Abstract
Coxsackievirus B3 (CVB3) induces cardiac inflammation (myocarditis) in male but not female C57BL/6 mice. Protection of females correlates with reduced expression of TNF-α and IL-1β at both the mRNA and protein levels in the heart. Treatment of females with 300 ng/mouse of recombinant TNF-α on days +1 and +3 relative to infection restores myocarditis susceptibility to levels approximating those of infected male mice, showing that TNF-α deficiency is central to disease resistance. Female mice express little CD1d on spleen lymphocytes or cardiac myocytes, while females treated with TNF-α show increased CD1d expression in both cell populations. TNF-α treatment of male or female CD1d knockout (CD1dKO) mice failed to restore myocarditis susceptibility, demonstrating that of the multiple potential TNF-α activities, its ability to upregulate this non-classical major histocompatibility complex antigen is its dominant function in myocarditis susceptibility. Bone marrow chimeric mice were produced between female C57BL/6 and C57BL/6 CD1dKO mice so that either hematopoietic or non-hematopoietic cells were CD1d deficient. TNF-α treatment of chimeric mice having wild-type (CD1d+) hematopoietic cells restored myocarditis susceptibility, while TNF-α treatment of chimeric mice having CD1dKO hematopoietic cells, but CD1d+ myocytes, failed to develop myocarditis, showing that CD1d expression in lymphoid cells controls disease susceptibility.
Introduction
Myocarditis is an inflammation of the myocardium that often follows microbial infections. Many types of infection (with virus, bacteria, fungi, or protozoa/helminths) can cause this disease; however, among viral etiological agents, enteroviruses of the picornavirus family and adenoviruses dominate (1,2). Although it is estimated from large unselected autopsy series that between 1% and 3% of humans may have myocarditis at any time, the incidence of clinical disease with symptoms severe enough to cause hospitalization is significantly lower (25). Up to 20% of patients with histological evidence of myocarditis will ultimately develop dilated cardiomyopathy (3), and individuals with enteroviral RNA in the heart as determined by endocardial biopsies were six times more likely to die in the subsequent 2 y (25% versus 4%) than virus-negative patients (24), indicating that myocarditis and dilated cardiomyopathy of viral etiology has a much worse prognosis than patients who develop these diseases from other causes. Sex and sex-associated hormones may play a role in myocarditis susceptibility. Although the earlier estimate of a twofold incidence of myocarditis in men compared to women, as reported by Woodruff in 1980 (25) is probably high, more recent clinical studies have reported that more men than women develop this disease (14,17).
A murine model of coxsackievirus B3 (CVB3)-induced myocarditis has been developed that shares many characteristics with human disease. Inflammation is primarily comprised of mononuclear cells, and necrotic myocytes are intimately associated with the inflammatory cells (5,26). Not all CVB3 infections result in myocarditis, however. Certain inbred mouse strains are relatively resistant to viral myocarditis (15), and even in a mouse strain in which CVB3-infected males are highly susceptible to developing myocarditis, females can be highly resistant to inflammation and cardiac injury despite virus infection and replication in the heart (6,7,11). The mechanism of sex bias in myocarditis susceptibility is not completely understood, but clearly testosterone is important to susceptibility in males, since castration of males is protective, while restoration of testosterone to castrated males restores susceptibility (6,7,11). Similarly, ovariectomy of females actually increases myocarditis susceptibility, which can be suppressed by exogenous replacement of 17-β-estradiol. Gonadal hormones influence both innate and adaptive immunity (14,15) in diverse and complex ways. 17-β-Estradiol is known to increase interferon-γ (IFN-γ) secretion (16,17), but it suppresses monocyte chemotactic protein-1 (MCP-1) (18) and tumor necrosis factor-α (TNF-α) (19,20) responses. Other effects of estrogen include: (1) promotion of antibody responses either through inhibition of B lymphocyte apoptosis (4), or an increase in antibody secretion (22,23); (2) augmentation of calcineurin signaling (24); and (3) promotion of dendritic cell differentiation and antigen presentation (19). In contrast, testosterone increases TNF-α, interleukin-1β (IL-1β), and IL-6 expression in the heart, indicating that this hormone is likely proinflammatory in cardiovascular and renal disease (18,23). The diverse actions of 17-β-estradiol and testosterone on TNF-α expression should be crucial to the relative susceptibility of males and resistance of females to CVB3-induced myocarditis, since TNF-α is a dominant factor controlling susceptibility. Treatment of myocarditis-resistant strains of mice with exogenous TNF-α is sufficient to convert the mice to myocarditis susceptibility (10,15).
