute to increased ductus arteriosus smooth muscle cell migration. Dev Biol. 1991 Feb;143(2):235–247. doi: 10.1016/0012-1606(91)90074-d. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clausell N., Molossi S., Rabinovitch M. Increased interleukin-1 beta and fibronectin expression are early features of the development of the postcardiac transplant coronary arteriopathy in piglets. Am J Pathol. 1993 Jun;142(6):1772–1786. [PMC free article] [PubMed] [Google Scholar]
- Copp A. J., Bernfield M. Accumulation of basement membrane-associated hyaluronate is reduced in the posterior neuropore region of mutant (curly tail) mouse embryos developing spinal neural tube defects. Dev Biol. 1988 Dec;130(2):583–590. doi: 10.1016/0012-1606(88)90353-3. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Clark G. J. Cytokines and their receptors in transplantation. Curr Opin Immunol. 1991 Oct;3(5):729–734. doi: 10.1016/0952-7915(91)90104-9. [DOI] [PubMed] [Google Scholar]
- Dasmahapatra H. K., Wilson G. J., David S. L., Kielmanowicz S., Kent G., Gokhale S. N., Gorczynski R., Shore A., Coles J. G. A new technique of experimental heterotopic cardiac transplantation. Cardiovasc Res. 1988 Apr;22(4):296–299. doi: 10.1093/cvr/22.4.296. [DOI] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Patterns of fibronectin gene expression and splicing during cell migration in chicken embryos. Development. 1988 Nov;104(3):369–382. doi: 10.1242/dev.104.3.369. [DOI] [PubMed] [Google Scholar]
- Glukhova M. A., Frid M. G., Shekhonin B. V., Vasilevskaya T. D., Grunwald J., Saginati M., Koteliansky V. E. Expression of extra domain A fibronectin sequence in vascular smooth muscle cells is phenotype dependent. J Cell Biol. 1989 Jul;109(1):357–366. doi: 10.1083/jcb.109.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Hedin U., Bottger B. A., Forsberg E., Johansson S., Thyberg J. Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol. 1988 Jul;107(1):307–319. doi: 10.1083/jcb.107.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A., Boyle J., Rabinovitch M. Vascular smooth muscle cell detachment from elastin and migration through elastic laminae is promoted by chondroitin sulfate-induced "shedding" of the 67-kDa cell surface elastin binding protein. Exp Cell Res. 1992 Dec;203(2):344–353. doi: 10.1016/0014-4827(92)90008-v. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Kirkman R. L., Colvin R. B., Flye M. W., Leight G. S., Rosenberg S. A., Williams G. M., Sachs D. H. Transplantation in miniature swine. VI. Factors influencing survival of renal allografts. Transplantation. 1979 Jul;28(1):18–23. [PubMed] [Google Scholar]
- Kramer R. H., Fuh G. M., Bensch K. G., Karasek M. A. Synthesis of extracellular matrix glycoproteins by cultured microvascular endothelial cells isolated from the dermis of neonatal and adult skin. J Cell Physiol. 1985 Apr;123(1):1–9. doi: 10.1002/jcp.1041230102. [DOI] [PubMed] [Google Scholar]
- Kuijpers T. W., Hakkert B. C., Hoogerwerf M., Leeuwenberg J. F., Roos D. Role of endothelial leukocyte adhesion molecule-1 and platelet-activating factor in neutrophil adherence to IL-1-prestimulated endothelial cells. Endothelial leukocyte adhesion molecule-1-mediated CD18 activation. J Immunol. 1991 Aug 15;147(4):1369–1376. [PubMed] [Google Scholar]
- Lee Y. J., Radhakrishnamurthy B., Dalferes E. R., Jr, Berenson G. S. Fractionation of aorta glycosaminoglycans by high-performance liquid chromatography. J Chromatogr. 1987 Aug 7;419:275–279. doi: 10.1016/0378-4347(87)80286-4. [DOI] [PubMed] [Google Scholar]
- Li Y. Y., Cheung H. T. Basement membrane and its components on lymphocyte adhesion, migration, and proliferation. J Immunol. 1992 Nov 15;149(10):3174–3181. [PubMed] [Google Scholar]
- Libby P., Warner S. J., Friedman G. B. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988 Feb;81(2):487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linask K. K., Lash J. W. A role for fibronectin in the migration of avian precardiac cells. I. Dose-dependent effects of fibronectin antibody. Dev Biol. 1988 Oct;129(2):315–323. doi: 10.1016/0012-1606(88)90378-8. [DOI] [PubMed] [Google Scholar]
- Loppnow H., Libby P. Functional significance of human vascular smooth muscle cell-derived interleukin 1 in paracrine and autocrine regulation pathways. Exp Cell Res. 1992 Feb;198(2):283–290. doi: 10.1016/0014-4827(92)90381-h. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Yamada A., Kay J., Yamada K. M., Akiyama S. K., Schlossman S. F., Morimoto C. Activation of CD4 cells by fibronectin and anti-CD3 antibody. A synergistic effect mediated by the VLA-5 fibronectin receptor complex. J Exp Med. 1989 Oct 1;170(4):1133–1148. doi: 10.1084/jem.170.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury C. P., Teppo A. M. Serum immunoreactive interleukin 1 in renal transplant recipients. Association of raised levels with graft rejection episodes. Transplantation. 1988 Jan;45(1):143–147. doi: 10.1097/00007890-198801000-00031. [DOI] [PubMed] [Google Scholar]
- Mobley J. L., Dailey M. O. Regulation of adhesion molecule expression by CD8 T cells in vivo. I. Differential regulation of gp90MEL-14 (LECAM-1), Pgp-1, LFA-1, and VLA-4 alpha during the differentiation of cytotoxic T lymphocytes induced by allografts. J Immunol. 1992 Apr 15;148(8):2348–2356. [PubMed] [Google Scholar]
