Abstract
Prostaglandins (PGs) play an important role in pulmonary physiology and various pathophysiological processes following infection. The initial step in the biosynthesis of PGs is regulated by two distinct cyclooxygenase enzymes, cyclooxygenase-1 (COX-1) and COX-2. The goal of this study was to investigate the pulmonary cellular localization and distribution of COX-1 and COX-2 in a neonatal lamb model following respiratory syncytial virus (RSV) and parainfluenza virus 3 (PI3) infection, organisms that also cause significant respiratory disease in children. No significant differences were seen in pulmonary COX-1 expression at various microanatomical locations following RSV or PI3 infection compared to controls. In contrast, COX-2 was upregulated following RSV and PI3 infection. Strong expression was restricted to bronchial and bronchiolar epithelial cells and macrophages, while minimal expression was present in the same microanatomical locations in the uninfected lungs. Other microanatomical locations in both the controls and the infected lungs lacked expression. This work suggests that during RSV or PI3 infection: (1) COX-1 cellular expression is not altered, (2) COX-2 cellular expression is upregulated in airway bronchiolar and bronchial epithelial cells and macrophages, (3) respiratory epithelium along with macrophages are important microanatomical compartments regulating the host inflammatory response during viral infection, and (4) COX-2 may be a potential target for RSV and PI3 therapy.
Introduction
Prostaglandins (PGs) are synthesized from arachidonic acid by enzymatic reactions. Cyclooxygenase-derived PGs have two distinct isoenzymes, COX-1 and COX-2. COX-1 is constitutively expressed, is found in most normal body tissues, and is involved in physiological processes. COX-2 is expressed in normal tissues at very low levels, but is highly induced by proinflammatory mediators in the setting of inflammation, injury, and pain (30).
In the lung, COX pathways are among the many different mediators involved in various physiological and pathophysiological processes, such as regulation of vascular tone (20), pulmonary fibrosis and tissue remodeling (45), surfactant homeostasis (2), alveolar macrophages and their role in pulmonary defense (31,46), bronchial mucus secretion (38), and the pathogenesis of asthma and chronic obstructive pulmonary disease (COPD) (29,33). Additionally, marked increases in prostaglandin E2 (PGE2) synthesis and upregulation of COX-2 have been reported in acid-induced epithelial injury (6), suggesting that COX plays an integral role in the pulmonary system.
Both respiratory syncytial virus (RSV) and parainfluenza viruses (PIs) are pathogens of human infants and perinatal lambs. RSV and PI are leading viral causes of respiratory disease in infants in their first year of life, and cause morbidity and mortality in transplant patients and older adults (15,41). Bovine and ovine strains of both RSV and paramyxoviruses have high homology to human strains and induce identical pulmonary lesions (9,16,17,23,25,27). Several proinflammatory cytokines and chemokines can be detected in patients with RSV and PI infection (5,7,15,21). Increased levels of PGE2 in the plasma or endotracheal aspirates have been demonstrated in animals and infants infected with RSV (14,37). In addition, RSV-induces PGE2 production in human alveolar type II-like epithelial cells (26). However, to the best of our knowledge, COX cellular expression and distribution following RSV or PI3 infection in human or animal models of RSV or PI3 pulmonary infection has not been evaluated.
Therefore, we investigated the pulmonary microanatomical location and cellular expression of COX-1 and COX-2 in lung tissues obtained from a lamb pulmonary model infected with RSV or PI3. This study is the first reported analysis of the pulmonary microanatomical expression of COX-1 and COX-2 in a neonatal lamb model of paramyxoviral infection.
Materials and Methods
Experimental animal model, respiratory viruses, and animal inoculations
A total of 20 neonatal lambs (3–5 d of age) were used in the study. These included: (1) control (saline) inoculated (n = 8), (2) respiratory syncytial virus–infected (bovine strain) lambs (n = 8), and (3) parainfluenza virus-3–infected (ovine strain) lambs (n = 4). The PI3 viral inoculum consisted of infectious supernatant prepared from a culture of ovine fetal turbinate cells previously infected with ovine PI3 virus strain DH-1, and the lambs received 10 mL of 106.9 tissue culture infective doses50 (TCID50) per milliliter intratracheally, and the lambs were inoculated with 10 mL of PI3. The RSV strain 375 was grown on bovine turbinate cells to a concentration of 107 TCID50 per milliliter, and the lambs received 10 mL of RSV (16,27). The control group was given saline.
