Abstract
Previous studies of epithelial immune responses to rotavirus infection have been conducted in transformed cell lines. In this study, we evaluated a non-transformed porcine jejunum epithelial cell line (IPEC-J2) as an in-vitro model of rotavirus infection and probiotic treatment. Cell-culture-adapted porcine rotavirus (PRV) OSU strain, or human rotavirus (HRV) Wa strain, along with Lactobacillus acidophilus (LA) or Lactobacillus rhamnosus GG (LGG) were used to inoculate IPEC-J2 cells. LA or LGG treatment was applied pre- or post-rotavirus infection. We demonstrated that IPEC-J2 cells were productively infected by PRV. LA or LGG treatment of the cells did not reduce virus replication. PRV infection increased MUC3 mucin secretion. LGG treatment post-rotavirus infection reduced the mucin secretion response induced by PRV; LGG alone increased the production of membrane-associated MUC3 mucin. LA treatment prior to rotavirus infection significantly increased PRV replication and the IL-6 response to PRV infection, which is consistent with the adjuvant effect of LA. LGG treatment post-rotavirus infection downregulated the IL-6 response, confirming the anti-inflammatory effect of LGG. IPEC-J2 cells expressed toll-like receptor (TLR) 2, TLR3, and TLR9 constitutively. TLR2 expression was upregulated by LGG and peptidoglycan, corresponding to the decreased IL-6 response, indicating that the protective effect of LGG is associated with upregulation of TLR2 expression on intestinal epithelial cells. The IPEC-J2 cell model of PRV infection is a completely homologous system. It is a valuable model for studying the interactions among rotavirus-host-probiotics, and the mechanisms behind the immunomodulating effect of probiotic bacteria on innate immune responses.
Introduction
Rotavirus is the most common cause of severe dehydrating diarrhea in infants and young children, is responsible for 22% of all hospitalizations attributed to acute diarrhea, and causes nearly 600,000 deaths annually in children less than 5 y old worldwide (42). Differentiated intestinal epithelial cells, primarily the epithelial cells of the ileum and jejunum, are the main targets of rotavirus (58), and thus intestinal epithelial cells serve as the first physical barrier against rotavirus infection. Aside from their barrier function, epithelial cells utilize a variety of innate immune mechanisms to reduce the risk of infection from invading foreign agents, including viruses and bacteria. These mechanisms of epithelial cells involve mucus secretion, cytokine/chemokine production, and toll-like receptor (TLR) expression (18,41).
Because of the limited availability of epithelial cell lines of small intestinal origin, knowledge concerning the role of epithelial cells in the innate immune response of the small intestine, which is the location of rotavirus replication, is limited. In-vivo studies of intestinal epithelial cell responses have been limited to the mouse model of murine rotavirus infection (6,7), even though rotavirus infection in mice does not induce the same pathological changes as those observed in humans and pigs (63). Recently, a cell line from jejunum epithelium isolated from a neonatal unsuckled piglet, the porcine small intestinal epithelial cell line (IPEC-J2), was characterized and used as an in-vitro model system for studying porcine intestinal pathogen-host interactions, porcine-specific pathogenesis, and innate immune responses (1,8–10,27,49,51). IPEC-J2 is a non-transformed, non-tumorigenic intestinal epithelial cell line, which maintains differentiated characteristics and exhibits strong similarities to primary intestinal epithelial cells (49). Therefore, IPEC-J2 represents a better model of normal intestinal epithelial cells than do transformed cell lines. IPEC-J2 cells express mRNAs encoding TLR1, TLR2, TLR3, TLR4, TLR6, TLR8, TLR9, TLR10, IL-1α, IL-6, IL-7, IL-8, IL-18, TNF-α, and GM-CSF (1,10,36,49). MUC3 mucin production in IPEC-J2 cells was preliminarily confirmed, but MUC2 gene expression was not detected (49). In the present study, our objectives were to establish the IPEC-J2 cell model of human and porcine rotavirus infection, and use the in-vitro model system to determine intestinal epithelial cell responses to rotavirus infection, and the effects of probiotic treatment pre- and post-rotavirus infection.
Probiotics are viable microorganisms, the consumption of which in sufficient amounts benefits gastrointestinal tract health by improving the intestinal microbial balance and modulating immune functions (5,17). Lactobacilli are one of the major gram-positive probiotics; they are also commensals found in human and porcine intestinal tracts. Specific beneficial effects of Lactobacillus rhamnosus GG strain (LGG) have been documented in a large number of clinical trials. These include shortening the duration of rotavirus diarrhea, reducing the number of diarrhea episodes, lessening rotavirus shedding, normalizing gut permeability, and increasing the production of rotavirus-specific antibodies (20–24,46,53). L. acidophilus NCFM strain (LA) has been used commercially as a probiotic in dietary supplements in the U.S. (47). We previously reported that LA had significant potentiating effects on the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. However, LA plus L. reuteri did not reduce rotavirus diarrhea in gnotobiotic pigs (68). We hypothesize that the mechanisms behind the diarrhea-alleviating effect of LGG versus the adjuvant effect of LA are related to their distinctly different modulating functions on innate immune responses in epithelial cells. Therefore LGG and LA strains were chosen for this study.
Gastrointestinal mucin production plays a critical role in the maintenance of mucosal homeostasis, and is involved in responses against a plethora of microorganisms, including commensals and pathogens (31). Thus mucins are also considered a major part of the innate immune response (38). Epithelial MUC2 mucin is expressed by goblet cells and exists in secreted form. MUC3 mucin is expressed by both goblet cells and intestinal epithelial cells, and exists in secreted and membrane-bound forms (14,32,60). It has been reported that intestinal mucins from mice, rats, and humans inhibit rotavirus replication in cell cultures (15,62). Additionally, during rotavirus infection, MUC2 mucin expression was increased in mice (6). However, there is no report about the response of MUC3 mucins in rotavirus infection, thus it was one of the targets of the present study. It has also been reported that probiotics (including LGG) increased mucin gene expression and mucin secretion in HT-29 cells (a human colon adenocarcinoma grade II cell line) (32,33), Caco-2 cells (human epithelial colorectal adenocarcinoma cells) (37), and rat colonic epithelial cells (11). Consequently, the effect of LA and LGG on rotavirus infection-induced mucin production in IPEC-J2 cells was another objective of the present study.
The production of cytokines/chemokines is one of the major innate immune responses against microorganisms in epithelial cells. Rotavirus or synthetic double-strand RNA (dsRNA) has been shown to stimulate IL-8 mRNA expression and IL-8 secretion in many cultured human intestinal epithelial cells, such as HT-29 (45,50) and T48 (a human colonic epithelial cell line) (56). In vivo, elevated IL-6 and TNF-α levels in serum have been associated with increased severity of rotavirus disease in humans (3,25) and gnotobiotic pigs (2). Rotavirus dsRNA also triggers the production of IL-15 in intestinal epithelial cells via the TLR3-activated pathway. IL-15 increased the frequency and cytotoxicity of intestinal CD8αα+ intraepithelial leukocytes, causing severe mucosal injury in the small intestines of mice (69). Additionally, mRNA expression of interferon (IFN)-γ-inducible protein of 10 kDa (IP-10/CXCL10) has also been induced by rotavirus infection in HT-29 cells and in mice <15 d old (45). IP-10 is a potent chemoattractant for activated T and NK cells (52). Thus rotavirus infection in intestinal epithelial cells induces production of cytokines/chemokines that promote infiltration of inflammatory cells that have direct and indirect antiviral effects, and stimulates adaptive B- and T-cell immune responses, but it may also contribute to the pathogenesis of rotavirus disease.
Epithelial TLR expression is also thought to be key to the host defense against pathogens by triggering innate immune responses (28,54). As principal TLRs implicated in innate immune responses to gram-positive bacteria (TLR2 and TLR9) and dsRNA (TLR3), the expression levels of TLR2, TLR3, and TLR9 in macrophages and dendritic cells of gnotobiotic pigs infected with human rotavirus infection and colonized with probiotic lactobacilli was previously studied in our lab (61). It has also been reported that TLR2 stimulation by peptidoglycan (PGN) enhanced intestinal epithelial barrier function and anti-inflammatory cytokine release (13,40). In the present study, our general hypothesis was that LGG's effect on rotavirus diarrhea is mediated, at least in part, by regulating mucin production and secretion, increasing TLR2 expression, and reducing the production of inflammatory cytokines in the intestinal epithelium. The IPEC-J2 cell model of rotavirus infection provided a good system to test this hypothesis.
Materials and Methods
Intestinal epithelial cell line
The IPEC-J2 cell line was a generous gift from Dr. Anthony Blikslager (North Carolina State University, Raleigh, NC). The cells were grown in Dulbecco's modified Eagle medium: nutrient mixture F-12 (Ham) (1:1) with GlutaAMAX™-I (DMEM/F12) (Invitrogen, Carlsbad. CA), supplemented with 5% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA), 1% insulin-transferring-selenium supplements (Invitrogen), 5 ng/mL epidermal growth factor (Invitrogen), 1% penicillin-streptomycin (penicillin 10,000 U/mL and streptomycin 100 mg/mL; Invitrogen), and 15 mM HEPES in a humidified atmosphere of 5% CO2 at 37°C. Cell culture media were changed every 2 d and the cells were passaged every 4–5 d by trypsinization with 0.25% trypsin-EDTA. To perform the following experiments, the cells were seeded at a suitable concentration, which was determined in pilot studies, and is defined in each of the following experiments. The cell concentration was determined by 0.4% trypan blue viability staining. Immediately prior to use, the confluent monolayers were washed 2–3 times with phosphate-buffered saline (PBS).
