Abstract
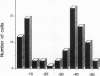
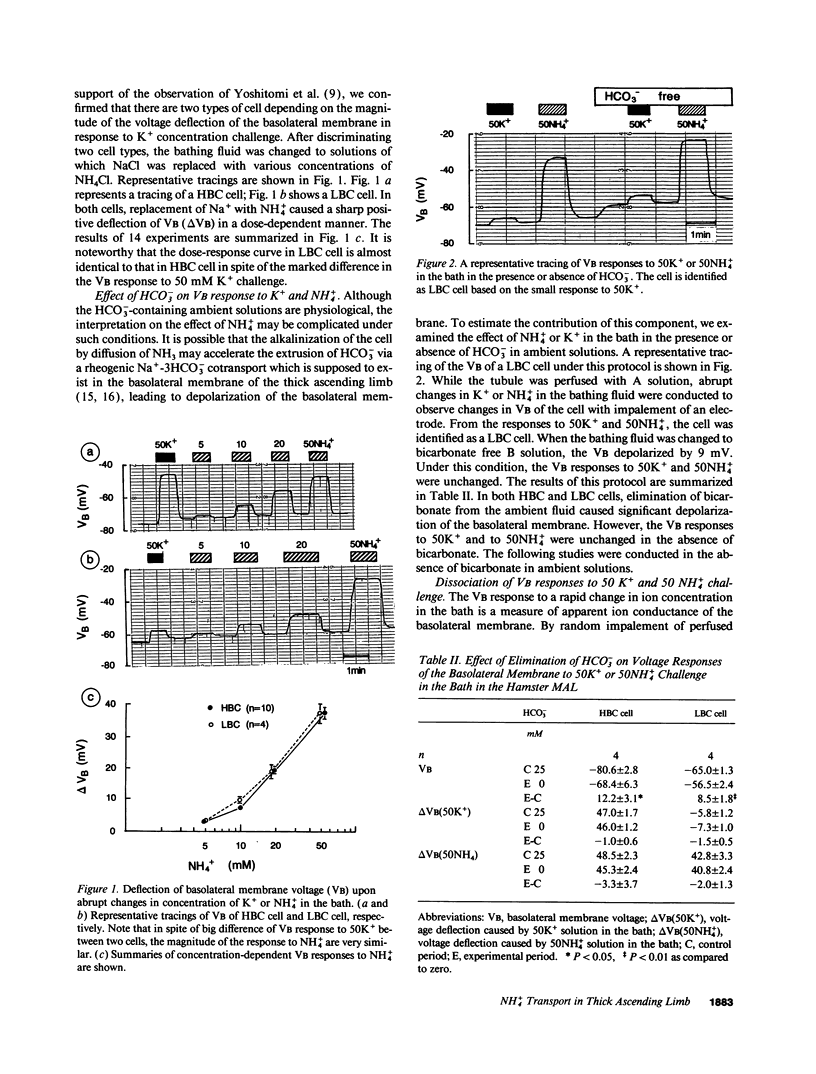
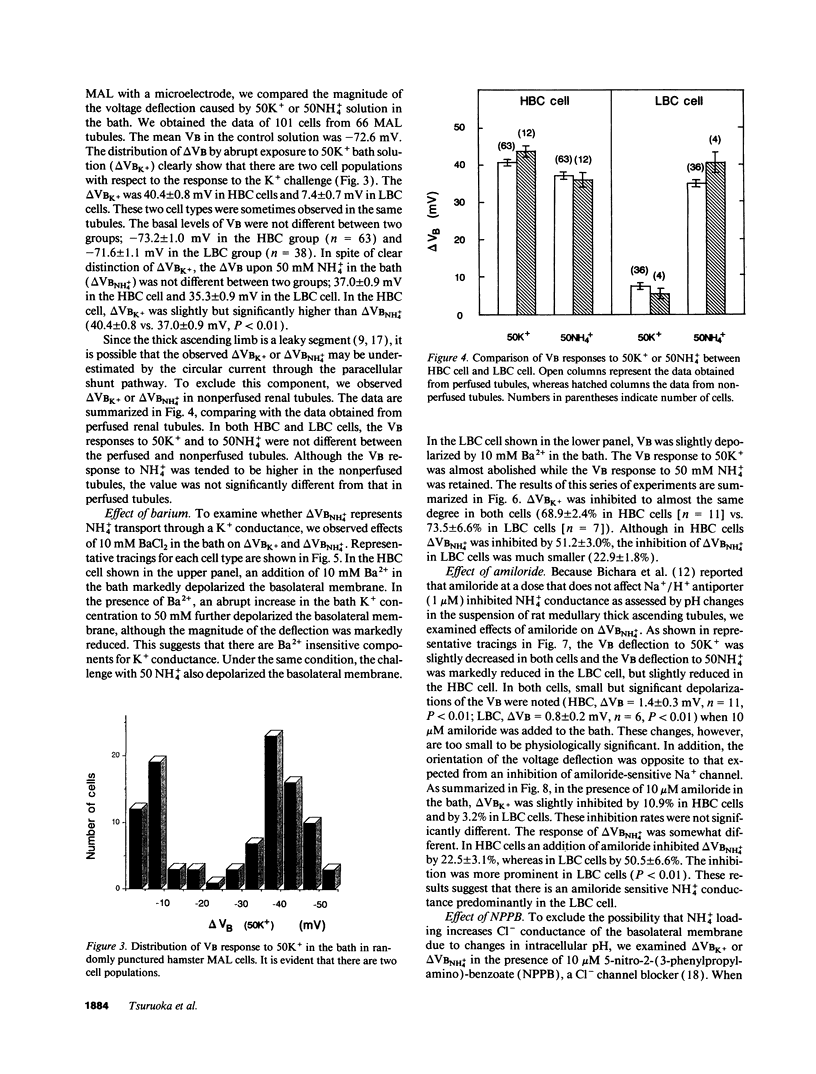
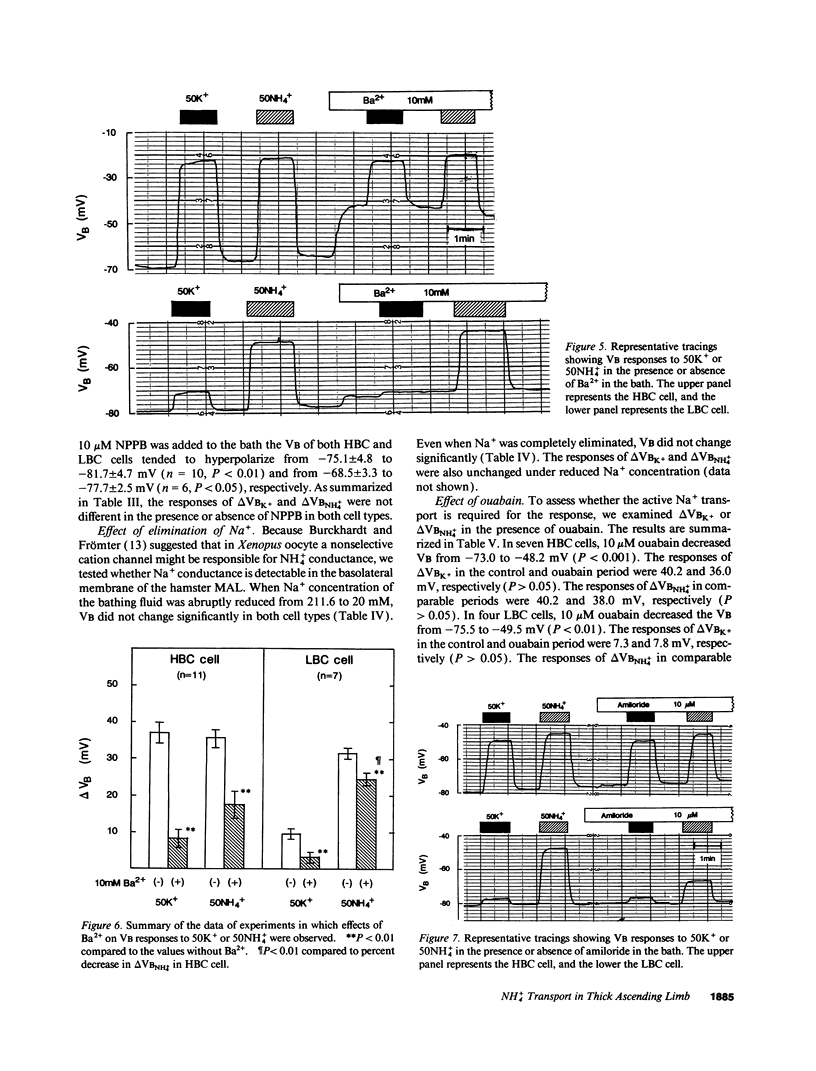
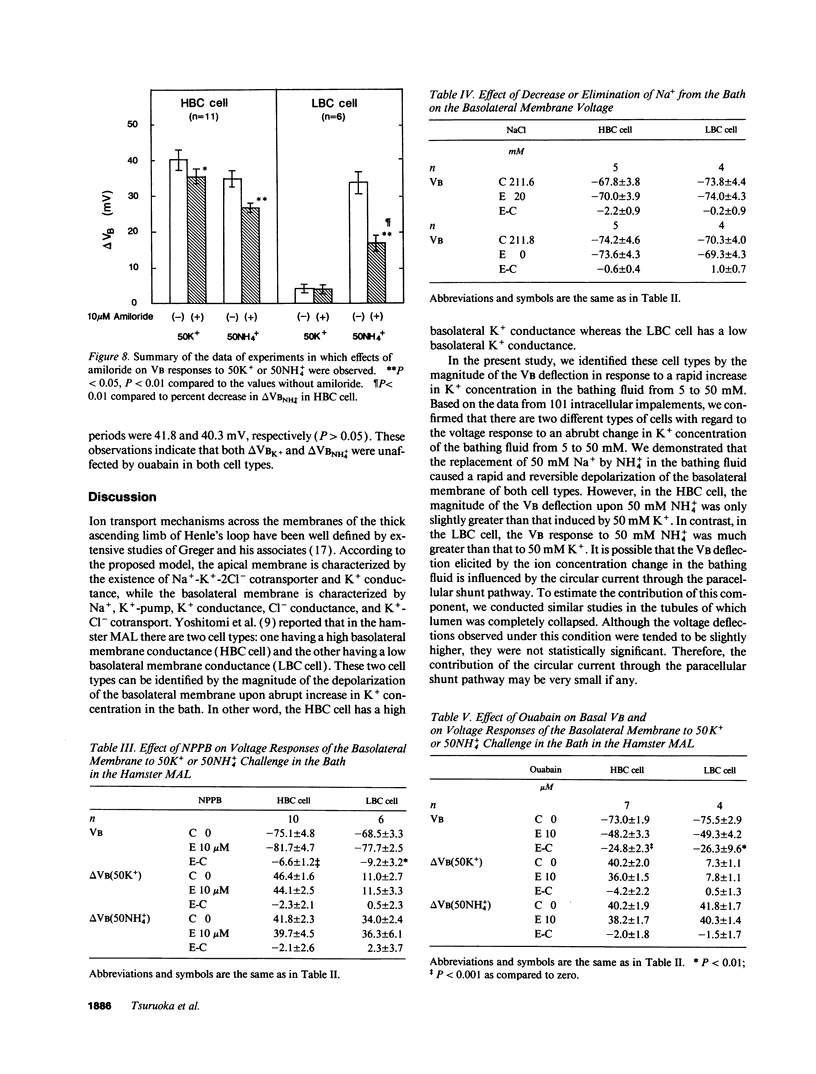
The epithelia of the medullary thick ascending limb (MAL) consists of two cell types, high (HBC) and low basolateral conductance (LBC) cell, depending on the K+ conductance of the basolateral membrane. The NH4+ conductance distinct from the K+ conductance has been suggested to exist in the proximal tubule, MAL cell, and Xenopus oocyte. The present study was designed to examine whether there is a conductive NH4+ transport system distinct from K+ conductance in two different cell types of the hamster MAL perfused in vitro. The basolateral membrane voltage (VB) was measured by impaling cells with conventional microelectrodes. Addition of NH4+ to the bath depolarized VB in a dose-dependent manner in both cell types. The response was maintained in the absence of HCO3-. When the VB deflection elicited upon 50 mM KCl or