Abstract
In this review of the chemistry, absorption, metabolism, and mechanisms of action of plant isoflavones, emphasis is placed on the isoflavones in soy and the food products derived from them. Soybeans have been part of food history in Asia for several millennia but did not reach the Americas and Europe until the eighteenth century. In the twentieth century, there was a tremendous increase in the cultivation of soybeans in the United States and more recently in South America. Soy foods have entered the U.S. food supply in ever-increasing amounts both in the form of traditional products (soy milk, tofu) and in more subtle ways in dairy and bread/cake products. The isoflavones in non-fermented foods are for the most part in the form of glycoside conjugates. These undergo changes due to different processing procedures. Isoflavones and their metabolites are well absorbed and undergo an enterohepatic circulation. They are often termed phytoestrogens because they bind to the estrogen receptors although weakly compared to physiologic estrogens. This estrogenicity is not the only mechanism by which isoflavones may have bioactivity—they inhibit tyrosine kinases, have antioxidant activity, bind to and activate peroxisome proliferator regulators α and γ, inhibit enzymes in steroid biosynthesis, strongly influence natural killer cell function and the activation of specific T-cell subsets, and inhibit metastasis. These various properties may explain the much lower incidence of hormonally-dependent breast cancer in Asian populations compared to Americans and Europeans.
History
The soybean is a member of the leguminosae family, plants that form root noodles that house nitrogen-fixing soil bacteria (Rhizobia) in a symbiotic relationship, an event that is essential for life on this planet. Legumes have been used in a crop rotation system to restore the nitrogen in the soil on ground used for agriculture. The soybean, now called Glycine max, has had a long history as a domesticated plant, with records of its use as far back as the eleventh century BC in China. Missionaries took it into Korea and Japan in the third and fourth centuries AD.1 It did not reach Europe until 1739 in Paris and 1790 in Kew gardens in London.1 The first recorded use in the United States was in 1765 in Savannah, Georgia.1 Evaluation of the soybean was carried out by the U.S. Department of Agriculture in the early 1900s to identify strains that grew best in each of the farming states.1,2 The value of the soybean as a source of protein and oils was realized by George Washington Carver at the Tuskegee Institute in Alabama and by Henry J. Ford who used soy extensively in production of cars. In 1960, the annual world production of soybeans was 27 million tons, with 69% being grown in the United States. By 2007, annual world production had risen to 206.4 million tons with nearly 90 million tons grown in Brazil and Argentina.
Isoflavone Biosynthesis
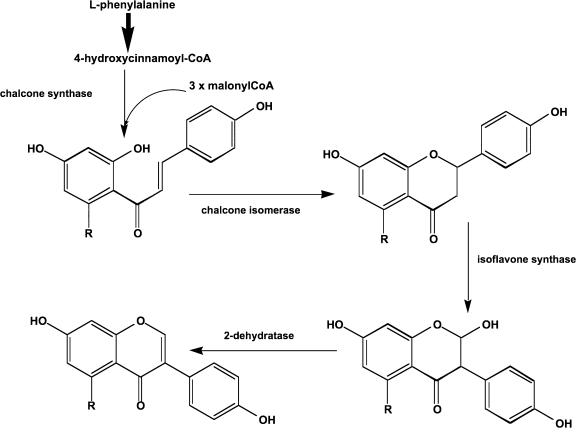
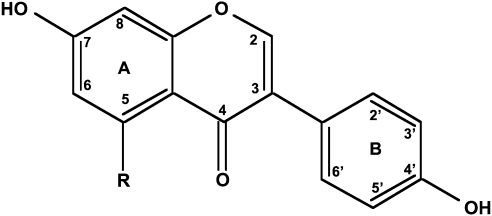
The signals released by the soybean that attract the rhizobial bacteria are the isoflavonoids.3 These are a subclass of the much more common flavonoids. These in turn are members of the large family of polyphenols that are widely found in plants. Isoflavonoids are formed by the same biosynthetic pathway for flavonoids.4 First, phenylalanine reacts with malonyl CoA to form 4-hydroxycinnamoyl CoA (Fig. 1). Chalcone synthase catalyzes the reaction of this intermediate with three more molecules of malonyl CoA to form isoliquiritigenin or naringenin chalcone. Chalcone isomerase catalyzes the ring closure of the heterocyclic ring. Isoflavone synthase introduces a 2-hydroxyl group, which in turn is removed by an isoflavone dehydratase to yield daidzein (7,4’-dihydroxyisoflavone) and genistein (5,7,4’-trihydroxyisoflavone) (Fig. 1). The biosynthesis of glycitein (7,4’-dihydroxy-6-methoxyisoflavone), a major isoflavone in the soy germ (hypocotyls) is not understood. The numbering scheme for isoflavones is shown in Figure 2.
FIG. 1.
The pathway for isoflavone biosynthesis. First phenylalanine reacts with malonyl CoA to produce 4-hydroxycinnamoyl CoA. Under the catalytic control of chalcone synthase 4-hydroxycinnamoyl CoA condenses with three molecules of malonyl CoA to form a chalcone. Chalcone isomerase closes the heterocyclic ring to form naringenin. The B-ring is moved from the 2-position to the 3-position by isoflavone synthase. Isoflavone dehydratase removes water to generate the 2,3 double bond in the heterocyclic ring (see Figure 2 for the numbering scheme).
FIG. 2.
The numbering scheme of isoflavones. The scheme starts from the ethereal oxygen in the heterocyclic ring. The B-ring ring has a separate numbering system (1’-6’).
