Abstract
The protein mammalian target of rapamycin (mTOR) plays a central role in cell growth and proliferation. Excessive mTOR activity is a prominent feature of many neoplasms and hamartoma syndromes, including lymphangioleiomyomatosis (LAM), a destructive lung disease that causes progressive respiratory failure in women. Although pharmacological inhibitors of mTOR should directly target the pathogenesis of these disorders, their clinical efficacy has been suboptimal. Recent scientific findings reviewed here have greatly improved our understanding of mTOR signaling mechanisms, provided new insights into the control of cell growth and proliferation, and facilitated the development of new therapeutic approaches in LAM, as well as other neoplastic disorders that exhibit excessive mTOR activity.
Introduction
Mammalian target of rapamycin (mTOR) is a highly-conserved serine-threonine kinase that plays a central role in the regulation of cell growth and proliferation. In all eukaryotic cells, TOR senses environmental stress or cellular metabolism, and then executes the appropriate anabolic or catabolic cellular responses. Conditions such as hypoxia, low ATP levels, amino acid withdrawal, or glucose deprivation inhibit mTOR activity. The resulting catabolic responses include cell cycle arrest, reduced protein synthesis, atrophy, autophagy, and attenuated mitochondrial respiration. In higher eukaryotes, mTOR can also integrate signals from growth factor-activated signaling pathways.1 The prototypical signaling sequence begins with ligation of the insulin-like growth factor-1 (IGF-1) receptor, the recruitment of insulin receptor substrate-1 (IRS-1), and the activation of phosphatidylinositol-3 kinase (PI3K). Elevated phosphatidylinositol 3-phosphate (PI3P) levels leads to the repression of tuberin, the protein encoded by the tuberous sclerosis complex-2 (TSC2) gene. mTOR can then be activated by the small G-protein Ras homologue enriched in brain (Rheb), which is normally suppressed by TSC2. Therefore, loss of TSC2 or gain of PI3K activity leads to excessive Rheb and mTOR activity, which contribute to the pathogenesis of LAM,2–4 as well as many neoplastic tumors.5–7 Subsequent anabolic responses include the synthesis of oncogenic and angiogenic proteins, ribosomal biogenesis, cell growth, and cell proliferation. Here, we discuss the mechanisms by which abnormalities in mTOR signaling might contribute to the pathogenesis of LAM, and other disorders of abnormal cell proliferation.
The Molecular Organization of mTOR
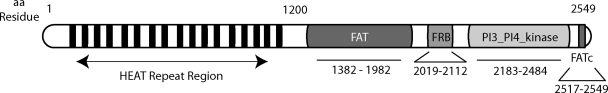
Initially reported in 1994,8–10 mTOR is a ∼240-kDa protein composed of structural domains that permit multiple protein–protein interactions, and the assembly of macromolecular signaling complexes that control anabolic functions11 (Fig. 1). A C-terminal PI3K-like domain contains the serine/threonine kinase active site. The heterogeneity of its target sequences suggests that the mTOR kinase domain directly phosphorylates certain effector substrates, but may control the phosphorylation of others via intermediary kinases or phosphatases.12 Of the few known post-translational modification sites in mTOR, all involve serine/threonine phosphorylation.13,14 The auto-phosphorylation of serine 2481 (S·2481) correlates with mTOR kinase activity, but not necessarily the ability to activate downstream effectors.15 Although it inhibits mTOR signaling, rapamycin does not necessarily reduce mTOR autokinase activity. Novel mTOR inhibitors are currently undergoing characterization.16–18 The phosphorylation of S·2448 also correlates with mTOR kinase activity, as well as with mitogen or nutrient levels.19,20 Phosphorylation of S·1261 in cells exposed to insulin is required for the activation of mTOR, its autophosphorylation at S·2481, and its regulation of cell size.21
FIG. 1.
Structural domains and motifs in mTOR. mTOR consists of 2549 amino acids organized in several structural domains. The N-terminal portion of mTOR contains multiple HEAT (Huntington, Elongation factor 3, A subunit of type 2A protein phosphatase (PP2A), and TOR). The FAT (FRAP, Ataxia telangiectasia mutated (ATM), Transformation/transcription domain associated protein (TRRAP)) and FAT c-terminal (FATc) domains flank the kinase domain, are required for kinase activity, and appear to cooperatively bind certain mTOR-interacting proteins.37 Highly conserved, and homologous to that in ATM and ATR, the c-terminal kinase domain (PI3_PI4_kinase) contains the serine/threonine kinase active site. The FKBP12-rapamycin-binding (FRB) domain binds the rapamycin/FKBP12 complex or phosphatidic acid.143,144
mTOR also contains amino acid residues that mediate its subcellular localization. mTOR nuclear trafficking requires two leucine residues in its HEAT repeat region (Fig. 1); constitutive nuclear localization of mTOR is observed in several tumor cell lines.22,23 A distinct set of residues in the HEAT repeat region determines mTOR localization to endoplasmic reticulum (ER) and/or Golgi membranes.24 The ER may provide a physical platform for mTOR metabolic sensing functions, control of the ER stress response,25 and coordination of mTOR signaling to the translational and transcriptional machinery.26,27 mTOR can also localize to mitochondrial membranes, although the molecular mechanism is unknown.28 mTOR mitochondrial localization was associated with an effect on ATP production and oxygen consumption.29 Furthermore, mTOR directly sensed changes in cell redox state, possibly via highly-conserved cysteine residues in its FATc domain.30,31
The Core mTOR Complexes
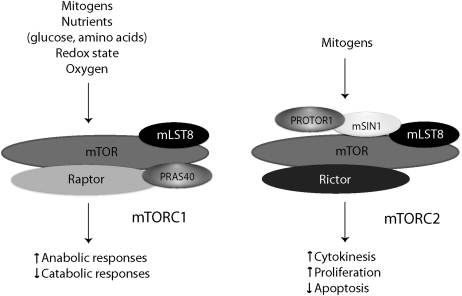
From yeast to mammals, TOR nucleates two distinct macromolecular complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2)32,33 (Fig. 2). mTORC1 is defined by the presence of ‘regulatory associated partner of mTOR’ (raptor). Raptor physically links mTOR to its effectors (e.g., 4E-BP1, p70 S6 kinase (S6K)) by binding their conserved ‘target of rapamycin signaling’ (TOS) motifs.34–36 mTORC1 enhances protein synthesis and ribosomal biogenesis, whereas it suppresses catabolic responses such as autophagy and the induction of cell cycle inhibitor genes.
