Abstract
Previous studies in rodents and people have demonstrated that repeated tick exposure is associated with reduced Borrelia burgdorferi transmission but the mechanism of prevention remains unclear. We examined the acute histopathologic reactions to initial and repeated Ixodes scapularis bites in BALB/c mice and in people. Skin biopsies of BALB/c mice infested for the first time by I. scapularis nymphs revealed vascular dilatation and an accumulation of inflammatory cells adjacent to the bite site but absent at the site of tick attachment. Such changes would enhance tick-borne pathogen transmission. Mice reexposed to I. scapularis nymphs experienced a decrease in vascular dilatation and a marked increase in inflammatory cells at the site of tick attachment. Skin biopsies of people with attached I. scapularis nymphs revealed similar histologic patterns. These results indicate that cellular changes at the tick-dermal interface following I. scapularis attachment are likely to allow for successful transmission of tick-borne pathogens in non-tick-immune hosts and to inhibit tick-borne pathogen transmission in hosts that have developed tick immunity.
Key Words: Ixodes, Tick(s), Immunology, Lyme disease
Introduction
Infectious agents that are transmitted by blood-feeding arthropods cause significant morbidity, mortality, and economic loss throughout the world (Hill et al. 2005). Although mosquitoes surpass ticks as vectors of public health importance, ticks transmit the greatest variety of microbial pathogens of any arthropod vector including established, emerging, and resurging infectious agents (Walker 1998, Childs and Paddock 2003, Jongejan and Uilenberg 2004, Telford and Goethert 2004, Dennis and Piesman 2005, Ginsberg and Stafford 2005, Hanincová et al. 2006, Walker 2005). The hard-bodied tick Ixodes scapularis transmits causative agents of Lyme disease, human babesiosis, and human granulocytic anaplasmosis (Krause 2002, Steere et al. 2004, Dumler et al. 2007). I. scapularis saliva contains a complex mixture of pharmacologically active molecules that potentiate transmission of these pathogens by modulating host immune defenses, itch responses, and hemostasis (Zeidner et al. 2002, Brossard and Wikel 2004, Steen et al. 2006). The complexity of the rich pharmacologic cocktail of I. scapularis saliva is further reflected in the salivary gland transcriptome that encodes several hundred potentially secreted proteins that are differentially expressed during the course of blood feeding (Ribeiro et al. 2006).
Hosts develop immune responses to salivary gland molecules during tick feeding. Host immunity is enhanced following repeated tick exposure and progressively reduces the ability of each subsequently attached tick to suppress the effects of the host immune response (Wikel 1982, 1996, Brossard and Wikel 2004). For example, I. scapularis–induced Th2 polarization of host clonotypic CD4+ T cells is significantly reduced by prior tick infestation (Müller-Doblies 2007). Development of acquired resistance to tick infestation depends upon the species of tick, host species, and history of prior exposure to tick feeding (Wikel 1996, Brossard and Wikel 2004). Laboratory mice infested with I. scapularis or Ixodes pacificus do not develop resistance to repeat infestations (Schoeler et al. 2000), while guinea pigs or cattle infested with Dermacentor andersoni develop intense acquired resistance (Allen 1973, Willadsen 1980). Elements of the host immune system implicated in acquired resistance include proinflammatory cytokines, antibodies, T cells, complement, histamine, neutrophils, basophils, and eosinophils (Allen 1973, Theis and Budwiser 1974, Wikel 1996, Brossard and Wikel 2004). This augmented host immune response also affects tick-borne pathogen transmission. Primary infestation of animal hosts with uninfected ticks induces a host response that blocks the ability of a subsequent infestation with infected ticks to transmit tularemia and B. burgdorferi (Bell et al. 1979, Wikel et al. 1997, Nazario et al. 1998). People exhibit an immune response to tick bites as evidenced by local redness and itching at the bite site and the development of antibody against specific tick salivary proteins (Alarcon-Chaidez et al. 2006). Those who experience repeated tick exposure and persistent itch from I. scapularis bites have a decreased risk of developing Lyme disease (Burke et al. 2005).
Distinct patterns of histopathologic change occur in mammalian hosts at the tick bite site that are dependent upon the frequency and intensity of infestation, as well as the host and tick species (Wikel 1983). The initial experimental study of tick bite histology reported that guinea pigs infested for the first time with Dermacentor variabilis larvae developed a small hemorrhagic pool beneath the mouth parts with essentially no cellular infiltrate (Trager 1939). A second infestation resulted in an intense accumulation of neutrophils, edema, and epidermal hyperplasia at the tick attachment site. Humans also develop inflammatory responses to tick bites in the dermis, including infiltrates of neutrophils, eosinophils, histiocytes, and lymphocytes, as well as vascular thrombi, extravasation of erythrocytes, and neutrophil damage of blood vessels consistent with vasculitis (Patterson et al. 1979, Beaudouin et al. 1997, Stefanato et al. 2002, Galaria et al. 2003, Pajvani et al. 2006). Despite these data, it remains unclear how tick-induced changes at the tick-dermal interface may enhance pathogen transmission and how host responses to repeated tick bites might inhibit such transmission, especially as a result of I. scapularis infestation. We therefore examined the cutaneous histopathologic responses of BALB/c mice given one or two infestations of pathogen-free I. scapularis nymphs and compared these responses to those of people naturally infested with I. scapularis.
Materials and Methods
Ticks
Pathogen-free nymphs of I. scapularis were obtained from a colony maintained at the University of Connecticut Health Center according to methods described by Bouchard and Wikel (2005). The colony was continuously maintained at 22°C, under a 14-hour light/10-hour dark photoperiod. Ticks were held in 16-mL glass vials (Wheaton Glass, Millville, NJ) with a mesh top over a supersaturated solution of potassium sulfate. Adult ticks were fed on rabbits for colony maintenance.
