Abstract
Small rodents are purported to be enzootic hosts of Yersinia pestis and may serve as sources of infection to prairie dogs or other epizootic hosts by direct or flea-mediated transmission. Recent research has shown that small rodent species composition and small rodent flea assemblages are influenced by the presence of prairie dogs, with higher relative abundance of both small rodents and fleas at prairie dog colony sites compared to grasslands without prairie dogs. However, it is unclear if increased rodent or flea abundance predisposes prairie dogs to infection with Y. pestis. We tracked rodent and flea occurrence for 3 years at a number of prairie dog colony sites in Boulder County, Colorado, before, during, and after a local plague epizootic to see if high rodent or flea abundance was associated with plague-affected colonies when compared to colonies that escaped infection. We found no difference in preepizootic rodent abundance or flea prevalence or abundance between plague-positive and plague-negative colonies. Further, we saw no significant before-plague/after-plague change in these metrics at either plague-positive or plague-negative sites. We did, however, find that small rodent species assemblages changed in the year following prairie dog die-offs at plague-affected colonies when compared to unaffected colonies. In light of previous research from this system that has shown that landscape features and proximity to recently plagued colonies are significant predictors of plague occurrence in prairie dogs, we suggest that landscape context is more important to local plague occurrence than are characteristics of rodent or flea species assemblages.
Key Words: Boulder County, Colorado, Cynomys ludovicianus, Disease ecology, Pathogen dispersal, Yersinia pestis
Introduction
Plague, the disease caused by the bacterium Yersinia pestis, is generally thought to be maintained in natural systems by reservoir species that are partially resistant to infection and that can serve as sources of infection to other species (Pollitzer and Meyer 1961). Plague epizootics are characterized by rapid transmission among individuals and widespread die-offs and occur when a particularly susceptible (epizootic) host species becomes infected (Pollitzer and Meyer 1961). In western North America, prairie dogs and other ground squirrels serve as epizootic hosts and suffer high rates of mortality upon infection with Y. pestis (Cully and Williams 2001), yet true reservoir (enzootic) hosts are unidentified in many systems (Gage and Kosoy 2005). Individuals of some small rodent species, such as deer mice (Peromyscus maniculatus), may become bacteremic upon exposure to Y. pestis and can serve as sources of infectious bloodmeals for the fleas that transmit the pathogen, whereas other individuals are resistant to infection (Eisen et al. 2008a). Therefore, populations of small rodent species, such as deer mice, that are associated with prairie dog colonies have long been considered to be enzootic hosts of Y. pestis (Pollitzer and Meyer 1961). If such species do, in fact, serve as pathogen reservoirs, it is reasonable to presume that changes in their abundance or changes in the prevalence and abundance of their fleas could trigger epizootic events.
Small rodent abundance (Collinge et al. 2008) as well as flea prevalence and abundance may be significantly higher on small rodents (Brinkerhoff et al. 2008) at black-tailed prairie dog (Cynomys ludovicianus) colony sites than at grassland sites where prairie dogs are absent. Increased flea prevalence has been associated with higher rates of host switching by fleas (Bossard 2006) and high flea abundance may be required for epizootic maintenance (Lorange et al. 2005). Therefore, positive effects of prairie dog presence on flea and rodent occurrence could predispose prairie dogs to plague transmission from enzootic sources. However, deer mice sampled on prairie dog colony sites rarely harbor fleas typically associated with prairie dogs (Salkeld and Stapp 2008a), and a recent study demonstrated that Y. pestis exposure in small rodents does not precede epizootic events in prairie dogs (Stapp et al. 2008), suggesting that small rodents may not serve as enzootic hosts for Y. pestis. Further, Webb et al. (2006) suggest that mechanisms other than flea-mediated transmission could drive epizootic plague events in prairie dogs, which would potentially discount the role of small rodents and their fleas in maintaining epizootics.