Isoflavones in the soybean are converted to 7-O-β-glucosides by a glucosyltransferase and then to their 6”-O-malonates by a malonyl transferase. This chemical form is stored in vacuoles until used by the plant and is the major form in harvested soybeans. Although the yellow or black soybeans are the most familiar forms, an early harvesting before ripening results in a green immature soybean. This is cooked by boiling still in the pod and is served as edamame. It has similar levels of isoflavones to the yellow and black soybeans.5,6
Soy Foods in Asia
Although soybean-containing foods have become more popular in the United States over the past 50 years, they are in general quite different from the forms of soy consumed in Asia.7 Unlike American soy foods, the latter are often fermented. Soybeans are converted using microorganisms to miso (added to soups and stews in Japan), soy paste (in Korea) and tempeh (with a texture like meat in Indonesia). Soy sauce is another familiar soy product and is made either by acid hydrolysis (no isoflavones) or by prolonged fermentation. The proteins and lipids in soybeans are extracted with boiling water to form soy milk, an important alternative to mother's milk in countries with a high incidence of lactase insufficiency. Soymilk is curdled to prepare tofu, which can be pressed to remove water. Tofu can be fried or added to numerous other dishes.
Soy Foods in the United States
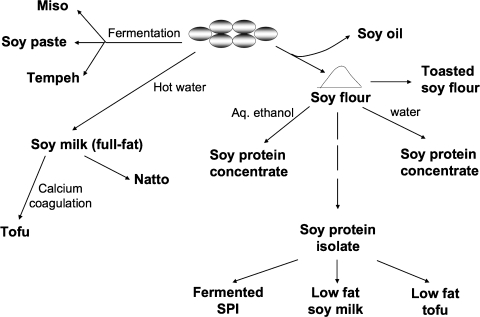
In the United States, soybeans are grown mostly as a source of edible oil using a hexane extraction approach (Fig. 3). The defatted soy flour is enriched in protein (50% by weight). This has been traditionally used as the protein source in domesticated and research animal diets.8 The soy flour is heated to produce a variety of related products—this includes toasting at 250°C. These are used in many bread and cake products, particularly in doughnuts. Soy flour is washed with water to remove soluble carbohydrates—this creates soy protein concentrate, which contains 70% protein by weight (Fig. 3). Alternatively, soy flour is extracted with hot, aqueous 65% alcohol to remove carbohydrates, lipids, and other small molecules, including the isoflavones (also color and taste). This is another form of soy protein concentrate. Both the soy protein concentrates can be extruded to form textured soy protein, another meat-like product. Soy protein isolate (SPI), >92% protein, is prepared by first solubilizing the proteins in soy flour with a mild alkaline extraction (leaving behind complex carbohydrates and lipids) and precipitating the proteins by lowering the pH to 4.5 (Fig. 3). The soluble sugars remain in solution. SPI is found in many canned food products. It's added to improve the appearance of the food. It is also widely used by serious athletes who are on low-fat, high-protein diets. It's also used to prepare low-fat forms of soymilk. As in Asia, these soymilks are converted to tofu. However, this is typically done aseptically producing shelf-stable products. In a recent development, soy products are being made where microorganisms that hydrolyze the isoflavones are added to soy protein preparations.
FIG. 3.
Soy food processing to commercially available items. The Asian products are largely on the left of the figure. There fermentation of soybeans is very common to make miso, soybean paste and tempeh, as well as soy sauce. Soybeans are used to manufacture full-fat soymilk and tofu. Tofu can be found in several forms depending how much water is squeezed out. Natto Is obtained from the surface layer when generating soymilk. The American products start with a solvent (hexane) extraction procedure to recover the oil from the soybean. The defatted protein-enriched soy flour (50% protein) is the source of soy protein concentrate (70% protein) and soy protein isolate (>90% protein). Soy protein isolate is used to make low-fat soymilk and tofu as well as a fermented isoflavone-protein enriched product.
Hidden Soy in Foods
Just as for those who suffer from peanut or milk allergies, there are those for whom avoiding soy is essential. And with the widespread presence of soy in food products, this can be a difficult task. Read the food labels carefully—a product having added “hydrolyzed vegetable protein,” or just “vegetable protein” contains soy. Besides the clearly labeled new “soy” foods (soy cheese, soy ice cream, soy yoghurt), soy can turn up in strange, but often familiar places. For instance, a soy-based batter is used to coat doughnuts—it provides the needed mouth-feel. When roasting the Christmas turkey, the expectation is that cooking will produce profuse gravy—to ensure that will happen, producers pump a solution of SPI into the turkey before it is sold. In some brands of canned tuna, the tuna meat is soaked in a soy broth. Chili often has added soy protein. Energy bars that are low-fat and high in protein may contain SPI. Licorice and licorice teas and most meatless products contain significant amounts of isoflavones. Extensive lists of common foods and their isoflavone content have been published.9,10 There is also a compilation of the isoflavone content of foods provided by a study funded by the U. S. Departments of Agriculture and the Army that can be obtained from the following website: http://www.nal.usda.gov/fnic/foodcomp/Data/isoflav/isoflav.html.
Changing Chemistry of the Isoflavones in Foods
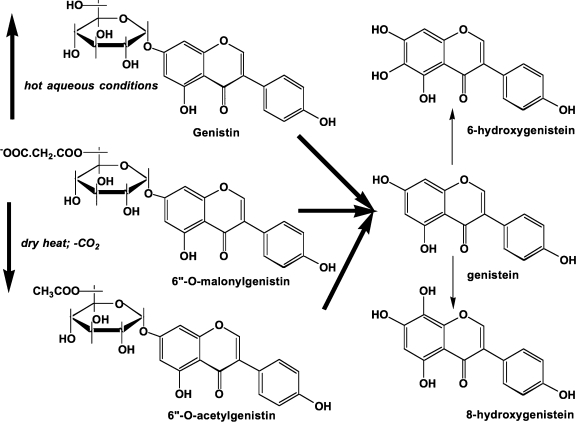
Processing soybeans to make foods results in changes in their chemistry (Fig. 4). In general, fermentation causes the removal of the glucosidic group releasing the isoflavone aglucone.11,12 If the fermentation is a lengthy process (for miso or some forms of soy sauce this can be up to 9 months), additional oxidative metabolism can occur introducing hydroxyl groups into the 6- and 8-positions on the A-ring (Fig. 4).13 Hexane extraction to recover the oil fraction does not alter the composition. However, the boiling water extraction of soybeans to make soymilk causes the hydrolysis of the malonyl group, yielding simple β-glucosides.14 This also occurs during the hot aqueous alcohol extraction of soy flour. The alcohol extract is evaporated to form soy molasses, a rich source of isoflavones. The isoflavones are recovered to make products such as NovaSoy™, a 40% by weight isoflavone material. When soy is heated in a dry format (extrusion of soy protein concentrate or toasting of soybeans, soy flour or the hypocotyls), the malonyl group is decarboxylated to form the 6”-O-acetyl-7-O-β-glucoside.14 These purified protein products can also be treated at the last stage of manufacturing with microorganisms to generate a soy protein material containing unconjugated isoflavones.