Viruses
The attenuated HRV Wa (G1P1A[8]) strain (acquired from Dr. Linda J. Saif, The Ohio State University, Wooster, OH) (58), and tissue-culture-adapted porcine rotavirus (PRV) OSU (G5P9[7]) strain (ATCC #VR-893) (4) were passaged in MA104 clone 1 cells (ATCC# CRL-2378.1™). The virus titer was determined using cell culture immunofluorescence (CCIF) assay, and was expressed as fluorescent focus-forming units (FFU) per mL. The virus stock was stored at −80°C until use.
Probiotic bacteria strains
Lactobacillus acidophilus (LA) NCFM™ and L. rhamnosus GG (LGG; ATCC# 53103) were propagated in lactobacilli MRS broth (Weber Scientific, Hamilton, NJ) overnight at 37°C anaerobically (85% nitrogen, 10% hydrogen, and 5% carbon dioxide) in sealed Gaspak jars containing anaerobic Gaspacks (BD Biosciences, San Jose, CA). The bacteria were harvested in log phase and the suspensions (in MRS and 15–20% glycerol) were stored at −80°C. Prior to use, the bacteria were thawed and washed two times with maintenance medium (DMEM/F12 with 15 mM HEPES) by centrifugation at 2000 rpm for 10 min at 4°C. The viability of the thawed and washed bacteria was determined by plating on MRS agar. The bacteria counts were expressed as colony-forming units (CFU) per mL.
Protocols for rotavirus infection of IPEC-J2 cells and treatment of the cells with probiotics
Monolayers of IPEC-J2 cells seeded at the density of 1 × 105/cm2, and then grown for 48 h in 25 cm2 flasks (about 3.5–4 × 106 cells/flask) were used in the two protocols as previously described (8) with some modifications, and in the other experiments, unless otherwise specified.
Protocol 1: Probiotic treatment prior to rotavirus infection (pre-rotavirus infection)
The monolayers were inoculated with LA (1 × 108 CFU/mL) or LGG (1 × 106 CFU/mL) in 2.5 mL maintenance medium for 24 h at 37°C with 5% CO2, followed by removal of the non-attached bacteria and washing of the cells three times with PBS. The treated monolayers were then challenged with 20 multiplicity of infection (MOI) of trypsin-activated rotavirus (pre-treated with 5 μg/mL trypsin at 37°C for 30 min). The cells were incubated for 1 h at 37°C with 5% CO2, followed by removal of the inoculums and washing of the cells twice with PBS. The infection was continued for 24 h at 37°C with 5% CO2 in 2.5 mL of maintenance medium containing 0.5 μg/mL trypsin.
Protocol 2: Probiotic treatment after rotavirus infection (post-rotavirus infection)
The monolayers were challenged with 20 MOI of activated rotavirus as described above. Following removal of the viral inoculums and two washings, the monolayers were incubated with probiotic bacteria LA (1 × 108 CFU/mL) or LGG (1 × 106 CFU/mL) in 2.5 mL of maintenance medium containing 0.5 μg/mL trypsin for 24 h.
The probiotic concentration and incubation time were determined in pilot experiments as previously described (8). Higher concentration (LA ≥ 1 × 109 and LGG ≥ 1 × 107), and increased incubation (48 h), significantly damaged the monolayers. All control cells were incubated with corresponding medium and treated in the same manner in all experiments. At the end of the experiments, the cells were checked for viability by trypan blue exclusion and monolayer integrity. Cell culture supernatants were collected and stored at −80°C until assayed.
Stimulation of IPEC-J2 cells with TLR agonists
Monolayers of IPEC-J2 cells were stimulated with 10 μg/mL of PGN from Bacillus subtilis (InvivoGen, San Diego, CA), 20 μg/mL of polyinosinic-polycytidylic acid (polyI:C; Sigma-Aldrich Co., St. Louis, MO), 10 μg/mL of unmethylated deoxycytidine-phosphate-deoxyguanosine (CpG) (5′-EETGCATCGATGCAEEEEEG-3′, type A; Invitrogen), or 10 μg/mL of total DNA purified from LA or LGG for 24 h. The total DNA of LA or LGG was purified by using a PurElute™ Bacterial Genomic Kit (Edge BioSystems, Gaithersburg, MD), and was quantified by using a NanoDrop™ 1000 spectrophotometer (Thermo Scientific, Rochester, NY).
Immunofluorescence staining for detection of rotavirus-infected cells
Rotavirus-infected cells were fixed with 80% acetone at room temperature for 10 min. A goat anti-rotavirus polyclonal antibody was added (Affinity BioReagents, Golden, CO; IgG 1:250 in PBS with 0.05% Tween 20 and 2% non-fat milk) to all except the controls. The plates were incubated at 37°C for 1 h. Following rinsing three times with PBS with 0.05% Tween, a rabbit anti-goat IgG conjugated with fluorescein isothiocyanate antibody (Sigma-Aldrich) was added to the monolayers (1:400 in PBS with 0.05% Tween 20 and 2% not-fat milk), and the plates were incubated at 37°C for 1 h. Finally, following rinsing three times with PBS, mounting medium (60% glycerol in PBS) was added and the cells were examined under an inverted fluorescence microscope.
Determination of rotavirus antigens and titers in cell culture supernatants by ELISA and CCIF
Rotavirus antigens in the supernatants of IPEC-J2 cells were detected by a sandwich ELISA. A goat anti-bovine rotavirus polyclonal antibody (Affinity BioReagents; 1:1000 in carbonate buffer, pH 9.6) was used to coat 96-well plates (100 μL/well) overnight at 4°C. The plates were blocked with 300 μL/well of blocking buffer (5% non-fat dry milk in PBS) for 1 h at 37°C. Then 100 μL of the supernatants in diluent (21–24 in PBS with 0.1% bovine serum albumin [BSA]) were added to each well, in duplicate. A rotavirus stock and supernatants of mock-infected MA104 cells served as a positive and a negative control, respectively. Following 1 h incubation at 37°C, an anti-rotavirus polyclonal antibody-HRP (Affinity BioReagents; 1:200 in diluent, 100 μL/well) was added and incubated for 1 h at 37°C as well. The plates were developed with 100 μL of ABTS (2,2′-azio-3-ethylbenzthiazoline-6-sulfonate) peroxidase substrate solution, and incubated for 5–10 min at room temperature. The reaction was stopped with 100 μL/well of ABTS peroxidase stop solution. The plates were washed three times with PBS containing 0.05% Tween 20 following each step. The optical density (OD) values of the wells were measured at the wavelength of 405 nm using a spectrophotometer (Safire II-basic; Tecan Austria GmbH, Grödig, Austria). Any non-specific reactions occurring in blank controls was subtracted from the OD values of all the samples.
The virus titers in the cell supernatants were determined using CCIF. Briefly, MA104 cells were grown in 96-well cell culture plates to reach confluence at 37°C in 5% CO2. The supernatants were diluted 10-fold from 1:101 to 1:106 with serum-free medium and were added to the cells (50 μL/well) in duplicate. The plates were centrifuged at 2800 rpm for 1 h at room temperature, followed by the addition of 50 μL of serum-free medium containing trypsin. The final concentration of trypsin was 0.5 μg/mL. The plates were incubated at 37°C for about 18–24 h before immunofluorescence staining. To calculate the virus titer, the number of isolated foci units (FU) were counted in each well. The virus titer was calculated as: titer = (FU × dilution factor)/sample volume in the well, and expressed as FFU per milliliter.
Detection of mucin production in IPEC-J2 cells and cell-culture supernatants by PAS and ELISA
IPEC-J2 cells were seeded at 0.5 × 105/cm2 and grown for 24 h in chamber slides (Nalge Nunc, Rochester, NY). The monolayers were fixed with 80% acetone at room temperature for 10 min. Mucin production (neutral mucopolysaccharides) in the IPEC-J2 cells was demonstrated using periodic acid-Schiff (PAS) staining, as previously described (49).
The MUC3 mucin production in the supernatants of IPEC-J2 cells was detected by a direct-binding ELISA. The supernatants were loaded onto 96-well plates in duplicate (100 μL/well). A MUC3 mucin peptide (AnaSpec Inc., Fremont, CA; 800 μg/mL in carbonate buffer, pH 9.6) was used as the positive control, and the carbonate buffer was used as the blank control. The plates were incubated at room temperature for 90 min, followed by blocking with 300 μL 10% FBS in PBS for 1 h. Mouse anti-human MUC3 (no cross-reactivity with human MUC2 mucin) peptide monoclonal antibody (AbD Serotec, Raleigh, NC; IgG2a, 2.5 μg/mL in blocking buffer) was added at 100 μL/well and incubated for 1 h. A biotinylated polyclonal goat anti-mouse IgG (Zymed, Carlsbad, CA; 1:5000 in blocking buffer) was then added (100 μL/well,) and the plates were incubated for 30 min. Then the plates were incubated with 100 μL/well of streptavidin conjugated with horseradish peroxidase (HRP) (Biosource International, Wilmington, NC; 1:5000 in blocking buffer) for 30 min. Finally, 100 μL/well of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution (Pierce Protein Research Products, Rockford, IL) were added and the plates were incubated for 5 min. The reaction was stopped by the addition of 100 μL of TMB stop solution (2 M H2SO4). The plates were washed three times with PBS containing 0.05% Tween 20 following each step. The OD of the wells was measured at a wavelength of 450 nm by the spectrophotometer within 30 min. Any non-specific reactions occurring in blank controls were subtracted from the OD value of all the samples.
