Abstract
Lymphangioleiomyomatosis (LAM), a rare multisystem disease, occurs primarily in women, with cystic destruction of the lungs, abdominal tumors, and involvement of the axial lymphatics in the thorax and abdomen. To understand the pathogenesis of LAM, we initiated a longitudinal study of patients with LAM; over 500 patients have been enrolled. LAM results from the proliferation of a neoplastic cell (LAM cell), which has mutations in the tuberous sclerosis complex (TSC) genes, TSC1 or TSC2. Consistent with their metastatic behavior, LAM cells were isolated from blood, urine, and chylous effusions. Surface proteins on LAM cells include those found on metastatic cells and those involved in cell migration. In the lung, LAM cells are found clustered in nodules, which appear in the walls of the cysts, and in the interstitium. LAM lung nodules are traversed by slit-like vascular structures, with lining cells showing reactivity with antibodies against components of lymphatic endothelial cells. The axial lymphatics appear to be infiltrated by LAM cells, which may result in obstruction and formation of chyle-filled lymphangioleiomyomas. LAM cell clusters have been isolated from chylous pleural effusions, and it is hypothesized that these clusters may be responsible for metastatic spread of LAM cells via lymphatic vessels. Consistent with a lymphangiogenic process, levels of VEGF-D, a lymphangiogenic factor, were higher in sera of patients with LAM and lymphatic involvement (i.e., lymphangioleiomyoma, adenopathy) than in healthy volunteers or LAM patients with cystic disease limited to the lung. These findings are consistent with an important function for lymphangiogenesis in LAM.
Introduction
Lymphangioleiomyomatosis (LAM), a disease of women, primarily of child-bearing age, is characterized by cystic lung destruction, abdominal tumors [e.g., angiomyolipomas (AMLs)], and involvement of axial lymphatics with lymphangioleiomyomas or adenopathy.1–5 The majority of patients present with dyspnea, or spontaneous pneumothorax.1–5 As these manifestations are shared by more common lung diseases, the diagnosis of LAM is often delayed by several years from the onset of symptoms. Because of this delay, survival rates are difficult to establish, but generally are accepted as ranging from 79%–91% at 10 years from the onset of symptoms.3,6 Progressive dyspnea may be associated with airway hyperreactivity and hemoptysis. Other symptoms include chylous pleural effusions, chylous ascites, and abdominal hemorrhage originating from angiomyolipomas.1–5 Cystic lung destruction may result in progressive decline in lung function, leading to respiratory failure, oxygen dependency, or lung transplantation.7 Abnormal pulmonary function in LAM includes airflow obstruction, reflected by a decline in expiratory flow (FEV1) and decreased lung diffusion capacity for carbon monoxide (DLco).1,5,7,8 Ventilation-perfusion scintigrams show evidence of air-trapping.2,9 Cardiopulmonary exercise testing and 6-minute walk test may reveal arterial desaturation.10,11 Because LAM is almost exclusively a disease of women, therapy for the disease has been based on suppression of estrogen activity (e.g., progesterone, oophorectomy), but the effectiveness of these treatments is unproven.12
Genetic Factors and Susceptibility
Lung cysts similar to those found in LAM are observed on computed tomography (CT) scans of approximately 30% of women with tuberous sclerosis complex (TSC),13–15 an inherited autosomal disorder characterized by hamartomatous lesions, seizures, and mental retardation,16 caused by mutations in the TSC1 or TSC2 suppressor genes.17 Proteins encoded by TSC1/2 (hamartin and tuberin, respectively) form a complex, which functions as a negative regulator of mammalian target of rapamycin (mTOR).18 mTOR regulates cell size and number via downstream signaling of p70 S6 and 4E-BP1.18–20 Loss of TSC function results in activation of the mTOR pathway via the guanine nucleotide-binding protein, Rheb,19 suggesting that rapamycin treatment could affect LAM progression. A Phase II rapamycin clinical trial for patients with AMLs (CAST trial) showed a 50% decline in the size of angiomyolipomas and improvement of lung function in some patients. A second trial, testing the effect of rapamycin on lung function in a larger cohort, is now in progress (MILES trial).21,22
Sporadic LAM results from the proliferation of the LAM cell, a neoplastic cell having mutations in the TSC1 or TSC2 genes.7,23,24 AMLs, lung and lymphatic lesions are composed of LAM cells, which appear to be of clonal origin.23 A germline TSC mutation has not been reported in patients with LAM, however, genetic analysis of sporadic LAM cells revealed mutations in the TSC genes.23–25 Angiomyolipomas (Fig. 1F) occur more frequently in patients with LAM/TSC than sporadic LAM (80% and 40%, respectively).26 These tumors, usually located in the kidney, are characterized by underdeveloped vasculature and LAM cells intermixed with adipose tissue.27–30 Though AMLs are benign tumors, hemorrhage, especially of larger AMLs, is a serious complication. Treatment is based on the size and progression of the tumor and includes monitoring of asymptomatic tumors, and, in case of hemorrhage, arterial embolization or surgery.31
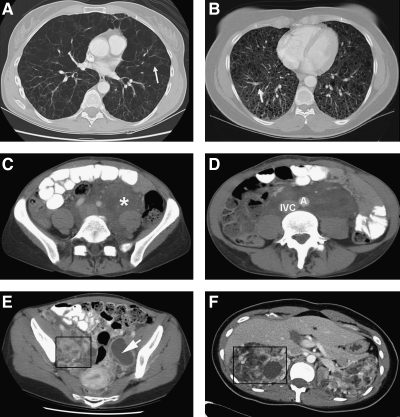
FIG. 1.