To address the uncertainty surrounding potential mechanisms by which plague epizootics are initiated and maintained in black-tailed prairie dogs, we tracked prairie dog and small rodent abundance as well as flea prevalence and abundance on these hosts at a number of prairie dog colony sites before, during, and after a plague epizootic. Our goal was to use longitudinal mammal and flea occurrence data to detect trends or changes that might precede plague epizootics in prairie dogs. Under the assumption that small rodents serve as enzootic hosts for Y. pestis and that flea prevalence and abundance are positively associated with inter- and intraspecific pathogen transmission, we predicted that prairie dog colonies experiencing plague would show increases in small mammal, prairie dog, and flea abundance in the years preceding a plague event. We also measured mammalian species diversity to determine if we could identify changes in mammalian species assemblages that potentially result from epizootic plague events in prairie dogs.
Materials and Methods
We sampled small mammals, prairie dogs, and fleas from active and recently plague-impacted black-tailed prairie dog colonies in Boulder County, Colorado, from 2003 to 2006. For the purpose of this study, we define small mammals as nonprairie dog rodents that are capable of being captured in 7.6 × 8.9 × 22.9 cm Sherman live traps (H.B. Sherman Traps, Tallahassee, FL). Trapping occurred at a total of 24 study sites (Fig. 1) ranging in elevation from 1550 to 1920 m and varying in landscape context from contiguous managed grassland to agriculture to urban and suburban development. All sites were trapped in 2004 and 2005. In 2003 trapping occurred at 20 of the 24 sites, and in 2006 we trapped small mammals and prairie dogs at a subset of 14 of the 24 colonies trapped in previous years (Fig. 1).
FIG. 1.
Approximate location of Boulder County, Colorado (inset), and locations of 24 study sites within Boulder County. Squares denote sites that were trapped beginning in 2003 and diamonds denote sites that were trapped beginning in 2004. Gray-filled shapes represent sites that were trapped until 2005 and black-filled shapes represent sites that were trapped until 2006. Broken circles surround sites that experienced plague in 2005 and solid circles surround sites that experienced plague in 2006.
For small mammal trapping we used square 120 × 120 m grids of 49 noncollapsible live traps (H.B. Sherman Traps) with 20 m spacing between traps. Prairie dog trapping grids were square or rectangular, consisting of 48, 49, or 50 12.5 × 12.5 × 40 cm wire box traps (Tomahawk Live Trap, Tomahawk, WI) arranged in 125 × 175 m, 150 × 150 m, or 100 × 225 m grids with 25 m spacing between traps. Small mammal traps were baited with rolled oats and set in the evening prior to sampling days and were closed during nonsampling periods. Small mammal trapping occurred during two 4-night trapping sessions (196 trap nights per session) in May/June and August/September of each year. Prairie dog traps were baited with a mixture of oats, barley, corn, and molasses, and set between 06:30 and 09:00 on each sampling day and were checked after 3 h. Prairie dog traps were prebaited for 3 (2003) or 5 (2004–2006) days prior to each trapping session. Prairie dog traps were locked open during all hours that they were not set during active trapping sessions. Prairie dogs were trapped at each site during 1 week (192–200 trap nights per site) in June and July of each year.
All captured individuals were anesthetized in a transparent plastic cylinder using isoflurane (Halocarbon Products Corporation, River Edge, NJ) administered on cotton balls to facilitate flea collection. Fleas were brushed from each host's body using a toothbrush (small mammals) or flea comb (prairie dogs) and were collected with forceps. Fleas were stored in 2 mL plastic vials containing a 2% saline solution containing a small amount of polysorbate 80 (ICI Americas, Wilmington, DE) at −20°C before identification with dichotomous keys (Hubbard 1947, Furman and Catts 1982).