TNF-α is a pleiotropic cytokine that not only induces cell death through apoptosis and necrosis, but also stimulates tissue repair/regeneration through activation of JNK, p38 MPK, NF-κB, and AP-1 (22). While often considered to be a proinflammatory cytokine that can upregulate expression of IL-6 and IL-1β, adhesion molecules, and major histocompatibility complex (MHC) antigens (20), it has also been reported to be anti-inflammatory and protective in autoimmune disease (16). Considering the multiple biological effects of TNF-α, it is not clear which of these is relevant to myocarditis. We have previously demonstrated that TNF-α is required for the upregulation of CD1d, a MHC class I-like molecule, and that CD1d knockout (CD1dKO) mice fail to develop myocarditis subsequent to CVB3 infection (8,9,12). Thus we hypothesize that female mice are protected from CVB3-induced myocarditis because low TNF-α concentrations in females prevent CD1d upregulation, and that treating female mice with exogenous TNF-α will restore myocarditis susceptibility through upregulation of CD1d expression.
Materials and Methods
Mice
Male and female C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). Breeding pairs of C57BL/6 CD1d knockout (KO) mice were kindly supplied by Dr. Jon Boyson, Department of Surgery, University of Vermont. All mice were 5–8 wk of age when infected. All of the studies were reviewed and approved by the University of Vermont Institutional Animal Care and Use Committee.
Virus
The H3 variant of CVB3 was made from an infectious cDNA clone as described previously (13).
Infection of mice
Mice were injected intraperitoneally (IP) with 102 plaque-forming units (PFU) of virus in 0.5 mL PBS. Animals were killed when moribund or at 7 d after infection. Controls were uninfected mice that were killed at the same time as the infected animals.
Organ virus titers
The hearts were aseptically removed from the animals, weighed, and homogenized in RPMI 1640 medium containing 5% fetal bovine serum (FBS), L-glutamine, streptomycin, and penicillin. Cellular debris was removed by centrifugation at 300 × g for 10 min. Supernatants were diluted serially using 10-fold dilutions and titered on HeLa cell monolayers by the plaque-forming assay (21).
Cytokines and ELISAs
Hearts from euthanized mice were perfused with PBS, dried, weighed, and homogenized in PBS. Cellular debris was removed by centrifugation at 325 × g and the supernatant was evaluated for TNF-α and IL-1β using a commercial ELISA kit according to the manufacturer's directions (Pierce Endogen, Rockford, IL). Recombinant mouse TNF-α was purchased from BD Pharmingen, San Diego, CA, diluted in PBS to 600 μg/mL, and injected (0.5 mL) IP on days +1 and +3 relative to infection. Controls received 0.5 mL PBS IP alone on the same days as cytokine injection.
Histology
Tissue was fixed in 10% buffered formalin for 48 h, paraffin embedded, sectioned, and stained with hematoxylin and eosin. Image analysis of cardiac inflammation was done as described previously (13).
RNA isolation and PCR arrays
The hearts were homogenized in Trizol containing 2 μL/mL Ribolock and centrifuged to remove cell debris. The RNA was extracted from the supernatant using chloroform/Trizol/ethanol and the Qiagen RNeasy Minikit (Qiagen, Valencia CA) according to the manufacturer's directions. Production of the cDNA from RNA was performed by the Vermont Cancer Center Microarray Facility using SA Biosciences (Frederick, MD) Profiler PCR Array Mouse Toll-Like Receptor Signaling Pathway plates. cDNA reactions were set up using the RT2 First Strand kit (SA Biosciences). Three reactions were set up for each sample, using 400 ng total RNA for each reaction. These were pooled and then divided among the three PCR array plates using RT2 Real-Time™ SYBR Green/Rox PCR Master Mix (SA Biosciences) according to the manufacturer's instructions. The PCR array plates were run on an ABI Prism 7900HT Sequence Detection System, and analyzed with SDS 2.2 software (Applied Biosystems, Foster City, CA).