Measurement of cytokine/chemokine concentrations in the cell culture supernatants of IPEC-J2 cells by ELISA
Concentrations of cytokines/chemokines in the supernatants were measured by sandwich ELISA as previously described (2). Porcine IL-6, IL-8, IP-10, and TNF-α ELISAs were performed according to the manufacturers' instructions (R&D Systems, Inc., Minneapolis, MN). The ELISA for IL-15 was conducted using a cross-reactive anti-human IL-15 polyclonal antibody (8 μg/mL), and biotinylated anti-human IL-15 antibody (0.25 μg/mL) (R&D Systems). A recombinant human IL-15 (R&D Systems) was used as a standard. The detection limits for IL-6, IL-8, IL-15, and IP-10 were 8.0 pg/mL, and for TNF-α was 3.0 pg/mL.
Detection of TLR2, TLR3, and TLR9 expression by flow cytometry
Expression levels of TLR2, TLR3, and TLR9 in IPEC-J2 cells were determined by flow cytometry as previously described (61). Briefly, the cells were collected by trypsinization and centrifugation. The live cell count was adjusted to ∼0.5–1 × 106 per Falcon tube, and the cells were fixed and permeabilized with the BD cytofix/cytoperm kit (BD Biosciences) according to the manufacturer's instructions. Then the fixed/permeabilized cells were resuspended and labeled with 1 μL of phycoerythrin (PE) anti-mouse/human TLR2 (eBioscience, San Diego, CA; clone T2.5, mouse IgG1), PE anti-human TLR3 (eBioscience; clone TLR3.7, mouse IgG1), and PE anti-human TLR9 (eBioscience; clone eB72-1665, rat IgG2a), respectively, in 50 μL perm/wash buffer for 15 min at room temperature. Cells stained with irrelevant PE mouse IgG1 or PE rat IgG2a isotype control antibodies (eBioscience) were included as controls. Next, the stained cells were washed once with 1 mL perm/wash buffer, followed by resuspension with 400–600 μL of staining buffer (PBS with 1% FBS and 0.09% NaN3); then they were kept at 4°C in the dark until flow cytometry analysis. Analysis of the stained cells was performed using a FACSAria flow cytometer, and at least 20,000 cells were acquired. Data analysis was performed using FlowJo 7.2.2 software (Tree Star, Inc., Ashland, OR). Data were presented as mean frequencies of TLR2-, TLR3-, and TLR9-expressing IPEC-J2 cells. Any non-specific staining occurring in the isotype-matched control tubes was subtracted from the frequencies of the TLR-positive cells.
Statistical analysis
Virus titers, ELISA OD values, cytokine concentrations, and frequencies of TLR-expressing cells were compared among treatment groups using the non-parametric test, Kruskal-Wallis rank sum test. Statistical significance was set at p < 0.05.
Results
Rotavirus infection of the IPEC-J2 cells
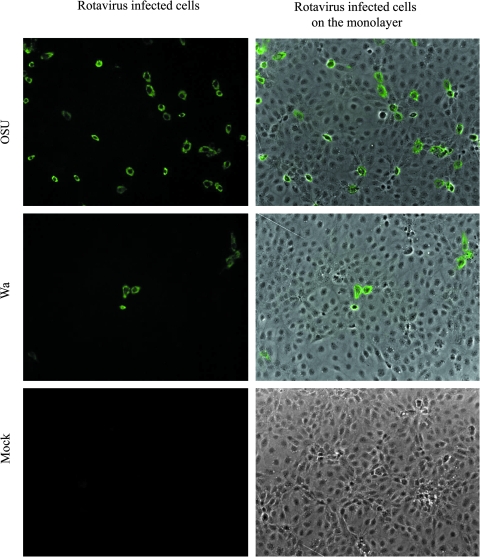
The OSU strain PRV and Wa strain HRV both were able to infect and replicate in the IPEC-J2 cells; however, the cell monolayers inoculated with OSU PRV had many more positively-stained cells than those inoculated with Wa HRV (Fig. 1). The virus titers reached 106–107 FFU/mL in the OSU PRV-infected cells, and 104–105 FFU/mL in the Wa HRV-infected cells. Thus the homologous OSU PRV was able to infect IPEC-J2 cells more productively than the heterologous Wa HRV. In the following studies, the effects of probiotic bacteria on rotavirus infectivity and mucin production were conducted using OSU PRV only. Cytokine and TLR responses to the homologous OSU PRV and the heterologous Wa HRV in IPEC-J2 cells were compared.
FIG. 1.
Rotavirus infection of IPEC-J2 cells. IPEC-J2 cells were infected with 20 MOI rotavirus for 24 h, and were then processed for immunofluorescence staining. Images are representative of three independent experiments (original magnification 100 ×). (Color image is available online at www.liebertonline.com/vim.)
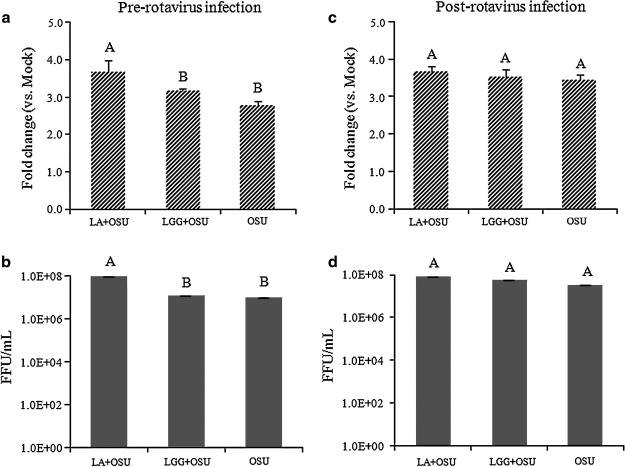
The effect of probiotics on rotavirus infectivity in the IPEC-J2 cells
To determine the effect of the probiotic bacteria LA or LGG on rotavirus infectivity, IPEC-J2 cells were treated using the pre- and post-rotavirus infection protocols. Rotavirus antigens in the supernatants were measured by ELISA, and rotavirus titers were measured by CCIF. Rotavirus was detected in LA + OSU, LGG + OSU, and OSU alone PRV-inoculated cells, but not in LA and LGG alone or mock-treated cells. LA treatment pre-rotavirus infection significantly increased the amount of rotavirus antigens (Fig. 2a), and the virus titers (Fig. 2b) in the supernatants, whereas LGG treatment did not alter the amount of rotavirus antigens or virus titers (Fig. 2a and 2b). LA or LGG treatment post-rotavirus infection did not change the amount of rotavirus antigens or virus titers (Fig. 2c and 2d). The virus-inoculated cells together with supernatants were frozen and thawed once, and then the supernatants were clarified by centrifugation and tested using the same methods. The same trend between the different treatments was obtained (data not shown).
FIG. 2.
The effect of probiotics on rotavirus infectivity in the IPEC-J2 cells. The cells were infected with OSU PRV and treated with the probiotics LA or LGG using the two protocols described in the materials and methods section. Rotavirus antigens in the supernatants were measured by ELISA, and the mean fold-increases in OD values of the treatment groups over the mock group are presented in the upper panels (a and c). The virus titers in the supernatants as measured by CCIF are presented as mean FFU/mL in panels b and d. Means were calculated from four to six independent experiments. Error bars indicate the standard error of the mean. The capital letters (A and B) indicate the results of significance testing for the difference between treatments. Unshared letters indicate significant difference between treatment groups (Kruskal-Wallis rank sum test, p < 0.05), while shared letters indicate no significant difference (LA+OSU, Lactobacillus acidophilus + OSU PRV; LGG+OSU, Lactobacillus rhamnosus + OSU PRV; OSU, OSU PRV alone).