BALB/c mouse infestation
Laboratory reared, pathogen-free I. scapularis nymphs were placed onto the skin of BALB/c mice. In the first set of experiments, mice were exposed to five ticks contained within a capsule affixed to the back to prevent host grooming (Bouchard and Wikel 2005). Skin biopsies were performed at the site of a single tick attachment at 6 hours and 3 days after feeding. In addition, ticks were allowed to feed for 3 days followed by a similar exposure 1 month later. An alternative approach was taken in a second set of experiments when mice were exposed to 10 ticks that were not contained within a capsule. The same times of attachment were used in these experiments as in the first experiment. Skin biopsies were obtained at the attachment site of a single tick. Skin biopsies also were obtained from control mice that were unexposed to ticks in both sets of experiments. All biopsies were assessed by dermatopathologists who were unaware of the tick exposure history of the mice.
Mouse skin biopsy
Skin biopsies (4 mm) were immediately fixed in 10% buffered formalin for 6 to 12 hours, dehydrated through graded alcohols, and embedded in paraffin blocks with an orientation that allows for sectioning perpendicular to the skin surface, according to an established standard protocol (Wikel 1996). Five-micron-thick sections were mounted on glass slides, deparaffinized in graded alcohols and xylene, and stained with hematoxylin and eosin for light microscopic evaluation. Mice were euthanized and biopsies were immediately obtained in accordance with an approved Institutional Animal Care and Use Committee protocol. Serial sections were performed, and the section with maximum inflammatory cell density was evaluated for the presence or absence of a paucicellular zone adjacent to the tick hypostome, vascular dilatation, red blood cell extravasation, and for the number of inflammatory cells per 4× to 20× objective lens field (HPF) and cell types present. The numbers of inflammatory cells per HPF were statistically compared between “naïve’’ and reinfested mice using the Wilcoxon two-sample test (SAS 9 for Windows, SAS Institute Inc., Cary, NC). Statistical comparison was attempted only for two samples of five or larger.
Human tick bite studies
Three people who resided in an I. scapularis endemic area in Connecticut and one in Massachusetts agreed to a skin biopsy to remove attached I. scapularis ticks. A 4-mm punch biopsy was performed at the site where the ticks were embedded. The biopsies were immediately placed in 10% buffered formalin for 6 to 12 hours, dehydrated through graded alcohols, embedded in paraffin blocks, and cut sections placed on glass slides. Biopsies were assessed by dermatopathologists who were unaware of the tick exposure history of the subjects. The procedures followed were approved by the Human Subjects Committee of the Connecticut Children’s Medical Center.
Results
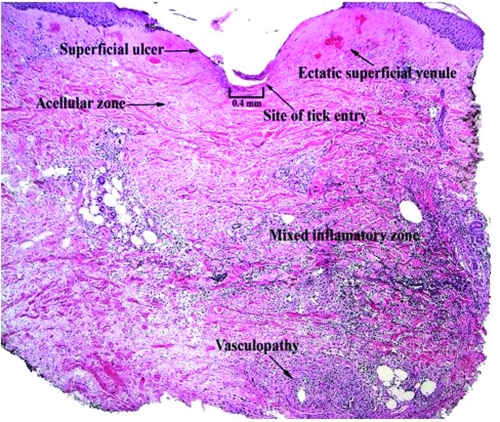
The inflammatory response of BALB/c mice to I. scapularis was directly associated with the number and duration of tick attachment (Table 1, Fig. 1). No inflammatory reaction occurred in control mice without an attached tick, although a mild inflammatory response occurred in some mice with an attached capsule but no attached tick. A mild dermal inflammatory response also occurred in mice exposed to I. scapularis ticks that were allowed to feed for 6 hours. The inflammatory response significantly increased when ticks were allowed to feed for 3 days. The response included a mixed inflammatory cell infiltrate composed of lymphocytes, neutrophils, and eosinophils. Of note, there were few such cells (paucicellular zone) immediately adjacent to the tick hypostome at the site of tick attachment. We also noted the presence of focal superficial ulceration with central invagination and necrosis at the point of tick entry and changes consistent with vascular injury in the mid-deep dermis, including vascular dilatation and red blood cell extravasation. The inflammatory response after an initial 3-day tick exposure followed by another 3-day exposure 1 month later demonstrated a further statistically significant increase in inflammatory cell density, especially adjacent to the tick hypostome at the site of tick attachment. Furthermore, there were about as many eosinophils as lymphocytes or neutrophils, and additional cell types including histiocytes. Histiocytes are tissue macrophages that ingest pathogenic microorganisms. Although vascular dilatation decreased markedly, there was no decrease in erythrocyte extravasation. Reinfestation resulted in an increased inflammatory response with a disappearance of the cell-free zone adjacent to the tick hypostome, the appearance of histiocytes, and a decrease in vascular dilatation.
Table 1.