Computer tomography scans of four patients with LAM. (A) shows numerous, relatively large, thin-walled cysts replacing the normal lungs. Arrow points to the wall of a cyst. (B) shows innumerous small thin-walled cysts distributed throughout the lungs replacing the normal lung parenchyma. (C) shows a large abdominal lymphangioleiomyoma (marked by the asterisk) surrounding the iliac vessels. (D) shows the same tumor surrounding the aorta (A) and inferior vena cava (IVC). (E) shows that lymphangioleiomyomas may appear as fluid-filled cystic structures (box and arrow). (F) shows large angiomyolipomas involving the right (inside box) and left kidneys. The normal kidney anatomy is distorted, making the kidney parenchyma almost completely unrecognizable.
Natural History
The National Heart, Lung and Blood Institute (NHLBI) initiated a LAM natural history study (protocol 95-H-0186) to facilitate research and treatment efforts. More than 500 patients with LAM or LAM/TSC from the United States, Canada, Europe, and Southeast Asia are enrolled in the longitudinal study. Approximately 250 patients have returned for five or more visits.5
Predictors of Prognosis and Survival
Pathological, radiologic, and physiological studies generated useful data for assessing the severity of disease, its rate of progression, and survival of patients with LAM. From microscopic analysis of open lung biopsy specimens, Matsui et al.32 developed the LAM Histologic Score (LHS) which is based on the percentage of lung tissue involved by cystic lesions and infiltration of LAM cells, with a grading scale of LHS-1, < 25%, LHS-2 25%–50%, and LHS-3 > 50% involvement. Using Kaplan–Meier analysis, there was a correlation between LHS and survival among patients with LAM (higher LHS associated with worse survival probability and time to transplantation).32 Data from the NHLBI longitudinal study, showed that DLco correlated with the LHS better than FEV1, making it a potential predictor of outcome. In addition, DLco was the best predictor of exercise-induced hypoxemia.10 A positive bronchodilator response was found to be associated with a predominance of LAM cell proliferation and a greater rate of decline in FEV1.8,33
Because the impairment in pulmonary function in some patients with LAM appears to be related to airflow obstruction, and in others predominantly due to gas exchange abnormalities, cardiopulmonary exercise testing (CPET) was studied as an alternative method for grading severity of disease.10 A correlation was observed between peak oxygen uptake (VO2 max), CT scan grade of severity, and LHS.10
Similar to the LHS grading system, Avila et al.,9 proposed assessing lung disease severity with computed tomography by semiquantitatively estimating the percentage of lung judged to be abnormal; CT grade I < 30%, CT grade II 30%–60%, and CT grade III > 60%. Disease severity, based on CT grade, correlated with FEV1, DLco, and VO2 max.9,10
Histopathology
CT scans of the chest demonstrated thin-walled cysts spread bilaterally throughout the lung parenchyma1,2 (Figs. 1A and 1B). Histological examination of lungs revealed abnormal smooth muscle-like LAM cells in the vicinity of vasculature, lymphatics, and bronchioles and in the lining of cysts,27 often forming nodules with slit-like lymphatic channels28 (Fig. 2A). Hyperplastic Type II pneumocytes line the cystic spaces and can also be found in alveoli not affected by LAM34 (Fig. 2A). Extrapulmonary LAM cells form fascicles and papillary patterns that are commonly found in lymph nodes along lymphatic vessels.28 Extrapulmonary and pulmonary LAM cells express both smooth muscle and melanoma antigens (alpha-smooth muscle actin and gp100).27,28 The morphologically heterogeneous LAM cells are either small and spindle-shaped, located inside the LAM nodule and reactive with antibodies against proliferating nuclear antigen (PCNA) or peripherally located large epithelioid cells, which react with HMB-45, a monoclonal antibody that reacts with the premelanosomal protein gp100.35 Receptors for estrogen, progesterone, and growth factors have been identified in LAM cells.36–38
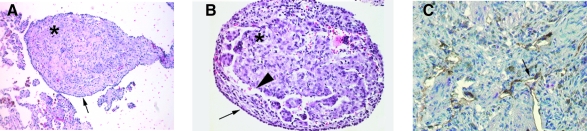
FIG. 2.
Lymphatics involvement in lymphangioleimyomatosis. (A) shows a hematoxylin and eosin (H & E) staining of LAM nodule that is characterized by proliferating smooth muscle-like cells (asterix, LAM cells) surrounded by type II pneumocytes (arrow). (B) shows close view of a LAM nodule; the arrowhead indicates the lymphatic-like structures. (C) shows reactivity of lymphatic nodules with anti D2-40 antibody, which recognizes an epitope of podoplanin (arrow) (Pacheco–Rodriguez et al. 2007).
Association of Modifier Genes and Biomarkers with Disease Severity and Progression
In the search for an effective treatment for LAM, studies focused on the discovery of modifier genes and biomarkers that are associated with diverse pathological, physiological, and radiographic characteristics of the disease. Further investigation of mechanisms through which candidate genes function in signal transduction, innate immunity, or extracellular matrix biological processes may illuminate the molecular basis of disease progression and severity and identify therapeutic targets.