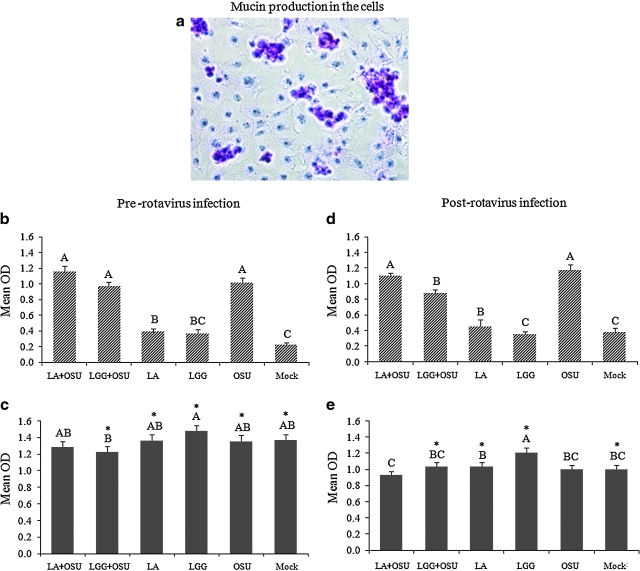
Mucin production in IPEC-J2 cells and the effect of rotavirus and probiotics on MUC3 mucin production and secretion
Mucin production was abundant in IPEC-J2 cells (Fig. 3a). To evaluate the effect of rotavirus and probiotics on mucin production and secretion, monolayers of IPEC-J2 cells were treated using the two protocols described above. MUC3 mucin concentrations in the cell culture supernatants before (Fig. 3b and 3d) and after a freezing and thawing cycle (Fig. 3c and 3e) were measured by ELISA and are presented as mean OD values. Before freezing and thawing, rotavirus-infected cell supernatants had significantly higher MUC3 mucin levels (secreted forms) compared to mock-treated and cells treated with LA or LGG alone (Fig. 3b and 3d). LA and LGG treatment pre-rotavirus infection, or LA treatment post-rotavirus infection, did not significantly alter the MUC3 mucin levels induced by OSU PRV infection, as the OD values were similar in the LA + OSU and LGG + OSU groups compared to the OSU group (Fig. 3b and 3d). Notably, however, LGG treatment post-rotavirus infection significantly decreased the MUC3 mucin level induced by OSU PRV (Fig. 3d).
FIG. 3.
Mucin production and the effect of LA and LGG on MUC3 mucin production in IPEC-J2 cells. (a) Mucin production (purple clusters) in IPEC-J2 cells was detected by PAS staining (original magnification 200 ×). (b and d) MUC3 mucin concentrations in the supernatants (secreted mucin), and (c and e) frozen and thawed cells along with the supernatants (total mucin) were measured by ELISA. Mean ELISA OD values of 5–10 independent experiments for each treatment group are presented. Error bars indicate the standard error of the mean. The capital letters (A, B, C) indicate the results of significance testing for the difference between treatments. Unshared letters indicate significant difference between treatment groups (Kruskal-Wallis rank sum test, p < 0.05), while shared letters indicate no significant difference Asterisks indicate significant difference between secreted and total mucin concentrations. (LA+OSU, Lactobacillus acidophilus + OSU PRV; LGG+OSU, Lactobacillus rhamnosus + OSU PRV; OSU, OSU PRV alone; Mock, mock infected). (Color image is available online at www.liebertonline.com/vim.)
Using another set of samples, we also froze and thawed the cells along with supernatants to release the membrane-associated mucin, and then collected the supernatants for measuring the total mucin concentration. Freezing and thawing significantly increased total MUC3 mucin levels in all treatment groups, except for the LA + OSU pre- and post-rotavirus infection groups, and the OSU alone post-rotavirus infection group (Fig. 3c and 3e). The increases were greatest in the mock, LA-alone and LGG-alone groups both pre- and post-rotavirus infection (Fig. 3b–e). Total mucin levels in the LGG-alone group were higher (pre-rotavirus infection), or significantly higher (post-rotavirus infection), compared to all other groups (Fig. 3c and 3e). Comparison of concentrations between secreted mucin and total mucin suggests that LGG treatment increased the production of membrane-associated mucin, but did not increase mucin secretion. On the other hand, OSU infection with or without LA/LGG significantly increased MUC3 mucin secretion, but not total mucin production, because the increase was no longer observed after the freezing and thawing process (Figs. 3b–e).
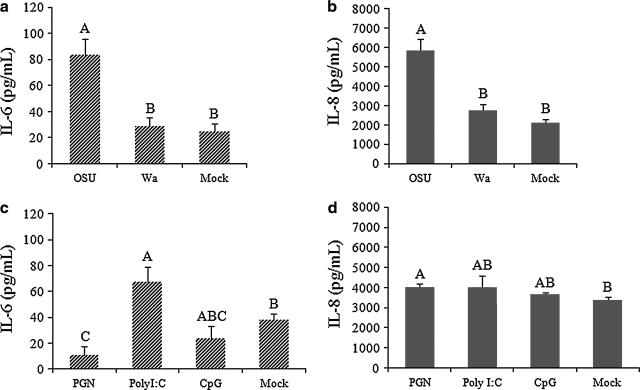
Cytokine/chemokine production in IPEC-J2 cells after infection with rotavirus or stimulation with the TLR agonists PGN, polyI:C, and CpG
Monolayers of IPEC-J2 cells were infected or mock-infected with 20 MOI rotavirus, or stimulated or mock-stimulated with 10 μg/mL of PGN, 20 μg/mL of polyI:C, or 10 μg/mL of CpG, for 24 h. IL-6 and IL-8 concentrations in the supernatants increased significantly after OSU PRV infection, and were significantly higher than those after Wa HRV infection and in the mock-infected cells (Fig. 4a and 4b). The increases in IL-6 and IL-8 production were not significant after Wa HRV infection compared to the mock-stimulated cells (Fig. 4a and 4b). Stimulation of the cells with the TLR2 agonist PGN significantly increased IL-8 production, but decreased the IL-6 production, compared to the mock-stimulated group (Fig. 4c and 4d). The TLR3 agonist polyI:C significantly increased IL-6 production; however, it had no significant effect on IL-8 production (Fig. 4c and 4d). The TLR9 agonist CpG had no significant effect on IL-6 or IL-8 production (Fig. 4a and 4b). The IL-15, TNF-α, and IP-10 concentrations were close to or below the detection limit of the ELISAs. Consequently, the following studies of the effects of probiotic bacteria on cytokine responses were focused on IL-6 and IL-8 induced by OSU PRV.
FIG. 4.
IL-6 and IL-8 responses in IPEC-J2 cells infected with rotavirus, or stimulated with PGN, polyI:C, or CpG. Concentrations of IL-6 and IL-8 in the cell supernatants were detected by ELISA. Mean cytokine concentrations from several independent experiments are presented (n = 14 in a and b; n = 4 in c and d). Error bars indicate the standard error of the mean. The capital letters (A, B, C) indicate the results of significance testing for the difference between treatments. Unshared letters indicate significant difference between treatment groups (Kruskal-Wallis rank sum test, p < 0.05), while shared letters indicate no significant difference.
The effect of probiotics on IL-6 and IL-8 responses induced by rotavirus in IPEC-J2 cells
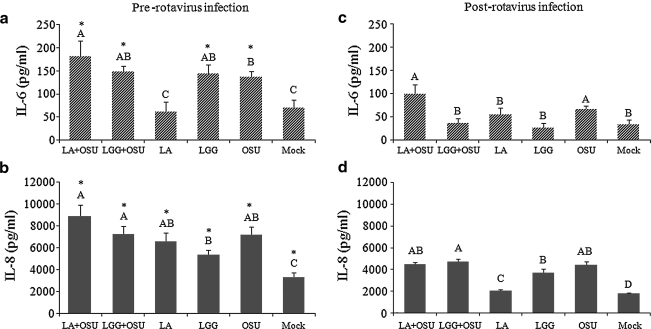
To determine the effect of the probiotic bacteria LA and LGG on IL-6 and IL-8 responses in small intestinal epithelial cells, monolayers of IPEC-J2 cells were treated with the two pre- and post-rotavirus infection protocols. Treatment of the cells for 24 h with LA, but not LGG, followed by OSU PRV infection (LA + OSU), significantly increased the IL-6 response compared to OSU PRV infection alone (Fig. 5a), indicating that LA primed the cells for a higher IL-6 response. However, treatment of rotavirus-infected cells with LGG (LGG + OSU) significantly decreased the IL-6 response compared to OSU PRV infection alone (Fig. 5c), indicating that LGG had an anti-inflammatory effect. LA alone did not significantly alter the IL-6 response in the cells (Fig. 5a and 5c), but LGG alone induced a significantly higher IL-6 response than mock-stimulated cells when the cells were incubated with LGG for 24 h and then continually incubated for 24 more hours after the LGG was removed by washing with PBS (Fig. 5a). LA and LGG treatment alone, OSU PRV infection alone, or the two combined (LA + OSU and LGG + OSU), induced significantly higher IL-8 production than the mock-stimulated group. LA or LGG did not change the IL-8 concentration induced by OSU PRV infection (Fig. 5b and 5d). LA and LGG treatment prior to rotavirus infection induced overall significantly higher IL-8 and IL-6 responses than those of rotavirus infection prior to probiotic treatment (Fig. 5), except for IL-6 in the LA and mock groups (Fig. 5a and 5c).
FIG. 5.
The effect of LA and LGG on IL-6 and IL-8 responses in IPEC-J2 cells. Mean cytokine concentrations of 6–10 independent experiments are presented. Error bars indicate the standard error of the mean. The capital letters (A, B, C, D) indicate the results of significance testing for the difference between treatments. Unshared letters indicate significant difference between treatment groups (Kruskal-Wallis rank sum test, p < 0.05), while shared letters indicate no significant difference. (LA+OSU, Lactobacillus acidophilus + OSU PRV; LGG+OSU, Lactobacillus rhamnosus + OSU PRV; LA, LA alone; LGG, LGG alone; OSU, OSU PRV alone; Mock, mock infected). Asterisks indicate significant difference between pre- and post-rotavirus infection.