Pulmonary tissues
On day 6 of infection, the time of peak lesion severity for both RSV and PI3 (16,27), the lambs were euthanized with an intravenous injection of sodium pentobarbital. The thorax was opened and the lungs were then removed from for tissue collection. The lungs were consistently sampled from the left and right cranial, middle, and caudal lobes. The tissues were fixed in 10% neutral buffered formalin for 24 h and embedded in paraffin wax. Sections were cut (3 μm) and stained immunohistochemically with antibodies to COX-1 and COX-2.
COX-1 and COX-2 expression by immunohistochemistry
Immunohistochemical (IHC) staining was conducted as previously described (31). Sections (3-μm thick) were mounted on positively-charged glass slides, dried, and then loaded on the automated immunostainer at 37°C, and we used a Ventana Discovery XT device (Ventana Medical Systems, Tucson, AZ) to analyze COX-1 and COX-2 expression. The slides were deparaffinized and rehydrated. The sections were incubated for 30 min with serum-free DakoCytomation protein blocker (Dako Corporation, Dako, CA), and then rinsed and incubated for 4 min with avidin-biotin blocking solution (Ventana Medical Systems). Heat-induced epitope antigen retrieval was completed using a Ventana specialty solution (pH = 8) (Ventana Medical Systems).
Automation included exposure to 100 μL of primary anti-COX-1 (1:200) or anti-COX-2 (1:50), rabbit polyclonal antibody (Cayman Chemical, Ann Arbor, MI) diluted using reagent diluent (Ventana Medical Systems) at 37°C for 60 min. Then 100 μL of the appropriate anti-rabbit biotinylated IgG (H + L) linking solution (Vector Laboratories, Burlingame, CA) was applied to each section at 1:1000 dilution for 30 min at room temperature. The sections were again rinsed and allowed to react with 100 μL of diaminobenzidine (DAB detection kit) substrate solution (Ventana Medical Systems) for 8 min, followed by counterstaining with hematoxylin then bluing reagent for 4 min each, removed from the autostainer, washed in warm water, dehydrated using graded alcohol, cleared in xylene, and cover-slipped. Control reactions included: (1) sections incubated with the omission of primary antibody and processed as mentioned above, and (2) sections incubated with normal rabbit serum instead of the primary antibody and processed as above. The IHC distribution and intensity scoring system consisted of: (–) = no expression, (+) = earliest weak minimal detectable expression; (++) = mild expression in up to 30% of pulmonary cell types; (+++) = moderate expression in 30–60% of pulmonary cell types; and (++++) = strong expression with >60% of pulmonary cell types.
Results
Clinical signs, gross pathology, and histopathology lesions from these animals were published previously (16). Briefly, both PI3- and RSV-infected animals had increased temperatures compared to controls. Gross pathology lesions were characterized by multifocal areas of red to light-red areas of consolidated regions (often 1–3 mm in size). Microscopically, the lambs had bronchiolitis characterized by slightly dilated bronchiolar lumens partially filled with neutrophils, cell debris, and occasional sloughed epithelial cells, with mild multifocal erosions. Occasional syncytial cells were present in multifocal bronchiolar lumens. Bronchiolar adventitia and alveolar septa contained mild infiltrates of lymphocytes, plasma cells, and occasional macrophages. Alveolar lumens were often partially collapsed and partially filled with seroproteinaceous fluid, alveolar macrophages, and cell debris.