Pathological, as well as genetic, evidence suggests that LAM cells possess metastatic properties. Mutations in the allograft of single lung transplants with recurrent LAM were found to be identical to mutations in the recipient before transplantations.39,40 TSC loss of heterozygosity (LOH) has been observed in circulating LAM cells isolated from chyle, blood, and urine41 and CD44v6, a metastatic cell surface receptor protein, was found in LAM cells that showed LOH for TSC2.42 In addition to the expression in LAM tissue of a specific group of chemokine receptors, CCL2 (MCP-1) has an in vitro chemotactic effect on LAM cells with TSC2 LOH, cells lacking TSC function produce greater amounts of MCP-1,43 and a polymorphism in the promoter region of the gene is associated with decline in lung function.44
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases which, in conjunction with tissue inhibitors of metalloproteinases (TIMPs), regulate turnover and remodeling of the extracellular matrix.45 Reactivity with antibodies against MMP-1, -2, -9, and -14 was demonstrated in LAM lesions, as was decreased expression of TIMP-3.27,46–49 The resulting unbalanced production of MMPs by LAM cells may, in part, lead to the formation of cysts in the lung parenchyma.27,46–49 Similarly, polymorphisms in MMP-1 and Types I and III collagen may contribute to susceptibility to pneumothorax in patients with LAM.50
Lymphatic Involvement in LAM
The presence of lymphangioleiomyomas and adenopathy, the clinical manifestations of chylous ascites and chylothorax in patients with LAM, and the metastatic behavior of LAM cells support the notion that lymphatics are important in determining the clinical phenotype of LAM.
Lymphangioleiomyomas and adenopathy occur most often in the retroperitoneal region. Adenopathy is found in about 39% of patients with sporadic LAM. CT scans revealed lymph nodes as large as 4.0 cm in diameter, some with areas of low attenuation presumed to be chylous lymph collections.51 Histologically, replacement of normal lymph node components with smooth muscle cells was seen. Lymphadenopathy was associated with severe lung involvement by computed tomography.51
Lymphangioleiomyomas appear on CT scans as clearly circumscribed lobular thin or thick-walled masses28,51 (Fig. 1C). Chyle-filled cystic lesions appear to result from the obstruction of lymphatic vasculature by proliferation of smooth muscle cells51 (Fig. 1E). LAM cells of lymphangioleiomyomas have been observed infiltrating the fatty capsule that surround the mass.28 Lymphangioleiomyomas occurred more frequently in patients with sporadic LAM than in LAM/TSC patients (29% sporadic LAM vs. 9% LAM/TSC).26 In some patients, there is a diurnal increase in the size of the lymphangioleiomyomas (visualized by CT or sonography), which can be an aid in the identification of a thick-walled lesion that resembles either a lymphangioleiomyoma, a sarcoma, or a lymphoma.52,53 Although characteristically benign, lesions that are large enough to displace abdominal viscera, may cause symptoms of abdominal pain, obstipation, and urinary frequency (Fig. 1D). Chylous ascites may result from the disruption of lymphatic flow.51 There is no effective treatment for lymphangioleiomyomas. An on-going clinical trial for patients with LAM includes treatment with octreotide, which has been used to retard the production of chyle in other disease states.22
Lymphatic Involvement in TSC
Patients with TSC may develop multiple types of skin lesions (i.e., facial angiofibromas, forehead plaques, ungual fibromas).54 AMLs occur more frequently in patients with LAM/TSC than in those with sporadic LAM, but LAM/TSC patients are less likely to present with other extrapulmonary manifestations (i.e., chylous effusions, lymphangioleiomyomas).26 Angiofibromas of patients with TSC demonstrated VEGF expression55 and cells cultured from AML tissue produced VEGF in vitro.56 Skin lesions also reacted with antibodies against CD31, a blood vascular endothelial marker, in some, but not all, dilated tubular structures.56 Reports of increased lymphatic vessels in TSC lesions57 suggests that the vessels not reactive to vascular endothelial markers may prove to be lymphatic vessels.
Histopathological Studies of Lymphatic Involvement in LAM
Microscopic studies revealed that the fine slit-like channels in LAM lung nodules and the cellular structures demarcating the extrapulmonary bundles of LAM cells are lymphatic vessels58 (Fig. 2B). The dilatation of some of these structures was believed to result from the proliferation of LAM cells,59,60 but further research was limited until the discovery of specific lymphatic endothelial markers useful for immunohistochemical studies, for example, podoplanin (a glomerular podocyte membrane mucoprotein recognized by the antibody D2-40), vascular endothelial growth factor receptor-3 (VEGFR-3, Flt-4, lymphatic growth factor receptor for ligands VEGF-C and VEGF-D and a mediator of lymphangiogenesis), and PROX-1 (transcription factor required for the differentiation of lymphatic endothelial cells).61,62
Using these newly discovered lymphatic markers, plus information regarding the metastatic properties of LAM cells and the association of lymphangiogenesis with metastatic processes, research focused on the mechanisms for dissemination of LAM cells. A study of 21 specimens from autopsies or explanted lungs, using antibodies against VEGFR-3, αSMA and HMB-45 showed extensive involvement of lymphatics in LAM.63 Immunoreactivity against VEGFR-3 lymphatic endothelial cell marker was observed in cells surrounding or infiltrating LAM cell foci (identified by positive reactivity to αSMA and HMB-45) in multiple sites, including the lung, retroperitoneal lymph nodes, ovaries, uterus, thoracic duct, and walls of lymphangioleiomyomas. Adjacent to areas of LAM cell proliferation, lymphatic vessels that penetrated vascular walls and tissue interstitium were found. LAM cell clusters (LCCs, LAM cells surrounded by lymphatic endothelial cells) were also seen in lymphatic vessels of the same areas. In contrast, there was very little reaction to those samples with anti-CD31 antibodies. CD31 is a putative vascular endothelial and angiogenic cell marker.63