FIG. 2.
Composition of the mTOR core complexes. mTOR complexes integrate mitogenic and metabolic signals, and control anabolic or catabolic cellular functions as appropriate. mTORC1, as defined by the presence of raptor, is shown to the left of mTORC2, which is defined by its association with rictor. Other adapter proteins that coordinate the functions of mTOR complexes are also shown.
mTORC2 is defined by the presence of ‘rapamycin insensitive companion of mTOR’ (rictor), and regulates cell proliferation, polarity, and cytokinesis by phosphorylating the hydrophobic and/or turn motifs on a subset of ‘cAMP-dependent protein kinase/protein kinase G/protein kinase C’ (AGC kinases; e.g., protein kinase Cα, Akt) kinases.37 Other known mTORC2-specific proteins include mammalian SAPK-interacting protein 1 (mSIN1), and ‘protein observed with rictor-1’ (PROTOR1). Rictor and mSIN1 determine mTORC2 substrate specificity, and are required for the phosphorylation of Akt or PKCα.38–41 PROTOR1, also known as PRR5, appears to regulate platelet-derived growth factor (PDGF) signaling and cellular apoptosis.42–44 Both mTOR complexes contain mLST8 (originally termed GβL), which is essential for the function of mTORC2, and modulates mTORC1 activity in nutrient-sensitive manner.45
Regulated mTORC–Protein Interactions
The small GTPase Rheb is an essential binding partner and activator of mTOR. GTP-bound Rheb stimulates mTORC1 activity. The GDP-bound (inactive) state is favored by the C-terminal GAP activity of tuberin (TSC2), but does not appear to affect the interaction between Rheb and mTOR.46–49 TSC2 is therefore an endogenous suppressor of mTORC1. In contrast, TSC2 appears to positively regulate mTORC2 independent of its GAP activity or Rheb.50,51
Additional mTOR binding partners act as ‘signal input receivers' for the mTOR complexes. The mTORC1 protein ‘proline-rich Akt/PKB Substrate 40’ (PRAS40) contains a TOS (i.e., raptor-binding) motif, and appears to be a bone fide mTORC1 substrate.52,53 However, PRAS40 also constitutively binds and inhibits mTORC1, dissociating from the complex in cells exposed to insulin or nutrients.43,54,55 The integral ER membrane protein FKBP38 constitutively inhibits mTORC1 activity by blocking the interaction between mTOR and Rheb.56–58 FKBP38 dissociates from Rheb in cells exposed to mitogens, and thus facilitates the activation of mTOR. Phospholipase D (PLD), a mitogen-activated Rheb- and mTOR-binding protein, catalyzes the synthesis of phosphatidic acid, which directly activates mTOR via binding to the FRB domain.59–61 The rapamycin/FKBP12 complex might inhibits mTOR in part by competing for the phosphatidic acid-binding site in the FRB domain.62 Ras-related GTP binding (Rag) GTPases were also recently identified as mTOR-associated proteins.63 Rag heterodimers targeted mTOR to cytoplasmic vesicular structures in response to amino acid repletion; their possible effects on mitogen-dependent mTOR signaling have not been defined.
mTOR interacts with, and regulates, the protein phosphatase 2A catalytic subunit (PP2Ac) via its associated adaptor subunit α4. In yeast, TORC1 controls the nuclear localization of transcription factors by associating with, and likely phosphorylating, the α4 homologue Tap42.64,65 α4, or Tap42, can be negative64,66,67 or positive68–70 regulators of PP2Ac, which in turn can dephosphorylate multiple targets, including AGC kinases.71 Some propose that mitogen-stimulated phosphorylation of S6K at its T·389 residue results from the inhibition of PP2Ac activity by mTOR.66 Genetic studies indicate a role for α4 in certain functions attributed to mTORC2, such as cytokinesis and cell spreading.72,73
Signaling Inputs to the mTOR Complexes
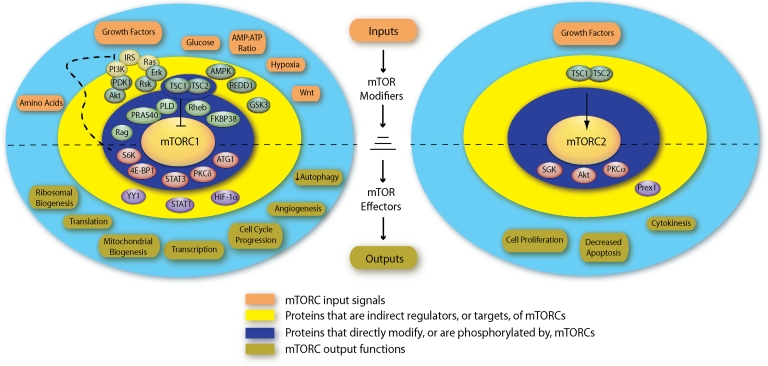
Mitogen-activated and metabolic sensing pathways converge upon the core mTOR signaling components at multiple levels (Fig. 3). The best studied is that involving PI3K, phosphatidylinositol-dependent kinase-1 (PDK1), and Akt. Ligation of growth factor receptors permits the recruitment and activation of IRS-1 and PI3K. Phosphorylated phosphatidylinositols (PI3Ps) bind PDK1, which then phosphorylates Akt in its activation loop (T·308). Akt phosphorylates TSC2, suppresses its GAP activity, and relieves the inhibitory effect of TSC2 on Rheb and mTORC1 activity.74,75 Commonly mutated in neoplastic disorders, the protein phosphatase and tensin homologue (PTEN) is a PI3P phosphatase; its loss results in elevated PDK1, Akt, and mTOR activity. PDK1 can also directly phosphorylate the activation loop of other AGC kinases that undergo phosphorylation by mTOR at their hydrophobic motifs (e.g., PKCα, PKCδ, S6K).38,39,76–78 Therefore, the PI3K/PDK1 pathway can activate AGC kinases independently of TSC2 or Rheb. The concurrent phosphorylation of AGC kinases by PDK1 and mTOR may be required for their maximal activation or stability.37 In agreement, the ability of rapamycin to block tumor progression or apoptosis in mice varied according to PI3P levels or Akt activity, suggesting that the net cellular growth and proliferation of neoplastic cells depends on the relative activities of the PI3K and mTOR signaling pathways.79–82
FIG. 3.