We measured rodent and prairie dog abundance at each study site as the number of unique individuals captured during a trapping session. We calculated flea prevalence (proportion of infested hosts), mean flea abundance (number of fleas collected divided by number of hosts), and mean flea intensity (number of fleas divided by number of infested hosts) for all small mammals, for P. maniculatus individually, and for C. ludovicianus at each site. To measure change in these metrics as a function of plague activity, we conducted before-after-control-impact analyses by calculating the difference in each metric at each site between year t and year t − 1, where year t is defined as the year in which a particular colony experienced a plague event. We then compared the distribution of means of the before–after difference values between sites that did and did not experience plague by Wilcoxon rank-sum tests. We did identical analyses to test for differences in mammal and flea occurrence in the years preceding plague events by comparing changes in the above-described metrics at plague-positive and plague-negative colonies between years t − 2 and t − 1. To characterize the effects of plague on small mammal species diversity, we calculated Shannon's index of diversity at each site in each year and compared the change in diversity index values from year t to year t + 1 at plague and nonplague sites with Wilcoxon rank-sum tests.
Results
We sampled a total of 3237 small mammals, representing 7 species, 1865 prairie dogs (Table 1), and collected a total of 19,962 fleas. Prairie dog die-offs due to plague were observed at three prairie dog colonies in 2005 and at another three colonies in 2006 (Fig. 1). Because fewer than half of our study colonies experienced plague in a given year, we randomly selected for analysis an equal number of control (plague-negative) sites to the number of plague-positive sites in a given year from the total number of plague-negative sites. At all plague-positive colonies, prairie dog die-offs were complete before small mammal and prairie dog trapping in the year of the plague event. Therefore, no prairie dogs were trapped in year t at plague-affected colonies and no before–after comparisons of flea occurrence on prairie dog hosts could be conducted for year t − 1 to year t. We were unable to sample small mammals at one plague-affected colony in 2006 (Fig. 1), reducing the number of colonies available for year t − 1 to year t comparisons to five.
Table 1.
Number of Each Mammal Species Sampled Per Year of Trapping
| Mammalian species | 2003 | 2004 | 2005 | 2006 |
|---|---|---|---|---|
| Chaetodipus hispidus | 21 | 21 | 10 | 16 |
| Cynomys ludovicianus | 323 | 625 | 697 | 220 |
| Microtus ochrogaster | 0 | 4 | 10 | 2 |
| M. pennsylvanicus | 0 | 7 | 4 | 2 |
| Peromyscus maniculatus | 497 | 1168 | 1038 | 400 |
| Reithrodontomys mega lotus | 2 | 6 | 0 | 1 |
| Spermophilus tridecemlineatus | 5 | 7 | 6 | 2 |
| Mus musculus | 0 | 4 | 4 | 0 |
The number of trap-nights was consistent per site, but the number of sampling sites varied among years; 20 sites were sampled in 2003, 24 sites in each 2004 and 2005, and 11 sites in 2006.
Mean flea prevalence, abundance, and intensity on small mammals increased between preplague (t − 1) and plague (t) years at plague-positive sites and decreased at plague-negative sites, although these differences were not significant (w = 24, p = 0.55; w = 20, p = 0.15; and w = 20, p = 0.15, for prevalence, abundance, and intensity, respectively). Mean small mammal abundance decreased at both plague-positive and plague-negative sites, and the difference in change between site classes was not significant (w = 23.5, p = 0.44). We also failed to detect significant differences in any metric of flea or mammal occurrence in years preceding plague occurrence (change from year t − 2 to year t − 1) between plague-positive and plague-negative sites at α = 0.05 (Table 2). Small mammal species assemblages were dominated by P. maniculatus (Table 1), and analyses of deer mice were qualitatively identical to analyses of all small mammal species combined.
Table 2.