Immunoreactive VEGF-C, a lymphatic growth factor, was found in lung, uterus, ovary, and lymph node tissue samples. In primary cell cultures from LAM lung explants, VEGF-C was present in cells that reacted with HMB-45.63
Correlations were found between the extent of lymphangiogenesis (intensity of VEGFR-3 staining), intensity of VEGF-C staining, and the LHS in patients with LAM. LAM tissue that reacted more with anti-VEGF-C antibodies also contained more immunoreactive VEGFR-3 and the presence of both VEGF-C and VEGFR-3 expression was associated with severe disease, expressed as LHS. Findings in this lymphatic study were all consistent with a lymphangiogenic process associated with LAM through the production of VEGF-C.63
The finding that LAM cell clusters were present in chylous effusions64,65 prompted immunocytologic and immunohistologic studies on samples of pleural and ascitic fluids, and lymphangioleiomyoma from 6 cases of LAM or LAM/TSC.66 LCCs were evident in all samples of chylous fluid. The inner spindle-shaped cells reacted with HMB-45 anti-αSMA, and the outer flattened endothelial cells reacted with antibodies against anti-VEGFR-3 antibodies.66 Cells from a resected diaphragmatic lesion of a patient with chylothorax and chylous ascites also expressed both lymphatic (Fig. 2C) and LAM cell markers and appeared to fragment into LCCs. Further, immunohistochemical characterization revealed LCCs similar to clusters observed in the stroma of the diaphragm that were also floating in the extralymphatic space of the pleural cavity. Consistent with these observations, double immunostaining for lymphatic endothelial cells and epithelial cells demonstrated a lymphatic channel within the diaphragmatic LAM lesion opening into the pleural cavity.66
As part of this study, a retrospective evaluation of autopsy samples from the axial lymphatic system confirmed the presence of LAM lesions in all regions of the axial lymphatics, but particularly in the retroperitoneal area.66 LAM lesions located in the thoracic duct wall and in retroperitoneal lymph nodes were observed to extend into adjacent fat tissue. LAM cells were also seen proximal to the junction of the lymphatic and venous blood systems. The identification of LCCs in chylous fluids and extralymphatic spaces and the extent of lymphatic involvement in LAM led to the premise that metastatic spread of LAM cells may be a consequence of the lymphangiogenesis-associated shedding of LCCs into the lymphatic circulation. Interaction of the lymphatic endothelial cells (LECs) of the clusters with LECs of the lymphatic channels could result in the fragmentation of the LAM cells clusters. In vitro, LCCs, separated into spindle-shaped cells reactive with anti-αSMA antibodies and large polygonal cells reactive with anti-VEGFR-3 antibodies. Freed from the LCCs and assisted by LAM cell-derived MMPs, LAM cells could then invade the extracellular matrix to form a new lesion.
As LAM cells have been recovered from circulating blood,41 serum levels of lymphatic growth factor levels in patients with LAM were studied to ascertain possible mediators of the lymphangiogenic process in LAM.67 Although VEGF-C had been demonstrated in LAM tissue,63 serum levels of VEGF-C were lower than those in age- and gender- matched levels of healthy volunteers, and VEGF-A levels were similar to those of controls. Serum levels of VEGF-D were, however, significantly higher than those in normal volunteers. Greater loss of pulmonary function was associated with higher levels of serum VEGF-D, indicating that VEGF-D may be the growth factor influencing lymphangiogenesis in LAM.67
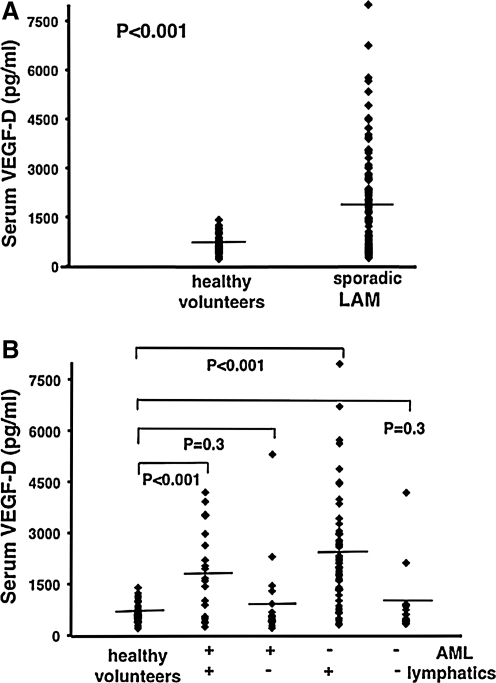
In another study of 38 patients with LAM and 29 healthy volunteers, serum VEGF-D levels were higher in LAM than in similar cystic or chylous lung diseases (i.e., pulmonary Langerhans-cell histiocytosis, n = 7; lymphangiomatosis, n = 7; and emphysema, n = 13).68 It was suggested that VEGF-D serum levels might be a diagnostic test for LAM.68 Data from a larger cohort of 111 patients with LAM grouped according to pulmonary and extrapulmonary manifestations, corroborated previous results comparing serum VEGF-D levels of patients with LAM to healthy volunteers (Fig. 3A) and the association between higher VEGF-D levels and lower pulmonary function (initial DLco % predicted, DLco/VA).69 Further, serum VEGF-D levels were associated with more severe disease by CT scan (CT grade). The correlation of VEGF-D levels to LAM disease was maintained, however, only for LAM patients with lymphatic involvement (defined in this study as the presence of adenopathy and/or lymphangioleiomyoma), regardless of the presence of AMLs69 (Fig. 3B). Thus, normal VEGF-D levels might be present in patients with disease limited to the lung. Statistical analysis of a predictive model for VEGF-D serum levels indicated that VEGF-D was a good biomarker for predicting lymphatic involvement, but not for diagnosing LAM.69 Consistent with these findings, extent of lymphatic involvement in LAM was also correlated with loss of pulmonary function and trended toward severe disease.69 Interestingly, in a study using cultured cells from Tsc2 wild-type (Tsc2+/+) and Tsc2 null (Tsc2−/−) mouse embryonic fibroblasts (MEFs), it was reported that inactivation of Tsc2 upregulated the expression of VEGF through increased accumulation of HIF-1a.70 Though Tsc2 null cells treated with rapamycin, an mTOR antagonist, lead to reduced secretion of VEGF, levels remained higher than VEGF levels in Tsc2+/+ cells.70 It is conceivable Tsc2 may also regulate VEGF-D in a similar manner in humans.
