Abstract
Background
Poor muscle strength is a major public health concern in older persons, predisposing to functional limitations, increased fall risk, and higher mortality. Understanding risk factors for muscle strength decline may offer opportunities for prevention and treatment. One of the possible causes of muscle strength decline is imbalance between catabolic and anabolic signaling. This study aims to examine whether high levels of multiple catabolic and low levels of multiple anabolic biomarkers predict accelerated decline of muscle strength.
Methods
In a representative sample of 716 men and women aged ≥65 years in the InCHIANTI study we measured C-reactive protein, interleukin-6 (IL-6), IL-1 receptor antagonist (IL-1RA), tumor necrosis factor-α receptor 1 as well as dehydroepiandrosterone sulfate (DHEA-S), insulin-like growth factor-1, and bioavailable testosterone. Biomarker values were divided into tertiles and the numbers of catabolic/anabolic biomarkers in the highest/lowest tertile were calculated. Hand-grip strength was measured at baseline and 3- and 6-year follow up.
Results
In adjusted linear mixed models, higher concentration of IL-6 (p = 0.02) and IL-1RA (p = 0.04) as well as lower levels of DHEA-S (p = 0.01) predicted muscle strength decline. After combining all inflammatory markers, the rate of decline in grip strength was progressively greater with the increasing number of dysregulated catabolic biomarkers (p = 0.01). No effect on accelerated muscle strength decline was seen according to number of dysregulated anabolic hormones.
Conclusions
Having multiple elevated catabolic biomarkers is a better predictor of muscle strength decline than a single biomarker alone, suggesting that a catabolic dysregulation is at the core of the mechanism leading to muscle strength decline with aging.
Introduction
Poor muscle strength is a major public health concern in older persons because it predisposes to functional limitations, increased fall risk, and higher mortality.1–3 Thus, understanding risk factors for muscle strength decline may offer opportunities for prevention and treatment. The most important cause for a decline in muscle strength with aging is the tendency to acquire a physically sedentary behavior. Beyond the effects of sedentary state, nutritional deficiencies, chronic diseases, age-related changes in immunological, and neural and hormonal signals also contribute to the progressive muscle impairment with aging.4
An extensive literature has shown that the proinflammatory state that often affects older persons is associated with decreased muscle strength,5–7 but longitudinal studies that addressed this relationship have produced inconsistent findings.5–8 High concentrations of inflammatory markers such as interleukin-6 (IL-6) and C-reactive protein (CRP) are associated with decline in physical function, measured by walking speed, and predict the development of mobility disability, disability in activities of daily living, and mortality.9,10 Visser et al.9 and Reuben et al.10 reported that multiple elevated inflammatory markers have an additive negative effect on muscle impairment and decline in physical function. These findings suggest that the effect of inflammatory markers is additive, perhaps through increasing catabolic signaling. If this hypothesis is correct, then the effect of inflammatory markers should be examined in the context of the multiple pathways that modulate the balance between anabolism and catabolism in the muscle tissue.
In addition to proinflammatory state, aging is associated with a gradual decline in several anabolic steroids and somatotrophic hormones, especially in men.11 Low levels of anabolic hormones have found to be associated with low muscle mass and strength in older persons,12–14 but whether the level of single hormones predicts accelerated decline of muscle strength and physical performance in older persons is not clear.15 In an analogous matter as multiple elevated inflammatory markers are associated with risk of accelerated physical performance decline, we hypothesize that the effect of anabolic hormones on muscle impairment can be detected only by looking at multiple hormones dysregulation. Recent studies have shown that multiple anabolic hormone deficiency, and not single hormone levels, is associated with frailty16 and predicts mortality in the older population.17,18 However, no study has examined the effect of multiple anabolic hormones dysregulation on muscle strength decline. To address a gap in the literature, the aim of this prospective study is to examine the hypotheses that high levels of multiple catabolic and low levels of multiple anabolic biomarkers predict muscle strength decline in older men and women.
Methods
Study population
The InCHIANTI is an epidemiological study of a representative sample of 500 men and 760 women aged 65 years and older living in Tuscany, Italy. We selected from this population 862 persons who had data on the biomarkers examined in this paper and hand-grip strength at baseline. Of these, 146 did not have either 3- or 6-year follow-up data: 96 died, 44 refused or were unable to participate in the study, and 6 moved away from the area during the 6 years of follow up. Thus, the final analytical sample included was 716 participants. The baseline data were collected in 1998–2000; the 3-year follow up took place in 2001–2003 and the 6-year follow up in 2004–2006. The design of the study and data collection have been previously described in detail.19
Participants received an extensive description of the study and participated after providing written informed consent. The Italian National Institute of Research and Care on Aging Ethical Committee approved the study protocol, which complied with the principles stated in the Declaration of Helsinki.