Coordination of mTORC1 and mTORC2 signaling activities. mTOR signaling can be organized into concentric spheres consisting from center to periphery of the mTORCs (center), their regulatory interaction partners, proteins that indirectly modify mTORC activity or mediate mTORC functions, and the mTORC-related physiological stimuli or functions. Proteins that modify mTORC activity are above the horizontal line, whereas those which mediate mTOR functions are below. Although multiple feedback and crosstalk mechanisms occur, the directionality of mTOR signaling is depicted in the center, with the mTOR complexes acting as signal integrators. Color images available online at www.liebertonline.com/lrb.
Whereas Akt enhances mTORC1 activity, its S·473 residue is also a substrate for mTORC2.83 The biological significance of Akt as both a substrate of mTORC2 and a kinase for mTOR signaling intermediates remains unclear. Akt appears to act as a signal transducer of growth factor signaling in the control of mTORC1 and cell growth, while simultaneously mediating mTORC2 suppression of apoptosis and enhancement of proliferation. Future studies investigating the intracellular regulators of mTORC2, as well as the subcellular localization and phosphorylation kinetics of the respective mTOR complexes may shed light on their relative contributions to the balance between cell proliferation and apoptosis.
mTORC1 simultaneously controls a negative feedback mechanism that dampens growth factor signaling. The mTORC1 effector S6K phosphorylates and inhibits IRS-1, thereby limiting the activation of mTOR in TSC2-deficient tumors84,85 (Fig. 3). This may in part explain the relatively benign nature of TSC and LAM hamartomas, as well as the limited clinical efficacy of rapamycin.86 That is, by inhibiting mTORC1, rapamycin relieves the S6K-dependent inhibition of IRS-1/PI3K/Akt signaling, and limits its therapeutic effect on tumor growth.
In addition to the PI3K pathway, receptor tyrosine kinases activate Ras, an oncogenic protein that promotes the transformation and growth of neoplasms. Ras activates intermediates, including p90 ribosomal S6 kinase (Rsk), the mitogen-activated protein kinase Erk, and phospholipase D, all of which can modulate mTOR signaling (Fig. 3). TSC2 and raptor contain Erk- or Rsk-phosphorylated residues.87–90 Like its negative feedback effect on the PI3K/Akt pathway, S6K also attenuated Erk activity, and Erk was ‘de-repressed’ in cells exposed to rapamycin.91
Other inputs to the TSC2/Rheb/mTOR signaling axis include metabolic sensing pathways, which are commonly dysregulated in cancer or hamartoma syndromes.6,92 The energy (ATP)-sensing protein AMP-dependent protein kinase (AMPK) can control TSC2 or raptor via direct phosphorylation.93–95 Its upstream kinase LKB1 is lost or mutated in a variety of cancers.5 Phosphorylation of TSC2 by AMPK permits its phosphorylation by GSK3, an intermediate in the Wnt signaling pathway.94,96 Thus, Wnt, an important controller of differentiation and proliferation, can activate mTORC1, cell growth, and tumor progression independent of its known effect on the β-catenin-mediated Wnt transcriptional program.97
mTORC Signaling Targets in LAM and Neoplasia
Translation and cell growth
Excessive cell growth and proliferation require a robust protein synthesis machinery.98,99 In response to mitogens, S6K and 4E-BP1 associate with mTORC1 via their respective TOS motifs. Phosphorylation of 4E-BP1 at multiple sites inhibits its interaction with the mRNA CAP-binding protein eIF4E, and permits the initiation of translation.100 This mechanism recognizes a subset of mRNAs, many of which are involved in cell growth, proliferation, and angiogenesis (e.g., ribosomal proteins, cyclin D1, HIF-1α). eIF4E was sufficient to transform cells,101 and overexpression of 4E-BP1 partially reversed v-src-induced transformation.102 S6K phosphorylates components of the translational machinery, and promotes ribosomal biogenesis via the transcriptional induction of ribosomal RNA and genes encoding ribosomal proteins.