Dermal Inflammation in BALB/c Mice Infested with Ixodes scapularis Ticks
| |
Number of |
Number of biopsies showing |
|||||
|---|---|---|---|---|---|---|---|
| Duration of tick attachment | Mice | Biopsies | Cells per HPF | Inflammatory cells | Paucicellular zone | Vascular dilatation | Erythrocyte extravasation |
| Experiment 1 | |||||||
| Reinfestation | |||||||
| 3 days and repeat for 3 days | 3 | 3 | 240–265 | 3/3 | 0/3 | 0/3 | 3/3 |
| No reinfestation | |||||||
| 3 days | 3 | 3 | 75–150 | 3/3 | 3/3 | 3/3 | 3/3 |
| 6 h | 2 | 2 | 0–75 | 1/2 | 0/2 | 0/2 | 0/2 |
| Control (no ticks, capsule) | 2 | 2 | 75 | 2/2 | 0/2 | 0/2 | 0/2 |
| Control (no ticks, no capsule) | 1 | 1 | 0 | 0/1 | 0/1 | 0/1 | 0/1 |
| Experiment 2 | |||||||
| Reinfestation | |||||||
| 3 days and repeat for 3 days | 6 | 7 | 8–100a | 7/7 | 0/7 | 4/7 | 3/7 |
| No reinfestation | |||||||
| 3 days | 6 | 11 | 0–50b | 9/11 | 3/11 | 8/11 | 3/11 |
| 6 h | 6 | 7 | 0 | 0/7 | 0/7 | 0/7 | 0/7 |
| Control (no ticks, no capsule) | 3 | 7 | 0 | 0/7 | 0/7 | 0/7 | 0/7 |
In experiment 1, a capsule was used to contain five ticks and the biopsy was obtained at the site of one attached tick. In experiment 2, 10 ticks were allowed to feed without a capsule and the biopsy was obtained at the site of 1 attached tick. HPF, high-power field.
Comparison of number of cells per HPF from biopsies of mice after 3 days’ infestation with that of reinfested mice: p = 0.0027.
Comparison of number of cells per HPF from biopsies of mice after 3 days’ infestation with that of mice after 6 hours’ infestation: p = 0.0027.
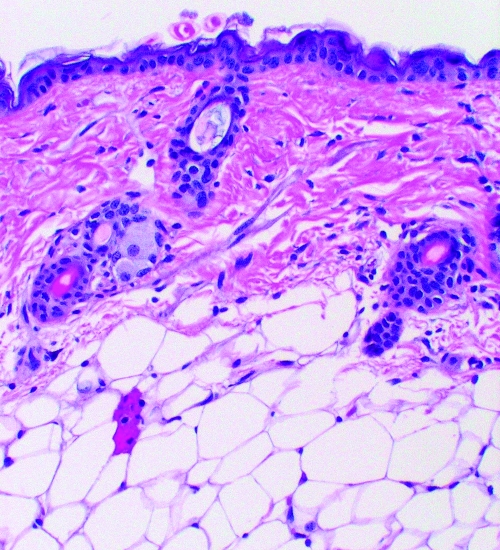
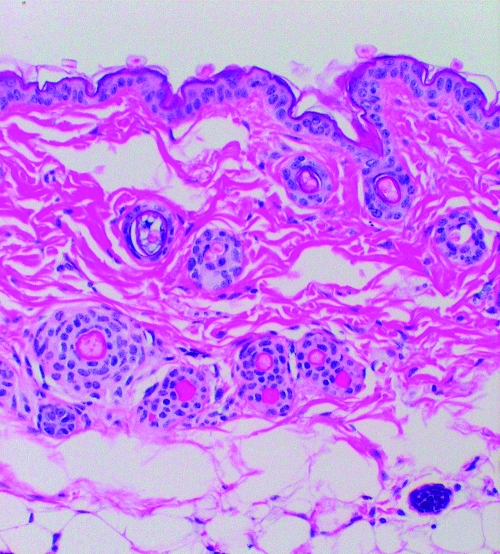
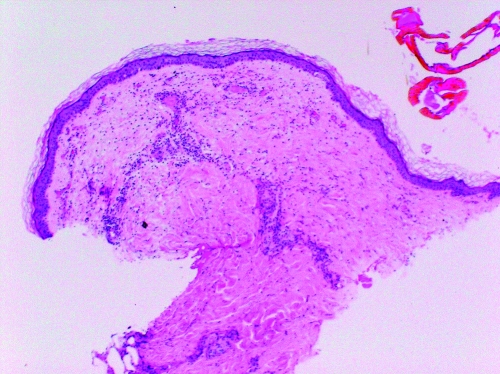
FIG. 1.
A: No tick exposure (control). The epidermis, dermis (containing a few blood vessels), and subdermal layer of fatty tissue show normal architecture and only occasional inflammatory cells.
B: Six-hour I. scapularis exposure. This section is similar to the control with few inflammatory cells.
C: Three-day I. scapularis exposure. The number of inflammatory cells has increased compared with the 6 hour tick exposure in the dermis and subcutaneous but few inflammatory cells are present in the area immediately adjacent to the hypostone (paucicellular zone).
D: Three-day I. scapularis exposure and repeat 3-day exposure 1 month later. The number of inflammatory cells has increased compared with the single 3-day tick exposure in the dermis and subcutaneous tissue and inflammatory cells are present in the area immediately adjacent to the hypostome.
Dermatologic changes (hematoxylin and eosin stained, 20× magnification) in representative skin biopsies from BALB/c mice showing increasing inflammatory cell presence with increasing exposure to Ixodes scapularis ticks and from a control mouse with no I. scapularis exposure. Figures 1B, 1C, 1D are the portion of the biopsy taken directly at the site of the tick attachment.
We examined the cutaneous histopathologic response of 4 people who underwent skin biopsies as a means of tick removal after the bite of I. scapularis ticks. Each had a different amount of tick exposure prior to biopsy (Table 2, Fig. 2). As noted with the BALB/c mice, a history of previous tick exposure was associated with an increased inflammatory cell response, including an increase in the number of eosinophils, as well as a decrease in vascular dilatation and erythrocyte extravasation. The patient who reported the greatest prior exposure to ticks (subject 2d) experienced the most intense dermal inflammatory reaction, including the presence of the greatest number of eosinophils. He also was the only patient to report tick-associated itch. Increased exposure to ticks in people is associated with increased cellular inflammation, disappearance of the paucicellular zone adjacent to the tick hypostome, and decreased vascular dilatation and erythrocyte extravasation at the tick bite site.
Table 2.