Confocal microscopy
The hearts were collected from euthanized animals, perfused with PBS, and flash frozen in OCT freezing medium (Sigma-Aldrich, St. Louis, MO) and stored at –80°C. The hearts were grossly cut through the ventricles and sectioned using a Triangle Biomedical Sciences, Durham, NC cryostat. The sections were placed on Fisherbrand Superfrost microscope slides (Fisher Scientific, Pittsburgh, PA). The sections were fixed in −20°C acetone for 5 min, rehydrated in distilled water for 10 min, blocked with Fc block (anti-mouse CD16/32, clone 2.4G2; Pharmingen) in PBS containing 1% bovine serum albumin (BSA), and stained with 1:100 dilutions of mouse anti-α actinin (sarcomeric) (clone EA-53; Sigma) and rat anti-mouse CD1d (clone 1B1; Pharmingen) for 90 min at room temperature. Then they were washed twice in PBS, and incubated with 1:100 dilutions of Alexa Fluor 488 F(ab)2 rabbit anti-mouse IgG (Invitrogen, Carlsbad, CA) and Alexa Fluor 568 goat anti-rat IgG (Invitrogen) at room temperature for 60 min. Nuclear staining was done with 4′,6-diamidino-2-phenylindole (Roche, Indianapolis, IN). The sections were imaged using a Zeiss LSM 510 META confocal laser scanning microscope (Zeiss Microimaging, Thornwood, NY) operating in multitrack mode, as previously described (9).
Flow cytometry
The spleens were removed and pressed through fine mesh screens. Lymphoid cells were isolated by centrifugation of cell suspensions on Histopaque (Sigma, St. Louis, MO), then 105 spleen cells were washed in PBS and 1% BSA containing a 1:100 dilution of Fc Block and FITC-rat-anti-mouse CD1d (clone 1B1) on ice for 30 min, washed once with PBS-BSA, and fixed in 2% paraformaldehyde. The cells were analyzed using a Coulter Epics Elite flow cytometer, Brea, CA with a single excitation wavelength (488 nm) and a band filter for FITC (525 nm). Controls were labeled with FITC-rat IgG2b isotype immunoglobulin (Pharmingen). The cell population was classified for cell size (forward scatter) and complexity (side scatter). At least 10,000 cells were evaluated. Positive staining was determined relative to isotype controls. Data were analyzed using FlowJo Experiment-Based Analysis for Flow Cytometry (Tree Star Inc., Ashland, OR).
Bone marrow transplantation
Four-week-old mice were give two 400-roentgen irradiations using a cesium irradiator 4 h apart. Radiation dose was confirmed by placing a model 746 dosimeter (serial number HB174953, calibration date 9/4/2008; Arrow-Tech Inc., Rolla, ND) in the cage during the irradiation procedure. Bone marrow donor mice were euthanized and the tibia and femur were removed. Bone marrow was flushed from the bones, treated with anti-CD3 antibody (1:50 dilution) and 20% rabbit complement (Sigma) for 40 min at 37°C, counted by trypan blue exclusion, and injected (5 × 107 cells/0.2 mL PBS) IV into the tail vein of irradiated recipients within 6 h of the last irradiation. Four weeks after bone marrow injection, the animals were infected.
Statistical analysis
Data were analyzed for skewness and kurtosis using SPSS for Windows (version 11.0; SPSS, Inc., Chicago, IL), and it showed that the variance was not normally distributed for several groups. Statistical analysis was done with the nonparametric Mann-Whitney test using SPSS for Windows. The threshold for significance was p < 0.05.