Apart from S6K and Akt, mTORCs can associate with and phosphorylate two other AGC kinases that modify cell proliferation and apoptosis: serum/glucocorticoid kinase-1(SGK1) and protein kinase Cδ.78,103,104 Genetic or biochemical studies in C. elegans and mammalian cell lines indicate phosphorylation and regulation of SGK by TORC2 and not mTORC1.105–107 In a cell line overexpressing mTOR, however, SGK was phosphorylated by mTORC1, and subsequently blocked cell cycle arrest.108 In contrast to the mTORC2 substrate PKCα, PKCδ is phosphorylated by mTORC1, phosphorylates 4E-BP1, and controls the initiation of translation.104 Interestingly, PKCδ is a kinase for the transcription factor STAT1 kinase, and was detected in a rapamycin-sensitive macromolecular complex with mTOR and STAT1.109–111
Gene transcription
Neoplasms exhibit transcriptomic programs that determine oncogenic potential and pathogenesis.112,113 Emerging data indicate that mammalian or yeast TOR can localize to the nucleus, bind DNA regulatory elements, and modify the transcription of nutrient and stress response genes.114–116 mTOR can physically associate with transcription factors that control cell proliferation, apoptosis, ribosomal biogenesis, and angiogenesis. In response to growth factors, mTORC1 can associate with and phosphorylate STAT3, an oncogenic transcription factor that is increased in LAM, and a variety of cancers.117–119 Conversely, inactivation of mTOR or its associated phosphatase complex, promoted its association with STAT1, enhanced STAT1 nuclear content, and amplified the transcription of STAT1-dependent anti-proliferative and pro-apoptotic genes.109 The balance between STAT1 and STAT3 is dysregulated in LAM, and is thought to control the fate of neoplastic cells.120 mTOR also activates NF-κB, a pro-proliferative transcription factor implicated in the pathogenesis of cancer.121 Others demonstrated a physical association between mTOR and IKKα, the IκB kinase required for NF-κB translocation to the nucleus.122 Loss of TSC2 inhibited NF-κB-dependent responses to apoptosis inducting agents.123
The metabolic switch from oxidative phosphorylation to glycolysis is a prominent feature of tumor biology.92 mTOR physically bound the promoters of mitochondrial biogenesis genes, and acted as a transcriptional co-activator via physical interactions with the transcription factors PGC-1α and YY1.124 mTORC1 also interacted with hypoxia inducible factor-1α (HIF-1α), a transcription factor that, in cells exposed to hypoxia, controls a gene transcription program of angiogenesis, proliferation, and metabolic switch to anaerobic metabolism.125 A TOS motif in HIF-1α mediated its interaction with raptor and enhanced transcriptional activity.126 mTORC1 also promoted the synthesis of HIF-1α protein.127 Moreover, in a negative feedback mechanism, the hypoxia-induced protein ‘regulated in development and DNA damage responses-1’ (REDD1) bound TSC2, thereby suppressing mTOR activity.128 The loss of REDD1 or TSC2 likely prevents hypoxic inhibition of cell growth and proliferation, an important tumor survival mechanism. Neoplastic disorders with elevated PI3K and mTOR activity often exhibit elevated HIF-1α levels, dysregulated angiogenesis, and therapeutic sensitivity to mTOR inhibitors.
Other emerging mTOR targets in LAM
Recent studies revealed an important role for autophagy in oncogenesis and tumor survival.129 mTORC1 interacts with, phosphorylates, and inhibits the activity of Ulk1, a protein that initiates autophagy.130,131 Whereas mTOR inhibitors block cell growth and proliferation, they may limit their own efficacy by promoting autophagy and reducing apoptosis in established tumors.
TSC2-deficient cells clearly exhibit abnormalities in cytoskeletal function consistent with their ability to invade and metastasize.132,133 mTORC2 directly interacted with and regulated Prex1, a Rac1 guanine nucleotide exchange factor that controls cytoskeletal rearrangement.134
Although this discussion has been restricted to direct mTOR signaling interactions (Fig. 3), certain indirect effects of mTOR on biological processes relevant to LAM are worthy of mention. TSC2-deficient cells exhibit an activated ER stress responses, consistent with their requirement for protein synthesis and cell growth.25 The ER stress-activated transcription factor ATF6 rendered dormant cancer cells resistant to rapamycin-induced apoptosis and tumor regression.135 ER stress-activated proteins (e.g., PERK, DAPK, eIF2α, ATF6) may represent potential targets for the sensitization of LAM and neoplastic cells to apoptotic stimuli.
Consistent with the restriction of LAM to women, estrogen promotes the proliferation and metastasis of TSC2-deficient tumors in vivo, or TSC2-deficient cells derived from renal angiomyolipomas, in mTOR-dependent fashion.136–139 Separate studies suggested that prolactin stimulates mTOR, and modifies STAT1 activity in mTOR-dependent fashion.140,141 Further studies are required to better understand how mTORCs interact with androgen or estrogen signaling pathways.
Although the cell of origin in LAM is unknown, the expression of both neuroendocrine and smooth muscle cell lineage markers in LAM cells suggests abnormalities in cellular differentiation. In TSC1-deficient mice, mTOR controlled hematopoietic stem cell quiescence, renewal, and lineage development. Further studies investigating the role of mTOR in the biology of LAM or cancer stem cells will permit a better understanding on the origins of LAM.
Conclusions
Since the identification of the core mitogen-activated mTOR pathway (i.e., PI3K/Akt/TSC2/mTOR), a large number of additional mTOR-interacting proteins and signaling pathways have been identified. It has become clear that neoplastic cells express oncogenes, or lack tumor suppressors, that can render them resistant to the antiproliferative effects of rapamycin. Moreover, the normal feedback mechanisms that limit cell proliferation can be blocked by rapamycin. Finally, TSC2 and Rheb participate in signaling mechanisms other than the mTOR pathway that contribute to the cytoskeletal,132 hormonal,137 and proliferative142 characteristics of neoplastic cells. Further characterization of the mTOR complexes and their signal integration functions will lead to therapeutic approaches for LAM and neoplasia that simultaneously target multiple receptors and signaling intermediates.
Abbreviations
- AGC
cAMP-dependent protein kinase/protein kinase G/protein kinase C
- ER
endoplasmic reticulum
- HEAT
Huntington, Elongation factor 3, A subunit of type 2A protein phosphatase (PP2A), and TOR
- LAM
lymphangioleiomyomatosis
- mTOR
mammalian target of rapamycin
- mTORC
mTOR complex
- PI3K
phosphatidylinositol-3 kinase
- PI3P
phosphatidylinositol-3 phosphate
- PKC
protein kinase C
- PP2A
protein phosphatase 2A
- Rheb
Ras homologue enriched in brain
- S6K
p70 S6 kinase
- TOS
target of rapamycin signaling
- TSC
tuberous sclerosis complex
- PDK1
phosphatidylinositol-dependent kinase-1
Acknowledgments
The author thanks Dr. Joel Moss and Dr. Stewart Levine (National Institutes of Health) for helpful comments and suggestions regarding the content of this manuscript. The author's work is supported by the National Institutes of Health (R01-CA125436) in conjunction with the Tuberous Sclerosis Alliance and Tuberous Sclerosis Canada, an ATS/LAM Foundation Award, and the Canadian Institutes of Health Research.
Disclosures
Dr. Kristof has no conflicts of interest or financial ties to disclose.