Dermal Inflammation and History of Tick Exposure among People Infested with Ixodes scapularis Ticksa
| |
|
|
|
Biopsies showing |
|||
|---|---|---|---|---|---|---|---|
| |
History |
Inflammatory change |
Vascular change |
||||
| Subject | No. tick bites in past 12 mo | Itch with tick bites | Tick-borne illness | Cells per HPF | Paucicellular zone | Vascular dilataionb | Erythrocyte extravasationc |
| 2a | 0 | No | No | 50–750 | Yes | 3+ | 2+ |
| 2b | 0 | Uncertain | No | 50–750 | Yes | 2+ | 2+ |
| 2c | 0 | No | Yes | 76–100 | No | 2+ | 2+ |
| 2d | 6 | Yes | No | 76–100 | No | 2+ | 1+ |
HPF, high-power field.
Patients are listed according to extent of previous tick exposure.
The intensity of vascular dilation is graded as follows: 0 = dermal blood vessels normal diameter, 1+ = dermal blood vessels dilated up to 2 times normal cross-sectional area in histologic sections, 2+ = dermal blood vessels dilated 2–3 times normal cross-sectional area in histologic sections, 3+ = dermal blood vessels dilated > 3 times normal cross-sectional area in histologic sections.
The extent of erythrocyte extravasation is graded as follows: 0 = no erythrocyte extravasation, 1+ = aggregates of up to 50 extravasated erythrocytes around blood vessels in histologic sections, 2+ = aggregates of 50–100 extravasated erythrocytes around blood vessels in histologic sections, 3+ = confluent aggregates of extravasated erythrocytes occupying at least 1 mm in greatest dimension in histologic sections.
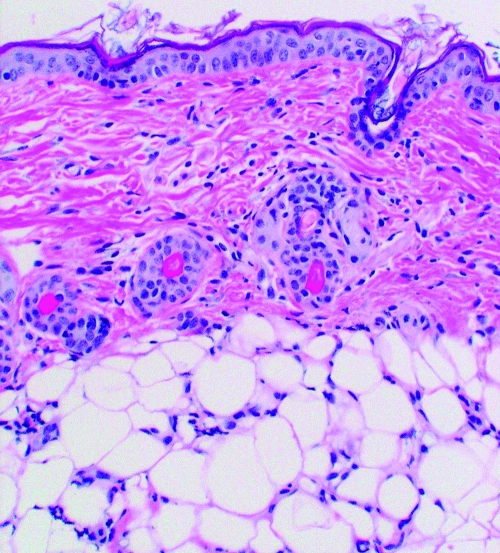
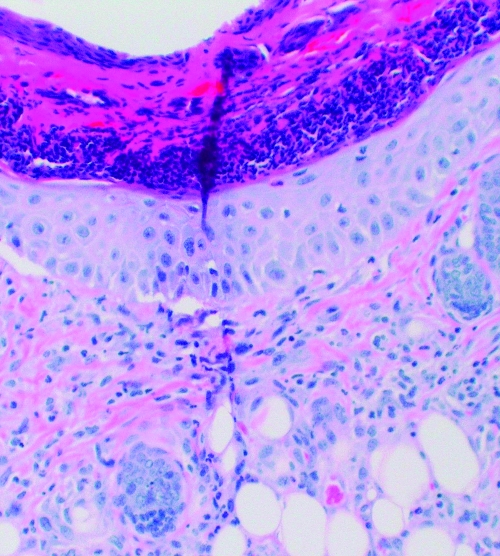
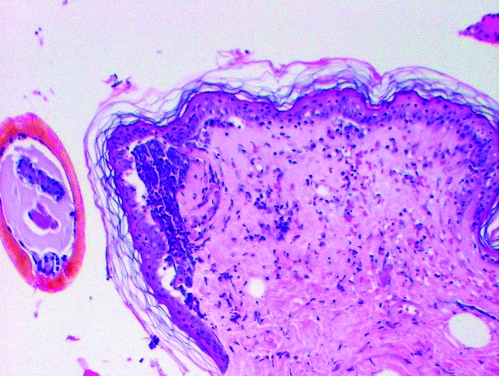
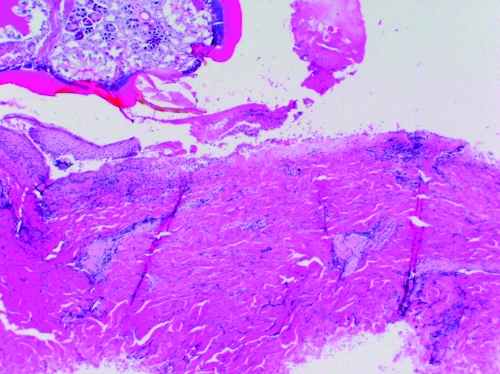
FIG. 2.
A: Subject a. This patient had no history of tick-borne infection and reported no tick bite in the year prior to this tick exposure. Tick mouthparts are visible at the top of the slide. Very few inflammatory cells are located adjacent to the site of tick attachment (paucicellular zone) but a moderate number of cells are located more distant to the site of tick entry. Vascular dilation is present.
B: Subject b. This patient was uncertain about the number of tick bites in the previous year but had no history of tick-borne infection. Tick mouthparts are not visible but the site of tick entry is labeled. A paucicellular area exists adjacent to the site of tick entry with a moderate number of inflammatory cells in the surrounding dermis. Vascular dilation is present.
C: Subject c. This patient had a history Lyme disease 2 years prior to this tick bite but reported no tick bite in the prior year. The tick mouthparts are visible on the left side of the section. Numerous inflammatory cells are noted at the site of tick entry. A greater number of inflammatory cells and less vascular dilation are present compared with subjects a and b.