FIG. 4.
Effect of processing on soy chemistry. The 6”-O-malonate ester of genistin is either hydrolyzed (hot water or aqueous solvent) to genistein or decarboxylated by dry heat to 6”-O-acetylgenistin. Fermentation to release genistein, the aglycone, can also be accompanied by 6- or 8-hydroxylation.
Other Foods or Supplements Containing Isoflavones
Soy is the principal plant that produces isoflavones (1–2 mg/g).13 Generally, only small amounts are found in most other plants.15 The chickpea has 1/100th of the amount in soy. However, there are two other rich plant sources of isoflavones, one of which is found in large amounts in dietary supplements. The tuber of the American groundnut, Apios Americana, contains copious amounts (as high as 8 mg/g) of the 7-O-glucosylglucoside of genistein.16 The tuber has been an important part of the Native American diet, particularly on the East Coast, and was reported to be in the first Thanksgiving feast. The other source is the root of the Kudzu (pueraria lobata), a tropical vine presented to the U.S. government in 1876 to celebrate 100 years of independence. Unfortunately, it has no natural enemies and grows prolifically particularly in the southeastern part of the United States. There it is regarded as a pest. However, the root of the Kudzu has been used as a traditional medicine in Asia.17 In addition to the O-glucosides, the Kudzu also contains large amounts of the C-glucosides of isoflavones.18 Daidzein-8-C-glucoside is known as puerarin (Fig. 5)and it is often the major constituent of isoflavone dietary supplements found on pharmacy shelves.
FIG. 5.
The C- and O-glucosides of daidzein. Daidzein undergoes conjugation with glucose either to form the 7-O-glucoside daidzin (as in soybeans) or the 8-C-glucoside puerarin.
Absorption, Metabolism and Excretion Of Isoflavones
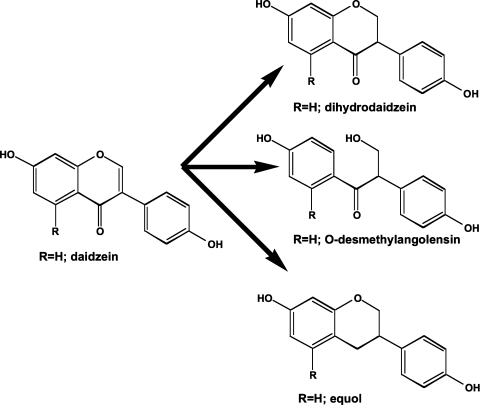
The isoflavone aglycones are readily absorbed in the upper small intestine by passive diffusion, peaking in the blood within an hour of being ingested.19,20 In contrast, the β-glucosides are not passively absorbed; however, they are easily hydrolyzed by β-glucosidases, either from intestinal bacteria, or an enzyme in the intestinal mucosa.21 The latter, lactase-phlorizin hydrolase, is also responsible for lactose hydrolysis.22 Interestingly, its absence in many Asians may mean that the isoflavone β-glucosides are not readily hydrolyzed in the small intestine. The 6”-O-malonyl- or 6”-O-acetyl-7-O-β-glucosides are poorly hydrolyzed by β-glucosidases and therefore enter the large bowel where the microorganism concentrations are much higher. In this more anaerobic environment, not only hydrolysis occurs, but also reductive modifications to the heterocyclic ring producing dihydrodaidzein, O-desmethylangolensin and equol (7,4’-dihydroxyisoflavan) (Fig. 6). Unlike daidzein and genistein, the reduced bacterial metabolites contain a chiral 3-carbon atom. In the case of equol, it is the S-(-)-isomer23 (Fig. 7). This is important since the R-(-)-isomer is 30 times weaker in its affinity for estrogen receptor beta (ERβ).24 Chemically synthesized equol is a racemic mixture of the R- and S-isomers.
FIG. 6.
Bacterial metabolism of daidzein. Daidzein is converted by intestinal microorganisms in most people to form dihydrodaidzein and O-desmethylangolensin. A limited group of people (∼30%) have microorganisms that reduce daidzein to the isoflavan S-(-)-equol. Each of the metabolites has a chiral center at C-3.
FIG. 7.
Orientation of the isoflavone genistein and 17β-estradiol. Genistein has been drawn using the convention of flavonoid investigators (A). This has the A-ring on the left and B-ring on the right. The keto group in the heterocyclic ring points down. Most investigators in isoflavone research have drawn genistein rotated so that the keto group faces up (B). The X-ray crystallographic studies36, however, suggest that the correct orientation when comparing genistein to 17β-estradiol (D), is that obtained by rotating structure horizontally, with the B-ring on the left and the A-ring on the right (C).