Results
TNF-α and IL-1β expression in the heart correlate with myocarditis susceptibility
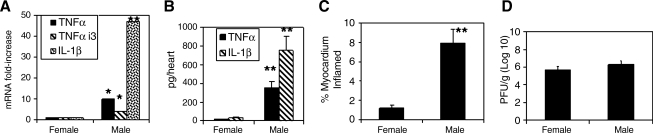
Male and female C57BL/6 mice were infected with 102 PFU of CVB3 IP and killed 7 d later. The hearts were removed and evaluated for cardiac virus titer, myocarditis, TNF-α, and IL-1β by ELISA, or extracted for RNA and evaluated for mRNA expression by PCR array (Fig. 1). Female mice showed significantly less myocarditis (Fig. 1C) and cytokine expression (Fig. 1B) in the heart than infected male mice. Expression levels of mRNA for TNF-α, TNF-α-induced protein 3 (TNFaip3), and IL-1β were also substantially elevated in males compared to females (Fig. 1A). No differences in mRNA or cytokine protein were observed between uninfected male and female mice (data not shown). No significant differences were found in cardiac virus titers between male and female mice (Fig. 1D).
FIG. 1.
Susceptibility to CVB3-induced myocarditis correlates with TNF-α expression in male and female mice. Male and female C57BL/6J mice were infected with 102 PFU of CVB3 and killed 7 d later. The hearts were removed and evaluated for (C) myocarditis by image analysis, and (D) cardiac virus titer per gram of heart tissue by plaque-forming assay. The hearts were also homogenized and the supernatants evaluated by (B) ELISA for TNF-α and IL-1β. RNA extracted from heart tissue was evaluated for mRNA expression by real-time PCR (A). Results represent mean ± SEM of 4–6 mice/group. Values for males were significantly different than those for females (*p < 0.05; **p < 0.01).
TNF-α upregulates expression of CD1d and promotes myocarditis susceptibility
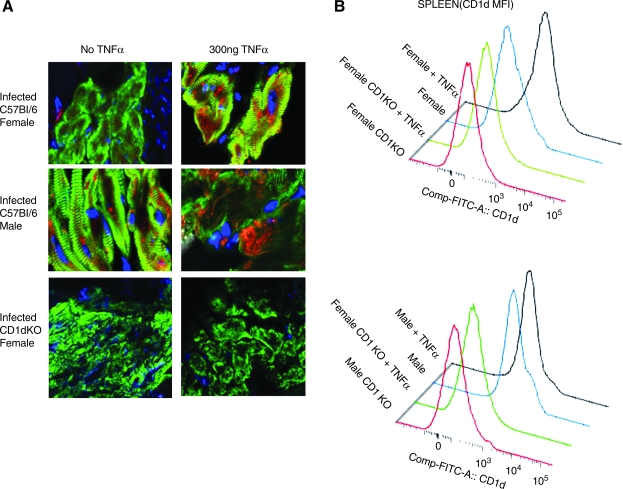
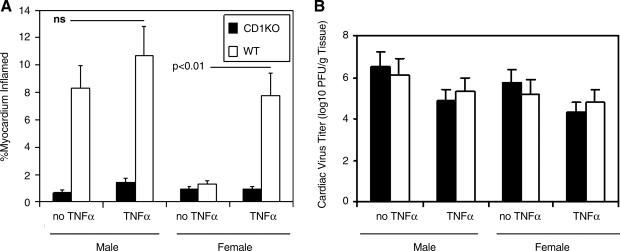
Previous studies have shown that TNF-α upregulates CD1d expression in CVB3-infected male mice and augments myocarditis susceptibility (10,12). To determine if increasing TNF-α in females would also enhance myocarditis susceptibility and upregulate CD1d, male and female mice were infected with virus and injected IP with 300 ng/mouse of recombinant mouse TNF-α on days +1 and +3 relative to infection. Fig. 2A demonstrates that while infected females show little CD1d expression on cardiac myocytes compared to infected male mice (red is CD1d, green is sarcomeric α-actinin, and blue is nuclear stain), exogenous treatment of both male TNFaip3 and female C57BL/6 mice with TNF-α significantly increases CD1d expression. As expected, neither infected female C57BL/6 CD1dKO mice with or without TNF-α treatment had any CD1d in the heart. CD1d was not expressed on myocytes in uninfected male or female mice, and TNF-α treatment of uninfected animals failed to upregulate its expression (data not shown). Flow analysis of spleen lymphocytes from infected male and female C57BL/6 and C57BL/6 CD1d KO mice demonstrates that infected female C57BL/6 mice expressed little CD1d (mean fluorescence intensity [MFI] 689 ± 76), but that after TNF-α treatment, CD1d expression was significantly increased in infected C57BL/6 animals (MFI 1124 ± 