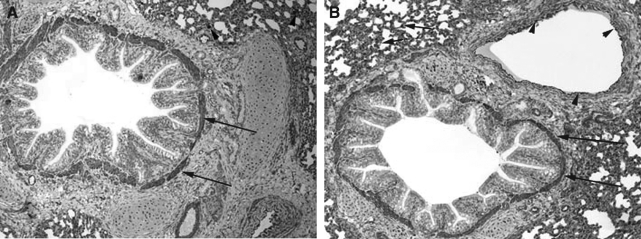
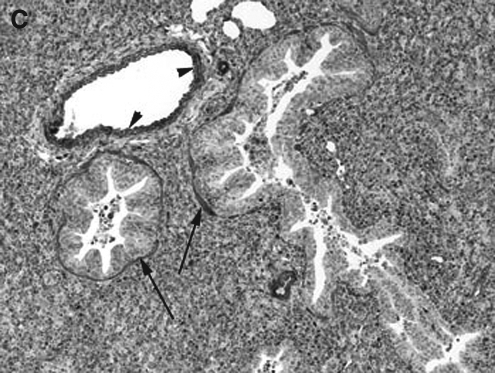
The pulmonary cellular expression and distribution of COX-1 and COX-2 during RSV and PI3 infection are summarized in Tables 1 and 2. Staining intensity ranged from none (–) to strong (++++). Strong (++++) COX-1 expression was present in macrophages, bronchial and bronchiolar smooth muscle cells, vascular endothelial cells, and vascular smooth muscle cells, in lungs from control, RSV-infected, and PI3-infected neonatal lambs (Fig. 1A, B, and C). Intense expression also occurred at sites of inflammation and injury, and correlated with the degree of inflammation and infection. Moderate (+++) COX-1 staining was present in alveolar septa in control and PI3-infected lungs. Alveolar septa from RSV-infected lungs had strong (++++) COX-1 expression. Mild (++) COX-1 expression was present in bronchial and bronchiolar epithelial cells from RSV-infected lungs, while minimal (+) expression was present in lungs from either control or PI3-infected lungs. Bronchiolar epithelium in sections from control and PI3-infected lungs had minimal staining, while mild staining was present in sections from RSV-infected lungs.
Table 1.
Pulmonary Cellular Expression of COX-1 After RSV and PI3 Infection
| Pulmonary compartment | Control | RSV | PI3 |
|---|---|---|---|
| Alveolar septae | +++ | ++++ | +++ |
| Bronchial epithelium | + | ++ | + |
| Bronchial SMC | ++++ | ++++ | ++++ |
| Bronchiolar epithelium | + | ++ | + |
| Bronchiolar SMC | ++++ | ++++ | ++++ |
| Macrophages | ++++ | ++++ | ++++ |
| Vascular EC | ++++ | ++++ | ++++ |
| Vascular SMC | ++++ | ++++ | ++++ |
(−) = No staining; (+) = minimal expression; (++) = mild expression; (+++) = moderate expression; (++++) = strong expression.
Abbreviations: EC, endothelial cells; SMC, smooth muscle cells.
Table 2.
Pulmonary Cellular Expression of COX-2 After RSV and PI3 Infection
| Pulmonary compartment | Control | RSV | PI3 |
|---|---|---|---|
| Alveolar septae | − | − | − |
| Bronchial epithelium | + | ++++ | ++++ |
| Bronchial SMC | − | − | − |
| Bronchiolar epithelium | + | ++++ | ++++ |
| Bronchiolar SMC | − | − | − |
| Macrophages | + | ++++ | ++++ |
| Vascular EC | − | − | − |
| Vascular SMC | − | − | − |
(−) = No staining; (+) = minimal expression; (++) = mild expression; (+++) = moderate expression; (++++) = strong expression.
Abbreviations: EC, endothelial cells; SMC, smooth muscle cells.
FIG. 1.
(A) COX-1 expression in the lung from a control lamb. Strong (++++) expression in bronchial smooth muscle cells (long arrows) and macrophages (arrowheads) can be seen (immunohistochemical stain, original magnification 10 ×). (B) COX-1 expression in the lung from a RSV-infected lamb. Strong (++++) expression in bronchial smooth muscle cells (long arrows), alveolar septa (short arrows), and vascular endothelial cells (arrowheads) can be seen (immunohistochemical stain, original magnification 10 ×). (C) COX-1 expression in the lung from a PI3-infected lamb. Strong (++++) expression in bronchial and bronchiolar smooth muscle cells (long arrows) and vascular endothelial cells (arrowheads) can be seen (immunohistochemical stain, original magnification 10 ×).