References
- 1.Pan D. Dong J. Zhang Y. Gao X. Tuberous sclerosis complex: from Drosophila to human disease. Trends Cell Biol. 2004;14:78–85. doi: 10.1016/j.tcb.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Goncharova EA. Goncharov DA. Eszterhas A, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 3.el Hashemite N. Zhang H. Henske EP. Kwiatkowski DJ. Mutation in TSC2 and activation of mammalian target of rapamycin signalling pathway in renal angiomyolipoma. Lancet. 2003;361:1348–1349. doi: 10.1016/S0140-6736(03)13044-9. [DOI] [PubMed] [Google Scholar]
- 4.Pacheco–Rodriguez G. Kristof AS. Stevens LA. Zhang Y. Crooks D. Moss J. Giles F. Filley Lecture. Genetics and gene expression in lymphangioleiomyomatosis. Chest. 2002;121:56S–60S. doi: 10.1378/chest.121.3_suppl.56s. [DOI] [PubMed] [Google Scholar]
- 5.Inoki K. Corradetti MN. Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 6.Shaw RJ. Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18:598–608. doi: 10.1016/j.ceb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 7.DeBerardinis RJ. Sayed N. Ditsworth D. Thompson CB. Brick by brick: Metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu MI. Katz H. Berlin V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc Natl Acad Sci USA. 1994;91:12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown EJ. Albers MW. Shin TB, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 10.Sabatini DM. Erdjument–Bromage H. Lui M. Tempst P. Snyder SH. RAFT1: A mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 11.Jacinto E. Hall MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003;4:117–126. doi: 10.1038/nrm1018. [DOI] [PubMed] [Google Scholar]
- 12.Alessi DR. Pearce LR. Garcia–Martinez JM. New insights into mTOR signaling: mTORC2 and beyond. Sci Signal. 2009;2:e27. doi: 10.1126/scisignal.267pe27. [DOI] [PubMed] [Google Scholar]
- 13.Daub H. Olsen JV. Bairlein M, et al. Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle. Mol Cell. 2008;3:438–448. doi: 10.1016/j.molcel.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Dephoure N. Zhou C. Villen J, et al. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson RT. Beal PA. Comb MJ. Schreiber SL. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem. 2000;275:7416–7423. doi: 10.1074/jbc.275.10.7416. [DOI] [PubMed] [Google Scholar]
- 16.Guertin DA. Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:e24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 17.Yu K. Toral–Barza L. Shi C, et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69:6232–6240. doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]
- 18.Kristof AS. Pacheco–Rodriguez G. Schremmer B. Moss J. LY303511 acts via PI3-kinase independent pathways to inhibit cell proliferation via mTOR- and non-mTOR-dependent mechanisms. J Pharmacol Exp Ther. 2005;314:1134–1143. doi: 10.1124/jpet.105.083550. [DOI] [PubMed] [Google Scholar]
- 19.Sekulic A. Hudson CC. Homme JL, et al. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 20.Nave BT. Ouwens M. Withers DJ. Alessi DR. Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: Identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- 21.Acosta–Jaquez HA. Keller JA. Foster KG, et al. Site-specific mTOR phosphorylation promotes mTORC1-mediated signaling and cell growth. Mol Cell Biol. 2009;29:4308–4324. doi: 10.1128/MCB.01665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X. Shu L. Hosoi H. Murti KG. Houghton PJ. Predominant nuclear localization of mammalian target of rapamycin in normal and malignant cells in culture. J Biol Chem. 2002;277:28127–28134. doi: 10.1074/jbc.M202625200. [DOI] [PubMed] [Google Scholar]
- 23.Bachmann RA. Kim JH. Wu AL. Park IH. Chen J. A nuclear transport signal in mammalian target of rapamycin is critical for its cytoplasmic signaling to S6 kinase 1. J Biol Chem. 2006;281:7357–7363. doi: 10.1074/jbc.M512218200. [DOI] [PubMed] [Google Scholar]
- 24.Liu X. Zheng XF. ER and Golgi localization sequences for mammalian target of rapamycin (mTOR) Mol Biol Cell. 2007;18:1073–1082. doi: 10.1091/mbc.E06-05-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozcan U. Ozcan L. Yilmaz E, et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drenan RM. Liu X. Bertram PG. Zheng XF. FKBP12-rapamycin-associated protein or mammalian target of rapamycin (FRAP/mTOR) localization in the endoplasmic reticulum and the Golgi apparatus. J Biol Chem. 2004;279:772–778. doi: 10.1074/jbc.M305912200. [DOI] [PubMed] [Google Scholar]
- 27.Puria R. Zurita–Martinez SA. Cardenas ME. Nuclear translocation of Gln3 in response to nutrient signals requires Golgi-to-endosome trafficking in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2008;105:7194–7199. doi: 10.1073/pnas.0801087105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai BN. Myers BR. Schreiber SL. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc Natl Acad Sci USA. 2002;99:4319–4324. doi: 10.1073/pnas.261702698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schieke SM. Phillips D. McCoy JP, Jr., et al. The mTOR pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- 30.Sarbassov DD. Sabatini DM. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J Biol Chem. 2005;280:39505–39509. doi: 10.1074/jbc.M506096200. [DOI] [PubMed] [Google Scholar]
- 31.Dames SA. Mulet JM. Rathgeb–Szabo K. Hall MN. Grzesiek S. The solution structure of the FATC domain of the protein kinase target of rapamycin suggests a role for redox-dependent structural and cellular stability. J Biol Chem. 2005;280:20558–20564. doi: 10.1074/jbc.M501116200. [DOI] [PubMed] [Google Scholar]
- 32.Jacinto E. What controls TOR? IUBMB Life. 2008;60:483–496. doi: 10.1002/iub.56. [DOI] [PubMed] [Google Scholar]
- 33.Loewith R. Jacinto E. Wullschleger S, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 34.Lee VH. Healy T. Fonseca BD. Hayashi A. Proud CG. Analysis of the regulatory motifs in eukaryotic initiation factor 4E-binding protein 1. FEBS J. 2008;275:2185–2199. doi: 10.1111/j.1742-4658.2008.06372.x. [DOI] [PubMed] [Google Scholar]
- 35.Schalm SS. Fingar DC. Sabatini DM. Blenis J. TOS Motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr Biol. 2003;13:797–806. doi: 10.1016/s0960-9822(03)00329-4. [DOI] [PubMed] [Google Scholar]