Assessment of physical performance
Maximal isometric hand grip strength was measured in kilograms using a hand-held dynamometer (hydraulic hand “BASELINE”; Smith & Nephew, Agrate Brianza, Milan, Italy). The subject was seated in front of a bench with the tested arm supported on the bench and the elbow flexed to 45°. Participants were asked to perform the task twice with each hand, and the maximum strength attained during the four trials was used for the present analyses. Grip strength was chosen as an indicator of overall muscle strength because of its excellent reproducibility.18 There is also strong evidence that grip strength is highly correlated with strength of other muscle groups19 and is a strong correlate of mobility disability in older persons.20
To measure walking speed, subjects were asked to walk 4 meters at their usual pace as if they were walking down the street, starting from a standing position. Use of a cane or walker was permitted. Walking speed is a valid and widely used measure of mobility limitation for both healthy and impaired older persons21 with high predictive validity for subsequent disability, hospitalization, and mortality.22,23
Grip strength and walking speed measurements were performed using identical methods at baseline and at 3- and 6-year follow ups, and all follow-up information was used in the analyses.
Assessment of biomarkers
Baseline blood samples were collected in the morning after a 12-h fast and after a 15-min rest. Serum and plasma were stored in a deep freezer at −80°C and were not thawed until analyzed. The catabolic and anabolic biomarkers used in this study were chosen a priori, based on the current knowledge about the association of different biomarkers and muscle strength.
Catabolic biomarkers
Catabolic biomarkers used in this study were all markers of inflammation. High-sensitivity (hs) CRP levels were measured by enzyme-linked immunoabsorbent assay (ELISA) using purified protein and polyclonal anti-CRP antibodies (Roche Diagnostics, GmbH, Mannheim, Germany). For hsCRP, the minimum detectable concentration (MDC) was 0.03 mg/L and the interassay coefficient of variation (CV) was 5%. Serum IL-6, interleukin-1 receptor antagonist (IL-1RA), and tumor necrosis factor-α receptor 1 (TNF-αR1) concentrations were determined by high-sensitivity ELISA using commercial kits (Human Ultrasensitive, BioSource International Inc., Camarillo, CA). For IL-6, the MDC was 0.1 pg/mL, and the interassay CV 4.5%. For IL-1RA the MDC was 4.00 pg/mL and the mean interassay CV 8.2%. For TNF-αR1, the MDC was approximately 8 pg/mL and the interassay CV 10%. IL-1RA and TNF-αR1 were used instead of IL-1 and TNF-α because measurements of soluble receptors are more sensitive and easier than those for the respective cytokines.24,25 All cytokine assays were done in duplicate and were repeated if the second measure was >10% or <10% compared to the first. The average of the two measures was used in the analyses.
Anabolic biomarkers
Dehydroepiandrosterone sulfate (DHEA-S), total insulin-like growth factor-1 (IGF-1), and total testosterone concentrations were measured in duplicate by immunoradiometric assays, using commercial reagents (Diagnostic Systems Laboratories Corporation, Webster, TX). For DHEA-S, the MDC was 1.7 μg/dL; intraassay and interassay CV values for three different concentrations were 4.1%, 5.3%, and 4.7%, and 4.8%, 7.0%, and 4.6%, respectively. For total IGF-1, the MDC was 0.80 ng/mL and interassay and intraassay CV values for three concentrations were all less than 10%. For total testosterone, the MDC was 0.86 ng/dL; intraassay and inter assay CV values for three different concentrations (low, medium, and high) were 9.6%, 8.1%, and 7.8% and 8.6%, 9.1%, and 8.4%, respectively. Concentrations of bioavailable testosterone (serum-free and albumin-bound testosterone, but not sex hormone-binding globulin) were calculated using the Vermeulen formula.
Because there are no commonly used cutoffs to describe elevated inflammation or deficiency in anabolic hormones in this age group, we divided values into tertiles based on their distribution. To take into account different levels of anabolic hormones in men and women, gender-specific tertiles of all biomarkers were used. Tertile limits for men and women are shown in Table 2 (below). In inflammatory markers, the highest tertile indicates the “highest catabolic state” and in anabolic markers the lowest tertile indicates the “lowest anabolic state.”
Table 2.