Puerarin (daidzein-8-C-glucoside) is different from the O-glucosides. It is resistant to enzymatic hydrolysis because of the C-C link between the glucose and isoflavone moieties. As a consequence, it is transported by glucose transporters in the intestinal mucosal cells. Its chemistry also means that it undergoes very little other metabolism prior to excretion.25
The isoflavone aglucones once they enter the intestinal cell are converted to their β-glucuronides by UDP-glucuronyltransferases20 and to a lesser extent to sulfate esters catalyzed by PAPS-sulfotransferases.26 Glucuronidation and sulfation also occur in the liver.27 These phase II metabolites are excreted in the bile and are deconjugated in the lower bowel allowing them to be reabsorbed again, creating an enterohepatic circulation.20 Sulfate ester formation can also occur in peripheral tissues, particularly in cells obtained from mammary tumors.28,29 Localized metabolism of isoflavones can occur in the immediate vicinity of inflammatory cells. Hypochlorous acid (HOCl) produced by activated neutrophils causes chlorination of isoflavones.30,31 These can also be glucuronidated and excreted into bile. Peroxynitrite (ONO2−), formed by the reaction of nitric oxide ( ) and superoxide (
) and superoxide (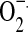 ) generated by activated macrophages, neutrophils and esinophils, causes 3’-nitration of isoflavones in a reaction similar to that observed on tyrosine residues in proteins.30,31 Interestingly, these modifications of genistein and daidzein substantially reduce their affinities to both ERα and ERβ in a reporter gene system.
) generated by activated macrophages, neutrophils and esinophils, causes 3’-nitration of isoflavones in a reaction similar to that observed on tyrosine residues in proteins.30,31 Interestingly, these modifications of genistein and daidzein substantially reduce their affinities to both ERα and ERβ in a reporter gene system.
Mechanisms of Action of Isoflavones
The binding of isoflavones to mammalian estrogen receptors has been known for over 40 years.32 However, compared to physiologic estrogens such as 17β-estradiol, isoflavones have approximately 100 times weaker affinities.33 This is in part offset by the higher circulating isoflavone concentrations. A major difference between endogenous and dietary estrogens is that once made in the ovaries, the former reach responsive tissues in the unconjugated, i.e., biologically active, form whereas dietary estrogens are almost entirely conjugated, even in portal blood just after their absorption from the intestine.20 The concentrations of unconjugated isoflavones are only 1–5% of the total isoflavones in the blood. A similar issue occurs for orally administered estradiol—it has little or no biological effect except at high doses. Pharmacologists overcame this “problem” by replacing the 17α-H with an acetylenic residue (to form ethinylestradiol) to prevent metabolism. This modified steroid is the estrogenic component of many oral contraceptives.
A new chapter in estrogen action was opened in 1996 with the discovery of ERβ.34 While it was related to ERα, which is located on chromosome 6, it was instead present on chromosome 14.35 The ligand-binding sites were highly homologous between ERα and ERβ. However, the few amino acid differences were such that genistein's binding affinity to ERβ was substantially enhanced and this was substantiated by X-ray crystal structure studies.36 It's worth noting that this isn't true for ERβ across all species—in the Zebra fish, a popular new animal model, the amino acid differences between ERα and ERβ do not lead to differences in the affinities of these receptors.37 The structural studies also revealed that the conventional way (as used in Figs. 1, 2, 4, 5, 6, 8, 9) of drawing the structure of genistein when comparing it to 17β-estradiol is wrong; genistein should be reversed so that it is the B-ring that enters the estrogen binding site, not the A-ring36,38 (Fig. 7).
FIG. 8.
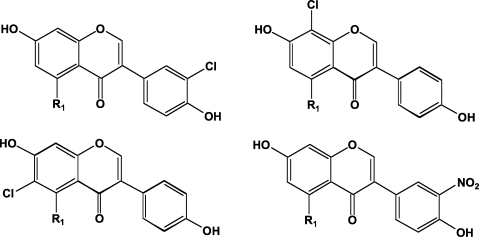
Products of reaction of isoflavones with neutrophil oxidants. Three monochloroisomers (6-, 8- and 3’-Cl) and one mononitro (3’-) have been synthesized30 and tested in biological experiments.31,46 Chloronitrogenistein has also been observed.30 Daidzein R1 = H; genistein R1 = OH.
FIG. 9.
Product of the in vitro reaction between genistein and thyroid peroxidase. Similar to the chloroisoflavones (Fig. 8), thyroid peroxidase iodinates genistein in the 6-, 8- and 3’-positions.
A key point about ERα and ERβ is that they are expressed in varying concentrations in different organs, as well as in different cell types. Some have suggested that isoflavone binding to ERβ is an inhibitory process, not a proliferative one.39 This presents a more complicated scenario for the likely mechanism of action of isoflavones.
A second mechanism of action of isoflavones, particularly of genistein, was discovered in the mid-1980s. Investigators were searching for “safe” inhibitors of protein tyrosine kinases (PTK). A Japanese group grew microorganisms in cell culture and tested the media for PTK activity. An inhibitory activity was detected40 and the following year was identified as genistein.41 Eventually, it was realized that genistein originated from the medium itself since a source of protein in the medium was soy protein. The microorganism caused hydrolysis of the genistein glucoside conjugates. However, although genistein is a PTK inhibitor, and is described as such in hundreds, if not thousands of papers, and there is a critical residue (Tyr537) on ERα that has to be phosphorylated for ERα to undergo nuclear binding42, in most circumstances the prevailing genistein concentrations at target sites are too low for this mechanism to be important in vivo.
Genistein, being a polyphenol, also has antioxidant properties. It can slow the rate of lipid peroxidation by reacting with lipid radicals.43 However, it is not a strong antioxidant since the genistein radical can react with a polyunsaturated lipid to reinitiate the oxidation cycle.43 Genistein may therefore be an antioxidant because of its effects on gene expression of enzymes that react with antioxidants or it can react with secondary oxidants such as HOCl or ONO2− (Fig. 8).