D: Subject d. This patient had no history of tick-borne illness but reported six tick bites in the year prior to this tick exposure. He also reported tick-associated itch. The tick mouthparts are visible at the top of the slide. Numerous inflammatory cells are noted at the site of tick entry. A greater number of inflammatory cells and less vascular dilation are present compared with subjects a and b.
Dermatologic changes (hematoxylin and eosin stained, 4× to 10× magnification) of four people showing increasing inflammatory cell presence with increasing exposure to Ixodes scapularis ticks.
Discussion
Our findings indicate that cellular changes at the tick-dermal interface following I. scapularis attachment are likely to allow for successful transmission of tick-borne pathogens in non-tick-immune hosts and to inhibit tick-borne pathogen transmission in hosts that have developed tick immunity. Skin biopsies at the site of I. scapularis attachment of non-tick-sensitized BALB/c mice and people showed vascular dilatation and a paucicellular zone adjacent to the tick hypostome. The biologic factors causing a zone of minimal cellular inflammation adjacent to the site of hypostome injection are not known but are likely due to the anti-inflammatory effect of tick salivary protein. These changes would be expected to help provide a blood meal and minimize tissue disruption as a result of any host immune response. Repeated tick exposure is associated with interruption of tick feeding, early tick detachment, and prevention of tick-borne pathogen transmission (Bell et al. 1979, Wikel et al. 1997, Nazario et al. 1998, Zeidner et al. 2002, Brossard and Wikel 2004). We found that repeated I. scapularis exposure in mice and people results in a decrease in vascular size and probable decreased blood flow to the feeding site. Reinfestation also was accompanied by infiltration of the cell-free zone with neutrophils, lymphocytes, eosinophils, and histiocytes. These changes disrupt tick feeding and cause premature tick detachment prior to pathogen transmission (Trager 1939, Allen 1973). Pathogen transmission requires tick attachment for at least 36 to 48 hours for Babesia microti or B. burgdorferi and at least 24 hours for Anaplasma phagocytophilum (Piesman and Spielman 1980, Piesman 1993, des Vignes et al. 2001). Furthermore, any pathogen that might be transmitted in tick-immune hosts would likely be destroyed by neutrophils and lymphocytes at the tick-dermal interface (Xu et al. 2007). Inflammatory and vascular changes in the dermis provide an explanation for the decreased incidence of tick-borne disease in tick-immune mammalian hosts.
People who experience repeated tick bites and tick-associated itch are less likely to develop Lyme disease (Burke et al. 2005). Itch can alert the host to the presence of a tick and cause its removal prior to pathogen transmission. Tick-associated itch reactions are well recognized in people and especially those who develop immunoglobulin E antibody (Alexander 1986, Beaudouin et al. 1997, Fisher et al. 2006). The abundant basophils, eosinophils, and neutrophils that we noted in tick-sensitized hosts have been shown to play a role in the development of itch reactions (Gill and Walker 1985, Alexander 1986). Molecules derived from neutrophils are known to mediate a cutaneous hypersensitivity itch response (Alexander 1986). Tick bite reactions of recurrently infested rabbits were reported to consist of degranulated mast cells and infiltrates of lymphocytes, macrophages, neutrophils, and eosinophils (Gill and Walker 1985). Of the four people who were biopsied with an attached I. scapularis in our study, the subject who experienced tick-associated itch reported the most tick bites in the previous year, experienced the most intense cutaneous inflammatory response, and lacked evidence of vasculitis with associated erythrocyte extravasation. This patient also had no history of tick-borne disease, perhaps as a result of repeated exposure to uninfected ticks and development of tick-associated immunity. Although previous studies have demonstrated an influx of inflammatory cells in human hosts experiencing tick bites, no one has correlated the amount of previous tick exposure with the extent or distribution of the dermal inflammatory response.
Tick-induced modifications of host defenses evolved to facilitate blood feeding. Tick transmitted pathogens have exploited these changes for their own survival. I. scapularis salivary gland extract down-regulates the expression of endothelial cell adhesion molecules P-selectin and vascular cell adhesion molecule-1 that can reduce leukocyte extravasation into dermal tissue (Maxwell et al. 2005). These observations help explain the presence of a cell-free zone adjacent to the tick hypostome that we noted after initial tick exposure in mice and human subjects. I. scapularis saliva also reduces the expression of β2-integrin (CD18) that is essential for interactions of human neutrophils with extracellular matrix and decreases the uptake and killing of B. burgdorferi (Montgomery et al. 2004). I. scapularis saliva inhibits T-cell proliferation (Urioste et al. 1994), contains an interleukin-2 binding protein (Gillespie et al. 2001) and polarizes CD4 T-cells to a Th2 cytokine profile (Schoeler et al. 1999, Müller-Doblies et al. 2007). CD4+ T-cell activation is inhibited by the I. scapularis protein, Salp 15, which binds to extracellular domains of CD4 (Garg et al. 2006). Prostaglandin E2 in I. scapularis saliva inhibits dendritic cell maturation and ability to stimulate CD4+ T-cell proliferation (Sá-Nunes et al. 2007). I. scapularis saliva contains a protein, Isac, which uncouples factor Bb of the alternative pathway C3 convertase (Valenzuela et al. 2000). Another I. scapularis salivary protein, Salp 20, directly binds to and displaces properdin from C3 convertase, accelerating the decay of the C3 convertase and leading to inhibition of the alternative complement pathway (Tyson et al. 2008). Salivary gland transcriptome analyses also identified gene products such as I. scapularis sialostatins L and L2, which are cysteine protease inhibitors that target cathepsins and possess anti-inflammatory activity (Kotsyfakis et al. 2006, 2007). Finally, I. scapularis salivary protein contains kinases that break down bradykinin and help prevent itch (Wikel 1983). Repeated tick exposure induces host immune factors that act to destroy these kinases and lead to the development of tick-associated itch.