The Effect of Catabolic and Anabolic Biomarkers on Grip Strength Decline: Linear Mixed-Effect Model
| |
|
Model 1a |
Model 2a |
||
|---|---|---|---|---|---|
| β Estimateb | SE | β Estimateb | SE | ||
| Catabolic markers | |||||
| Interleukin-6, pg/mL | |||||
| I | M: <1.1 | 0.02 | 0.08 | 0.01 | 0.09 |
| F: <1.0 | |||||
| II | M: 1.1–1.9 | −0.29 | 0.09 | −0.23 | 0.09 |
| F: 1.0–1.7 | |||||
| III | M: >1.9 | −0.33 | 0.09 | −0.33 | 0.09 |
| F: >1.7 | |||||
| p for trend | 0.01 | 0.02 | |||
| Interleukin-1 receptor antagonist, pg/mL | |||||
| I | M: <107.9 | −0.25 | 0.09 | −0.24 | 0.09 |
| F: <105.1 | |||||
| II | M: 107.9–161.4 | −0.02 | 0.09 | 0.005 | 0.09 |
| F: 105.1–163.4 | |||||
| III | M: >161.4 | −0.31 | 0.08 | −0.29 | 0.09 |
| F: >163.4 | |||||
| p for trend | 0.04 | 0.04 | |||
| C-reactive protein, mg/L | |||||
| I | M: <1.5 | −0.19 | 0.09 | −0.17 | 0.09 |
| F: <1.6 | |||||
| II | M: 1.5–3.9 | −0.14 | 0.09 | −0.13 | 0.09 |
| F: 1.6–4.2 | |||||
| III | M: >3.9 | −0.23 | 0.09 | −0.19 | 0.09 |
| F: >4.2 | |||||
| p for trend | 0.78 | 0.88 | |||
| Tumor necrosis factor-α receptor 1, pg/mL | |||||
| I | M: <1166.6 | −0.12 | 0.09 | −0.12 | 0.09 |
| F: <1128.3 | |||||
| II | M: 1166.6–1569.2 | −0.21 | 0.09 | −0.19 | 0.09 |
| F: 1128.3–1479.7 | |||||
| III | M: >1569.2 | −0.23 | 0.09 | −0.20 | 0.09 |
| F: >1479.7 | |||||
| p for trend | 0.62 | 0.80 | |||
| Anabolic markers | |||||
| Dehydroepiandrosterone sulfate, μg/dL | |||||
| I | M: <53.9 | −0.39 | 0.09 | −0.41 | 0.09 |
| F: <42.7 | |||||
| II | M: 53.9–93.7 | −0.12 | 0.09 | −0.07 | 0.09 |
| F: 42.7–92.3 | |||||
| III | M: >93.7 | −0.10 | 0.08 | −0.07 | 0.09 |
| F: >92.3 | |||||
| p for trend | 0.03 | 0.01 | |||
| Total insulin-like growth factor 1, ng/mL | |||||
| I | M: <100.6 | −0.15 | 0.09 | −0.12 | 0.09 |
| F: <85.7 | |||||
| II | M: 100.6–148.4 | −0.06 | 0.09 | −0.04 | 0.09 |
| F: 85.7–128.4 | |||||
| III | M: >148.4 | −0.34 | 0.09 | −0.35 | 0.09 |
| F: >128.4 | |||||
| p for trend | 0.06 | 0.04 | |||
| Bioavailable testosterone, ng/dL | |||||
| I | M: <77.0 | −0.09 | 0.09 | −0.02 | 0.09 |
| F: <7.1 | |||||
| II | M: 77.0–109.0 | −0.32 | 0.09 | −0.36 | 0.09 |
| F: 7.1–13.3 | |||||
| III | M: >109.0 | −0.19 | 0.09 | −0.17 | 0.09 |
| F: >13.3 | |||||
| p for trend | 0.18 | 0.03 | |||
Model 1 is adjusted for age and sex. Model 2 is additionally adjusted for physical activity, daily energy intake, smoking, body mass index, coronary heart disease, diabetes, asthma, chronic bronchitis, and knee osteoarthritis.
Beta estimates for different biomarker tertile are derived from linear mixed-effect models and they indicate the average change in hand grip strength change per year in that specific category.
Abbreviations: M, Male; F, female; SE, Standard error.
Covariates and other variables
Diseases were ascertained by a trained geriatrician according to standard, preestablished criteria and algorithms, based on those used in the Women's Health and Aging Study, that combine information from self-reported physician diagnoses, current pharmacological treatment, medical records, clinical examinations, and blood tests.24 The following diseases were included in the current analyses: Coronary heart disease, diabetes, asthma, chronic bronchitis, and knee osteoarthritis.
Body mass index (BMI) was calculated as measured weight in kilograms divided by measured height in meters squared (kg/m2). Waist circumference was measured at the midpoint between the lower rib margin and the iliac crest. The level of physical activity in the 12 months prior to the interview was assessed through an interviewer-administered questionnaire25 and was coded as sedentary (inactivity or light-intensity activity less than 1 h/week), light physical activity (light-intensity activity 2–4 h/week), and moderate-high physical activity (light-intensity activity at least 5 h/week or moderate activity at least 1–2 h/week). Average daily intake of energy (kcal) was estimated using the European Prospective Investigation into Cancer and Nutrition food frequency questionnaire. Smoking history was determined based on self-reports and categorized into never smokers, former smokers, and current smokers.
Statistical analysis
Study population characteristics according to sex are reported as mean and median values for continuous variables and proportions for categorical variables. Sex differences were examined with the chi-squared test for categorical variables, Kruskal–Wallis test for skewed continuous variables, and t-test for normally distributed continuous variables. The association of individual catabolic and anabolic biomarkers and the combination of these biomarkers on the magnitude of muscle strength decline over the follow-up time was calculated with linear mixed-effect regression models by using compound symmetry as the covariance structure. The analysis was completed with the MIXED procedure in SAS. The major advantages of using mixed models in longitudinal studies are that the technique takes into account the correlation between serial measures obtained in the same subjects as well as the missing observations, thus allowing all available data on each subject to be used.26,27 Thus, in this study, we were able to use muscle strength information from baseline, 3-year follow-up, and 6 year follow-up measurements. In the results, we will present the beta estimates for each comparable group indicating the slope (change per year). Models were adjusted for baseline age, sex, physical activity, daily energy intake, smoking, BMI, coronary heart disease, diabetes, asthma, chronic bronchitis, and knee osteoarthritis.
Furthermore, to describe the change in absolute terms, the change in muscle strength over 6-year follow up was calculated by subtracting the 6-year follow-up value from the baseline value. The association of multiple inflammatory and anabolic biomarkers on absolute change in muscle strength was calculated with analysis of covariance using generalized linear models (GLM). The models were adjusted for baseline muscle strength as well as for the above-mentioned covariates. Adjusted means are reported as the least-square means estimating the marginal means over a balanced population. To test for trend, categorical variables were entered in the linear regression model as ordinal variables.