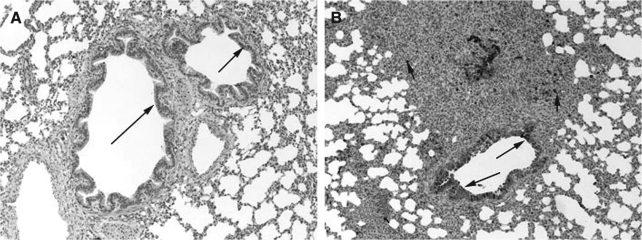
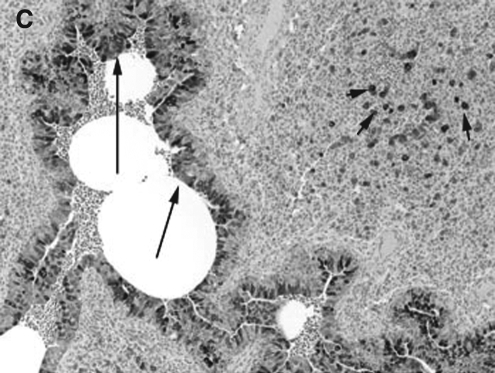
COX-2 expression was minimally present in macrophages from control lungs, while strong (++++) expression was present in macrophages in RSV- and PI3-infected lungs (Fig. 2A, B, and C). Strong (++++) COX-2 expression was present in bronchial epithelium from both PI3-infected and RSV-infected lungs. Bronchial epithelium from control lungs had minimal COX-2 expression. Similarly, strong (++++) COX-2 expression was present in the bronchiolar epithelium of both RSV-infected and PI3-infected lungs, with control lungs had minimal (+) COX-2 expression. Alveolar septa, bronchial and bronchiolar smooth muscle cells, and vascular endothelial and smooth muscle cells lacked COX-2 expression in control and infected lungs (–). Intense expression was present at sites of infection and inflammation and correlated with the degree of inflammation and infection.
FIG. 2.
(A) COX-2 expression in the lung from a control lamb. Minimal (+) expression in bronchial and bronchiolar epithelial cells (long arrows) can be seen (immunohistochemical stain, original magnification 10 ×). (B) COX-2 expression in the lung from an RSV-infected lamb. Strong (++++) expression in bronchiolar epithelial cells (long arrows) and macrophages (short arrows) can be seen (immunohistochemical stain, original magnification 10 ×). (C) COX-2 expression in the lung from a PI3-infected lamb. Strong (++++) expression in bronchial epithelial cells (long arrows) and macrophages (short arrows) can be seen (immunohistochemical stain, original magnification 10 ×).
Discussion
Pulmonary epithelial cells play an integral role in airway homeostasis, perform numerous biological functions, and represent the first line of defense against infection (39). Prostanoids, generated by COX, are present in high concentrations during various pulmonary disease conditions, such as acute respiratory distress syndrome (ARDS), asthma, COPD, and sepsis (13,29,42). In addition, increased COX-2 expression has been demonstrated in various viral infections (36). Prostanoids are important mediators in both normal and pathological pulmonary functions. All microanatomical locations in control, RSV-infected, and PI3-infected lungs had some degree of COX-1 expression in our study. This finding is consistent with other studies demonstrating COX-1 expression in various pulmonary microanatomical locations in normal human lungs (18). COX-1-dependent prostanoid generation has also been associated with regulation of bronchial tone (11). In lungs from normal animal models, COX-1 was present in bronchiolar epithelium and smooth muscle, alveolar macrophages, endothelial cells, and vascular smooth muscle cells of the rat (11), and in vascular endothelium and alveolar epithelial cells in the non-human primate (24). In lungs of sheep, COX-1 was present in endothelial cells and airway epithelium, and have critical involvement in vasodilatation, bronchodilation, and surfactant synthesis (8). The strong COX-1 expression in macrophages seen in our study is in accordance with results of other studies in rats, indicating that COX-1 was largely responsible for the enzyme activity displayed by alveolar macrophages (46).