The effect of probiotics on TLR2, TLR3, and TLR9 expression in IPEC-J2 cells
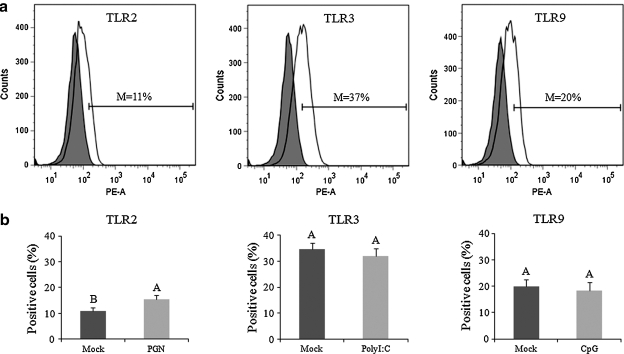
TLR2, TLR3, and TLR9 expression protein levels in IPEC-J2 cells were evaluated by flow cytometry. The mean frequencies of TLR2, TLR3, and TLR9 expression in mock-stimulated IPEC-J2 cells were about 11%, 37%, and 20%, respectively (Fig. 6a). PGN significantly increased the TL2 expression in the cells; however, polyI:C and CpG had no significant effect on TLR3 and TLR9 expression (Fig. 6b).
FIG. 6.
TLR2, TLR3, and TLR9 expression on IPEC-J2 cells. The PGN-, polyI:C-, or CpG-stimulated or mock-stimulated cells were labeled with TLR2, TLR3, or TLR9 antibodies, respectively (white histograms in a), and assayed by flow cytometry. Cells labeled with isotype-matched irrelevant antibodies served as background controls (shaded histograms in a). Histogram plots were generated by gating on live single cells. Data presented in a and b are the means of percentages of TLR-positive cells minus the percentages of non-specific staining in isotype-matched background controls. The histograms in 6a are representative of 9–10 independent experiments; 6b presents the means of 9–10 independent experiments. Error bars indicate the standard error of the mean. The capital letters (A and B) indicate the results of significance testing for the difference between treatments. Unshared letters indicate significant difference between treatment groups (Kruskal-Wallis rank sum test, p < 0.05), while shared letters indicate no significant difference (M, mean frequencies).
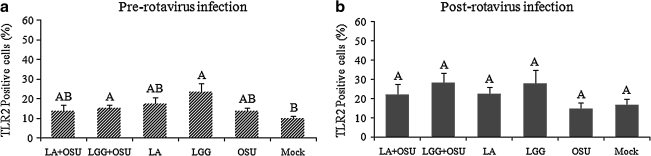
To evaluate the effects of probiotics on the expression of TLR2, TLR3, and TLR9 in the cells, monolayers of IPEC-J2 cells were treated using the two protocols. As expected, OSU PRV had no significant effect on TLR2 expression on the cells (Fig. 7). When the cells were pre-treated with LGG, but not LA, for 24 h, and then infected with OSU PRV or mock-infected, TLR2 expression was significantly increased (Fig. 7a). In contrast, LA or LGG treatment following rotavirus infection or mock infection had no significant effect on TLR2 expression (Fig. 7b). There was a trend that LGG induced higher TLR2 expression than other treatment groups, but it was not statistically significant (Fig. 7b). OSU PRV infection had no significant effect on TLR3 and TLR9 expression in the cells, and LA and LGG treatment did not alter TLR3 and TLR9 expression in the cells, with or without OSU PRV infection (data not shown). We also measured the effect of Wa HRV on TLR2, TLR3, and TLR9 expression, and the effect of LA and LGG on TLR2, TLR3, and TLR9 expression in Wa HRV-infected cells. The results were the same as for the OSU PRV-infected cells (data not shown).
FIG. 7.
The effect of probiotics on TLR2 expression by IPEC-J2 cells. Mean frequencies of each treatment group from 5–10 independent experiments are presented. Error bars indicate the standard error of the mean. The capital letters (A and B) indicate the results of significance testing for the difference between treatments. Unshared letters indicate significant difference between treatment groups (Kruskal-Wallis rank sum test, p < 0.05), while shared letters indicate no significant difference (LA+OSU, Lactobacillus acidophilus + OSU PRV; LGG+OSU, Lactobacillus rhamnosus + OSU PRV; LA, LA alone; LGG, LGG alone; OSU, OSU PRV alone; Mock, mock infected).
The effect of probiotic bacterial total DNA on TLR2 and TLR9 expression by IPEC-J2 cells
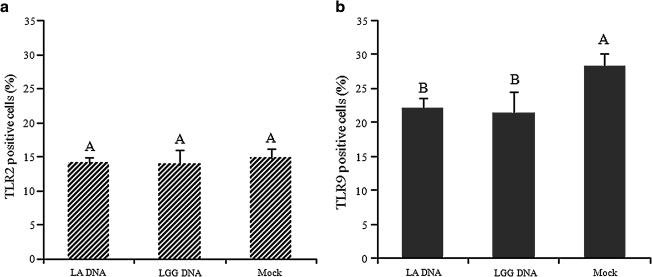
To determine if probiotics exert their effects via stimulating TLR2 with PGN in the bacterial cell wall, or by stimulating TLR9 with CpG from the genome, we used 10 μg/mL of the total DNA purified from LA or LGG to stimulate the IPEC-J2 cell monolayers for 24 h, then analyzed TLR2 and TLR9 expression by flow cytometry. As expected, DNA from LA and LGG had no significant effect on TLR2 expression (Fig. 8a). Surprisingly, however, they significantly decreased TLR9 expression in the cells (Fig. 8b), suggesting a regulatory effect of probiotic DNA via the TLR9 pathway.
FIG. 8.
The effect of probiotic genome DNA on TLR2 and TLR9 expression by IPEC-J2 cells. The cells stimulated or mock-stimulated with total DNA from LA or LGG were collected for detection of TLR2 and TLR9 expression by flow cytometry. Fig. 8a presents the mean of three independent experiments, and Fig. 8b presents the mean of six independent experiments. Error bars indicate the standard error of the mean. The capital letters (A and B) indicate the results of significance testing for the difference between treatments. Unshared letters indicate significant difference between treatment groups (Kruskal-Wallis rank sum test, p < 0.05), while shared letters indicate no significant difference.
Discussion
The IPEC-J2 cell line, a non-transformed, non-tumorigenic small intestinal cell line, can secrete mucin, produce cytokines/chemokines, and express TLRs similar to those of the original tissues, thus it conserves its epithelial nature and can serve as a convenient model to simulate innate immune functions of the intestinal epithelium. An increasing number of studies have used IPEC-J2 cells as models for studying infective processes of enteric pathogens in porcine intestinal epithelium (1,8–10,27,35,49,51); however, most of those studies were on bacterial pathogens. Only one study used the IPEC-J2 cell line as a model to study interactions among vesicular stomatitis virus (VSV), host, and probiotic bacteria. Also, the study focused on the antiviral activity of probiotic bacteria (8). In the present study, we established for the first time the IPEC-J2 cell line model of rotavirus infection. Our objective was to use the cell line as an in-vitro model system to investigate the effect of the probiotic bacteria LA and LGG on virus infectivity and the innate immune responses to rotaviruses.
We first demonstrated and compared the replication capacity of homologous PRV and heterologous HRV in IPEC-J2 cells. Although neither OSU PRV nor Wa HRV induced a significant cytopathic effect on the cells, homologous OSU PRV was able to replicate in the cells to a higher titer. The virus titers of OSU PRV in the supernatants of infected IPEC-J2 cells reached 106–107 FFU/mL and the virus liters is 104–105 FFU/mL in the Wa HRV-infected cells. Both the OSU PRV and Wa HRV are well adapted to cell culture in MA104 cells (derived from embryonic kidney of the African green monkey), and the OSU PRV grows to a slightly higher titer in MA104 cells than does Wa HRV (2.1 × 108 FFU/mL versus 9.1 × 107 FFU/mL). Therefore the significantly different infectivity of OSU PRV versus Wa HRV in IPEC-J2 cells is likely a reflection of host specificity. The establishment of this rotavirus infection model of the IPEC-J2 cell line thus may provide an excellent opportunity for studying the mechanisms behind host-restriction of rotavirus infection. In addition, the IPEC-J2 cell line infected with PRV presents a completely homologous and virtually optimal system for investigating rotavirus replication in vitro.