Besides the estrogen receptor, genistein and other isoflavones also interact with the peroxisome proliferator-activated receptors, PPARα/γ.44,45 These are nuclear receptors that are activated by fatty acids (PPARα) and prostaglandins (PPARγ) and serve as transcription factors. Genistein inhibits the tumor necrosis factor α-stimulation of monocyte adhesion to the vascular wall by upregulating PPARγ.45 Daidzein is less active than genistein. Interestingly, 3’-chlorination increased daidzein's agonist effects on PPARγ to those comparable to genistein.46 In contrast, 6- and 8-chlorination of daidzein substantially reduced the agonist activity on PPARγ.46
Several other mechanisms have been proposed for the activities of isoflavones. These include stimulation/inhibition of enzyme activities involved in steroid synthesis and metabolism.47,48 There are extragonadal sites of estrogen synthesis stimulated by genistein.49 However, the effects of isoflavones on plasma estrogen or androgen concentrations are generally small.50 Another enzyme target is thyroid peroxidase—this enzyme converts tri-iodothyronine (T3) to thyroxine (T4). Isoflavones are competitive inhibitors of this reaction.51 Genistein is converted to a tri-iodo derivative (Fig. 9). However, all polyphenols from fruits and vegetables are inhibitors of thyroid peroxidase, many with lower Kms than genistein.52 It is possible in environments where iodine intake is very low that polyphenol-induced reductions in thyroid hormones could occur. However, in the United States table salt and soy-based protein products are supplemented with sodium iodide and the Japanese and Koreans include seaweed and kelp in their diets. Therefore, attempts to demonstrate thyroid deficiency while consuming soy or isoflavones have not been successful.53,54
The use of the ovariectomized athymic nude mouse to address the question of how does isoflavones influence breast cancer cell growth has been of great interest. In this model, addition of genistein to the diet provokes the growth of human, estrogen receptor-positive MCF-7 cells55, whereas daidzein and its bacterial metabolite S-(-)-equol have no effect.56,57 A limitation of this model is that to be able to introduce the human cells in the mouse, the immune system has to be disabled. In intact young mice, transplanted mouse mammary tumor cells are recognized by the immune system and are not allowed to grow. However, in older mice immune tolerance allows the tumor cells to grow.58 Fractionation of the cell medium in which the tumor cells were grown demonstrated that an exosome fraction from the tumor inhibited activation of natural killer (NK) cells by interleukin-2 (IL-2).59 The exosomes fused with the NK cells allowing the proteins in these lipid particles to enter the NK cells. Subsequent investigations revealed that polyphenols, particularly curcumin, restored NK cell response to IL-2.59,60 The mechanism for curcumin's effect appeared to be increase protein ubiquitination in the exosome.59 Hence, when the exosome proteins where transferred to the NK cells they had lowered or no activity since they would be transferred to the proteasome for degradation.
Finally, in attempts to understand genistein's role in the clinical phase of breast cancer, it is important to consider metastasis. Clinically, it is metastasis that is life threatening, not the presence of the primary tumor in the breast. Most of the animal models in which mammary tumors are induced by carcinogens do not have a metastatic component. In an interesting experiment in mice, human breast cancer cells expressing the green fluorescent protein were introduced and allowed to grow.61 The animals after 5 weeks were placed on either an isoflavone-free diet or a diet containing 500 ppm genistein. After five weeks, the lungs of the animals were examined for the presence of the GFP-labeled tumor cells. Genistein had no effect on the size of the tumor, but reduced the number of tumor cells in the lung by 95%.61 This result may explain why unlike American or European women, the incidence of breast cancer in Asian women hardly changes once they reach menopause, a result of soy and its associated isoflavones in their diet.62 These studies need to be urgently carried out in the model of metastasis using breast cancer cells containing an estrogen receptor to see if this interesting result is generalizable to all breast cancers. Comparable studies are being pursued to ascertain the value of genistein in the prevention of metastasis in prostate cancer.63,64
Footnotes
Research in the Barnes' lab at the University of Alabama at Birmingham is supported in part from grants to the Center for Nutrient-Gene Interaction from the National Cancer Institute (U54 CA100949, S. Barnes, PI), to the Purdue University-University of Alabama at Birmingham Botanicals Center for Age-Related Disease from the National Center for Complementary and Alternative Medicine and the National Institutes of Health Office of Dietary Supplements (P50 AT00477, C. Weaver, PI), and the National Center for Complementary and Alternative Medicine (R21 AT004661, S. Barnes, PI).
Acknowledgments
The efforts of Ali Arabshahi, Lori Coward, Dr. Tracy D'Alessandro, Marion Kirk, Dr. T. Greg Peterson, Dr. Jeevan Prasain, Dr. Jeffrey Sfakianos, Michelle Smith-Johnson, Dr. Chao-Cheng Wang and Landon Wilson in the development of methods for the analysis of isoflavones are warmly appreciated. Thanks are also given to Dr. Brenda Boersma, Dr. Victor Darley-Usmar, Dr Helen Kim, Dr. Rakesh Patel and Dr. Huang-Ge Zhang for their contributions to the mechanisms of action of isoflavones.
Author Disclosure Statement
Dr. Barnes is a grant reviewer for the United Soybean Board and serves on the National Advisory Council of the National Center for Complementary and Alternative Medicine. He also holds a U.S. patent on the use of conjugated isoflavones in the prevention of osteoporosis. In the past year he has received honoraria for research presentations organized by the Center for Emerging Issues in Science of the Life Sciences Research Office, the American Institute for Cancer Research, and the Society for Free Radical Biology and Medicine.