To examine whether catabolic and anabolic biomarkers have different effect of muscle strength decline in men and women, an interaction term “sex*biomarker” was included in the linear mixed-effect models. All of the interaction terms with individual biomarkers and with an ordinal variable for multiple dysregulated catabolic and anabolic biomarkers were nonsignificant. Thus, men and women were combined in our analysis, and analyses were adjusted for sex. Finally, interactions between cumulative anabolic and catabolic biomarkers on muscle strength decline were tested, as well as potential age, physical activity, and BMI differences in the studied relationship. No statistically significant interactions were found. The SAS 9.1 Statistical Package was used for all analyses (SAS Institute, Inc., Cary, NC).
Results
Mean ages of male and female study participants were 73.1 [standard deviation (SD) 6.4] and 74.2 (6.6) years, respectively. In general, men had more chronic diseases than women, except for knee osteoarthritis, which was more common in women. The median level of catabolic biomarkers was similar for men and women, except that IL-6 was higher in men than in women (p = 0.02). The median level of the anabolic biomarkers was higher in men than in women. The average annual decline in grip strength was −0.4 kg (SD 1.9) in men and −0.02 kg (1.5) in women. Other baseline characteristics are shown in Table 1.
Table 1.
Baseline Characteristics of the Study Population by gender (N = 716)
| |
Men (n = 310) |
Women (n = 406) |
|
||
|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | pa |
| Age, years | 73.1 | 6.4 | 74.2 | 6.6 | 0.03 |
| Body mass index, kg/m2 | 26.8 | 3.2 | 28.0 | 4.5 | <0.001 |
| Waist circumference, cm | 94.8 | 8.7 | 90.9 | 10.7 | <0.001 |
| Total energy intake, kcal/day | 2224.6 | 545.3 | 1760.4 | 488.6 | <0.001 |
| Baseline grip strength, kg | 38.8 | 10.2 | 22.2 | 7.6 | <0.001 |
| Grip strength decline, kg |
−0.4 |
1.9 |
−0.02 |
1.5 |
<0.001 |
| |
Median |
IQR |
Median |
IQR |
|
| Interleukin-6, pg/mL | 1.5 | 0.9–2.3 | 1.3 | 0.8–2.0 | 0.02 |
| Interleukin-1 receptor antagonist, pg/mL | 133.0 | 98.0–180.6 | 131.9 | 97.5–178.7 | 0.98 |
| C-reactive protein, mg/L | 2.8 | 1.2–5.0 | 2.4 | 1.3–5.4 | 0.64 |
| Tumor necrosis factor-α receptor 1, pg/mL | 1337.5 | 1080.2–1686.6 | 1273.4 | 1056.7–1563.8 | 0.96 |
| Dehydroepiandrosterone sulfate, μg/dL | 71.3 | 44.2–112.3 | 61.6 | 34.7–112.4 | 0.07 |
| Insulin-like growth factor 1, ng/mL | 124.5 | 90.0–160.5 | 103.4 | 74.6–142.7 | <0.001 |
| Bioavailable testosterone, ng/dL |
92.1 |
71.4–120.4 |
9.5 |
5.9–15.2 |
<0.001 |
| |
% |
|
% |
|
|
| Current smoking | 23 | 7.6 | |||
| Physical activity | <0.001 | ||||
| Sedentary | 8.4 | 21.6 | <0.001 | ||
| Moderate | 34.6 | 53.3 | |||
| Active | 57.0 | 25.1 | |||
| Coronary heart disease | 9.5 | 4.9 | 0.02 | ||
| Diabetes | 14.5 | 11.1 | 0.17 | ||
| Asthma | 8.1 | 2.5 | 0.001 | ||
| Chronic bronchitis | 16.1 | 1.0 | <0.001 | ||
| Knee osteoarthritis | 3.6 | 11.1 | <0.001 | ||
Values are shown in mean (SD) and medians (IQR) for continuous variables and N (%) for categorical variables.
Comparisons across groups were examined with chi-squared test for categorical variables, Kruskal–Wallis test for skewed continuous variables, and t-test for normally distributed continuous variables.
Abbreviations: SD, Standard deviation; IQR, interquartile range.
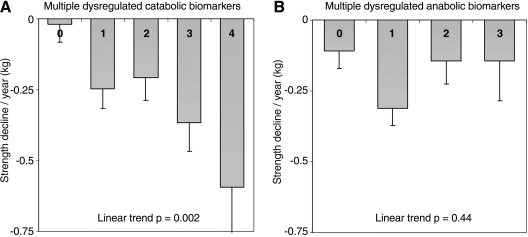
In Table 2 the annual grip strength decline in different biomarker tertile is presented based on the linear mixed-effect models adjusted for multiple lifestyle factors and diseases. Of the catabolic biomarkers, increasing levels of IL-6 was significantly associated with greater grip strength decline: tertile I, 0.01 kg/year; tertile II, −0.23 kg/year; and tertile III, −0.33 kg/year (p for trend 0.02). A higher concentration of IL-1RA was also associated with greater grip strength decline (p for trend 0.04). Although both CRP and TNF-αR1 showed a slight trend toward greater strength decline, the trends were not statistically significant. After combining all catabolic biomarkers, the rate of decline in grip strength was progressively greater with the increasing number of catabolic biomarkers in the highest serum tertile levels (Table 3 and Fig. 1A). Participants with high levels in one or more biomarkers experienced significantly greater (p < 0.05) decline in grip strength compared to those without increased catabolic biomarkers. The effect remained statistically significant after adjusting for lifestyle factors and chronic diseases.