159; p < 0.01) (Fig. 2B). As expected, spleen cells from CD1d KO animals did not express CD1d above isotype control values (MFI 178 ± 89), and TNF-α treatment had no effect on CD1d expression (MFI 195 ± 45). Infected male mice had high CD1d expression in the spleen (MFI 1267 ± 211), and this level of CD1d expression was only slightly increased with TNF-α treatment (MFI 1311 ± 301). Fig. 3 illustrates the cardiac inflammation seen in male and female C57BL/6 and C57BL/6 CD1dKO mice with and without TNF-α treatment, and shows that while TNF-α treatment of infected female wild-type mice restored myocarditis susceptibility, the cytokine was ineffective in CD1d KO animals, indicating that the primary effect of TNF-α on myocarditis susceptibility is through its ability to upregulate CD1d expression. Mortality was increased in both male and female C57BL/6 mice treated with exogenous TNF-α (4/8 [dead/total infected] or 50% of males, and 7/12 or 58% of males with TNF-α [not significant]; 1/7 or 14% of infected females, and 5/10 or 50% of infected females with TNF-α; p < 0.05). There was no significant increase in mortality in CD1dKO mice treated with TNF-α compared to those not treated with the cytokine (1/6 or 17% of infected males, and 1/8 or 13% of infected males with TNF-α [not significant]; 0/6 of infected females, and 0/6 of infected females with TNF-α). Images of representative heart histology from each experimental group are shown in Fig. 4. Arrows indicate areas of cardiac inflammation.
FIG. 2.
TNF-α increases CD1d expression on cardiac myocytes and spleen lymphocytes. Male and female C57BL/6 and C57BL/6 CD1d KO mice were infected with 102 PFU of CVB3. Half the mice were injected on days + 1 and +3 relative to infection with 300 ng/mouse of recombinant TNF-α. The mice were killed 7 d later and evaluated for CD1d expression in the (A) heart by confocal microscopy, and for (B) CD1d expression on spleen lymphocytes by flow cytometry. For confocal microscopy, the hearts were labeled with mouse anti-α-actinin and Alexa Fluor 488-anti-mouse IgG (green), rat anti-CD1d and Alexa Fluor 568-anti-rat IgG (red), and DAPI (blue nuclei). For flow cytometry, the lymphocyte population was gated by forward versus side scatter, and mean fluorescence intensity (MFI) of the total lymphocyte population was determined. Representative data are from one mouse of the 4–6 mice per experimental group.
FIG. 3.
TNF-α promotes myocarditis susceptibility through CD1d upregulation. Hearts from infected male and female wild-type (WT) C57BL/6 and C57BL/6 CD1d KO mice were evaluated for (A) myocarditis by image analysis, and for (B) cardiac virus titers by the plaque-forming assay on HeLa cells. Half of the mice received 300 ng/mouse of recombinant TNF-α on days +1 and +3 relative to infection. Results represent mean ± SEM of 4–6 mice/group.
FIG. 4.
Images of the representative histology of the mice described in Fig. 3. The hearts were fixed in formalin, sectioned, and stained with hematoxylin and eosin. Areas of inflammation are indicated by the arrows.