Mean Year-to-Year Changes in Metrics of Mammal and Flea Occurrence at Prairie Dog Colony Sites that Did Experience Plague Events in Year t
| |
Year t − 1 to year t |
Year t − 2 to year t − 1 |
||
|---|---|---|---|---|
| Metric | Plague (5) | Nonplague (5) | Plague (6) | Nonplague (6) |
| Small mammal (SM) abundance | −13.8 | −6 | +5.5 | +6.67 |
| Flea prevalence–SM | +0.04 | −0.03 | +0.21 | −0.06 |
| Flea abundance–SM | +0.27 | −0.45 | +0.32 | −0.05 |
| Flea intensity–SM | +0.13 | −0.74 | +0.22 | +0.30 |
| Prairie dog (PD) abundance | – | – | +1.33 | −2.83 |
| Flea prevalence–PD | – | – | +0.23 | +0.14 |
| Flea abundance–PD | – | – | +3.60 | +6.01 |
| Flea intensity–PD | – | – | +2.48 | +6.30 |
None of these metrics differed significantly between plague and nonplague sites.
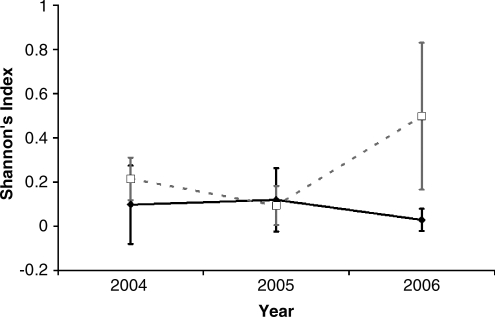
For the three prairie dog colonies that were affected by plague in 2005, we were able to track changes in small mammal species diversity (Shannon's index) in years before, during, and after the plague event. Small mammal diversity was significantly higher (w = 27, p = 0.017) in the year following a plague event at plague-positive sites (n = 3) than at plague-negative sites (n = 6) (Fig. 2) and changed significantly at plague-positive sites relative to plague-negative sites from years t and t + 1 at w = 27, p = 0.017.
FIG. 2.
Year-to-year change in small mammal diversity at sites that did (open squares, gray line) and did not (filled diamonds, black line) experience prairie dog die-offs in 2005. Error bars represent one standard deviation from the mean.
Discussion
We detected no significant evidence of differential changes in mammal or flea occurrence on black-tailed prairie dog colonies in the years preceding a prairie dog plague epizootic when comparing plague-positive versus plague-negative sites. At plague-positive colonies, all metrics of flea occurrence on small mammals increased relative to plague-negative colonies, yet these changes did not differ significantly between the site classes. Small mammal species diversity increased significantly at prairie dog colony sites that recently experienced plague activity compared to sites that were unaffected by plague. These results suggest that small mammal species are affected by plague events, but that changes in mammal abundance or flea prevalence, abundance, or intensity do not precede plague epizootics. Our results also suggest that high abundance of prairie dogs and small mammals and high flea prevalence and abundance do not predispose a given prairie dog colony to plague.
P. maniculatus was, by far, the most commonly occurring small mammal species prairie dog colony sites in this system (Table 1). The occurrence of Y. pestis–positive fleas on P. maniculatus during and following epizootic events (Thiagarajan et al. 2008), as well as the ubiquity of this species in grassland ecosystems where Y. pestis occurs (Collinge et al. 2008), makes P. maniculatus an attractive candidate as an enzootic host of Y. pestis. However, fleas typically associated with P. maniculatus are rarely recovered from black-tailed prairie dogs (Brinkerhoff et al. unpublished data) or prairie dog burrows (Salkeld and Stapp 2008b) just as prairie dog–specific fleas are rarely collected from P. maniculatus (Salkeld and Stapp 2008a). Further, the timing of Y. pestis exposure in P. maniulatus suggests that this species becomes infected following prairie dog die-offs and does not serve as a source of infection to prairie dogs (Stapp et al. 2008). These results, combined with the observed lack of increase in the abundance of P. maniculatus prior to plague epizootic events, are inconsistent with the assumption that this species acts as an enzootic host for Y. pestis.