To assess the potential prophylactic or therapeutic effect of the probiotic bacteria LA and LGG on rotavirus infectivity in intestinal epithelial cells, we treated IPEC-J2 cells with two protocols: probiotic treatment prior to rotavirus infection (pre-rotavirus infection), and probiotic treatment after rotavirus infection (post-rotavirus infection). It was reported that the reduction of VSV infectivity in IPEC-J2 cells by probiotics was independent of the virus titer, but was dependent on the probiotic dose and incubation time (8). A dose of 105 CFU/mL of probiotic bacteria was needed to exert an antiviral effect, with the maximal effect seen at 108 CFU/mL (8). However, we found that higher-dose LA (≥109 CFU/mL) or LGG (≥107 CFU/mL) can damage the integrity of cell monolayers due to reductions in the pH of the media over the incubation period. Long incubation times (48 h) with 108 CFU/mL of LA or 106 CFU/mL of LGG, particularly LGG, also damaged the integrity of the monolayers for the same reason. Thus we used 108 CFU/mL of LA, 106 CFU/mL of LGG, and 20 MOI of rotavirus, and incubated the cells with rotavirus or probiotics for 24 h in our study. In our system, probiotics did not show any direct anti-rotavirus effect. On the contrary, we found that LA treatment significantly increased the amount of OSU PRV antigens and virus titers (pre-rotavirus infection) in the cell culture supernatants. This finding, although surprising, is in agreement with in-vivo results from gnotobiotic pigs. In two earlier studies, we found that neonatal gnotobiotic pigs inoculated with the virulent Wa HRV and fed with LA plus L. reuteri, or inoculated with the attenuated Wa HRV and fed with LA, shed higher titers of the viruses in feces than the pigs not receiving lactobacilli feeding (Yuan unpublished data, 68). The mean peak virus titers shed by the virulent and attenuated Wa HRV-inoculated and lactobacilli-fed pigs were 6.4 × 104 and 3.9 × 103 FFU/mL, respectively, versus those in the virus-inoculated pigs without lactobacilli, which were 3.3 × 104 and 1 × 103 FFU/mL, respectively. Although the differences (a 1.9-fold and a 3.9-fold increase, respectively) were not statistically significant, the trend is consistent between the virulent and attenuated Wa HRV in gnotobiotic pigs in vivo, and it corroborates the effect of LA on OSU PRV infection in IPEC-J2 cells in vitro. The mechanism behind the observed enhancement of rotavirus replication by LA is unknown, and further studies are needed. Nevertheless, the enhanced virus replication seen in gnotobiotic pigs may have contributed to the increased immunogenicity of the attenuated Wa HRV vaccine in pigs fed with LA (68). In other words, LA given as an adjuvant for the live attenuated HRV vaccine may have increased the amount of the vaccine antigens available to the gut-associated lymphoid tissues (GALT).
It has been demonstrated using PAS staining of the cell membrane and glycocalyx-bound layers that the IPEC-J2 cells produce mucins (49). It was also confirmed using RT-PCR that the mucin was not MUC2 mucin (49). MUC2 mucin is expressed by goblet cells, but no goblet cells were detected among the IPEC-J2 cells. MUC3 mucin is expressed by both goblet cells and intestinal epithelial cells (14,32,60). Thus the mucin produced by the IPEC-J2 cells was implied, but not confirmed, to be MUC3 mucin (49). We confirmed using PAS staining in the present study that IPEC-J2 cells produce abundant mucins (neutral mucopolysaccharides) in normal culture conditions. More importantly, we directly confirmed that the mucin produced by IPEC-J2 cells is MUC3 mucin using an anti-human MUC3 mucin monoclonal antibody, which does not cross-react with MUC2 mucin in an ELISA assay.
In our study, LGG treatment increased the total MUC3 mucin levels. This finding agrees with the previous report that LGG stimulated the increase of MUC3 mucin mRNA expression by HT-29 cells (32). However, LGG did not increase the extracellular secretion of MUC3 mucin in IPEC-J2 cells. On the other hand, LGG significantly decreased the mucin secretion induced by PRV infection, suggesting that LGG exerts a protective effect on the epithelial cells by maintaining and increasing membrane-bound forms of MUC3 mucin, which can function to reduce the adherence of rotavirus particles to the epithelial cells.
In the present study, LGG downregulated OSU PRV-induced IL-6; LGG also inhibited IL-8 induced by IL-1β (16) and TNF-α (65). IL-6 and IL-8 responses in intestinal epithelial cells play important roles in the pathogenesis and immune defense against enteric pathogens. We compared IL-6 and IL-8 production after homologous OSU PRV or heterogeneous Wa HRV infection of IPEC-J2 cells. We found that IL-6 and IL-8 concentrations were significantly increased after OSU PRV infection, and were significantly higher than those seen after the Wa HRV infection. The IL-6 and IL-8 production did not differ in Wa HRV-infected IPEC-J2 cells compared to the mock controls, likely due to the low infectivity of Wa HRV. IL-15, TNF-α, and IP-10 also are very important cytokines/chemokines involved in rotavirus infection (45,48,50,69). However, in IPEC-J2 cells, IL-15, TNF-α, and IP-10 concentrations were near or below the detection limit of the ELISA assays. Our pilot study of the IL-15, TNF-α, and IP-10 mRNA by RT-PCR (data not shown), and a published report (36), indicated that IPEC-J2 cells do not express IL-15 mRNA. Thus we mainly focused on IL-6 and IL-8 in the experiments investigating the effect of probiotics on cytokine/chemokine responses induced by rotavirus. We found that LA and LGG had significant and differential influence on the innate cytokine IL-6 production in OSU PRV-infected IPEC-J2 cells. LA increased the IL-6 response to rotavirus infection, which is consistent with the immunostimulatory effect of LA on B- and T-cell immune responses (68). It suggests that LA primed the intestinal epithelial cell for a higher immune response upon rotavirus infection. On the other hand, LGG significantly decreased the IL-6 production by the IPEC-J2 cells, suggesting an immunoregulatory effect of this lactobacillus strain. Treatment of normal IPEC-J2 cells with LGG enhanced the IL-6 production (Fig. 5a), whereas LGG treatment of rotavirus-infected cells reduced the IL-6 response to rotavirus (Fig. 5c). LA plus LGG also decreased the production of IL-6 (data not shown). These observations support the findings that LGG has differential regulatory and stimulatory effects in hosts with different immune status (44). A number of studies have documented that LGG has significant anti-inflammatory effects. LGG suppressed E. coli- and Bacteroides ovatus-induced proinflammatory cytokine responses in primary murine colonic epithelial cells (30). LGG downregulated LPS-induced proinflammatory mediators in rat (66), and E. coli-induced IL-6 production in pigs (67). Clinical studies showed that LGG upregulated the Th-1 response, including serum IL-6 levels in infants with IgE-associated atopic eczema-dermatitis syndrome (Th-2-biased immune status) (59), whereas it downregulated the inflammatory response in milk-hypersensitive subjects (over-reactive phagocytes) (44). In-vitro studies of dendritic cells showed that LGG is a weak inducer of Th-1 cytokines, but when combined with strong inducers (e.g., LA), it suppressed the production of Th-1 cytokines (64). Taken together, these findings suggest that LGG functions to maintain the homeostasis of a balanced, healthy immune system.
Epithelial TLR expression has been described as being fundamental in the host defense against pathogenic challenges and linking the innate and adaptive immune responses (1). TLR2, TLR3, and TLR9 mRNA expression have been confirmed in the IPEC-J2 cells (1,10,36). For the first time, we determined the TLR2, TLR3, and TLR9 expression in protein levels in the cells by flow cytometry. One potential mechanism for the effect of gram-positive probiotic bacteria on intestinal epithelial cell responses to rotavirus infection is to regulate TLR expression. In the present study, PGN significantly increased the TLR2 expression in the cells; however, polyI:C and CpG had no significant effect on TLR3 and TLR9 expression. In agreement with this unresponsiveness, TLR9 is known to be stably expressed in human and porcine intestinal epithelial cells; however, it is unresponsive to CpG stimulation (39,43). We also confirmed that stimulation of IPEC-J2 cells with the TLR2 agonist PGN significantly increased IL-8 production, but significantly decreased IL-6 production. The TLR3 agonist polyI:C significantly increased IL-6 production. Taken together, this suggests that stimulation of TLR2 by probiotics has an anti-inflammatory effect, whereas stimulation of TLR3 has a proinflammatory effect. However, the IL-6 and IL-8 responses to rotavirus are apparently not mediated by TLR3 in IPEC-J2 cells.
In evaluating whether LA and LGG exert their effect by modulating TLR expression in IPEC-J2 cells, we found that LGG, but not LA, treatment, with or without subsequent rotavirus infection, increased the frequencies of TLR2 expression on the cells compared to basal levels. It was reported that commensals exert host defense mechanisms through TLR2 to maintain the barrier integrity and function of intestinal epithelium (12,13). Although LGG treatment post-rotavirus infection did not significantly increase the TLR2 expression on the infected cells or on the mock-infected cells, there was a clear trend toward higher TLR2 expression in the LGG-treated cells than the basal levels. These observations suggest that LGG may confer protection to the intestinal epithelial barrier via the TLR2 pathway.
It was known in human endometrial epithelial cells that TLR3 responds to dsRNA stimulation and initiates production of proinflammatory and antiviral cytokines (26,55); however, in our study, TLR3 expression on IPEC-J2 cells did not change from basal levels after polyI:C stimulation and rotavirus infection. These data further suggest that IL-6 and/or IL-8 responses induced by rotavirus and polyI:C are not mediated by TLR3 (possibly by RIG-I or MDA5) in IPEC-J2 cells.
Consistent with our findings in IPEC-J2 cells, others also showed that lactobacilli, including LGG, did not significantly change TLR9 expression in protein levels in HT-29 cells (57). Expression of TLR9 was upregulated on HT-29 cells in response to pathogenic bacterial DNA, but not probiotic bacterial Bifidobacterium breve DNA, suggesting that intestinal epithelial cells are capable of distinguishing between probiotic bacterial DNA and pathogenic bacterial DNA (19). In the present study we found that although live LA and LGG did not alter the TLR9 expression in IPEC-J2 cells, the total DNA of LA or LGG significantly decreased the TLR9 expression. This finding indicates that there are different mechanisms used by intestinal epithelial cells to respond to live probiotic bacteria and probiotic bacterial DNA. Indeed, DNA from probiotic bacteria has been shown to enhance intestinal barrier function (34), reduce proinflammatory cytokine secretion, and reduce disease severity in mouse models of colitis (59). Additionally, in mouse models of autoimmunity, deficiency in TLR9 showed a protective effect for the disease (29). Thus, probiotic bacterial DNA may confer protection to intestinal epithelial cells by TLR9 downregulation.