Our study was subject to several limitations. The capsule attached to the backs of mice that was used in one set of experiments causes slight inflammatory changes that might have contributed to the total immune response. We found similar results in a second set of mouse experiments, however, where capsules were not used. The number of human subjects was small, and we were only able to estimate the extent of previous tick exposure in these subjects. Thus, we were unable to determine with precision the number of ticks, tick species, intervals between infestations, and how much a given tick fed. These limitations are mitigated by the fact that no previous studies have compared animal and human histology at the tick-dermal interface, few have examined both inflammatory and vascular changes associated with tick bite, and none has studied histologic changes associated with I. scapularis exposure. The results of this study suggest that traditional preventative approaches for tick-transmitted diseases, including application of acaricides to mice or deer, reduction in the deer population, prompt removal of ticks, use of protective clothing, personal application of acaricides, and antibiotic prophylaxis for Lyme disease following an I. scapularis bite, might usefully be augmented by the addition of a pathogen transmission blocking vaccine (Hayes and Piesman 2003, Willadsen 2004, Wikel et al. 2005). A tick salivary gland vaccine could prevent infection by inducing antibodies and/or cell-mediated immune factors against tick proteins that modulate host hemostasis, wound healing, pain/itch responses, and immune defenses. Although the first study to report that host immunity to the bite of pathogen-free blood-feeding arthropods conferred resistance to subsequent pathogen transmission was one that involved ticks (Bell 1979), the same principles of arthropod-induced immunity also might be extended to vaccination for other arthropod-borne agents, including the strong delayed-type cutaneous hypersensitivity response to sand fly bites that is associated with reduced transmission of Leishmania parasites (Valenzuela et al. 2000). A clear link exists between mosquito modulation of host innate and specific acquired immune defenses and enhanced transmission and establishment of arboviruses (Schneider and Higgs 2008). Prior exposure of BALB/c mice to bites of uninfected Anopheles stephensi led to reduced parasitemia following subsequent mosquito transmission of Plasmodium yoelii malaria parasites (Donovan et al. 2007). Transmission-inhibiting responses are not always elicited by the bites of uninfected blood-feeding arthropods. Prior exposure to bites of uninfected Aedes aegypti exacerbated mosquito transmitted West Nile virus infection (Schneider et al. 2007).
The potential advantage of vector-specific vaccines over pathogen-specific vaccines (such as those for Lyme disease or tick-borne encephalitis) is the protection a single vaccine may afford against multiple infections, including Lyme disease, babesiosis, human granulocytic anaplasmosis, and tick-borne encephalitis. Further development of an I. scapularis vaccine will depend in part on additional development of a BALB/c mouse model that mimics human histopathologic reaction to I. scapularis bites and a more complete immunologic dissection of the events following tick bite in people.
Disclosure Statement
This work was supported by grants from the National Institutes of Health: RO1 AI062735 (S.W.) and the University of Connecticut Health Center General Clinical Research Center MO1RR06192 (P.J.K. and S.W.)
References
- Alarcon-Chaidez F. Ryan R. Wikel S. Dardick K, et al. Confirmation of tick bite by detecting antibody to Ixodes calreticulin salivary protein. Clin Vaccine Immunol. 2006;13:1217–1222. doi: 10.1128/CVI.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JO. The physiology of itch. Parasitol Today. 1986;2:345–351. doi: 10.1016/0169-4758(86)90055-4. [DOI] [PubMed] [Google Scholar]
- Allen JR. Tick resistance: basophils in skin reactions of resistant guinea pigs. Int J Parasitol. 1973;3:195–200. doi: 10.1016/0020-7519(73)90024-6. [DOI] [PubMed] [Google Scholar]
- Beaudouin E. Kanny G. Guerin B. Guerin L, et al. Unusual manifestations of hypersensitivity after a tick bite: report of two cases. Ann Allergy Asthma Immunol. 1997;79:43–46. doi: 10.1016/S1081-1206(10)63082-7. [DOI] [PubMed] [Google Scholar]
- Bell JF. Stewart SJ. Wikel SK. Resistance to tick-borne Francisella tularensis by tick-sensitized rabbits: allergic klendusity. Am J Trop Med Hyg. 1979;28:876–880. [PubMed] [Google Scholar]
- Bouchard KR. Wikel SK. Care, maintenance, and experimental infestation of ticks in the laboratory setting. In: Marquardt WC, editor; Black WC IV, et al., editors. Biology of Disease Vectors. San Diego: Elsevier Academic Press; 2005. pp. 705–711. [Google Scholar]
- Brossard M. Wikel SK. Tick immunobiology. Parasitology. 2004;129:S161–S176. doi: 10.1017/s0031182004004834. [DOI] [PubMed] [Google Scholar]
- Burke G. Wikel SK. Spielman A. Pollack R, et al. Cutaneous tick hypersensitivity in humans is associated with decreased Lyme disease risk. Emerg Infect Dis. 2005;11:36–41. doi: 10.3201/eid1101.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs JE. Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- Dennis DT. Piesman JF. Overview of tick-borne infections of humans. In: Goodman JL, editor; Dennis DT, editor; Sonenshine DE, editor. Tick-borne Diseases of Humans. Washington, D.C.: ASM Press; 2005. pp. 3–11. [Google Scholar]