The effect of TNF-α is primarily mediated through hematopoietic cells
Since TNF-α upregulated CD1d expression on both hematopoietic and non-hematopoietic cardiac myocytes, the next question was whether the primary effect of TNF-α treatment was on CD1d expression in hematopoietic or non-hematopoietic cell populations. To address this question, bone marrow chimeric mice were produced using female C57BL/6 and C57BL/6 CD1dKO mice. Recipient female C57BL/6 and C57BL/6 CD1dKO mice were given two irradiation treatments of 400 roentgens each, and were then injected IV with 5 × 107 bone marrow (BM) cells from donor mice as indicated in Fig. 5. After 6 wk, irradiated mice were infected with CVB3 and injected on days +1 and +3 relative to infection with 300 ng/mouse of TNF-α. The following bone marrow/recipient groups were evaluated: female C57BL/6 recipients given female C57BL/6 bone marrow (WT BM → WT recipients); female C57BL/6 recipients given female C57BL/6 CD1d bone marrow (CD1dKO BM → WT recipients); female C57BL/6 CD1dKO recipients given female C57BL/6 CD1dKO bone marrow (CD1dKO BM → CD1dKO recipients); and female C57BL/6 CD1dKO recipients given female C57BL/6 bone marrow (WT BM → CD1dKO recipients). Female C57BL/6 mice given C57BL/6 bone marrow developed minimal myocarditis, unless the recipients also received TNF-α. However, TNF-α treatment was not capable of promoting myocarditis when female C57BL/6 recipients were given bone marrow from CD1dKO donor mice, indicating that the hematopoietic cells must have the ability to express CD1d in order for TNF-α to promote myocarditis. Similarly, CD1dKO recipients given C57BL/6 bone marrow showed aggravated myocarditis with TNF-α treatment, again indicating that hematopoietic cells must have the ability to express CD1d for TNF-α to promote myocarditis, but that the ability of non-hematopoietic cells to express CD1d was not necessary. No significant differences were observed in cardiac virus titers between the groups (data not shown).
FIG. 5.
TNF-α promotes myocarditis susceptibility through its effects on hematopoietic cells. Bone marrow chimeric mice were produced by irradiating recipient female mice and injecting bone marrow from female donors IV into the tail vein. The bone marrow recipients were rested for 6 wk, then injected with 102 PFU of CVB3 IP. Some mice also received 300 ng/mouse of recombinant TNF-α IP on days +1 and +3 relative to infection. All the mice were euthanized 7 d after infection, and the hearts were evaluated by image analysis for myocarditis. The first bar indicates bone marrow donors, and the second bar represents bone marrow recipients (C57BL/6 wild-type [WT] bone marrow injected into irradiated C57BL/6 WT recipients; e.g., WT → WT). Results represent mean ± SEM of 4–5 mice/group (ns, not significant; **p < 0.01).
Discussion
This article demonstrates that CVB3 infection of female mice results in substantially less TNF-α induction than infection of male mice. The lack of TNF-α induction is a major factor in female resistance to disease, since exogenous administration of this cytokine restores myocarditis susceptibility. Thus there is a strong similarity between different conditions of CVB3 myocarditis resistance. Three such conditions of resistance have been identified. These are: (1) the genetics of inbred mouse strains (15); (2) the genetics of the CVB3 variant (21); and the sex of the mice (6). Exogenous administration of TNF-α to normally myocarditis-resistant B10.A mice results in significant enhancement of disease (15), as does TNF-α treatment of normally myocarditis-susceptible BALB/c male mice infected with a non-myocarditic variant of CVB3 designated H310A1 (10,12). Therefore, with the demonstration here that exogenous TNF-α also makes normally CVB3 myocarditis-resistant female mice susceptible, this indicates that the lack of TNF-α is the cause of most forms of myocarditis resistance. The major question is how TNF-α promotes this disease. TNF-α is a pleiotropic cytokine that not only promotes cell apoptosis, but also is highly proinflammatory though the activation of multiple signal pathways, including NF-κB, JNK, and p38 MAPK (22); this also leads to increases in cytokines such as IL-1β, adhesion molecules, and MHC (15,22). This laboratory has previously shown that upregulation of CD1d, a non-classical MHC class I molecule, is essential to myocarditis susceptibility (9), and that TNF-α promotes CD1d upregulation through the TNF-receptor 1 (TNFR1) (10,12). Also as shown here, increased CD1d expression in female mice treated with TNF-α correlates with enhanced myocarditis in this gender. However, this alone does not prove that the primary effect of TNF-α in myocarditis susceptibility results from its modulation of CD1d. To demonstrate this, mice lacking CD1d were treated with TNF-α. If this cytokine promotes myocarditis through an CD1d-independent mechanism, one would expect that the CD1dKO mice would have developed substantial myocarditis, as TNF-α signaling through both TNFR1 and TNFR2 should remain intact. As shown in Fig. 3, both female and male CD1dKO mice develop slightly, but not significantly, more cardiac inflammation when treated with TNF-α than untreated CD1dKO mice of the same gender. However, the slight increase in myocarditis seen in cytokine-treated CD1dKO mice is minor compared to the strongly enhanced myocarditis in cytokine-treated wild-type (CD1d+) female animals, indicating that the non-CD1d-dependent effects of TNF-α are not sufficient to promote myocarditis.