It is noteworthy that all metrics of flea occurrence on small mammals decreased during plague years at plague-negative sites and increased at plague-positive sites (Table 2) even though these differences are not statistically significant. However, because prairie dogs at each plague-positive site had already died off in year t, it is impossible to determine whether potential increases in flea prevalence or abundance might have been a cause or effect of prairie dog, and potentially small mammal, mortality from plague. With relatively fewer individual small mammals at a plague-positive site in year t (Table 1), increases in flea occurrence might be an artifact of a smaller pool of available hosts rather than an indication of preplague changes that increased the likelihood of a plague event.
A number of studies have linked climatic variable to the timing of plague epizootics (Parmenter et al. 1999, Stapp et al. 2004, Collinge et al. 2005a, Kausrud et al. 2007), and some (e.g., Collinge et al. 2005a) have suggested that factors such as increased precipitation and relatively cool summers are conducive to increases in flea and small mammal populations. These increases, in turn, are thought to raise the likelihood of Y. pestis transmission to prairie dogs and cause the initiation of widespread epizootic events. Our data, however, indicate that changes in flea and mammal occurrence metrics are not predictive on a site-by-site basis. Overall, mean flea prevalence roughly doubled (29% to 57%) and abundance nearly tripled (0.68 to 1.65 fleas per host) on small mammals from 2003 to 2005, but these increases were not statistically different between plague-positive and plague-negative sites. It is possible that the overall increase in flea occurrence on small mammals facilitated the plague events of 2005 and 2006 but that landscape factors and proximity to other plague-positive colonies were more important in plague emergence at a given site (Collinge et al. 2005b).
The only dramatic relationship we found between prairie dog plague epizootics and small mammal occurrence was the increase in small mammal diversity following prairie dog die-offs (Fig. 2). In this study system, grassland sites without prairie dogs support richer assemblages of small mammals (Collinge et al. 2008). Our results demonstrate that within 1 year of a prairie dog die-off, small mammal diversity begins to trend toward that which is found in the absence of prairie dogs. The presence of prairie dogs variably influences small mammal abundance and species richness among studies (Agnew et al. 1986, Shipley and Reading 2006, Stapp 2007) although, in this study, the effective removal of prairie dogs caused a significant change in small mammal diversity that is consistent with differences in small mammal occurrence at prairie dog colonies and off-colony grassland sites (Collinge et al. 2008).
Because we could not predict which colonies would and would not experience plague when we designed this study, it was impossible to decide on and plan for adequate sample sizes for statistical testing a priori, and the power of our testing was ultimately limited by low sample size. We detected a great deal of variation in all the metrics we tested and, as a result, low sample size may be masking any biologically significant differences between plague-positive and plague-negative sites in flea and mammal occurrence. We do not suggest that mammal abundance and flea prevalence and abundance are unimportant to Y. pestis transmission, but we believe other factors have equivalent or greater influence on the dynamics of plague-associated prairie dog die-offs. In this study system, it is likely that landscape features and spatial proximity to other plague-affected colonies are more important predictors of plague emergence at a given site than characteristics of the host and vector community. Collinge et al. (2005b) found that prairie dog colonies that did not experience die-offs during widespread epizootics were farther from plague-positive colonies and were associated with higher cover of roads, streams, and lakes than were colonies that did experience die-offs. Thus, isolation by distance and effective isolation by landscape boundaries influence plague establishment at a site irrespective of mammal and flea abundance.
The complexity of the ecological systems in which Y. pestis occurs makes predicting its emergence problematic. Characterization of mammalian species as epizootic and enzootic hosts may not be appropriate (Gage and Kosoy 2005, Hanson et al. 2007), and it is possible that a nonmammalian Y. pestis reservoir exists. Low overall rates of plague exposure during and preceding epizootics among rodent (Stapp et al. 2008) and nonrodent (Brinkerhoff et al. 2009) mammalian species point to a nonmammalian source of Y. pestis infection to prairie dogs. Recent demonstrations of Y. pestis persistence in soils (Ayyadurai et al. 2008, Eisen et al. 2008b) indicate that a mammalian reservoir may not be required for epizootic initiation, although it has not been demonstrated that soils contaminated with Y. pestis can initiate epizootic events in prairie dogs.