- des Vignes F. Piesman J. Heffernan R. Schulze TL, et al. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J Infect Dis. 2001;183:773–778. doi: 10.1086/318818. [DOI] [PubMed] [Google Scholar]
- Donovan MJ. Messmore AS. Scafford DA. Sacks DL. Kamhawi S. McDowell MA. Uninfected mosquito bites confer protection against infection with malaria parasites. Infect Immun. 2007;75:2523–2530. doi: 10.1128/IAI.01928-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler JS. Madigan JE. Pusterla N. Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45:S45–S51. doi: 10.1086/518146. [DOI] [PubMed] [Google Scholar]
- Fisher EJ. Mo J. Lucky AW. Multiple pruritic papules from Lone Star tick larvae bites. Arch Dermatol. 2006;142:491–494. doi: 10.1001/archderm.142.4.491. [DOI] [PubMed] [Google Scholar]
- Galaria NA. Chaudhary O. Magro CM. Tick mouthparts occlusive vasculopathy: a localized cryoglobulinemic vasculitic response. J Cutan Pathol. 2003;30:303–306. doi: 10.1034/j.1600-0560.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Garg R. Juncadella IJ. Ramamoorthi N, et al. Cutting edge: CD4 is the receptor for the tick saliva immunosuppressor, Salp15. J Immunol. 2006;177:6579–6583. doi: 10.4049/jimmunol.177.10.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill HS. Walker AR. Differential cellular responses at Hyalomma anatolicum anatolicum feeding sites on susceptible and tick-resistant rabbits. J Immunol. 1985;91:591–607. doi: 10.1017/s0031182000062831. [DOI] [PubMed] [Google Scholar]
- Gillespie RD. Dolan MC. Piesman J. Titus RG. Identification of an IL-2 binding protein in the saliva of the Lyme disease vector tick, Ixodes scapularis. J Immunol. 2001;166:4319–4327. doi: 10.4049/jimmunol.166.7.4319. [DOI] [PubMed] [Google Scholar]
- Ginsberg HS. Stafford K., III . Management of ticks and tick-borne diseases. In: Goodman JL, editor; Dennis DT, editor; Sonenshine DE, editor. Tick-borne Diseases of Humans. Washington, D.C.: ASM Press; 2005. pp. 65–86. [Google Scholar]
- Hanincová K. Kurtenbach K. Diuk-Wasser M. Brei B. Fish D. Epidemic spread of Lyme borreliosis, northeastern United States. Emerg Infect Dis. 2006;12:604–611. doi: 10.3201/eid1204.051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes EB. Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348:2424–2430. doi: 10.1056/NEJMra021397. [DOI] [PubMed] [Google Scholar]
- Hill CA. Kafatos FC. Stansfield SK. Collins FH. Arthropod-borne diseases: vector control in the genomics era. Nat Rev Microbiol. 2005;3:262–268. doi: 10.1038/nrmicro1101. [DOI] [PubMed] [Google Scholar]
- Jongejan F. Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–S14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- Kotsyfakis M. Karim S. Andersen JF. Mather TN. Ribeiro JMC. Selective cysteine protease inhibition contributes to blood-feeding success of the tick Ixodes scapularis. J Biol Chem. 2007;282:29156–29263. doi: 10.1074/jbc.M703143200. [DOI] [PubMed] [Google Scholar]
- Kotsyfakis M. Sa-Nunes A. Francischetti IMB. Mather TN. Andersen JF. Ribeiro JMC. Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick, Ixodes scapularis. J Biol Chem. 2006;281:26298–26307. doi: 10.1074/jbc.M513010200. [DOI] [PubMed] [Google Scholar]
- Krause PJ. Babesiosis. Med Clin North Am. 2002;86:361–373. doi: 10.1016/s0025-7125(03)00092-0. [DOI] [PubMed] [Google Scholar]
- Maxwell SS. Stoklasek TA. Dash Y. Macaluso KR, et al. Tick modulation of the in-vitro expression of adhesion molecules by skin-derived endothelial cells. Ann Trop Med Parasitol. 2005;99:661–672. doi: 10.1179/136485905X51490. [DOI] [PubMed] [Google Scholar]
- Montgomery RR. Lusitani D. De Boisfleury. Chevance A. Malawista SE. Tick saliva reduces adherence and area of human neutrophils. Infect Immun. 2004;72:2989–2994. doi: 10.1128/IAI.72.5.2989-2994.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Doblies UU. Maxwell SS. Boppana VD. Mihalyo MA, et al. Feeding by the tick, Ixodes scapularis, causes CD4+ T cells responding to cognate antigen to develop the capacity to express IL-4. Parasite Immunol. 2007;29:485–499. doi: 10.1111/j.1365-3024.2007.00966.x. [DOI] [PubMed] [Google Scholar]
- Nazario S. Das S. de Silva AM. Deponte K, et al. Prevention of Borrelia burgdorferi transmission in guinea pigs by tick immunity. Am J Trop Med Hyg. 1998;58:780–785. doi: 10.4269/ajtmh.1998.58.780. [DOI] [PubMed] [Google Scholar]
- Patterson JW. Fitzwater JE. Connell J. Localized tick bite reaction. Cutis. 1979;24:168–169. [PubMed] [Google Scholar]
- Pajvani U. Zeikus S. Basile O. Toback N, et al. Thrombogenic vasculopathy with diffuse neutrophilic inflammation: a histologic manifestation of a tick bite. Cutis. 2006;78:321–324. [PubMed] [Google Scholar]
- Piesman J. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J Infect Dis. 1993;167:1082–1085. doi: 10.1093/infdis/167.5.1082. [DOI] [PubMed] [Google Scholar]
- Piesman J. Spielman A. Human babesiosis on Nantucket Island: prevalence of Babesia microti in ticks. Am J Trop Med Hyg. 1980;29:742–746. doi: 10.4269/ajtmh.1980.29.742. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. Alarcon-Chaidez F. Francischetti IMB. Mans B, et al. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol. 2006;36:111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Sá-Nunes A. Bafica A. Lucas DA, et al. Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva. J Immunol. 2007;179:1497–1505. doi: 10.4049/jimmunol.179.3.1497. [DOI] [PubMed] [Google Scholar]