FIG. 3.
Serum Levels of VEGF-D in lymphangioleiomyomatosis. In (A), serum VEGF-D levels in all patients with sporadic lymphangioleiomyomatosis (LAM) (n = 111) were compared to those of healthy volunteers (n = 40). (B) shows patient samples further grouped and compared on the basis of thoracic or abdominal lymphatic involvement (presence (n = 77) or absence (n = 34) of lymphangioleiomyomas and/or adenopathy), and the presence (n = 40) or absence (n = 71) of renal angiomyolipomas (AMLs). All groups were compared to healthy volunteers (n = 40). (+) = presence of, (−) = absence of. Each ♦ represents serum measurement of VEGF-D from one patient or healthy volunteer. Lines represent mean values. (From Reference 69).
Conclusion
Lymphatic involvement in LAM patients, which can be widespread, may be associated with metastatic spread of LAM cells and a negative indicator of prognosis. VEGF-D, a lymphangiogenic growth factor, may be elevated in sera of patients with LAM, and disease progression may be slowed by anti-VEGF-D therapy.
Footnotes
Research was supported in part by the Intramural Research Program, NIH, NHLBI.
For review and references to contributions of LAM investigators, please see: Glasgow et al. Lymphatic involvement in lymphangioleiomyomatosis, Ann NY Acad Sci 1131, 206–214, 2008.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institutes of Health, NHLBI. We thank Dr. Martha Vaughan for helpful discussions and critical review of the manuscript. We thank the patients with LAM for their commitment and inspiration.
Disclosure Statement
Ms. Glasgow and Drs. Taveira–DaSilva, Pacheco–Rodriguez, Steagall, Tsukada, Cai, El–Chemaly, and Moss have no conflicts of interest or financial ties to disclose.
References
- 1.Kitaichi M. Nishimura K. Itoh H. Izumi T. Pulmonary lymphangioleiomyomatosis: A report of patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med. 1995;151:527–533. doi: 10.1164/ajrccm.151.2.7842216. [DOI] [PubMed] [Google Scholar]
- 2.Chu S. Horiba K. Usuki J. Avila N. Chen C. Travis W. Ferrans V. Moss J. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest. 1999;115:1041–1052. doi: 10.1378/chest.115.4.1041. [DOI] [PubMed] [Google Scholar]
- 3.Urban T. Lazor R. Lacronique J. Murris M. Labrune S. Valeyre D. Cordier J–F. Pulmonary lymphangioleiomyomatosis: A study of 69 patients. Medicine. 1999;78:321–337. doi: 10.1097/00005792-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Johnson S. Tattersfield A. Clinical experience of lymphangioleiomyomatosis in the UK. Thorax. 2000;55:1052–1057. doi: 10.1136/thorax.55.12.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryu J. Moss J. Beck G. Lee J–C. Brown K. Chapman J. Finlay G. Olson E. Ross S. Maurer J. Raffin T. Peavy H. McCarthy K. Taveira–DaSilva A. McCormack F. Avila N. DeCastro R. Jacobs S. Stylianou M. Fanburg B for the NHLBI LAM Registry Group. The NHLBI lymphangioleiomyomatosis registry: Characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173:105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson S. Whale C. Hubbard R. Lewis S. Tattersfield A. Survival and disease progression in UK patients with lymphangioleiomyomatosis. Thorax. 2004;59:800–803. doi: 10.1136/thx.2004.023283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taveira–DaSilva A. Steagall WK. Moss J. Lymphangioleiomyomatosis. Cancer Control. 2006;13:276–285. doi: 10.1177/107327480601300405. [DOI] [PubMed] [Google Scholar]
- 8.Taveira–DaSilva A. Hedin C. Stylianou M. Travis W. Matsui K. Ferrans F. Moss J. Reversible airflow obstruction, proliferation of abnormal smooth muscle cells, and impairment of gas exchange as predictors of outcome in lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2001;164:1072–1076. doi: 10.1164/ajrccm.164.6.2102125. [DOI] [PubMed] [Google Scholar]
- 9.Avila N. Chen C. Chu S. Wu M. Jones E. Neumann R. Moss J. Pulmonary lymphangioleiomyomatosis: Correlation of ventilaton-perfusion scintigraphy, chest radiography, and CT with pulmonary function tests. Radiology. 2000;214:441–446. doi: 10.1148/radiology.214.2.r00fe41441. [DOI] [PubMed] [Google Scholar]
- 10.Taveira–DaSilva A. Stylianou M. Hedin C. Kristof A. Avila N. Rabel A. Travis W. Moss J. Maximal oxygen uptake and severity of disease in lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2003;168:1427–1431. doi: 10.1164/rccm.200206-593OC. [DOI] [PubMed] [Google Scholar]
- 11.Taveira–DaSilva A. Hathaway O. Sachdev V. Shizukuda Y. Birdsall C. Moss J. Pulmonary artery pressure in lymphangioleiomyomatosis: An echocardiographic study. Chest. 2007;1332:1573–1578. doi: 10.1378/chest.07-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taveira–DaSilva A. Stylianou M. Hedin C. Hathaway O. Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126:1867–1874. doi: 10.1378/chest.126.6.1867. [DOI] [PubMed] [Google Scholar]