Table 3.
The Effect of Multiple Dysregulated Catabolic and Anabolic Biomarkers on Grip Strength Decline. Linear Mixed-Effect Model
| |
|
Model 1a |
Model 2a |
||
|---|---|---|---|---|---|
| N | β Estimateb | SE | β Estimateb | SE | |
| Catabolic biomarkersc | |||||
| 0 | 227 | −0.05 | 0.09 | −0.07 | 0.09 |
| 1 | 204 | −0.25 | 0.09 | −0.19 | 0.09 |
| 2 | 153 | −0.24 | 0.11 | −0.22 | 0.11 |
| 3 | 95 | −0.26 | 0.14 | −0.25 | 0.14 |
| 4 | 37 | −0.44 | 0.22 | −0.41 | 0.22 |
| p for trend | 0.03 | 0.01 | |||
| Anabolic biomarkersd | |||||
| 0 | 263 | −0.09 | 0.08 | −0.07 | 0.08 |
| 1 | 247 | −0.35 | 0.08 | −0.37 | 0.09 |
| 2 | 150 | −0.19 | 0.11 | −0.13 | 0.11 |
| 3 | 51 | −0.01 | 0.19 | 0.02 | 0.20 |
| p for trend | 0.11 | 0.05 | |||
Model 1 is adjusted for age and sex. Model 2 is additionally adjusted for physical activity, daily energy intake, smoking, body mass index, coronary heart disease, diabetes, asthma, chronic bronchitis, and knee osteoarthritis.
Beta estimates for different biomarker tertile are derived from linear mixed-effect models and they indicate the average change in hand grip strength change per year in that specific category.
Number of catabolic biomarkers in the highest tertile (CRP, IL-6, IL-1RA, TNF-αR1).
Number of anabolic biomarkers in the lowest tertile (DHEA-S, IGF-1, bioavailable testosterone).
Abbreviations: SE, Standard error; CRP, C-reactive protein; IL-1RA, interleukin-1 receptor antagonist; TNF-αR1, tumor necrosis factor-α receptor.
FIG. 1.
The mean strength decline according to the number of dysregulated biomarkers. Adjusted for age, sex, baseline grip strength, physical activity, daily energy intake, smoking, body mass index, coronary heart disease, diabetes, asthma, chronic bronchitis, and knee osteoarthritis. (A) Multiple dysregulated catabolic biomarkers. (B) Multiple dysregulated anabolic biomarkers.
Similar analyses were conducted with anabolic hormones (Tables 2 and 3). Of the anabolic biomarkers, only decreasing levels of DHEA-S were associated with greater muscle strength decline (p for trend 0.01). Increasing IGF-1 and bioavailable testosterone values were not consistently associated with greater decline in muscle strength. Subjects who had deficiencies in one or more anabolic hormones did not show any greater decline in grip strength compared to those who did not have low anabolic hormones at all (Table 3 and Fig. 1B).
Finally, to examine whether the effect of multiple dysregulated catabolic and anabolic biomarkers on muscle strength translates into an accelerated decline in functional performance, a similar analysis was carried out using walking speed as outcome. Of the individual catabolic and anabolic markers, only increased level of TNF-αRI and lower levels of DHEA-S were associated with significant walking speed decline (p < 0.05). After combining all catabolic biomarkers, the number of catabolic markers in the highest tertile levels was associated with greater walking speed decline (p = 0.18). Similarly, the number of anabolic hormones in the lowest tertile was not significantly associated with greater walking speed decline (p = 0.06).
Discussion
This study shows that accumulated burden of multiple inflammatory markers predicts grip strength decline more than single inflammatory marker alone in older community-dwelling persons. Unexpectedly, simultaneous deficiencies in several anabolic hormones were not associated with greater strength decline than deficiency in one anabolic hormone.
Our study is based on a view that aging is intrinsically a dysregulation in multiple systems in the body. Thus, it is unlikely that much of the variability between individuals in muscle strength decline may be accounted for by changes in a single biomarker alone. By combining four different catabolic markers, we were able to show that elevation in several inflammatory factors is associated with greater decline in muscle strength. A few studies have reported similar, although cross-sectional, associations with muscle strength.6,10 The advantage of using a combination of inflammatory markers instead of only one marker is to increase the chance of capturing the severity of inflammation and possibly also its overall and interactive effect on the catabolic equilibrium.28
To explain the additive effect of multiple inflammatory markers on muscle strength, it is important to consider that inflammatory markers are not independent from each other. For example, IL-1, IL-6, and TNF-α expression is modulated mainly by the nuclear factor-κB (NF-κB),29,30 and increasing cytokines are responsible for increasing CRP levels in circulation.30 We may hypothesize that the additive effect of multiple inflammatory markers is due to different converging catabolic pathways. Another possibility is that by measuring multiple biomarkers that address the same pathway, the catabolic signal is assessed more precisely, and therefore the relationship with muscle strength decline is more robust.