- Moyer C. F., Sajuthi D., Tulli H., Williams J. K. Synthesis of IL-1 alpha and IL-1 beta by arterial cells in atherosclerosis. Am J Pathol. 1991 Apr;138(4):951–960. [PMC free article] [PubMed] [Google Scholar]
- Muller D. W., Ellis S. G., Topol E. J. Experimental models of coronary artery restenosis. J Am Coll Cardiol. 1992 Feb;19(2):418–432. doi: 10.1016/0735-1097(92)90500-m. [DOI] [PubMed] [Google Scholar]
- Navab M., Liao F., Hough G. P., Ross L. A., Van Lenten B. J., Rajavashisth T. B., Lusis A. J., Laks H., Drinkwater D. C., Fogelman A. M. Interaction of monocytes with cocultures of human aortic wall cells involves interleukins 1 and 6 with marked increases in connexin43 message. J Clin Invest. 1991 May;87(5):1763–1772. doi: 10.1172/JCI115195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K., Björk P., Bergenfeldt M., Hageman R., Thompson R. C. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990 Dec 6;348(6301):550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- Pescovitz M. D., Thistlethwaite J. R., Jr, Auchincloss H., Jr, Ildstad S. T., Sharp T. G., Terrill R., Sachs D. H. Effect of class II antigen matching on renal allograft survival in miniature swine. J Exp Med. 1984 Nov 1;160(5):1495–1508. doi: 10.1084/jem.160.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Smith G. N., Jr, Lachman L. B., Endres R. O., Poppleton H. M., Hasty K. A., Seyer J. M., Kang A. H. Stimulation of glycosaminoglycan synthesis in cultured human dermal fibroblasts by interleukin 1. Induction of hyaluronic acid synthesis by natural and recombinant interleukin 1s and synthetic interleukin 1 beta peptide 163-171. J Clin Invest. 1989 Feb;83(2):629–636. doi: 10.1172/JCI113927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M., Beharry S., Bothwell T., Jackowski G. Qualitative and quantitative differences in protein synthesis comparing fetal lamb ductus arteriosus endothelium and smooth muscle with cells from adjacent vascular sites. Dev Biol. 1988 Nov;130(1):250–258. doi: 10.1016/0012-1606(88)90431-9. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Rizzino A. Transforming growth factor-beta: multiple effects on cell differentiation and extracellular matrices. Dev Biol. 1988 Dec;130(2):411–422. doi: 10.1016/0012-1606(88)90337-5. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J., Sarsfield S. J., Townsend Y. Pig interleukin 1. Purification of two immunologically different leukocyte proteins that cause cartilage resorption, lymphocyte activation, and fever. J Exp Med. 1985 Oct 1;162(4):1208–1222. doi: 10.1084/jem.162.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhonin B. V., Domogatsky S. P., Idelson G. L., Koteliansky V. E., Rukosuev V. S. Relative distribution of fibronectin and type I, III, IV, V collagens in normal and atherosclerotic intima of human arteries. Atherosclerosis. 1987 Sep;67(1):9–16. doi: 10.1016/0021-9150(87)90259-0. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J Immunol. 1990 Jul 1;145(1):59–67. [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Roles of adhesion molecules in T-cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990 Apr;114:109–143. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Shingu M., Nobunaga M., Ezaki I., Yoshioka K. Recombinant human IL-1 beta and TNF-alpha stimulate production of IL-1 alpha and IL-1 beta by vascular smooth muscle cells and IL-1 alpha by vascular endothelial cells. Life Sci. 1991;49(3):241–246. doi: 10.1016/0024-3205(91)90009-z. [DOI] [PubMed] [Google Scholar]
- Uretsky B. F., Murali S., Reddy P. S., Rabin B., Lee A., Griffith B. P., Hardesty R. L., Trento A., Bahnson H. T. Development of coronary artery disease in cardiac transplant patients receiving immunosuppressive therapy with cyclosporine and prednisone. Circulation. 1987 Oct;76(4):827–834. doi: 10.1161/01.cir.76.4.827. [DOI] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J Exp Med. 1987 May 1;165(5):1316–1331. doi: 10.1084/jem.165.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West D. C., Sattar A., Kumar S. A simplified in situ solubilization procedure for the determination of DNA and cell number in tissue cultured mammalian cells. Anal Biochem. 1985 Jun;147(2):289–295. doi: 10.1016/0003-2697(85)90274-x. [DOI] [PubMed] [Google Scholar]
- Wight T. N. Cell biology of arterial proteoglycans. Arteriosclerosis. 1989 Jan-Feb;9(1):1–20. doi: 10.1161/01.atv.9.1.1. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Maeno M., Hawrylyshyn B., Yao K. L., Domenicucci C., Sodek J. Differential effects of transforming growth factor-beta on the synthesis of extracellular matrix proteins by normal fetal rat calvarial bone cell populations. J Cell Biol. 1988 Mar;106(3):915–924. doi: 10.1083/jcb.106.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa M., Tilney N. L., Kupiec-Weglinski J. W. Recognition of self within self: specific lymphocyte positioning and the extracellular matrix. Immunol Today. 1991 Aug;12(8):262–266. doi: 10.1016/0167-5699(91)90123-B. [DOI] [PubMed] [Google Scholar]
- van Seventer G. A., Shimizu Y., Shaw S. Roles of multiple accessory molecules in T-cell activation. Curr Opin Immunol. 1991 Jun;3(3):294–303. doi: 10.1016/0952-7915(91)90027-x. [DOI] [PubMed] [Google Scholar]