- 13.Moss J. Avila N. Barnes P. Litzenberger R. Bechtle J. Brooks P. Hedin C. Hunsberger S. Kristof A. Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am J Respir Crit Care Med. 2001;163:669–671. doi: 10.1164/ajrccm.164.4.2101154. [DOI] [PubMed] [Google Scholar]
- 14.Franz D. Brody A. Meyer C. Leonard J. Chuck G. Dabora S. Sethuraman G. Colby T. Kwiatkowski D. McCormack F. Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis. Am J Respir Crit Care Med. 2001;164:661–668. doi: 10.1164/ajrccm.164.4.2011025. [DOI] [PubMed] [Google Scholar]
- 15.Costello L. Hartman T. Ryu J. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc. 2000;75:591–594. doi: 10.4065/75.6.591. [DOI] [PubMed] [Google Scholar]
- 16.Osborne J. Fryer A. Webb D. Epidemiology of tuberous sclerosis. Ann NY Acad Sci. 1991;615:125–127. doi: 10.1111/j.1749-6632.1991.tb37754.x. [DOI] [PubMed] [Google Scholar]
- 17.Sampson J. Harris P. The molecular genetics of tuberous sclerosis. Hum Mol Genetics. 1994;3:1477–1480. doi: 10.1093/hmg/3.suppl_1.1477. [DOI] [PubMed] [Google Scholar]
- 18.Tee A. Fingar D. Manning B. Kwiatkowski D. Cantley L. Blenis J. Tuberous sclerosis complex-1 and −2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tee A. Manning B. Roux P. Cantley L. Blenis J. Tuberous sclerosis complex gene products, tuberin and hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 20.Goncharova E. Goncharov D. Eszterhas A. Hunter D. Glassberg M. Yeung R. Walker C. Noonan D. Kwiatkowski D. Chou M. Panettieri R., Jr. Krymskaya V. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 21.Bissler J. McCormack F. Young L. Elwing J. Chuck G. Leonard J. Schmithorst V. Laor T. Brody A. Bean J. Salisbury S. Franz D. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormack F. Lymphangioleiomyomatosis: A clinical update. Chest. 2008;133:507–516. doi: 10.1378/chest.07-0898. [DOI] [PubMed] [Google Scholar]
- 23.Smolarek T. Wessner L. McCormack F. Mylet J. Menon A. Henske E. Evidence that lymphangiomyomatosis is caused by TSC2 Mutations: Chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet. 1998;62:810–815. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carsillo T. Astrinidis A. Henske E. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato T. Seyama K. Fujii H. Maruyama H. Setoguchi Y. Iwakami S-i. Fukuchi Y. Hino O. Mutation analysis of the TSC1 and TSC2 genes in Japanese patients with pulmonary lymphangioleiomyomatosis. J Hum Genet. 2002;47:20–28. doi: 10.1007/s10038-002-8651-8. [DOI] [PubMed] [Google Scholar]
- 26.Avila N. Dwyer A. Rabel A. Moss J. Sporadic lymphangioleiomyomatosis and tuberous sclerosis complex with lymphangioleiomyomatosis: Comparison of CT features. Radiology. 2007;242:277–285. doi: 10.1148/radiol.2421051767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrans V. Yu Z–X. Nelson W. Valencia J. Tatsuguchi A. Avila N. Riemenschneider W. Matsui K. Travis W. Moss J. Lymphangioleiomyomatosis (LAM): A review of clinical and morphological features. J Nippon Med Sch. 200;67:311–329. doi: 10.1272/jnms.67.311. [DOI] [PubMed] [Google Scholar]
- 28.Matsui K. Tatsuguchi A. Valencia J. Yu Z–X. Bechtle J. Beasley M. Avila N. Travis W. Moss J. Ferrans V. Extrapulmonary lymphangioleiomyomatosis (LAM): Clinicopathologic features in 22 cases. Hum Pathol. 2000;31:1242–1248. doi: 10.1053/hupa.2000.18500. [DOI] [PubMed] [Google Scholar]
- 29.Maziak D. Kesten S. Rappaport D. Maurer J. Extrathoracic angiomyolipomas in lymphangioleimyomatosis. Eur Respir J. 1996;9:402–405. doi: 10.1183/09031936.96.09030402. [DOI] [PubMed] [Google Scholar]
- 30.Kelly J. Moss J. Lymphangioleiomyomatosis. Am J Med Sci. 2001;321:17–25. doi: 10.1097/00000441-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Bissler J. Kingswood J. Renal angiomyolipomata. Kidney Int. 2004;66:924–934. doi: 10.1111/j.1523-1755.2004.00838.x. [DOI] [PubMed] [Google Scholar]
- 32.Matsui K. Beasley M. Nelson W. Barnes P. Bechtle J. Falk R. Ferrans V. Moss J. Travis W. Prognostic significance of pulmonary lymphangioleiomyomatosis histologic score. Am J Surg Pathol. 2001;29:1356–1366. doi: 10.1097/00000478-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Taveira–DaSilva A. Steagall WK. Rabel A. Hathaway O. Harari S. Cassandro R. Stylianou M. Moss J. Reversible airflow obstruction in lymphangioleiomyomatosis. Chest. 2009 May 15; doi: 10.1378/chest.09-0624. [Epub ahead of print] PMID: 19447921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsui K. Riemenschneider W. Hilbert S. Yu Z–X. Takeda K. Travis W. Moss J. Ferrans V. Hyperplasia of type II pneumocytes in pulmonary lymphangioleimyomatosis: Immunohistochemical and electron microscopic study. Arch Pathol Lab Med. 2000;124:1642–1648. doi: 10.5858/2000-124-1642-HOTIPI. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto Y. Horiba K. Usuki J. Chu S. Ferrans V. Moss J. Markers of cell proliferation and expression of melanosomal antigen in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 1999;21:327–336. doi: 10.1165/ajrcmb.21.3.3693. [DOI] [PubMed] [Google Scholar]