References
- 1.Hymowitz T. Soybeans: The success story. In: Janick J., editor; Simon J.E., editor. Advances in new crops. Timber Press; Portland, OR: 1990. pp. 159–163. [Google Scholar]
- 2.Sundquist WB. Cheng C-G. Norton GW. Measuring the Returns to Agricultural Experiment Station Research Expenditures for Corn, Wheat and Soybeans. Department of Agricultural and Applied Economics; 1980. pp. 1–43. Staff Paper P80-20. [Google Scholar]
- 3.Rolfe BG. Flavones and isoflavones as inducing substances of legume nodulation. Biofactors. 1988;1:3–10. [PubMed] [Google Scholar]
- 4.Deavours BE. Dixon RA. Metabolic engineering of isoflavonoid biosynthesis in alfalfa. Plant Physiol. 2005;138:2245–2259. doi: 10.1104/pp.105.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonne AH. Smith M. Weaver DB. Vail T. Barnes S. Wei CI. Retention and changes of soy isoflavones and carotenoids in immature soybean seeds (Edamame) during processing. J Agric Food Chem. 2000;48:6061–6069. doi: 10.1021/jf000247f. [DOI] [PubMed] [Google Scholar]
- 6.Wu Q. Wang M. Sciarappa WJ. Simon JE. LC/UV/ESI-MS analysis of isoflavones in Edamame and Tofu soybeans. J Agric Food Chem. 2004;52:2763–2769. doi: 10.1021/jf035053p. [DOI] [PubMed] [Google Scholar]
- 7.Synder HE. Kwon TW. Soybean utilization. Van Nostrand Reinhold; New York: 1987. Chapter 7. Oriental Soy Food Products; pp. 218–241. [Google Scholar]
- 8.Synder HE. Kwon TW. Soybean utilization. Van Nostrand Reinhold; New York: 1987. Chapter 3. Processing of soybeans; pp. 74–144. [Google Scholar]
- 9.Coward L. Barnes NC. Setchell KDR. Barnes S. The antitumor isoflavones, genistein and daidzein, in soybean foods of American and Asian diets. J Agric Food Chem. 1993;41:1961–1967. [Google Scholar]
- 10.Horn-Ross PL. Barnes S. Lee M. Coward L. Mandel JE. Koo J. John EM. Smith M. Assessing phytoestrogen exposure in epidemiologic studies: development of a database (United States) Cancer Causes Control. 2000;11:289–298. doi: 10.1023/a:1008995606699. [DOI] [PubMed] [Google Scholar]
- 11.Kuo LC. Cheng WY. Wu RY. Huang CJ. Lee KT. Hydrolysis of black soybean isoflavone glycosides by Bacillus subtilis natto. Appl Microbiol Biotechnol. 2006;73:314–320. doi: 10.1007/s00253-006-0474-7. [DOI] [PubMed] [Google Scholar]
- 12.Chun J. Kim GM. Lee KW. Choi ID. Kwon GH. Park JY. Jeong SJ. Kim JS. Kim JH. Conversion of isoflavone glucosides to aglycones in soymilk by fermentation with lactic acid bacteria. J Food Sci. 2007;72:M39–M44. doi: 10.1111/j.1750-3841.2007.00276.x. [DOI] [PubMed] [Google Scholar]
- 13.Esaki H. Kawakishi S. Morimitsu Y. Osawa T. New potent antioxidative o-dihydroxyisoflavones in fermented Japanese soybean products. Biosci Biotechnol Biochem. 1999;63:1637–1639. doi: 10.1271/bbb.63.1637. [DOI] [PubMed] [Google Scholar]
- 14.Barnes S. Kirk M. Coward L. Isoflavones and their conjugates in soy foods: extraction conditions and analysis by HPLC-mass spectrometry. J Agric Food Chem. 1994;42:2466–2474. [Google Scholar]
- 15.Dixon RA. Phytoestrogens. Annu. Rev. Plant Biol. 2004;55:225–261. doi: 10.1146/annurev.arplant.55.031903.141729. [DOI] [PubMed] [Google Scholar]
- 16.Barnes S. Wang C-C. Kirk M. Smith-Johnson M. Coward L. Barnes N.C. Vance G. Boersma B. HPLC-Mass Spectrometry of Isoflavonoids in Soy and the American Groundnut, Apios americana. In: Béla S. Buslig., editor; Manthey John A., editor. “Flavonoids in Cell function”. Kluwer Academic/Plenum Publishers; New York, NY: 2002. pp. 77–88. [DOI] [PubMed] [Google Scholar]
- 17.Qicheng F. Some current study and research approaches relating to the use of plants in the traditional Chinese medicine. J Ethnopharmacol. 1980;2:57–63. doi: 10.1016/0378-8741(80)90031-8. [DOI] [PubMed] [Google Scholar]
- 18.Prasain JK. Jones K. Kirk M. Wilson L. Smith-Johnson M. Weaver C. Barnes S. Profiling and quantification of isoflavonoids in kudzu dietary supplements by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. J Agric Food Chem. 2003;51:4213–4218. doi: 10.1021/jf030174a. [DOI] [PubMed] [Google Scholar]
- 19.King RA. Broadbent JL. Head RJ. Absorption and excretion of the soy isoflavone genistein in rats. J Nutr. 1996;126:176–182. doi: 10.1093/jn/126.1.176. [DOI] [PubMed] [Google Scholar]
- 20.Sfakianos J. Coward L. Kirk M. Barnes S. Intestinal uptake and biliary excretion of the isoflavone genistein in the rat. J Nutr. 1997;127:1260–1268. doi: 10.1093/jn/127.7.1260. [DOI] [PubMed] [Google Scholar]
- 21.Day AJ. Cañada FJ. Díaz JC. Kroon PA. Mclauchlan R. Faulds CB. Plumb GW. Morgan MR. Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468:166–170. doi: 10.1016/s0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- 22.Rivera-Sagredo A. Cañada FJ. Nieto O. Jimenez-Barbero J. Martín-Lomas M. Substrate specificity of small-intestinal lactase. Assessment of the role of the substrate hydroxyl groups. Eur J Biochem. 1992;209:415–422. doi: 10.1111/j.1432-1033.1992.tb17304.x. [DOI] [PubMed] [Google Scholar]