Acknowledgments
This research was funded by the NSF/NIH joint program in Ecology of Infectious Diseases (DEB-0224328) and the National Center for Environmental Research (NCER) STAR program of the US-EPA (R-82909101-0). We would like to thank both Boulder City Open Space and Boulder County Parks and Open Space departments for allowing us to conduct this study on their properties. We would also like to acknowledge the data collection efforts of graduate students D. Conlin and A. Markeson and a large number of field technicians. This manuscript benefited from the comments of two anonymous reviewers.
Disclosure Statement
No competing financial interests exist.
References
- Agnew W. Uresk DW. Hansen RM. Flora and fauna associated with prairie dog colonies and adjacent ungrazed mixed-grass prairie in western South Dakota. J Range Manag. 1986;39:135–139. [Google Scholar]
- Ayyadurai S. Houhamdi L. Lepidi H. Nappez C, et al. Long term persistence of virulent Yersinia pestis in soil. Microbiology. 2008;154:2865–2871. doi: 10.1099/mic.0.2007/016154-0. [DOI] [PubMed] [Google Scholar]
- Bossard RL. Mammal and flea relationships in the Great Basin desert: from H.J. Egoscue's collections. J Parasitol. 2006;92:260–266. doi: 10.1645/GE-3545.1. [DOI] [PubMed] [Google Scholar]
- Brinkerhoff RJ. Collinge SK. Bai Y. Ray C. Are carnivores universally good sentinels of plague? Vector Borne Zoonot. 2009;9:491–497. doi: 10.1089/vbz.2008.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkerhoff RJ. Ray C. Thiagarajan B. Cully JF, et al. Prairie dog presence affects disease vector occurrence on small rodents. Ecography. 2008;31:654–662. [Google Scholar]
- Collinge SK. Johnson WC. Ray C. Matchett R, et al. Testing the generality of a trophic cascade model for plague. EcoHealth. 2005a;2:102–112. [Google Scholar]
- Collinge SK. Johnson WC. Ray C. Matchett R, et al. Landscape structure and plague occurrence in black-tailed prairie dogs on grasslands of the western USA. Landsc Ecol. 2005b;20:941–955. [Google Scholar]
- Collinge SK. Ray C. Cully JF. Effects of disease on keystone species, dominant species, and their communities. In: Ostfeld RS, editor; Keesing F, editor; Evines VT, editor. Infectious Disease Ecology: Effects of Ecosystems on Disease and of Disease on Ecosystems. Princeton Univ. Press; Princeton, NJ: 2008. pp. 129–144. [Google Scholar]
- Cully JF. Williams ES. Interspecific comparisons of sylvatic plague in prairie dogs. J Mammal. 2001;82:894–905. [Google Scholar]
- Eisen RJ. Holmes JL. Schotthoefer AM. Vetter SM, et al. Demonstration of early-phase transmission of Yersinia pestis by the mouse flea, Aetheca wagneri (Siphonaptera: Ceratophylidae), and implications for the role of deer mice as enzootic reservoirs. J Med Entomol. 2008a;45:1160–1164. doi: 10.1603/0022-2585(2008)45[1160:doetoy]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Eisen RJ. Petersen JM. Higgins CL. Wong D, et al. Persistence of Yersinia pestis in soils under natural conditions. Emerg Infect Dis. 2008b;14:941–943. doi: 10.3201/eid1406.080029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman DP. Catts EP. Manual of Medical Entomology. 4th. New York: Cambridge University Press; 1982. [Google Scholar]