- 36.Inoue Y. King T., Jr Barker E. Daniloff E. Newman L. Basic fibroblast growth factor and its receptors in idiopathic pulmonary fibrosis and lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2002;166:765–773. doi: 10.1164/rccm.2010014. [DOI] [PubMed] [Google Scholar]
- 37.Valencia J. Matsui K. Bondy C. Zhou J. Rasmussen A. Cullen K. Yu Z–X. Moss J. Ferrans V. Distribution and mRNA expression of insulin-like growth factor system in pulmonary lymphangioleiomyomatosis. 2001;49:421–433. doi: 10.2310/6650.2001.33787. [DOI] [PubMed] [Google Scholar]
- 38.Valencia J. Pacheco–Rodriguez G. Carmona A. Xavier J. Bruneval P. Riemenschneider W. Ikeda Y. Yu Z–X. Ferrans V. Moss J. Tissue-specific rennin-angiotensin system in pulmonary lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 2006;35:40–47. doi: 10.1165/rcmb.2005-0387OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bittmann I. Burkhard R. Amann G. Löhrs U. Recurrence of lymphangioleiomyomatosis after single lung transplantation: New insights into pathogenesis. Hum Pathol. 2003;34:95–98. doi: 10.1053/hupa.2003.50. [DOI] [PubMed] [Google Scholar]
- 40.Karbowniczek M. Astrinidis M. Balsara B. Testa J. Lium J. Colby T. McCormack F. Henske E. Recurrent lymphangiomyomatosis after transplantation: Genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med. 2003;167:976–982. doi: 10.1164/rccm.200208-969OC. [DOI] [PubMed] [Google Scholar]
- 41.Crooks D. Pacheco–Rodriguez G. deCastro R. McCoy P., Jr Wang J. Kumaki F. Darling T. Moss J. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleimyomatosis. Proc Natl Acad Sci USA. 2004;101:17462–17467. doi: 10.1073/pnas.0407971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pacheco–Rodriguez G. Steagall WK. Crooks D. Stevens L. Hashimoto H. Li S. Wang J–A. Darling T. Moss J. TSC2 loss in lymphangioleiomyomatosis cells correlated with expression of CD44v6, a molecular determinant of metastasis. Cancer Res. 2007;67:10573–10581. doi: 10.1158/0008-5472.CAN-07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S. Takeuchi F. Wang J–A. Fuller C. Pacheco–Rodriguez G. Moss J. Darling T. MCP-1 overexpressed in tuberous sclerosis lesions acts as a paracrine factor for tumor development. JEM. 2005;202:617–624. doi: 10.1084/jem.20042469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacheco–Rodriguez G. Kumaki F. Steagall WK. Zhang Y. Ikeda Y. Lin J–P. Billings E. Moss J. Chemokine-enhanced chemotaxis of lymphangioleiomyomatosis cells with mutations in the tumor suppressor TSC2 gene. J Immunol. 2009;182:1270–1277. doi: 10.4049/jimmunol.182.3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parks W. Shapiro S. Review; Matrix metalloproteinases in lung biology. Respir Res. 2001;2:10–19. doi: 10.1186/rr33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsui K. Takeda K. Yu Z–X. Travis W. Moss J. Ferrans V. Role for activation of matrix metalloproteinases in the pathogenesis of pulmonary lymphangioleimyomatosis. Arch Pathol Lab Med. 2000;124:267–275. doi: 10.5858/2000-124-0267-RFAOMM. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi T. Fleming M. Stetler–Stevenson W. Liotta L. Moss J. Ferrans V. Travis W. Immunohistochemical study of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in pulmonary lymphangioleiomyomatosis (LAM) Hum Pathol. 1997;28:1071–1078. doi: 10.1016/s0046-8177(97)90061-7. [DOI] [PubMed] [Google Scholar]
- 48.Zhe S. Yang Y. Jakkaraju S. Schuger L. Tissue inhibitor of metalloproteinase-3 downregulation in lymphangioleiomyomatosis: Potential consequence of abnormal serum response factor expression. Am J Respir Cell Mol Biol. 2003;28:504–511. doi: 10.1165/rcmb.2002-0124OC. [DOI] [PubMed] [Google Scholar]
- 49.Krymskaya V. Shipley J. Lymphangioleiomyomatosis: Aa complex tale of serum response factor-mediated tissue inhibitor of metalloproteinase-3 regulation. Am J Respir Cell Mol Biol. 2003;28:546–550. doi: 10.1165/rcmb.F267. [DOI] [PubMed] [Google Scholar]
- 50.Steagall WK. Glasgow CG. Hathaway O. Avila N. Taveira–DaSilva A. Rabel A. Stylianou M. Lin J–P. Chen X. Moss J. Genetic and morphologic determinants of pneumothorax in lymphangioleiomyomatosis. Am J Physiol Lung Cell Mol Physiol. 2007;293:L800–L808. doi: 10.1152/ajplung.00176.2007. [DOI] [PubMed] [Google Scholar]