Bronchial epithelial cells are known to play an integral role in airway defenses via mucociliary clearance, and they also constitute a mechanical barrier. In addition, bronchial epithelial cells produce and release biologically active compounds, including lipid mediators, growth factors, and a variety of cytokines important in the pathogenesis of airway disorders (40). Some in-vitro studies have demonstrated that cultured human bronchial epithelial cells express COX-2 constitutively (3). In addition, COX-2 expression was found in bronchial epithelium obtained from normal human subjects (10). RSV is a leading cause of respiratory infection and hospitalization in young children, especially those born preterm (17). Likewise, PI1 and PI3 are important causes of seasonal respiratory disease in human infants (17). Sheep are also susceptible to ovine and bovine strains of RSV and ovine strains of PI3. All of these strains have a very high degree of homology and induce similar lung lesions in both perinatal lambs and humans (9,16,17,23,27). The role of prostanoids in modulating RSV or PI3 infection in vivo is unknown. A slightly higher level of COX-1 expression was present in bronchial and bronchiolar epithelial cells from RSV-infected lungs compared to controls and PI3-infected lungs. The marked increase in COX-2 expression in pulmonary bronchial and bronchiolar epithelium and macrophages following RSV or PI3 infection found in our study is consistent with the known role of respiratory epithelia as the first line of defense during infection.
The airway epithelium forms a continuous barrier that limits the access of luminal substances to the systemic circulation (43). In a human airway epithelial cell culture system, COX-2 induction has been observed in the setting of inflammatory cytokine stimulation (44). Significant induction of COX-2 expression and activity was also observed in an experimental mouse model of acute lung injury (12). Minimal COX-2 expression was present in bronchial and bronchiolar epithelial cells from control animals, but was increased in RSV- and PI3-infected animals in our study. This observation corroborates results of other studies that have demonstrated low levels of COX-2 in untreated human airway epithelial cells in culture (28). It has been shown in in-vitro human alveolar type II-like epithelial cells that RSV infection induces a time-dependent increase in COX-2 expression, is a potent inducer of PGE2, and that viral replication is required for PGE2 secretion (26). In addition, strong induction of COX-2 expression during RSV infection in vitro, in human lung alveolar epithelial cells, and in vivo in lungs of cotton rats infected with RSV has been demonstrated (32). However, the exact pulmonary cellular source of the increased COX-2 was not determined in the study by Richardson et al. (32). The lack of COX-2 expression in pulmonary vascular endothelial cells found in our study is consistent with other in-vitro studies in which no COX-2 was detected in these cells under basal conditions (22).
The marked increase in COX-2 expression in macrophages is consistent with the finding that alveolar macrophages are a major source of PGE2 (19). Macrophages are postulated to be the primary cell type expressing COX-2 and producing PGs at sites of inflammation (34). In a mouse model of oxygen-induced ARDS, COX-2 was expressed in alveolar macrophages (1). Increased COX-2 levels in macrophages have been demonstrated in humans after rhinovirus infection (35). COX-2 was induced in RSV-infected cotton rat alveolar and peritoneal macrophages (32). In addition, human cord blood-derived macrophages and dendritic cells have been shown to secrete PGE2 following exposure to RSV (4).
Conclusion
Collectively, the findings in this work suggest that in an in-vivo neonatal lamb model: (1) COX-1 expression is not altered after RSV or PI3 pulmonary infection, (2) COX-2 expression is upregulated in bronchiolar and bronchial epithelial cells and macrophages after RSV and PI3 infection, (3) respiratory epithelia along with macrophages are important microanatomical compartments regulating the host inflammatory response during viral infection, and (4) COX-2 may be a potential target for RSV and PI3 therapy.
Acknowledgments
The work is supported in part by National Institutes of Health grants no. R01 AI062787 (M.R.A.) and KO8AI055499 (D.K.M. and M.R.A.).
Author Disclosure Statement
This work was supported by Pfizer.