- 23.Muthyala RS. Ju YH. Sheng S. Williams LD. Doerge DR. Katzenellenbogen BS. Helferich WG. Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–1567. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 24.Setchell KD. Clerici C. Lephart ED. Cole SJ. Heenan C. Castellani D. Wolfe BE. Nechemias-Zimmer L. Brown NM. Lund TD. Handa RJ. Heubi JE. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–1079. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- 25.Prasain JK. Jones K. Brissie N. Moore R. Wyss JM. Barnes S. Identification of puerarin and its metabolites in rats by liquid chromatography-tandem mass spectrometry. J Agric Food Chem. 2004;52:3708–3712. doi: 10.1021/jf040037t. [DOI] [PubMed] [Google Scholar]
- 26.Ronis MJ. Little JM. Barone GW. Chen G. Radominska-Pandya A. Badger TM. Sulfation of the isoflavones genistein and daidzein in human and rat liver and gastrointestinal tract. J Med Food. 2006;9:348–355. doi: 10.1089/jmf.2006.9.348. [DOI] [PubMed] [Google Scholar]
- 27.Nakano H. Ogura K. Takahashi E. Harada T. Nishiyama T. Muro K. Hiratsuka A. Kadota S. Watabe T. Regioselective monosulfation, disulfation of the phytoestrogens daidzein, genistein by human liver sulfotransferases. Drug Metab Pharmacokinet. 2004;19:216–226. doi: 10.2133/dmpk.19.216. [DOI] [PubMed] [Google Scholar]
- 28.Peterson TG. Coward L. Kirk M. Falany CN. Barnes S. Isoflavones and breast epithelial cell growth: the importance of genistein and biochanin A metabolism in the breast. Carcinogenesis. 1996;17:1861–1869. doi: 10.1093/carcin/17.9.1861. [DOI] [PubMed] [Google Scholar]
- 29.Peterson TG. Ji G-P. Kirk M. Coward L. Falany CN. Barnes S. Metabolism of the isoflavones genistein and biochanin A in human breast cancer cell lines. Am J Clin Nutr. 1998;68:1505–1511. doi: 10.1093/ajcn/68.6.1505S. [DOI] [PubMed] [Google Scholar]
- 30.Boersma BJ. Patel RP. Kirk M. Darley-Usmar VM. Barnes S. Chlorination and Nitration of Soy Isoflavones. Arch of Biochem Biophys. 1999;368:265–275. doi: 10.1006/abbi.1999.1330. [DOI] [PubMed] [Google Scholar]
- 31.Boersma BJ. D'Alessandro T. Benton MR. Kirk M. Wilson LS. Prasain J. Botting NP. Barnes S. Darley-Usmar VM. Patel RP. Neutrophil myeloperoxidase chlorinates soy isoflavones and enhances their antioxidant properties. Free Rad Biol Med. 2003;35:1417–1430. doi: 10.1016/j.freeradbiomed.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Martin PM. Horwitz KB. Ryan DS. McGuire WL. Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology. 1978;103:1860–1867. doi: 10.1210/endo-103-5-1860. [DOI] [PubMed] [Google Scholar]
- 33.Kuiper GG. Carlsson B. Grandien K. Enmark E. Häggblad J. Nilsson S. Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 34.Kuiper GG. Enmark E. Pelto-Huikko M. Nilsson S. Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enmark E. Pelto-Huikko M. Grandien K. Lagercrantz S. Lagercrantz J. Fried G. Nordenskjöld M. Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 36.Pike AC. Brzozowski AM. Hubbard RE. Bonn T. Thorsell AG. Engström O. Ljunggren J. Gustafsson JA. Carlquist M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 1999;18:4608–4618. doi: 10.1093/emboj/18.17.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sassi-Messai S. Gibert Y. Bernard L. Nishio S. Ferri Lagneau KF. Molina J. Andersson-Lendahl M. Benoit G. Balaguer P. Laudet V. The phytoestrogen genistein affects zebrafish development through two different pathways. PLoS One. 2009;4:e4935. doi: 10.1371/journal.pone.0004935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D'Alessandro T. Boersma B.J. Peterson T.G. Sfakianos J. Prasain J.K. Patel R. Darley-Usmar V.M. Botting N. Barnes S. Metabolism of phytoestrogen conjugates. Meth Enzymol. 2005;400:316–342. doi: 10.1016/S0076-6879(05)00019-4. [DOI] [PubMed] [Google Scholar]
- 39.Matthews J. Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 40.Ogawara H. Akiyama T. Ishida J. Watanabe S. Suzuki K. A specific inhibitor for tyrosine protein kinase from Pseudomonas. J Antibiot (Tokyo). 1986;39:606–608. doi: 10.7164/antibiotics.39.606. [DOI] [PubMed] [Google Scholar]
- 41.Akiyama T. Ishida J. Nakagawa S. Ogawara H. Watanabe S. Itoh N. Shibuya M. Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 42.Arnold SF. Vorojeikina DP. Notides AC. Phosphorylation of tyrosine 537 on the human estrogen receptor is required for binding to an estrogen response element. J Biol Chem. 1995;270:30205–30212. doi: 10.1074/jbc.270.50.30205. [DOI] [PubMed] [Google Scholar]
- 43.Patel RP. Boersma B. Crawford JH. Hogg N. Kirk M. Kalyanaraman B. Parks D. Barnes S. Darley-Usmar V. Antioxidant mechanisms of isoflavones in lipid systems: Paradoxical effects of peroxyl radical scavenging. Free Rad Biol Med, 2001;31:1570–1581. doi: 10.1016/s0891-5849(01)00737-7. [DOI] [PubMed] [Google Scholar]