We also observed in this study that the effect of multiple dysregulated catabolic biomarkers on muscle strength does not translate into an accelerated decline in more advanced physical performance, such as walking speed. Changes in walking speed are probably observed with delay, and we would have needed a longer follow up to see significant changes. In addition to muscle strength, there are many other factors affecting walking performance, such as balance and joint flexibility. This allows older people to compensate for impairment in one subsystem with good performance in other subsystems.
Although the results of the present study support the idea of catabolic markers as a prognostic tool for identifying persons at increased risk of muscle strength decline, it is still not clear whether intervention aimed at lowering these markers can prevent or alleviate the muscle strength loss that normally occurs over the aging process. So far, muscle strength training has been proved to be the most effective way to improve muscle strength or reduce strength decline.31 Furthermore, physical activity has found to be associated with lower levels of inflammatory markers.32 Although intervention studies have not provided clear evidence about the effectiveness of physical exercise in lowering inflammation,33,34 the research in this field is very active.35 Second, there is evidence that weight loss in obese persons produces a decrease in inflammatory markers through reduction of metabolically active adipose tissue. A combination of diet and exercise has proven to be most effective strategy to reduce inflammation.35
Among the tested anabolic hormones, DHEA-S was the only hormone that showed a significant association with muscle strength decline as well as decline in walking speed. This is the first population-based study confirming the longitudinal association with anabolic hormone and muscle strength decline, broadening our knowledge based on cross-sectional studies.12 DHEA and DHEA-S concentrations decline significantly with aging,36 and such a decline has complex consequences because DHEA-S acts as a prohormone to biologically active androgens and estrogens.37 Because of these premises, we expected to see an association between bioavailable testosterone and muscle strength decline as well. The nonsignificant linear association of bioavailable testosterone on muscle strength decline found in this study, although unpredicted, confirms previous findings from Schaap et al.15 In spite of the replicated evidence, this finding remains puzzling and difficult to explain with our current understanding of muscle biology.
In the light of recent research, it was unexpected that we did not see stronger association of multiple anabolic hormone deficiency on muscle strength decline. In the Women's Health and Aging Studies, Cappola et al.16 found that persons who had deficiencies in two or three hormones, including DHEA-S, IGF-1, and free testosterone, were more likely to be frail than those with deficiency in one or zero anabolic hormones. Furthermore, on the basis of the InCHIANTI Study, Maggio et al.18 showed that deficiency in multiple anabolic hormones is an independent predictor of mortality.
One explanation for why we did not see the association between burden of anabolic hormone deficiency and muscle strength decline in this study may partly be due to our outcome measure. Although the grip strength measurement in this study was performed in a maximal manner and using a highly standardized protocol, the measure may not be sensitive enough to capture the ‘true’ maximal voluntary strength and thus changes in it. In exercise physiology, maximal voluntary strength is usually defined and measured as the one repetition maximum (1 RM) in which a subject is able to lift/press only one time. However, as a proxy measure of maximal strength, grip strength has shown to be highly correlated with maximal isometric strength of other muscle groups.19 Moreover, another potential explanation for why we could not truly see a progressive effect in rate of muscle strength decline with an increasing number of deficient anabolic hormones could be that our method for summarizing the effect of multiple anabolic hormones is relatively crude. Although the same method has also been used in previous studies,17,18 detecting changes in muscle strength may require more advanced techniques to combine the effects of multiple biomarkers. The relationships and biological processes of these anabolic biomarkers that underlie the decline of muscle strength are very complex. Finally, due to the nonsignificant sex*biomarker interaction on strength decline, we combined men and women together in our analysis. Thus, despite the fact that women had significantly lower baseline levels of anabolic hormones compared to men (see Table 1), the effect on strength appears to be similar in men and women. However, in separate analyses including only men, we did not also see significant effect of multiple anabolic hormones on strength decline.
Although longitudinal design with repeated muscle strength measurements comprise the main strengths of this study, longitudinal studies especially those with long follow up have intrinsic limitations. Compared with the 716 participants included in the longitudinal analysis, those who were lost to follow up (n = 146) were older (p < 0.001) and had lower baseline muscle strength (p < 0.001), as well higher levels of CRP, IL-6, IL-1RA, and TNF-αR1 (p for all <0.01). Thus, the oldest and most disabled persons lost to follow up were also participants with the highest proinflammatory cytokine levels at baseline, probably causing underestimation of the studied associations. It is possible that with a larger sample size we could have detected a stronger association between multiple anabolic hormones and muscle strength decline.
Second, in our analyses we were only able to use the baseline information about the biomarker levels. Changes over time in biomarker levels may be stronger predictors than baseline levels, especially given the relatively long follow up. The effect of change in biomarkers on muscle strength decline should be examined in future studies and may help us in revealing the mechanisms that may lead to muscle strength decline. In addition, this being only an observational study, there is need for interventional studies to confirm the causality of elevated levels of inflammatory markers and muscle strength decline. Our findings clearly warrant further studies examining the effect of multiple catabolic and anabolic biomarkers on muscle strength decline in larger study population, possible with numerable measures of biomarkers and longer follow-up.