References
- 1.Adawi A. Zhang Y. Baggs R. Finkelstein J. Phipps RP. Disruption of the CD40-CD40 ligand system prevents an oxygen-induced respiratory distress syndrome. Am J Pathol. 1998;152:651–657. [PMC free article] [PubMed] [Google Scholar]
- 2.Arias-Diaz J. Vara E. Garcia C. Balibrea JL. Tumor necrosis factor-alpha-induced inhibition of phosphatidylcholine synthesis by human type II pneumocytes is partially mediated by prostaglandins. J Clin Invest. 1994;94:244–250. doi: 10.1172/JCI117313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asano K. Lilly CM. Drazen JM. Prostaglandin G/H synthase-2 is the constitutive and dominant isoform in cultured human lung epithelial cells. Am J Physiol. 1996;271:L126–L131. doi: 10.1152/ajplung.1996.271.1.L126. [DOI] [PubMed] [Google Scholar]
- 4.Bartz H. Buning-Pfaue F. Turkel O. Schauer U. Respiratory syncytial virus induces prostaglandin E2, IL-10 and IL-11 generation in antigen presenting cells. Clin Exp Immunol. 2002;129:438–445. doi: 10.1046/j.1365-2249.2002.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett BL. Garofalo RP. Cron SG. Hosakote YM. Atmar RL. Macias CG. Piedra PA. Immunopathogenesis of respiratory syncytial virus bronchiolitis. J Infect Dis. 2007;195:1532–1540. doi: 10.1086/515575. [DOI] [PubMed] [Google Scholar]
- 6.Bonnans C. Fukunaga K. Levy MA. Levy BD. Lipoxin A4 regulates bronchial epithelial cell responses to acid injury. Am J Pathol. 2006;168:1064–1072. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonville CA. Rosenberg HF. Domachowske JB. Macrophage inflammatory protein-1alpha and RANTES are present in nasal secretions during ongoing upper respiratory tract infection. Pediatr Allergy Immunol. 1999;10:39–44. doi: 10.1034/j.1399-3038.1999.101005.x. [DOI] [PubMed] [Google Scholar]
- 8.Brannon TS. MacRitchie AN. Jaramillo MA. Sherman TS. Yuhanna IS. Margraf LR. Shaul PW. Ontogeny of cyclooxygenase-1 and cyclooxygenase-2 gene expression in ovine lung. Am J Physiol. 1998;274:L66–L71. doi: 10.1152/ajplung.1998.274.1.L66. [DOI] [PubMed] [Google Scholar]
- 9.Cutlip RC. Lehmkuhl HD. Lesions in lambs experimentally infected with bovine respiratory syncytial virus. Am J Vet Res. 1979;40:1479–1482. [PubMed] [Google Scholar]
- 10.Demoly P. Jaffuel D. Lequeux N, et al. Prostaglandin H synthase 1 and 2 immunoreactivities in the bronchial mucosa of asthmatics. Am J Respir Crit Care Med. 1997;155:670–675. doi: 10.1164/ajrccm.155.2.9032211. [DOI] [PubMed] [Google Scholar]
- 11.Ermert L. Ermert M. Goppelt-Struebe M, et al. Cyclooxygenase isoenzyme localization and mRNA expression in rat lungs. Am J Respir Cell Mol Biol. 1998;18:479–488. doi: 10.1165/ajrcmb.18.4.2939. [DOI] [PubMed] [Google Scholar]
- 12.Fukunaga K. Kohli P. Bonnans C. Fredenburgh LE. Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005;174:5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 13.Fulkerson WJ. MacIntyre N. Stamler J. Crapo JD. Pathogenesis and treatment of the adult respiratory distress syndrome. Arch Intern Med. 1996;156:29–38. [PubMed] [Google Scholar]
- 14.Gershwin LJ. Giri SN. Stewart RS. Chen J. Prostaglandin and thromboxane concentrations in plasma and lung lavage fluids during sequential infection of vaccinated and nonvaccinated calves with bovine respiratory syncytial virus. Am J Vet Res. 1989;50:1254–1262. [PubMed] [Google Scholar]
- 15.Graham BS. Rutigliano JA. Johnson TR. Respiratory syncytial virus immunobiology and pathogenesis. Virology. 2002;297:1–7. doi: 10.1006/viro.2002.1431. [DOI] [PubMed] [Google Scholar]