- Schneider BS. Higgs S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med Hyg. 2008;102:400–408. doi: 10.1016/j.trstmh.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BS. McGee CE. Jordan JM. Stevenson HL. Soong L. Higgs S. Prior exposure to uninfected mosquitoes enhances mortality in naturally-transmitted West Nile virus infection. PLoS One. 2007;2:e1171. doi: 10.1371/journal.pone.0001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeler GB. Manweiler SA. Wikel SK. Cytokine responses of C3H/HeN mice infested with Ixodes scapularis or Ixodes pacificus nymphs. Parasite Immunol. 2000;22:31–40. doi: 10.1046/j.1365-3024.2000.00272.x. [DOI] [PubMed] [Google Scholar]
- Schoeler GB. Manweiler SA. Wikel SK. Ixodes scapularis: effects of repeated infestations with pathogen-free nymphs on macrophage and T lymphocyte cytokine responses of BALB/c and C3H/HeN mice. Exp Parasitol. 1999;92:239–248. doi: 10.1006/expr.1999.4426. [DOI] [PubMed] [Google Scholar]
- Steen NA. Barker SC. Alewood PF. Proteins in the saliva of the Ixodidae (ticks): pharmacological features and biological significance. Toxicon. 2006;47:1–20. doi: 10.1016/j.toxicon.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Steere AC. Coburn J. Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanato CM. Phelps RG. Goldberg LJ. Perry AE, et al. Type-I cryoglobulinemia-like histopathologic changes in tick bites: a useful clue for diagnosis in the absence of tick parts. J Cutan Pathol. 2002;29:101–106. doi: 10.1034/j.1600-0560.2001.290207.x. [DOI] [PubMed] [Google Scholar]
- Telford SR., III Goethert HK. Emerging tick-borne infections: rediscovered and better characterized, or truly “new”? Parasitology. 2004;129:S301–S327. doi: 10.1017/s0031182003004669. [DOI] [PubMed] [Google Scholar]
- Theis JH. Budwiser PD. Rhipicephalus sanguineus: sequential histopathology at the host-arthropod interface. Exp Parasitol. 1974;36:77–105. doi: 10.1016/0014-4894(74)90115-5. [DOI] [PubMed] [Google Scholar]
- Trager W. Acquired immunity to ticks. J Parasitol. 1939;25:57–81. [Google Scholar]
- Tyson KR. Elkins C. de Silva AM. A novel mechanism of complement inhibition unmasked by a tick salivary protein that binds to properdin. J Immunol. 2008;180:3964–3968. doi: 10.4049/jimmunol.180.6.3964. [DOI] [PubMed] [Google Scholar]
- Urioste S. Hall LR. Telford SR., III Titus RG. Saliva of the Lyme disease vector, Ixodes dammini, blocks cell activation by a nonprostaglandin E2-dependent mechanism. J Exp Med. 1994;180:1077–1085. doi: 10.1084/jem.180.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela JG. Charlab R. Mather TN. Ribeiro JMC. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J Biol Chem. 2000;275:18717–18723. doi: 10.1074/jbc.M001486200. [DOI] [PubMed] [Google Scholar]
- Walker DH. Ehrlichia under our noses and no one notices. Arch Virol. 2005;19:S147–S156. doi: 10.1007/3-211-29981-5_12. [DOI] [PubMed] [Google Scholar]
- Walker DH. Tick-transmitted infectious diseases in the United States. Annu Rev Public Health. 1998;19:237–269. doi: 10.1146/annurev.publhealth.19.1.237. [DOI] [PubMed] [Google Scholar]
- Wikel SK. Host immunity to tick bite. In: Harris K, editor. Current Topics in Vector Research. Praeger Publishers; 1983. pp. 249–269. [Google Scholar]
- Wikel SK. Host immunity to ticks. Annu Rev Entomol. 1996;41:1–22. doi: 10.1146/annurev.en.41.010196.000245. [DOI] [PubMed] [Google Scholar]
- Wikel SK. Influence of Dermacentor andersoni infestation on lymphocyte responsiveness to mitogens. Ann Trop Med Parasitol. 1982;76:627–632. doi: 10.1080/00034983.1982.11687593. [DOI] [PubMed] [Google Scholar]
- Wikel SK. Alarcon-Chaidez F. Müller-Doblies U. Immunological control of vectors. In: Marquardt WC, editor; Black WC IV, editor; Freier JE, et al., editors. Biology of Disease Vectors. San Diego: Elsevier Academic Press; 2005. pp. 671–682. [Google Scholar]
- Wikel SK. Ramachandra RN. Bergman DK. Burkot TR, et al. Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect Immun. 1997;65:335–338. doi: 10.1128/iai.65.1.335-338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willadsen P. Anti-tick vaccines. Parasitology. 2004;129:S367–S387. doi: 10.1017/s0031182003004657. [DOI] [PubMed] [Google Scholar]
- Willadsen P. Immunity to ticks. Adv Parasitol. 1980;18:293–313. doi: 10.1016/s0065-308x(08)60402-9. [DOI] [PubMed] [Google Scholar]
- Xu Q. Seemanapalli SV. Reif KE. Brown CR, et al. Increasing the recruitment of neutrophils to the site of infection dramatically attenuates infectivity. J Immunol. 2007;178:5109–5115. doi: 10.4049/jimmunol.178.8.5109. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG. Belkaid Y. Garfield MK. Mendez S, et al. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp Med. 2001;194:F7–F9. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidner NS. Schneider BS. Nuncio MS. Gern L. Piesman J. Coinoculation of Borrelia spp. with tick salivary gland lysate enhances spirochete load in mice and is tick species-specific. J Parasitol. 2002;88:1276–1278. doi: 10.1645/0022-3395(2002)088[1276:COBSWT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]