Conclusions
In summary, IPEC-J2 cells were productively infected by PRV. LA and LGG treatment of the cells did not reduce virus replication. PRV infection stimulated increased MUC3 mucin secretion, whereas LGG treatment of rotavirus-infected cells reduced the mucin secretion response induced by PRV. LGG treatment alone increased the production of membrane-bound MUC3 mucin, but not mucin secretion. LA treatment prior to rotavirus infection significantly increased the IL-6 response to rotavirus infection; LGG treatment post-rotavirus infection downregulated the IL-6 response. IPEC-J2 cells expressed TLR2, TLR3, and TLR9 constitutively. TLR2 expression was upregulated by LGG and PGN, corresponding to the increased IL-8 and decreased IL-6 responses, which suggests a mechanism of regulating TLR2 expression on intestinal epithelial cells by probiotics to achieve protective effects. The total DNA of LA or LGG also significantly decreased the TLR9 expression; thus probiotic bacterial DNA may confer protection to intestinal epithelial cells by TLR9 downregulation. Further studies are needed to confirm the differential effect of live probiotics and purified DNA from probiotic bacteria. In conclusion, the IPEC-J2 cell line is a valuable model for studying the interactions among rotavirus-host-probiotics. The results of this study improved our understanding of the mechanisms behind the immunomodulating effect of probiotic bacteria on epithelial cell innate immune responses, via use of a completely homologous system of porcine small intestinal epithelial cells infected with a porcine rotavirus.
Acknowledgments
This work was partially supported by a grant from the National Institutes of Health (1R01AT004789-01), and the start-up fund to L. Yuan from Virginia Tech. F. Liu is supported by the International Graduate Research Program, China Scholarship Council, P.R. China.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Arce C. Ramirez-Boo M. Lucena C. Garrido JJ. Innate immune activation of swine intestinal epithelial cell lines (IPEC-J2, IPI-2I) in response to LPS from Salmonella typhimurium. Comp Immunol Microbiol Infect Dis. doi: 10.1016/j.cimid.2008.1008.1003. [DOI] [PubMed]
- 2.Azevedo MS. Yuan L. Pouly S. Gonzales AM. Jeong KI. Nguyen TV. Saif LJ. Cytokine responses in gnotobiotic pigs after infection with virulent or attenuated human rotavirus. J Virol. 2006;80:372–382. doi: 10.1128/JVI.80.1.372-382.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azim T. Zaki MH. Podder G, et al. Rotavirus-specific subclass antibody and cytokine responses in Bangladeshi children with rotavirus diarrhoea. J Med Virol. 2003;69:286–295. doi: 10.1002/jmv.10280. [DOI] [PubMed] [Google Scholar]
- 4.Bohl EH. Theil KW. Saif LJ. Isolation and serotyping of porcine rotaviruses and antigenic comparison with other rotaviruses. J Clin Microbiol. 1984;19:105–111. doi: 10.1128/jcm.19.2.105-111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borchers AT. Selmi C. Meyers FJ. Keen CL. Gershwin ME. Probiotics and immunity. J Gastroenterol. 2009;44:26–46. doi: 10.1007/s00535-008-2296-0. [DOI] [PubMed] [Google Scholar]
- 6.Boshuizen JA. Reimerink JH. Korteland-Van Male AM, et al. Homeostasis and function of goblet cells during rotavirus infection in mice. Virology. 2005;337:210–221. doi: 10.1016/j.virol.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 7.Boshuizen JA. Reimerink JH. Korteland-Van Male AM, et al. Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice. J Virol. 2003;77:13005–13016. doi: 10.1128/JVI.77.24.13005-13016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Botic T. Klingberg TD. Weingartl H. Cencic A. A novel eukaryotic cell culture model to study antiviral activity of potential probiotic bacteria. Int J Food Microbiol. 2007;115:227–234. doi: 10.1016/j.ijfoodmicro.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 9.Brown DR. Price LD. Characterization of Salmonella enterica serovar Typhimurium DT104 invasion in an epithelial cell line (IPEC J2) from porcine small intestine. Vet Microbiol. 2007;120:328–333. doi: 10.1016/j.vetmic.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burkey TE. Skjolaas KA. Dritz SS. Minton JE. Expression of porcine Toll-like receptor 2, 4 and 9 gene transcripts in the presence of lipopolysaccharide and Salmonella enterica serovars Typhimurium and Choleraesuis. Vet Immunol Immunopathol. 2009;130:96–101. doi: 10.1016/j.vetimm.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Caballero-Franco C. Keller K. De Simone C. Chadee K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G315–G322. doi: 10.1152/ajpgi.00265.2006. [DOI] [PubMed] [Google Scholar]
- 12.Cario E. Barrier-protective function of intestinal epithelial Toll-like receptor 2. Mucosal Immunol. 2008;1(Suppl 1):S62–S66. doi: 10.1038/mi.2008.47. [DOI] [PubMed] [Google Scholar]
- 13.Cario E. Gerken G. Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 14.Chang SK. Dohrman AF. Basbaum CB, et al. Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer. Gastroenterology. 1994;107:28–36. doi: 10.1016/0016-5085(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 15.Chen CC. Baylor M. Bass DM. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology. 1993;105:84–92. doi: 10.1016/0016-5085(93)90013-3. [DOI] [PubMed] [Google Scholar]
- 16.Choi CH. Kim TI. Lee SK. Yang KM. Kim WH. Effect of Lactobacillus GG and conditioned media on IL-1beta-induced IL-8 production in Caco-2 cells. Scand J Gastroenterol. 2008;43:938–947. doi: 10.1080/00365520801965373. [DOI] [PubMed] [Google Scholar]
- 17.De Vrese M. Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- 18.Didierlaurent A. Sirard JC. Kraehenbuhl JP. Neutra MR. How the gut senses its content. Cell Microbiol. 2002;4:61–72. doi: 10.1046/j.1462-5822.2002.00177.x. [DOI] [PubMed] [Google Scholar]
- 19.Ewaschuk JB. Backer JL. Churchill TA. Obermeier F. Krause DO. Madsen KL. Surface expression of Toll-like receptor 9 is upregulated on intestinal epithelial cells in response to pathogenic bacterial DNA. Infect Immun. 2007;75:2572–2579. doi: 10.1128/IAI.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guandalini S. Probiotics for children: use in diarrhea. J Clin Gastroenterol. 2006;40:244–248. doi: 10.1097/00004836-200603000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Guandalini S. Probiotics for children with diarrhea: an update. J Clin Gastroenterol. 2008;42(Suppl 2):S53–S57. doi: 10.1097/MCG.0b013e3181674087. [DOI] [PubMed] [Google Scholar]
- 22.Guandalini S. Pensabene L. Zikri MA, et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30:54–60. doi: 10.1097/00005176-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Guarino A. Canani RB. Spagnuolo MI. Albano F. Di Benedetto L. Oral bacterial therapy reduces the duration of symptoms and of viral excretion in children with mild diarrhea. J Pediatr Gastroenterol Nutr. 1997;25:516–519. doi: 10.1097/00005176-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Isolauri E. Juntunen M. Rautanen T. Sillanaukee P. Koivula T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics. 1991;88:90–97. [PubMed] [Google Scholar]
- 25.Jiang B. Snipes-Magaldi L. Dennehy P, et al. Cytokines as mediators for or effectors against rotavirus disease in children. Clin Diagn Lab Immunol. 2003;10:995–1001. doi: 10.1128/CDLI.10.6.995-1001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorgenson RL. Young SL. Lesmeister MJ. Lyddon TD. Misfeldt ML. Human endometrial epithelial cells cyclically express Toll-like receptor 3 (TLR3) and exhibit TLR3-dependent responses to dsRNA. Hum Immunol. 2005;66:469–482. doi: 10.1016/j.humimm.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koh SY. George S. Brozel V. Moxley R. Francis D. Kaushik RS. Porcine intestinal epithelial cell lines as a new in vitro model for studying adherence and pathogenesis of enterotoxigenic Escherichia coli. Vet Microbiol. 2008;130:191–197. doi: 10.1016/j.vetmic.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Kopp E. Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 29.Kumagai Y. Takeuchi O. Akira S. TLR9 as a key receptor for the recognition of DNA. Adv Drug Deliv Rev. 2008;60:795–804. doi: 10.1016/j.addr.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Lan JG. Cruickshank SM. Singh JC. Farrar M. Lodge JP. Felsburg PJ. Carding SR. Different cytokine response of primary colonic epithelial cells to commensal bacteria. World J Gastroenterol. 2005;11:3375–3384. doi: 10.3748/wjg.v11.i22.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lievin-Le Moal V. Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315–337. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mack DR. Ahrne S. Hyde L. Wei S. Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52:827–833. doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mack DR. Michail S. Wei S. McDougall L. Hollingsworth MA. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol. 1999;276:G941–G950. doi: 10.1152/ajpgi.1999.276.4.G941. [DOI] [PubMed] [Google Scholar]