- 51.Avila N. Kelly J. Chu S. Dwyer A. Moss J. Lymphangioleiomyomatosis: Abdominopelvic CT and US findings. Radiology. 2000;216:147–153. doi: 10.1148/radiology.216.1.r00jl42147. [DOI] [PubMed] [Google Scholar]
- 52.Avila N. Bechtle J. Dwyer A. Ferrrans V. Moss J. Lymphangioleiomyomatosis: CT of diurnal variation of lymphangioleiomyomas. Radiology. 2001;221:415–421. doi: 10.1148/radiol.2212001448. [DOI] [PubMed] [Google Scholar]
- 53.Avila N. Dwyer A. Murphy–Johnson D. Brooks P. Moss J. Sonography of lymphangioleiomyoma in lymphangioleiomyomatosis: Demonstration of diurnal variation in lesion size. Am J Roentgenol. 2005;184:459–464. doi: 10.2214/ajr.184.2.01840459. [DOI] [PubMed] [Google Scholar]
- 54.Webb D. Clarke A. Fryer A. Osborne J. The cutaneous features of tuberous sclerosis: A population study. Br J Dermatol. 1996;135:1–5. [PubMed] [Google Scholar]
- 55.Nguyen–Vu P–A. Fackler I. Rust A. DeClue J. Sander C. Volkenandt M. Flaig M. Yeung R. Wienecke R. Loss of tuberin, the tuberous-sclerosis-complex-2 gene is associated with angiogenesis. J Cutan Pathol. 2001;28:470–475. doi: 10.1034/j.1600-0560.2001.028009470.x. [DOI] [PubMed] [Google Scholar]
- 56.Arbiser J. Brat D. Hunter S. D'Armiento J. Henske E. Arbiser Z. Bai X. Goldberg G. Cohen C. Weiss S. Tuberous sclerosis-associated lesions of the kidney, brain, and skin are angiogenic neoplasms. J Am Acad Dermatol. 2002;46:376–380. doi: 10.1067/mjd.2002.120530. [DOI] [PubMed] [Google Scholar]
- 57.Sanchez N. Wick M. Perry H. Adenoma sebaceum of Pringle: A clinicopathologic review, with a discussion of related pathologic entities. J Cutan Pathol. 1981;8:395–403. doi: 10.1111/j.1600-0560.1981.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 58.Wolff M. Lymphangiomyoma: Clinicopathologic study and ultrastructural confirmation of its histogenesis. Cancer. 1973;31:988–1007. doi: 10.1002/1097-0142(197304)31:4<988::aid-cncr2820310433>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 59.Corrin B. Liebow A. Friedman P. Pulmonary lymphangiomyomatosis, a review. Am J Pathol. 1975;79:348–368. [PMC free article] [PubMed] [Google Scholar]
- 60.Basset F. Soler P. Marsac J. Corrin B. Pulmonary lymphangiomyomatosis: Three new cases studied with electron microscopy. Cancer. 1976;38:2357–2366. doi: 10.1002/1097-0142(197612)38:6<2357::aid-cncr2820380623>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 61.Stacker S. Baldwin M. Achen M. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002;16:922–934. doi: 10.1096/fj.01-0945rev. [DOI] [PubMed] [Google Scholar]
- 62.Jussila L. Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol. Rev. 2002;82:673–700. doi: 10.1152/physrev.00005.2002. [DOI] [PubMed] [Google Scholar]
- 63.Kumasaka T. Seyama K. Mitani K. Sato T. Souma S. Kondo T. Hayashi S. Minami M. Uekusa T. Fukuchi Y. Suda K. Lymphangiogenesis in lymphangioleiomyomatosis: Its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol. 2004;289:1007–1016. doi: 10.1097/01.pas.0000126859.70814.6d. [DOI] [PubMed] [Google Scholar]
- 64.Itami M. Teshima S. Asakuma Y. Hino H. Aoyama K. Fukushima N. Pulmonary lymphangiomyomatosis diagnosed by effusion cytology. A case report. Acta Cytol. 1997;41:522–528. doi: 10.1159/000332550. [DOI] [PubMed] [Google Scholar]
- 65.Yamauchi M. Nakahara H. Uyama K. Tsumjimoto A. Tamai M. Aozasa K. Cytologic finding of chyloascites in lymphangioleiomyomatosis. A case report. Acta Cytol. 2000;44:1081–1084. doi: 10.1159/000328602. [DOI] [PubMed] [Google Scholar]
- 66.Kumasaka T. Seyama K. Mitani K. Souma S. Kashiwagi S. Hebisawa A. Sato T. Kubo H. Gomi K. Shibuya K. Fukuchi Y. Suda K. Lymphangiogenesis-mediated shedding of LAM cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am J Surg Pathol. 2005;29:13556–1366. doi: 10.1097/01.pas.0000172192.25295.45. [DOI] [PubMed] [Google Scholar]
- 67.Seyama K. Kumasaka T. Souma S. Sato T. Kurihara M. Mitani K. Tominaga S. Fukuchi Y. Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat Res Biol. 2006;3:143–152. doi: 10.1089/lrb.2006.4.143. [DOI] [PubMed] [Google Scholar]
- 68.Young L. Inoue Y. McCormack F. Diagnostic potential of serum VEGF-D for lymphangioleiomyomatosis. N Engl J Med. 2008;358:199–200. doi: 10.1056/NEJMc0707517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glasgow CG. Nilo A. Lin J–P. Stylianou M. Moss J. Serum vascular endothelial growth factor-D levels in patients with lymphangioleiomyomatosis reflect lymphatic involvement. Chest. 2009;135:1293–1300. doi: 10.1378/chest.08-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brugarolas J. Vazquez F. Reddy A. Sellers W. Kaelin W., Jr. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 2003;4:147–158. doi: 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]