- 36.Nojima H. Tokunaga C. Eguchi S, et al. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem. 2003;278:15461–15464. doi: 10.1074/jbc.C200665200. [DOI] [PubMed] [Google Scholar]
- 37.Jacinto E. Lorberg A. TOR regulation of AGC kinases in yeast and mammals. Biochem J. 2008;410:19–37. doi: 10.1042/BJ20071518. [DOI] [PubMed] [Google Scholar]
- 38.Ikenoue T. Inoki K. Yang Q. Zhou X. Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Facchinetti V. Ouyang W. Wei H, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–1943. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacinto E. Facchinetti V. Liu D, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt pPhosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 41.Frias MA. Thoreen CC. Jaffe JD, et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Pearce LR. Huang X. Boudeau J, et al. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J. 2007;405:513–522. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thedieck K. Polak P. Kim ML, et al. PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS ONE. 2007;2:e1217. doi: 10.1371/journal.pone.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woo SY. Kim DH. Jun CB, et al. PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling. J Biol Chem. 2007;282:25604–25612. doi: 10.1074/jbc.M704343200. [DOI] [PubMed] [Google Scholar]
- 45.Kim dH. Sarbassov dD. Ali SM, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 46.Long X. Lin Y. Ortiz–Vega S. Yonezawa K. Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 47.Li Y. Inoki K. Guan KL. Biochemical and functional characterizations of small GTPase Rheb and TSC2 GAP activity. Mol Cell Biol. 2004;24:7965–7975. doi: 10.1128/MCB.24.18.7965-7975.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garami A. Zwartkruis FJ. Nobukuni T, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 49.Tee AR. Manning BD. Roux PP. Cantley LC. Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 50.Huang J. Dibble CC. Matsuzaki M. Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang J. Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oshiro N. Takahashi R. Yoshino K, et al. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J Biol Chem. 2007;282:20329–20339. doi: 10.1074/jbc.M702636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fonseca BD. Smith EM. Lee VH. MacKintosh C. Proud CG. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem. 2007;282:24514–24524. doi: 10.1074/jbc.M704406200. [DOI] [PubMed] [Google Scholar]
- 54.Sancak Y. Thoreen CC. Peterson TR, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Vander HE. Lee SI. Bandhakavi S. Griffin TJ. Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 56.Bai X. Ma D. Liu A, et al. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 57.Rosner M. Hofer K. Kubista M. Hengstschlager M. Cell size regulation by the human TSC tumor suppressor proteins depends on PI3K and FKBP38. Oncogene. 2003;22:4786–4798. doi: 10.1038/sj.onc.1206776. [DOI] [PubMed] [Google Scholar]
- 58.Ma D. Bai X. Guo S. Jiang Y. The switch I region of Rheb is critical for its interaction with FKBP38. J Biol Chem. 2008;283:25963–25970. doi: 10.1074/jbc.M802356200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fang Y. Vilella–Bach M. Bachmann R. Flanigan A. Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 60.Fang Y. Park IH. Wu AL, et al. PLD1 regulates mTOR signaling and mediates Cdc42 activation of S6K1. Curr Biol. 2003;13:2037–2044. doi: 10.1016/j.cub.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 61.Sun Y. Chen J. mTOR signaling: PLD takes center stage. Cell Cycle. 2008;7:3118–3123. doi: 10.4161/cc.7.20.6881. [DOI] [PubMed] [Google Scholar]
- 62.Toschi A. Lee E. Xu L. Garcia A. Gadir N. Foster DA. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: A competition with rapamycin. Mol Cell Biol. 2009;29:1411–1420. doi: 10.1128/MCB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sancak Y. Sabatini DM. Rag proteins regulate amino-acid-induced mTORC1 signalling. Biochem Soc Trans. 2009;37:289–290. doi: 10.1042/BST0370289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan G. Shen X. Jiang Y. Rapamycin activates Tap42-associated phosphatases by abrogating their association with Tor complex 1. EMBO J. 2006;25:3546–3555. doi: 10.1038/sj.emboj.7601239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rohde JR. Bastidas R. Puria R. Cardenas ME. Nutritional control via Tor signaling in Saccharomyces cerevisiae. Curr Opin Microbiol. 2008;11:153–160. doi: 10.1016/j.mib.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peterson RT. Desai BN. Hardwick JS. Schreiber SL. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proc Natl Acad Sci USA. 1999;96:4438–4442. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nanahoshi M. Nishiuma T. Tsujishita Y, et al. Regulation of protein phosphatase 2A catalytic activity by alpha4 protein and its yeast homolog Tap42. Biochem Biophys Res Commun. 1998;251:520–526. doi: 10.1006/bbrc.1998.9493. [DOI] [PubMed] [Google Scholar]
- 68.Di Como CJ. Arndt KT. Nutrients, via the Tor proteins, stimulate the association of Tap42 with type 2A phosphatases. Genes Dev. 1996;10:1904–1916. doi: 10.1101/gad.10.15.1904. [DOI] [PubMed] [Google Scholar]
- 69.Inui S. Sanjo H. Maeda K. Yamamoto H. Miyamoto E. Sakaguchi N. Ig receptor binding protein 1 (alpha4) is associated with a rapamycin-sensitive signal transduction in lymphocytes through direct binding to the catalytic subunit of protein phosphatase 2A. Blood. 1998;92:539–546. [PubMed] [Google Scholar]
- 70.Murata K. Wu J. Brautigan DL. B cell receptor-associated protein alpha4 displays rapamycin-sensitive binding directly to the catalytic subunit of protein phosphatase 2A. Proc Natl Acad Sci USA. 1997;94:10624–10629. doi: 10.1073/pnas.94.20.10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eichhorn PJ. Creyghton MP. Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795:1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 72.Wang H. Jiang Y. The Tap42-protein phosphatase type 2A catalytic subunit complex is required for cell cycle-dependent distribution of actin in yeast. Mol Cell Biol. 2003;23:3116–3125. doi: 10.1128/MCB.23.9.3116-3125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kong M. Bui TV. Ditsworth D, et al. The PP2A-associated protein alpha 4 plays a critical role in the regulation of cell spreading and migration. J Biol Chem. 2007;282:29712–29720. doi: 10.1074/jbc.M703159200. [DOI] [PubMed] [Google Scholar]