TNF-α upregulates CD1d expression both on lymphoid cells and on cardiac myocytes. A separate question is whether CD1d upregulation on hematopoietic or non-hematopoietic cells primarily contributes to disease susceptibility. To answer this question, bone marrow chimeric mice were produced between wild-type and CD1d KO animals, such that CD1d would only be expressed on hematopoietic cells or on non-hematopoietic cells. In this experiment, the data clearly demonstrated that CD1d expression on hematopoietic cells is required for myocarditis susceptibility. CD1d is primarily known as a target for invariant NKT cell recognition (2). While this may lead to killing of virus-infected cells by the innate effectors, recognition of CD1d on antigen-presenting cells such as dendritic cells and macrophages can also alter the developing adaptive immune response (1). Although CD1d and iNKT cells have been shown to suppress virus load in encephalomyocarditis virus infections (another picornavirus), iNKT cells are apparently not important in controlling CVB3 in the heart (9). This is presumably why CD1d expression on cardiac myocytes plays little role in myocarditis. However, the increased CD1d expression seen on hematopoietic cells should promote pathogenicity due to the enhanced immune response, particularly since T cells are the major cause of cardiac injury in this disease (26).
Conclusion
CVB3 induces myocarditis in male but not female C57BL/6 mice, and the protection induced in females correlates with reduced expression of TNF-α and IL-1β at both the mRNA and protein levels in the heart. Treatment of females with recombinant TNF-α restores myocarditis susceptibility to levels close to those of infected male mice, showing that TNF-α deficiency is central to disease resistance.
Acknowledgments
This work was supported by National Institutes of Health grant no. HL80594.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Cerundolo V. Silk JD. Masri SH. Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 2.Exley M. Bigley N. Cheng O, et al. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J Leukoc Biol. 2001;69:713–718. [PubMed] [Google Scholar]
- 3.Feldman AM. McNamara D. Myocarditis. N Engl J Med. 2000;343:1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 4.Grimaldi CM. Cleary J. Dagtas AS. Moussai D. Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. 2002;109:1625–1633. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber S. Coxsackievirus-induced myocarditis is dependent on distinct immunopathogenic responses in different strains of mice. Lab Invest. 1997;76:691–701. [PubMed] [Google Scholar]
- 6.Huber S. Job L. Auld K. Influence of sex hormones on coxsackie B3 virus infection in Balb/c mice. Cell Immunol. 1982;67:173–179. doi: 10.1016/0008-8749(82)90210-6. [DOI] [PubMed] [Google Scholar]
- 7.Huber S. Polgar J. Schultheiss P. Schwimmbeck P. Augmentation of pathogenesis of coxsackievirus B3 infections in mice by exogenous administration of interleukin-1 and interleukin-2. J Virol. 1994;68:195–206. doi: 10.1128/jvi.68.1.195-206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber S. Sartini D. T cells expressing the Vgamma1 T-cell receptor enhance virus-neutralizing antibody response during coxsackievirus B3 infection of BALB/c mice: differences in male and female mice. Viral Immunol. 2005;18:730–739. doi: 10.1089/vim.2005.18.730. [DOI] [PubMed] [Google Scholar]
- 9.Huber S. Sartini D. Exley M. Role of CD1d in coxsackievirus B3-induced myocarditis. J Immunol. 2003;170:3147–3153. doi: 10.4049/jimmunol.170.6.3147. [DOI] [PubMed] [Google Scholar]