In conclusion, having multiple elevated inflammatory markers was found to be better predictor of muscle strength decline over a 6-year follow up than single inflammatory markers alone. These findings suggest that a catabolic dysregulation is at the core of the mechanism leading to muscle strength decline with aging. A better understanding of the potential interactions between biomarkers and their effect on muscle strength and physical performance in the older population is needed.
Acknowledgments
The InCHIANTI study baseline (1998–2000) was supported as a “targeted project” (ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336); the InCHIANTI Follow-up 1 (2001–2003) was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1-1 and N.1-AG-1-2111); the InCHIANTI Follow-up 2 study (2004–2006) was financed by the U.S. National Institute on Aging (Contract: N01-AG-5-0002);supported in part by the Intramural research program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland. This work was also supported by grant from the Finnish Academy (no. 125494 SS). The authors have no financial or personal conflicts of interest. None of the sponsoring institutions interfered with the design, methods, subject recruitment, data collections, analysis and preparation of paper.
References
- 1.Rantanen T. Harris T. Leveille SG. Visser M. Foley D. Masaki K. Guralnik JM. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55A:M168–M173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 2.Visser M. Goodpaster BH. Kritchevsky SB. Newman AB. Nevitt M. Rubin SM. Simonsick EM. Harris TB for the Health ABC Study. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:M324–M333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 3.Chan BK. Marshall LM. Winters KM. Faulkner KA. Schwartz AV. Orwoll ES. Incident fall risk and physical activity and physical performance among older men: The Osteoporotic Fractures in Men Study. Am J Epidemiol. 2007;165:696–703. doi: 10.1093/aje/kwk050. [DOI] [PubMed] [Google Scholar]
- 4.Rolland Y. Czerwinski S. Abellan Van Kan G. Morley JE. Cesari M. Onder G. Woo J. Baumgartner R. Pillard F. Boirie Y. Chumlea WMC. Vellas B. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaap LA. Pluijm SM. Deeg DJ. Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526.e529–526.e517. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 6.Taaffe DR. Harris TB. Ferrucci L. Rowe J. Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55A:M709–M715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 7.Ferrucci L. Penninx BWJH. Volpato S. Harris T. Bandeen-Roche K. Balfour J. Leveille SG. Fried LP. Guralnik JM. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 8.Schaap LA. Pluijm SM. Deeg DJ. Harris TB. Kritchevsky SB. Newman AB. Colbert LH. Pahor M. Rubin SM. Tylavsky FA, Visser M, for the Health ABC Study. Higher inflammatory marker levels in older persons: Associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009 doi: 10.1093/gerona/glp097. doi:10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visser M. Pahor M. Taaffe DR. Goodpaster BH. Simonsick EM. Newman AB. Nevitt M. Harris TB. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57A:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 10.Reuben DB.Cheh AI.Harris TB.Ferrucci L.Rowe JW.Tracy RP. SeemanTE.Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons J Am Geriatr Soc 200250638–644. [DOI] [PubMed] [Google Scholar]
- 11.Lamberts SW. van den Beld AW. van der Lely AJ. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 12.Valenti G. Denti L. Maggio M. Ceda G. Volpato S. Bandinelli S. Ceresini G. Anne Cappola A. Guralnik JM. Ferrucci L. Effect of DHEAS on skeletal muscle over the life span: The InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:466–472. doi: 10.1093/gerona/59.5.m466. [DOI] [PubMed] [Google Scholar]
- 13.Chu LW. Tam S. Kung AW. Lo S. Fan S. Wong RL. Morley JE. Lam KSL. Serum total and bioavailable testosterone levels, central obesity, and muscle strength changes with aging in healthy Chinese men. J Am Geriatr Soc. 2008;56:1286–1291. doi: 10.1111/j.1532-5415.2008.01746.x. [DOI] [PubMed] [Google Scholar]
- 14.Araujo AB. Travison TG. Bhasin S. Esche GR. Williams RE. Clark RV. McKinlay JB. Association between testosterone and estradiol and age-related decline in physical function in a diverse sample of men. J Am Geriatr Soc. 2008;56:2000–2008. doi: 10.1111/j.1532-5415.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaap LA. Pluijm SM. Deeg DJ. Penninx BW. Nicklas BJ. Lips P. Harris TB. Newman AB. Kritchevsky SB. Cauley JA. Goodpaster BH. Tylavsky FA. Yaffe K. Visser M. for the Health ABC Study. Low testosterone levels and decline in physical performance and muscle strength in older men: findings from two prospective cohort studies. Clin Endocrinol (Oxf) 2008;68:42–50. doi: 10.1111/j.1365-2265.2007.02997.x. [DOI] [PubMed] [Google Scholar]