- 74.Manning BD. Tee AR. Logsdon MN. Blenis J. Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 75.Inoki K. Li Y. Zhu T. Wu J. Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 76.Parker PJ. Protein kinase C phosphorylation: An introduction. Methods Mol Biol. 2003;233:159–162. doi: 10.1385/1-59259-397-6:159. [DOI] [PubMed] [Google Scholar]
- 77.Romanelli A. Dreisbach VC. Blenis J. Characterization of phosphatidylinositol 3-kinase-dependent phosphorylation of the hydrophobic motif site Thr(389) in p70 S6 kinase 1. J Biol Chem. 2002;277:40281–40289. doi: 10.1074/jbc.M205168200. [DOI] [PubMed] [Google Scholar]
- 78.Parekh D. Ziegler W. Yonezawa K. Hara K. Parker PJ. Mammalian TOR controls one of two kinase pathways acting upon nPKCdelta and nPKCepsilon. J Biol Chem. 1999;274:34758–34764. doi: 10.1074/jbc.274.49.34758. [DOI] [PubMed] [Google Scholar]
- 79.Sarbassov DD. Ali SM. Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 80.Neshat MS. Mellinghoff IK. Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA. 2001;98:10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Podsypanina K. Lee RT. Politis C, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/- mice. Proc Natl Acad Sci USA. 2001;98:10320–10325. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wendel HG. De SE. Fridman JS, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 83.Sarbassov DD. Guertin DA. Ali SM. Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 84.Manning BD. Logsdon MN. Lipovsky AI. Abbott D. Kwiatkowski DJ. Cantley LC. Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev. 2005;19:1773–1778. doi: 10.1101/gad.1314605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shah OJ. Wang Z. Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 86.Easton JB. Kurmasheva RT. Houghton PJ. IRS-1: Auditing the effectiveness of mTOR inhibitors. Cancer Cell. 2006;9:153–155. doi: 10.1016/j.ccr.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 87.Roux PP. Shahbazian D. Vu H, et al. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282:14056–14064. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carriere A. Cargnello M. Julien LA, et al. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr Biol. 2008;18:1269–1277. doi: 10.1016/j.cub.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 89.Ma L. Chen Z. Erdjument–Bromage H. Tempst P. Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 90.Distefano G. Boca M. Rowe I, et al. Polycystin-1 regulates ERKs-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream rffectors S6K and 4EBP1. Mol Cell Biol. 2009;29:2359–2371. doi: 10.1128/MCB.01259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carracedo A. Ma L. Teruya–Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jones RG. Thompson CB. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gwinn DM. Shackelford DB. Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Inoki K. Ouyang H. Zhu T, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 95.Inoki K. Zhu T. Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 96.Mak BC. Kenerson HL. Aicher LD. Barnes EA. Yeung RS. Aberrant beta-catenin signaling in tuberous sclerosis. Am J Pathol. 2005;167:107–116. doi: 10.1016/s0002-9440(10)62958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klaus A. Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 98.Ma MX. Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 99.Petroulakis E. Mamane Y. Le Bacquer O. Shahbazian D. Sonenberg N. mTOR signaling: Implications for cancer and anticancer therapy. Br J Cancer. 2006;94:195–199. doi: 10.1038/sj.bjc.6602902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sonenberg N. eIF4E, the mRNA cap-binding protein: From basic discovery to translational research. Biochem Cell Biol. 2008;86:178–183. doi: 10.1139/O08-034. [DOI] [PubMed] [Google Scholar]
- 101.Lazaris–Karatzas A. Montine KS. Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990;345:544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- 102.Rousseau D. Gingras AC. Pause A. Sonenberg N. The eIF4E-binding proteins 1 and 2 are negative regulators of cell growth. Oncogene. 1996;13:2415–2420. [PubMed] [Google Scholar]
- 103.Brunet A. Park J. Tran H. Hu LS. Hemmings BA. Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kumar V. Pandey P. Sabatini D, et al. Functional interaction between RAFT1/FRAP/mTOR and protein kinase cdelta in the regulation of cap-dependent initiation of translation. EMBO J. 2000;19:1087–1097. doi: 10.1093/emboj/19.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garcia–Martinez JM. Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 106.Jones KT. Greer ER. Pearce D. Ashrafi K. Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1. PLoS Biol. 2009;7:e60. doi: 10.1371/journal.pbio.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Soukas AA. Kane EA. Carr CE. Melo JA. Ruvkun G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009;23:496–511. doi: 10.1101/gad.1775409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hong F. Larrea MD. Doughty C. Kwiatkowski DJ. Squillace R. Slingerland JM. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–711. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 109.Fielhaber JA. Han YS. Tan j, et al. Inactivation of mammalian target of rapamycin increases stat1 nuclear content and transcriptional activity in {alpha}4- and protein phosphatase 2A-dependent fashion. J Biol Chem. 2009;284:24341–24353. doi: 10.1074/jbc.M109.033530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kristof AS. Marks–Konczalik J. Billings E. Moss J. Stimulation of STAT1-dependent gene transcription by lipopolysaccharide and interferon-gamma is regulated by mammalian target of rapamycin. J Biol Chem. 2003;278:33637–33644. doi: 10.1074/jbc.M301053200. [DOI] [PubMed] [Google Scholar]
- 111.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 112.Nguyen DX. Bos PD. Massague J. Metastasis: From dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 113.Vogelstein B. Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 114.Peng T. Golub TR. Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol. 2002;22:5575–5584. doi: 10.1128/MCB.22.15.5575-5584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsang CK. Zheng XF. TOR-in(g) the nucleus. Cell Cycle. 2007;6:25–29. doi: 10.4161/cc.6.1.3675. [DOI] [PubMed] [Google Scholar]