- Gage KL. Kosoy MY. Natural history of plague: perspectives from more than a century of research. Annu Rev Entomol. 2005;50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. [DOI] [PubMed] [Google Scholar]
- Hanson DA. Britton HB. Restani M. Washburn LR. High prevalence of Yersinia pestis in black-tailed prairie dog colonies during an apparent enzootic phase of sylvatic plague. Conserv Genet. 2007;8:798–795. [Google Scholar]
- Hubbard CA. Fleas of Western North America. Ames, IA: Iowa State College Press; 1947. p. 533. [Google Scholar]
- Kausrud KL. Viljugrein H. Frigessi A. Begon M, et al. Climatically-driven synchrony of gerbil populations allows large-scale plague outbreaks. Proc R Soc B Biol Sci. 2007;274:1963–1969. doi: 10.1098/rspb.2007.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorange EA. Race BL. Sebbane F. Hinnebusch BJ. Poor vector competence of fleas and the evolution of hypervirulence in Yersinia pestis. J Infect Dis. 2005;191:1907–1912. doi: 10.1086/429931. [DOI] [PubMed] [Google Scholar]
- Parmenter RR. Yadav EP. Parmenter CA. Ettestad P, et al. Incidence of plague associated with increased winter-spring precipitation in New Mexico. Am J Trop Med Hygiene. 1999;61:814–821. doi: 10.4269/ajtmh.1999.61.814. [DOI] [PubMed] [Google Scholar]
- Pollitzer R. Meyer KF. The ecology of plague. In: May JF, editor. Studies of Disease Ecology. New York: Hafner Publishing Co.; 1961. pp. 433–501. [Google Scholar]
- Salkeld DJ. Stapp P. No evidence of deer mouse involvement in plague (Yersinia pestis) epizootics in prairie dogs. Vector Borne Zoonot Dis. 2008a;8:331–337. doi: 10.1089/vbz.2007.0199. [DOI] [PubMed] [Google Scholar]
- Salkeld DJ. Stapp P. Prevalence and abundance of fleas in black-tailed prairie dog burrows: implications for the transmission of plague (Yersinia pestis) J Parasitol. 2008b;94:616–621. doi: 10.1645/GE-1368.1. [DOI] [PubMed] [Google Scholar]
- Shipley BK. Reading RP. A comparison of herpetofauna and small mammal diversity on black-tailed prairie dog (Cynomys ludovicianus) colonies and non-colonized grasslands in Colorado. J Arid Environ. 2006;66:27–41. [Google Scholar]
- Stapp P. Rodent communities in active and inactive colonies of black-tailed prairie dogs in short grass steppe. J Mammal. 2007;88:241–249. [Google Scholar]
- Stapp P. Antolin MF. Ball M. Patterns of extinction in prairie-dog metapopulations: plague outbreaks follow El Nino events. Front Ecol Environ. 2004;2:235–240. [Google Scholar]
- Stapp P. Salkeld DJ. Eisen RJ. Pappert R, et al. Exposure of small rodents to plague during epizootics in black-tailed prairie dogs. J Wildl Dis. 2008;44:724–730. doi: 10.7589/0090-3558-44.3.724. [DOI] [PubMed] [Google Scholar]
- Thiagarajan B. Bai Y. Gage KL. Cully JF. Prevalence of Yersinia pestis in rodents and fleas associated with black-tailed prairie dog (Cynomys ludovicianus) at Thunder Basin National Grassland, Wyoming. J Wildl Dis. 2008;44:731–736. doi: 10.7589/0090-3558-44.3.731. [DOI] [PubMed] [Google Scholar]
- Webb CT. Brooks CP. Gage KL. Antolin MF, et al. Classic flea-borne transmission does not drive plague epizootics in prairie dogs. Proc Natl Acad Sci. 2006;103:6236–6241. doi: 10.1073/pnas.0510090103. [DOI] [PMC free article] [PubMed] [Google Scholar]