- 16.Cappola AR. Xue QL. Fried LP. Multiple hormonal deficiencies in anabolic hormones are found in frail older women: The Women's Health and Aging studies. J Gerontol A Biol Sci Med Sci. 2009;64:243–248. doi: 10.1093/gerona/gln026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peolsson A. Hedlund R. Oberg B. Intra- and inter-tester reliability and reference values for hand strength. J Rehabil Med. 2001;33:36–41. doi: 10.1080/165019701300006524. [DOI] [PubMed] [Google Scholar]
- 18.Rantanen T. Era P. Kauppinen M. Heikkinen E. Maximal isometric muscle strength and socioeconomic status, health, and physical activity in 75-year-old persons. J Aging Phys Act. 1994;2:206–220. [Google Scholar]
- 19.Ferrucci L. Bandinelli S. Benvenuti E. Di Iorio A. Macci C. Harris TB. Guralnik JM. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 20.Lauretani F. Russo C. Bandinelli S. Bartali B. Cavazzini C. Di Iorio A. Corsi AM. Rantanen T. Guralnik JM. Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 21.Guralnik JM. Ferrucci L. Simonsick EM. Salive ME. Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guralnik JM. Ferrucci L. Pieper CF. Leveille SG. Markides KS. Ostir GV. Stephanie Studenski S. Berkman LF. Wallace RB. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A Biol Sci Med Sci. 2000;55A:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cesari M. Kritchevsky SB. Penninx BW. Nicklas BJ. Simonsick EM. Newman AB. Tylavsky FA. Brach JS. Satterfield S. Bauer DC. Visser M. Rubin SM. Harris TB. Pahor M. Prognostic value of usual gait speed in well-functioning older people: Results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik J. Fried L. Simonsick E. Kasper J. Lafferty M. National Institute on Aging, NIH Publication No. 95-4009. Bethesda, MD: 1995. The Women's Health and Aging Study: Health and Social Characteristics of Older Women with Disability. [Google Scholar]
- 25.Wareham NJ. Jakes RW. Rennie KL. Mitchell J. Hennings S. Day NE. Validity and repeatability of the EPIC-Norfolk Physical Activity Questionnaire. Int J Epidemiol. 2002;31:168–174. doi: 10.1093/ije/31.1.168. [DOI] [PubMed] [Google Scholar]
- 26.Verbeke G. Molenberghs G. Linear Mixed Models for Longitudinal Data. 2nd. Springer-Verlag; New York: 2001. [Google Scholar]
- 27.Edwards LJ. Modern statistical techniques for the analysis of longitudinal data in biomedical research. Pediatr Pulmonol. 2000;30:330–344. doi: 10.1002/1099-0496(200010)30:4<330::aid-ppul10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 28.Roth SM. Metter EJ. Ling S. Ferrucci L. Inflammatory factors in age-related muscle wasting. Curr Opin Rheumatol. 2006;18:625–630. doi: 10.1097/01.bor.0000245722.10136.6d. [DOI] [PubMed] [Google Scholar]
- 29.Ishii-Yonemoto T. Masuzaki H. Yasue S. Okada S. Kozuka C. Tanaka T. Noguchi M. Tomita T. JFujikura J. Yamamoto Y. Ebihara K. Hosoda K. Nakao K. Glucocorticoid reamplification within cells intensifies NF{kappa}B and MAPK signaling and reinforces inflammation in activated preadipocytes. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00320.2009. doi:10.1152/ajpendo.00320.2009. [DOI] [PubMed] [Google Scholar]
- 30.Abeywardena MY. Leifert WR. Warnes KE. Varghese JN. Head RJ. Cardiovascular biology of interleukin-6. Curr Pharm Des. 2009;15:1809–1821. doi: 10.2174/138161209788186290. [DOI] [PubMed] [Google Scholar]
- 31.Latham N. Anderson C. Bennett D. Stretton C. Progressive resistance strength training for physical disability in older people. Cochrane Database Syst Rev. 2003:CD002759. doi: 10.1002/14651858.CD002759. [DOI] [PubMed] [Google Scholar]
- 32.Geffken DF. Cushman M. Burke GL. Polak JF. Sakkinen PA. Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 33.Kelley GA. Kelley KS. Effects of aerobic exercise on C-reactive protein, body composition, and maximum oxygen consumption in adults: A meta-analysis of randomized controlled trials. Metabolism. 2006;55:1500–1507. doi: 10.1016/j.metabol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Lakka TA. Lakka HM. Rankinen T. Leon AS. Rao DC. Skinner JS. Wilmore JH. Bouchard C. Effect of exercise training on plasma levels of C-reactive protein in healthy adults: The HERITAGE Family Study. Eur Heart J. 2005;26:2018–2025. doi: 10.1093/eurheartj/ehi394. [DOI] [PubMed] [Google Scholar]
- 35.Thompson AM. Mikus CR. Rodarte RQ. Distefano B. Priest EL. Sinclair E. Earnest CP. Blair SN. Church TS. Inflammation and exercise (INFLAME): Study rationale, design, and methods. Contemp Clin Trials. 2008;29:418–427. doi: 10.1016/j.cct.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nafziger AN. Bowlin SJ. Jenkins PL. Pearson TA. Longitudinal changes in dehydroepiandrosterone concentrations in men and women. J Lab Clin Med. 1998;131:316–323. doi: 10.1016/s0022-2143(98)90181-0. [DOI] [PubMed] [Google Scholar]
- 37.Yen SS. Dehydroepiandrosterone sulfate and longevity: New clues for an old friend. Proc Natl Acad Sci USA. 2001;98:8167–8169. doi: 10.1073/pnas.161278698. [DOI] [PMC free article] [PubMed] [Google Scholar]