- 116.Li H. Tsang CK. Watkins M. Bertram PG. Zheng XF. Nutrient regulates Tor1 nuclear localization and association with rDNA promoter. Nature. 2006;442:1058–1061. doi: 10.1038/nature05020. [DOI] [PubMed] [Google Scholar]
- 117.Yokogami K. Wakisaka S. Avruch J. Reeves SA. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol. 2000;10:47–50. doi: 10.1016/s0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]
- 118.Zhou J. Wulfkuhle J. Zhang H, et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci USA. 2007;104:16158–16563. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goncharova EA. Goncharov DA. Chisolm A, et al. Interferon {beta} augments TSC2-dependent inhibition of TSC2-null ELT3 and human LAM-derived cell proliferation. Mol Pharmacol. 2007;73:778–788. doi: 10.1124/mol.107.040824. [DOI] [PubMed] [Google Scholar]
- 120.Regis G. Pensa S. Boselli D. Novelli F. Poli V. Ups and downs: The STAT1:STAT3 seesaw of interferon and gp130 receptor signalling. Semin Cell Dev Biol. 2008;19:351–359. doi: 10.1016/j.semcdb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 121.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 122.Dan HC. Cooper MJ. Cogswell PC. Duncan JA. Ting JP. Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–1500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ghosh S. Tergaonkar V. Rothlin CV, et al. Essential role of tuberous sclerosis genes TSC1 and TSC2 in NF-kappaB activation and cell survival. Cancer Cell. 2006;10:215–226. doi: 10.1016/j.ccr.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 124.Cunningham JT. Rodgers JT. Arlow DH. Vazquez F. Mootha VK. Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 125.Semenza GL. Hypoxia-inducible factor 1 and cancer pathogenesis. IUBMB Life. 2008;60:591–597. doi: 10.1002/iub.93. [DOI] [PubMed] [Google Scholar]
- 126.Land SC. Tee AR. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem. 2007;282:20534–20543. doi: 10.1074/jbc.M611782200. [DOI] [PubMed] [Google Scholar]
- 127.Brugarolas JB. Vazquez F. Reddy A. Sellers WR. Kaelin WG., Jr TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 2003;4:147–158. doi: 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]
- 128.Brugarolas J. Lei K. Hurley RL, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morselli E. Galluzzi L. Kepp O, et al. Anti- and pro-tumor functions of autophagy. Biochim Biophys Acta. 2009;1793:1524–1532. doi: 10.1016/j.bbamcr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 130.Jung CH. Jun CB. Ro SH, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ganley IG. Lam dH. Wang J. Ding X. Chen S. Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Goncharova EA. Goncharov DA. Lim PN. Noonan D. Krymskaya VP. Modulation of cell migration and invasiveness by tumor suppressor TSC2 in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 2006;34:473–480. doi: 10.1165/rcmb.2005-0374OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goncharova E. Goncharov D. Noonan D. Krymskaya VP. TSC2 modulates actin cytoskeleton and focal adhesion through TSC1-binding domain and the Rac1 GTPase. J Cell Biol. 2004;167:1171–1182. doi: 10.1083/jcb.200405130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hernandez–Negrete I. Carretero–Ortega J. Rosenfeldt H, et al. P-Rex1 links mTOR signaling to Rac activation and cell migration. J Biol Chem. 2007;282:23708–23715. doi: 10.1074/jbc.M703771200. [DOI] [PubMed] [Google Scholar]
- 135.Schewe DM. guirre-Ghiso JA. ATF6{alpha}-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proc Natl Acad Sci USA. 2008;105:10519–10524. doi: 10.1073/pnas.0800939105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu JJ. Robb VA. Morrison TA, et al. Estrogen promotes the survival and pulmonary metastasis of tuberin-null cells. Proc Natl Acad Sci USA. 2009;106:2635–2640. doi: 10.1073/pnas.0810790106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Finlay GA. York B. Karas RH, et al. Estrogen-induced smooth muscle cell growth is regulated by tuberin and associated with altered activation of platelet-derived growth factor receptor-beta and ERK-1/2. J Biol Chem. 2004;279:23114–23122. doi: 10.1074/jbc.M401912200. [DOI] [PubMed] [Google Scholar]
- 138.Yu J. Astrinidis A. Howard S. Henske EP. Estradiol and tamoxifen stimulate LAM-associated angiomyolipoma cell growth and activate both genomic and nongenomic signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2004;286:L694–L700. doi: 10.1152/ajplung.00204.2003. [DOI] [PubMed] [Google Scholar]
- 139.Yu J. Henske EP. Estrogen-induced activation of mammalian target of rapamycin is mediated via tuberin and the small GTPase Ras homologue enriched in brain. Cancer Res. 2006;66:9461–9466. doi: 10.1158/0008-5472.CAN-06-1895. [DOI] [PubMed] [Google Scholar]
- 140.Nien WL. Dauphinee SM. Moffat LD. Too CK. Overexpression of the mTOR alpha4 phosphoprotein activates protein phosphatase 2A and increases Stat1alpha binding to PIAS1. Mol Cell Endocrinol. 2006;263:10–17. doi: 10.1016/j.mce.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 141.Boudreau RT. Sangster SM. Johnson LM. Dauphinee S. Li AW. Too CK. Implication of alpha4 phosphoprotein and the rapamycin-sensitive mammalian target-of-rapamycin pathway in prolactin receptor signalling. J Endocrinol. 2002;173:493–506. doi: 10.1677/joe.0.1730493. [DOI] [PubMed] [Google Scholar]
- 142.Karbowniczek M. Robertson GP. Henske EP. Rheb inhibits C-raf activity and B-raf/C-raf heterodimerization. J Biol Chem. 2006;281:25447–25456. doi: 10.1074/jbc.M605273200. [DOI] [PubMed] [Google Scholar]
- 143.Chen J. Zheng XF. Brown EJ. Schreiber SL. Identification of an 11-kDa FKBP12-rapamycin-binding domain within the 289-kDa FKBP12-rapamycin-associated protein and characterization of a critical serine residue. Proc Natl Acad Sci USA. 1995;92:4947–4951. doi: 10.1073/pnas.92.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Choi J. Chen J. Schreiber SL. Clardy J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science. 1996;273:239–242. doi: 10.1126/science.273.5272.239. [DOI] [PubMed] [Google Scholar]