- 16.Grubor B. Gallup JM. Meyerholz DK. Crouch E. Evans RB. Brogden KA. Lehmkuhl HD. Ackermann MR. Enhanced surfactant protein and defensin mRNA levels and reduced viral replication during paramyxoviral pneumonia in neonatal lambs. Clin Diag Lab Immunol. 2004;11:599–607. doi: 10.1128/CDLI.11.3.599-607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 18.Hasturk S. Kemp B. Kalapurakal SK. Kurie JM. Hong WK. Lee JS. Expression of cyclooxygenase-1 and cyclooxygenase-2 in bronchial epithelium and non-small cell lung carcinoma. Cancer. 2002;94:1023–10231. [PubMed] [Google Scholar]
- 19.Hempel SL. Monick MM. Hunninghake GW. Lipopolysaccharide induces prostaglandin H synthase-2 protein and mRNA in human alveolar macrophages and blood monocytes. J Clin Invest. 1994;93:391–396. doi: 10.1172/JCI116971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holtzman MJ. Arachidonic acid metabolism. Implications of biological chemistry for lung function and disease. Am Rev Respir Dis. 1991;143:188–203. doi: 10.1164/ajrccm/143.1.188. [DOI] [PubMed] [Google Scholar]
- 21.Hornsleth A. Loland L. Larsen LB. Cytokines and chemokines in respiratory secretion and severity of disease in infants with respiratory syncytial virus (RSV) infection. Cytokines and chemokines in respiratory secretion and severity of disease in infants with respiratory syncytial virus (RSV) infection. J Clin Virol. 2001;21:163–170. doi: 10.1016/s1386-6532(01)00159-7. [DOI] [PubMed] [Google Scholar]
- 22.Jackson BA. Goldstein RH. Roy R. Cozzani M. Taylor L. Polgar P. Effects of transforming growth factor beta and interleukin-1 beta on expression of cyclooxygenase 1 and 2 and phospholipase A2 mRNA in lung fibroblasts and endothelial cells in culture. Biochem Biophys Res Commun. 1993;197:1465–1474. doi: 10.1006/bbrc.1993.2642. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JE. Gonzales RA. Olson SJ. Wright PF. Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 24.Khan KN. Stanfield K. Trajkovic D. Harris RK. Cyclooxygenase-2 expression in inflammatory lung lesions of nonhuman primates. Vet Pathol. 2000;37:512–516. doi: 10.1354/vp.37-5-512. [DOI] [PubMed] [Google Scholar]
- 25.Lehmkuhl HD. Cutlip RC. Experimental parainfluenza type 3 infection in young lambs: clinical, microbiological, and serological response. Vet Microbiol. 1983;8:437–442. doi: 10.1016/0378-1135(83)90038-x. [DOI] [PubMed] [Google Scholar]
- 26.Liu T. Zaman W. Kaphalia BS. Ansari GA. Garofalo RP. Casola A. RSV-induced prostaglandin E2 production occurs via cPLA2 activation: role in viral replication. Virology. 2005;343:12–24. doi: 10.1016/j.virol.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Meyerholz DK. Grubor B. Fach SJ. Sacco RE. Lehmkuhl HD. Gallup JM. Ackermann MR. Reduced clearance of respiratory syncytial virus in a preterm lamb model. Microbes Infect. 2004;6:1312–1319. doi: 10.1016/j.micinf.2004.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell JA. Belvisi MG. Akarasereenont P, et al. Induction of cyclo-oxygenase-2 by cytokines in human pulmonary epithelial cells: regulation by dexamethasone. Br J Pharmacol. 1994;113:1008–1014. doi: 10.1111/j.1476-5381.1994.tb17093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Profita M. Sala A. Bonanno A, et al. Increased prostaglandin E2 concentrations and cyclooxygenase-2 expression in asthmatic subjects with sputum eosinophilia. J Allergy Clin Immunol. 2003;112:709–716. doi: 10.1016/s0091-6749(03)01889-x. [DOI] [PubMed] [Google Scholar]