- 44.Kim S. Shin HJ. Kim SY. Kim JH. Lee YS. Kim DH. Lee MO. Genistein enhances expression of genes involved in fatty acid catabolism through activation of PPARalpha. Mol Cell Endocrinol. 2004;220:51–58. doi: 10.1016/j.mce.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Chacko BK. Chandler RT. Mundhekar A. Pruitt HM. Kucik DF. Kevil CG. Barnes S. Patel RP. Revealing anti-inflammatory mechanisms of soy-isoflavones by flow: modulation of leukocyte-endothelial cell interactions. Am J Physiol. 2005;289:H908–H915. doi: 10.1152/ajpheart.00781.2004. [DOI] [PubMed] [Google Scholar]
- 46.Chacko BK. Chandler RT. D'Alessandro TL. Mundhekar A. Khoo NK. Botting N. Barnes S. Patel RP. Anti-inflammatory effects of isoflavones are dependent on flow and human endothelial cell PPARγ. J Nutr. 2007;137:351–356. doi: 10.1093/jn/137.2.351. [DOI] [PubMed] [Google Scholar]
- 47.Mäkelä S. Poutanen M. Lehtimäki J. Kostian ML. Santti R. Vihko R. Estrogen-specific 17 beta-hydroxysteroid oxidoreductase type 1 (E.C. 1.1.1.62) as a possible target for the action of phytoestrogens. Proc Soc Exp Biol Med. 1995;208:51–59. doi: 10.3181/00379727-208-43831. [DOI] [PubMed] [Google Scholar]
- 48.Brooks JD. Thompson LU. Mammalian lignans and genistein decrease the activities of aromatase and 17beta-hydroxysteroid dehydrogenase in MCF-7 cells. J Steroid Biochem Mol Biol. 2005;94:461–467. doi: 10.1016/j.jsbmb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Ye L. Chan MY. Leung LK. The soy isoflavone genistein induces estrogen synthesis in an extragonadal pathway. Mol Cell Endocrinol. 2009;302:73–80. doi: 10.1016/j.mce.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Hooper L. Ryder JJ. Kurzer MS. Lampe JW. Messina MJ. Phipps WR. Cassidy A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:423–440. doi: 10.1093/humupd/dmp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Divi RL. Chang HC. Doerge DR. Anti-thyroid isoflavones from soybean: isolation, characterization, and mechanisms of action. Biochem Pharmacol. 1997;54:1087–1096. doi: 10.1016/s0006-2952(97)00301-8. [DOI] [PubMed] [Google Scholar]
- 52.Divi RL. Doerge DR. Inhibition of thyroid peroxidase by dietary flavonoids. Chem Res Toxicol. 1996;9:16–23. doi: 10.1021/tx950076m. [DOI] [PubMed] [Google Scholar]
- 53.Chang HC. Doerge DR. Dietary genistein inactivates rat thyroid peroxidase in vivo without an apparent hypothyroid effect. Toxicol Appl Pharmacol. 2000;168:244–252. doi: 10.1006/taap.2000.9019. [DOI] [PubMed] [Google Scholar]
- 54.Messina M. Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid. 2006;16:249–258. doi: 10.1089/thy.2006.16.249. [DOI] [PubMed] [Google Scholar]
- 55.Hsieh CY. Santell RC. Haslam SZ. Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998;58:3833–3838. [PubMed] [Google Scholar]
- 56.Allred CD. Allred KF. Ju YH. Virant SM. Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61:5045–5050. [PubMed] [Google Scholar]
- 57.Ju YH. Fultz J. Allred KF. Doerge DR. Helferich WG. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis. 2006;27:856–863. doi: 10.1093/carcin/bgi320. [DOI] [PubMed] [Google Scholar]
- 58.Grizzle WE. Xu X. Zhang S. Stockard CR. Liu C. Yu S. Wang J. Mountz JD. Zhang HG. Age-related increase of tumor susceptibility is associated with myeloid-derived suppressor cell mediated suppression of T cell cytotoxicity in recombinant inbred BXD12 mice. Mech Ageing Dev. 2007;128:672–680. doi: 10.1016/j.mad.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H-G. Kim H. Liu C. Yu S. Wang J. Zhang L. Grizzle WE. Kimberly RP. Barnes S. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochimica et Biophysica Acta. 2007;1773:1116–1123. doi: 10.1016/j.bbamcr.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnes S. Zhang HG. Liu CR. Liu P. Yu SH. Prasain JK. Polyphenols inhibit breast cancer cell exosome regulation of T cell and natural killer cell response. FASEB J. 2005;19:A1461. [Google Scholar]
- 61.Vantyghem SA. Wilson SM. Postenka CO. Al-Katib W. Tuck AB. Chambers AF. Dietary genistein reduces metastasis in a postsurgical orthotopic breast cancer model. Cancer Res. 2005;65:3396–3403. doi: 10.1158/0008-5472.CAN-04-4109. [DOI] [PubMed] [Google Scholar]
- 62.Chia KS. Reilly M. Tan CS. Lee J. Pawitan Y. Adami HO. Hall P. Mow B. Profound changes in breast cancer incidence may reflect changes into a Westernized lifestyle: a comparative population-based study in Singapore and Sweden. Int J Cancer. 2005;113:302–306. doi: 10.1002/ijc.20561. [DOI] [PubMed] [Google Scholar]
- 63.Lakshman M. Xu L. Ananthanarayanan V. Cooper J. Takimoto CH. Helenowski I. Pelling JC. Bergan RC. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008;68:2024–2032. doi: 10.1158/0008-5472.CAN-07-1246. [DOI] [PubMed] [Google Scholar]
- 64.Chambers AF. Influence of diet on metastasis and tumor dormancy. Clin Exp Metastasis. 2009;26:61–66. doi: 10.1007/s10585-008-9164-4. [DOI] [PubMed] [Google Scholar]