- 34.Madsen K. Cornish A. Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 35.Manta S. Agelis G. Botic T. Cencic A. Komiotis D. Fluoro-ketopyranosyl nucleosides: synthesis and biological evaluation of 3-fluoro-2-keto-beta-D-glucopyranosyl derivatives of N4-benzoyl cytosine. Bioorg Med Chem. 2007;15:980–987. doi: 10.1016/j.bmc.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 36.Mariani V. Palermo S. Fiorentini S. Lanubile A. Giuffra E. Gene expression study of two widely used pig intestinal epithelial cell lines: IPEC-J2, IPI-2I. Vet Immunol Immunopathol. doi:10.1016/j.vetimm.2009.1004.1006. [DOI] [PubMed]
- 37.Mattar AF. Teitelbaum DH. Drongowski RA. Yongyi F. Harmon CM. Coran AG. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr Surg Int. 2002;18:586–590. doi: 10.1007/s00383-002-0855-7. [DOI] [PubMed] [Google Scholar]
- 38.Moncada DM. Kammanadiminti SJ. Chadee K. Mucin and Toll-like receptors in host defense against intestinal parasites. Trends Parasitol. 2003;19:305–311. doi: 10.1016/s1471-4922(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 39.Moue M. Tohno M. Shimazu T. Kido T. Aso H. Saito T. Kitazawa H. Toll-like receptor 4 and cytokine expression involved in functional immune response in an originally established porcine intestinal epitheliocyte cell line. Biochim Biophys Acta. 2008;1780:134–144. doi: 10.1016/j.bbagen.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Netea MG. Van Der Meer JW. Kullberg BJ. Toll-like receptors as an escape mechanism from the host defense. Trends Microbiol. 2004;12:484–488. doi: 10.1016/j.tim.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Oswald IP. Role of intestinal epithelial cells in the innate immune defence of the pig intestine. Vet Res. 2006;37:359–368. doi: 10.1051/vetres:2006006. [DOI] [PubMed] [Google Scholar]
- 42.Parashar UD. Gibson CJ. Bresse JS. Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedersen G. Andresen L. Matthiessen MW. Rask-Madsen J. Brynskov J. Expression of Toll-like receptor 9 and response to bacterial CpG oligodeoxynucleotides in human intestinal epithelium. Clin Exp Immunol. 2005;141:298–306. doi: 10.1111/j.1365-2249.2005.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pelto L. Isolauri E. Lilius EM. Nuutila J. Salminen S. Probiotic bacteria down-regulate the milk-induced inflammatory response in milk-hypersensitive subjects but have an immunostimulatory effect in healthy subjects. Clin Exp Allergy. 1998;28:1474–1479. doi: 10.1046/j.1365-2222.1998.00449.x. [DOI] [PubMed] [Google Scholar]
- 45.Rollo EE. Kumar KP. Reich NC, et al. The epithelial cell response to rotavirus infection. J Immunol. 1999;163:4442–4452. [PubMed] [Google Scholar]
- 46.Rosenfeldt V. Michaelsen K. Jakobsen M, et al. Effect of probiotic Lactobacillus strains in young children hospitalized with acute diarrhea. Pediatr Infect Dis J. 2002;21:411–416. doi: 10.1097/00006454-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Sanders ME. Klaenhammer TR. Invited review: the scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J Dairy Sci. 2001;84:319–331. doi: 10.3168/jds.S0022-0302(01)74481-5. [DOI] [PubMed] [Google Scholar]
- 48.Sato A. Iizuka M. Nakagomi O, et al. Rotavirus double-stranded RNA induces apoptosis and diminishes wound repair in rat intestinal epithelial cells. J Gastroenterol Hepatol. 2006;21:521–530. doi: 10.1111/j.1440-1746.2005.03977.x. [DOI] [PubMed] [Google Scholar]
- 49.Schierack P. Nordhoff M. Pollmann M, et al. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem Cell Biol. 2006;125:293–305. doi: 10.1007/s00418-005-0067-z. [DOI] [PubMed] [Google Scholar]
- 50.Sheth R. Anderson J. Sato T, et al. Rotavirus stimulates IL-8 secretion from cultured epithelial cells. Virology. 1996;221:251–259. doi: 10.1006/viro.1996.0374. [DOI] [PubMed] [Google Scholar]
- 51.Skjolaas KA. Burkey TE. Dritz SS. Minton JE. Effects of Salmonella enterica serovar Typhimurium, or serovar Choleraesuis, Lactobacillus reuteri and Bacillus licheniformis on chemokine and cytokine expression in the swine jejunal epithelial cell line, IPEC-J2. Vet Immunol Immunopathol. 2007;115:299–308. doi: 10.1016/j.vetimm.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 52.Taima K. Imaizumi T. Yamashita K, et al. Expression of IP-10/CXCL10 is upregulated by double-stranded RNA in BEAS-2B bronchial epithelial cells. Respiration. 2006;73:360–364. doi: 10.1159/000091646. [DOI] [PubMed] [Google Scholar]
- 53.Teran CG. Teran-Escalera CN. Villarroel P. Nitazoxanide vs. probiotics for the treatment of acute rotavirus diarrhea in children: a randomized, single-blind, controlled trial in Bolivian children. Int J Infect Dis. 2009;13:518–523. doi: 10.1016/j.ijid.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 54.Uematsu S. Akira S. Toll-like receptors and Type I interferons. J Biol Chem. 2007;282:15319–15323. doi: 10.1074/jbc.R700009200. [DOI] [PubMed] [Google Scholar]
- 55.Vercammen E. Staal J. Beyaert R. Sensing of viral infection and activation of innate immunity by toll-like receptor 3. Clin Microbiol Rev. 2008;21:13–25. doi: 10.1128/CMR.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vijay-Kumar M. Gentsch JR. Kaiser WJ. Borregaard N. Offermann MK. Neish AS. Gewirtz AT. Protein kinase R mediates intestinal epithelial gene remodeling in response to double-stranded RNA and live rotavirus. J Immunol. 2005;174:6322–6331. doi: 10.4049/jimmunol.174.10.6322. [DOI] [PubMed] [Google Scholar]
- 57.Vizoso Pinto MG. Rodriguez Gomez M. Seifert S. Watzl B. Holzapfel WH. Franz CM. Lactobacilli stimulate the innate immune response and modulate the TLR expression of HT29 intestinal epithelial cells in vitro. Int J Food Microbiol. 2009;133:86–93. doi: 10.1016/j.ijfoodmicro.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 58.Ward LA. Rosen BI. Yuan L. Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77(Pt 7):1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 59.Watson JL. McKay DM. The immunophysiological impact of bacterial CpG DNA on the gut. Clin Chim Acta. 2006;364:1–11. doi: 10.1016/j.cca.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 60.Weiss AA. Babyatsky MW. Ogata S. Chen A. Itzkowitz SH. Expression of MUC2 and MUC3 mRNA in human normal, malignant, and inflammatory intestinal tissues. J Histochem Cytochem. 1996;44:1161–1166. doi: 10.1177/44.10.8813081. [DOI] [PubMed] [Google Scholar]
- 61.Wen K. Azevedo MS. Gonzalez A, et al. Toll-like receptor and innate cytokine responses induced by lactobacilli colonization and human rotavirus infection in gnotobiotic pigs. Vet Immunol Immunopathol. 2009;127:304–315. doi: 10.1016/j.vetimm.2008.10.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yolken RH. Ojeh C. Khatri I. Sajjan U. Forstner JF. Intestinal mucins inhibit rotavirus replication in an oligosaccharide-dependent manner. J Infect Dis. 1994;169:1002–1006. doi: 10.1093/infdis/169.5.1002. [DOI] [PubMed] [Google Scholar]
- 63.Yuan L. Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol. 2002;87:147–160. doi: 10.1016/S0165-2427(02)00046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeuthen LH. Christensen HR. Frokiaer H. Lactic acid bacteria inducing a weak interleukin-12 and tumor necrosis factor alpha response in human dendritic cells inhibit strongly stimulating lactic acid bacteria but act synergistically with gram-negative bacteria. Clin Vaccine Immunol. 2006;13:365–375. doi: 10.1128/CVI.13.3.365-375.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L. Li N. Caicedo R. Neu J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha-induced interleukin-8 production in Caco-2 cells. J Nutr. 2005;135:1752–1756. doi: 10.1093/jn/135.7.1752. [DOI] [PubMed] [Google Scholar]
- 66.Zhang L. Li N. Des Robert C. Fang M. Liboni K. McMahon R. Caicedo RA. Neu J. Lactobacillus rhamnosus GG decreases lipopolysaccharide-induced systemic inflammation in a gastrostomy-fed infant rat model. J Pediatr Gastroenterol Nutr. 2006;42:545–552. doi: 10.1097/01.mpg.0000221905.68781.4a. [DOI] [PubMed] [Google Scholar]
- 67.Zhang L. Xu YQ. Liu HY. Lai T. Ma JL. Wang JF. Zhu YH. Evaluation of Lactobacillus rhamnosus GG using an Escherichia coli K88 model of piglet diarrhoea: Effects on diarrhoea incidence, faecal microflora, immune responses. Vet Microbiol. 141:142–148. doi: 10.1016/j.vetmic.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Zhang W. Azevedo MS. Wen K, et al. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008;26:3655–3661. doi: 10.1016/j.vaccine.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou R. Wei H. Sun R. Tian Z. Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice. J Immunol. 2007;178:4548–4556. doi: 10.4049/jimmunol.178.7.4548. [DOI] [PubMed] [Google Scholar]