- 30.Radi ZA. Pathophysiology of cyclooxygenease inhibition in animal models. Toxicologic Pathol. 2009;37:34–46. doi: 10.1177/0192623308329474. [DOI] [PubMed] [Google Scholar]
- 31.Radi ZA. Ostroski R. Pulmonary and cardiorenal cyclooxygenase-1 (COX-1), −2 (COX-2), and microsomal prostaglandin E synthase-1 (mPGES-1) and −2 (mPGES-2) expression in a hypertension model. Mediators Inflamm. 2007;2007:85091. doi: 10.1155/2007/85091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson JY. Ottolini MG. Pletneva L, et al. Respiratory syncytial virus (RSV) infection induces cyclooxygenase 2: a potential target for RSV therapy. J Immunol. 2005;174:4356–4364. doi: 10.4049/jimmunol.174.7.4356. [DOI] [PubMed] [Google Scholar]
- 33.Rolin S. Masereel B. Dogne JM. Prostanoids as pharmacological targets in COPD and asthma. Eur J Pharmacol. 2006;533:89–100. doi: 10.1016/j.ejphar.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 34.Seibert K. Zhang Y. Leahy K, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seymour ML. Gilby N. Bardin PG, et al. Rhinovirus infection increases 5-lipoxygenase and cyclooxygenase-2 in bronchial biopsy specimens from nonatopic subjects. J Infect Dis. 2002;185:540–544. doi: 10.1086/338570. [DOI] [PubMed] [Google Scholar]
- 36.Steer SA. Corbett JA. The role and regulation of COX-2 during viral infection. Viral Immunol. 2003;16:447–460. doi: 10.1089/088282403771926283. [DOI] [PubMed] [Google Scholar]
- 37.Sznajer Y. Westcott JY. Wenzel SE. Mazer B. Tucci M. Toledano BJ. Airway eicosanoids in acute severe respiratory syncytial virus bronchiolitis. J Pediatr. 2004;145:115–118. doi: 10.1016/j.jpeds.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 38.Tamaoki J. Chiyotani A. Kobayashi K. Sakai N. Kanemura T. Takizawa T. Effect of indomethacin on bronchorrhea in patients with chronic bronchitis, diffuse panbronchiolitis, or bronchiectasis. Am Rev Respir Dis. 1992;145:548–552. doi: 10.1164/ajrccm/145.3.548. [DOI] [PubMed] [Google Scholar]
- 39.Takizawa H. Airway epithelial cells as regulators of airway inflammation. Int J Mol Med. 1998;1:367–378. doi: 10.3892/ijmm.1.2.367. [DOI] [PubMed] [Google Scholar]
- 40.Takizawa H. Bronchial epithelial cells in allergic reactions. Curr Drug Targets Inflamm Allergy. 2005;4:305–311. doi: 10.2174/1568010054022123. [DOI] [PubMed] [Google Scholar]
- 41.Thompson WW. Shay DK. Weintraub E. Brammer L. Cox N. Anderson LJ. Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 42.Vigano T. Toia A. Crivellari MT. Galli G. Mezzetti M. Folco GC. Prostaglandin synthetase inhibition and formation of lipoxygenase products in immunologically challenged normal human lung parenchyma. Eicosanoids. 1998;1:73–77. [PubMed] [Google Scholar]
- 43.Wallace JL. Commonality of defensive roles of COX-2 in the lung and gut. Am J Pathol. 2006;168:1060–1063. doi: 10.2353/ajpath.2006.060023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watkins DN. Garlepp MJ. Thompson PJ. Regulation of the inducible cyclo-oxygenase pathway in human cultured airway epithelial (A549) cells by nitric oxide. Br J Pharmacol. 1997;121:1482–1488. doi: 10.1038/sj.bjp.0701283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilborn J. Crofford LJ. Burdick MD. Kunkel SL. Strieter RM. Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest. 1995;95:1861–1868. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilborn J. DeWitt DL. Peters-Golden M. Expression and role of cyclooxygenase isoforms in alveolar and peritoneal macrophages. Am J Physiol. 1995;268:L294–L301. doi: 10.1152/ajplung.1995.268.2.L294. [DOI] [PubMed] [Google Scholar]