- 10.Huber SA. Born W. O'Brien R. Dual functions of murine gammadelta cells in inflammation and autoimmunity in coxsackievirus B3-induced myocarditis: role of Vgamma1+ and Vgamma4+ cells. Microbes Infect. 2005;7:537–543. doi: 10.1016/j.micinf.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Huber SA. Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber SA. Sartini D. Roles of tumor necrosis factor alpha (TNF-alpha) and the p55 TNF receptor in CD1d induction and coxsackievirus B3-induced myocarditis. J Virol. 2005;79:2659–2665. doi: 10.1128/JVI.79.5.2659-2665.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knowlton KU. Jeon ES. Berkley N. Wessely R. Huber S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996;70:7811–7818. doi: 10.1128/jvi.70.11.7811-7818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhl U. Pauschinger M. Schwimmbeck PL, et al. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003;107:2793–2798. doi: 10.1161/01.CIR.0000072766.67150.51. [DOI] [PubMed] [Google Scholar]
- 15.Lane J. Neumann D. Lafond-Walker A. Herkowitz A. Rose N. Interleukin 1 or tumor necrosis factor can promote coxsackie B3-induced myocarditis in resistant B10.A mice. J Exp Med. 1992;175:1123–1129. doi: 10.1084/jem.175.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J. Marino MW. Wong G, et al. TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nat Med. 1998;4:78–83. doi: 10.1038/nm0198-078. [DOI] [PubMed] [Google Scholar]
- 17.Mason JW. O'Connell JB. Herskowitz A. Rose NR. McManus BM. Billingham ME. Moon TE. A clinical trial of immunosuppressive therapy for myocarditis. The myocarditis treatment trial investigators. N Engl J Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 18.Metcalfe PD. Leslie JA. Campbell MT. Meldrum DR. Hile KL. Meldrum KK. Testosterone exacerbates obstructive renal injury by stimulating TNF-alpha production and increasing proapoptotic and profibrotic signaling. Am J Physiol Endocrinol Metab. 2008;294:E435–E443. doi: 10.1152/ajpendo.00704.2006. [DOI] [PubMed] [Google Scholar]
- 19.Paharkova-Vatchkova V. Maldonado R. Kovats S. Estrogen preferentially promotes the differentiation of CD11c + CD11b(intermediate) dendritic cells from bone marrow precursors. J Immunol. 2004;172:1426–1436. doi: 10.4049/jimmunol.172.3.1426. [DOI] [PubMed] [Google Scholar]
- 20.Pober JS. Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990;50:537–544. doi: 10.1097/00007890-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Van Houten N. Bouchard P. Moraska A. Huber S. Selection of an attenuated coxsackievirus B3 variant using a monoclonal antibody reactive to myocyte antigen. J Virol. 1991;65:1286–1290. doi: 10.1128/jvi.65.3.1286-1290.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wajant H. Pfizenmaier K. Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 23.Wang M. Tsai BM. Kher A. Baker LB. Wairiuko GM. Meldrum DR. Role of endogenous testosterone in myocardial proinflammatory and proapoptotic signaling after acute ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H221–H226. doi: 10.1152/ajpheart.00784.2004. [DOI] [PubMed] [Google Scholar]
- 24.Why H. Meany T. Richardson P, et al. Clinical and prognostic significance of detection of enteroviral RNA in the myocardium of patients with myocarditis or dilated cardiomyopathy. Circulation. 1994;89:2582–2589. doi: 10.1161/01.cir.89.6.2582. [DOI] [PubMed] [Google Scholar]
- 25.Woodruff J. Viral myocarditis. Am J Pathol. 1980;101:425–483. [PMC free article] [PubMed] [Google Scholar]
- 26.Woodruff J. Woodruff J. Involvement of T lymphocytes in the pathogenesis of coxsackievirus B3 heart disease. J Immunol. 1974;113:1726–1734. [PubMed] [Google Scholar]