NH4Cl in the bath (delta VBK+ or delta VBNH4+) were compared, delta VBNH4+ was almost the same as delta VBK+ in the HBC cell, whereas the former was greater than the latter in the LBC. In the HBC cell, 10 mM Ba2+ in the bath equally suppressed both delta VBK+ and delta VBNH4+, whereas in the LBC cell it suppressed delta VBK+ with a small effect on delta VBNH4+, indicating that NH4+ is transported via a pathway distinct from Ba(2+)-sensitive K+ conductance. The VB deflection by NH4+ was unaffected by addition of 0.1 mM ouabain or 10 microM 5-nitro-2-(3-phenylpropylamino)-benzoate (a Cl- channel blocker) to the bath, excluding the contribution of the Na+, K+ pump or Cl- channel. An abrupt reduction of Na+ in the bath from 200 to 20 mM did not cause any changes in VB, suggesting that a nonselective cation channel may not account for the NH4+ transport. Amiloride at 10 microM inhibited delta VBNH4+ with a higher efficacy in the LBC cell. We conclude that a rheogenic NH4+ transport system independent from the K+ conductance exists in the basolateral membrane of the LBC cell of the hamster MAL, and may play some roles in the regulation of NH4+ transport.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleich M., Schlatter E., Greger R. The luminal K+ channel of the thick ascending limb of Henle's loop. Pflugers Arch. 1990 Jan;415(4):449–460. doi: 10.1007/BF00373623. [DOI] [PubMed] [Google Scholar]
- Burckhardt B. C., Frömter E. Pathways of NH3/NH4+ permeation across Xenopus laevis oocyte cell membrane. Pflugers Arch. 1992 Jan;420(1):83–86. doi: 10.1007/BF00378645. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Discala F., Hulin P., Belachgar F., Planelles G., Edelman A., Anagnostopoulos T. Millimolar amiloride concentrations block K conductance in proximal tubular cells. Br J Pharmacol. 1992 Oct;107(2):532–538. doi: 10.1111/j.1476-5381.1992.tb12779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin J. L., Burg M. B., Knepper M. A. Active NH4+ absorption by the thick ascending limb. Am J Physiol. 1988 Jul;255(1 Pt 2):F57–F65. doi: 10.1152/ajprenal.1988.255.1.F57. [DOI] [PubMed] [Google Scholar]
- Good D. W. Active absorption of NH4+ by rat medullary thick ascending limb: inhibition by potassium. Am J Physiol. 1988 Jul;255(1 Pt 2):F78–F87. doi: 10.1152/ajprenal.1988.255.1.F78. [DOI] [PubMed] [Google Scholar]
- Good D. W., Knepper M. A. Ammonia transport in the mammalian kidney. Am J Physiol. 1985 Apr;248(4 Pt 2):F459–F471. doi: 10.1152/ajprenal.1985.248.4.F459. [DOI] [PubMed] [Google Scholar]
- Good D. W., Knepper M. A., Burg M. B. Ammonia and bicarbonate transport by thick ascending limb of rat kidney. Am J Physiol. 1984 Jul;247(1 Pt 2):F35–F44. doi: 10.1152/ajprenal.1984.247.1.F35. [DOI] [PubMed] [Google Scholar]
- Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev. 1985 Jul;65(3):760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- Guggino W. B. Functional heterogeneity in the early distal tubule of the Amphiuma kidney: evidence for two modes of Cl- and K+ transport across the basolateral cell membrane. Am J Physiol. 1986 Mar;250(3 Pt 2):F430–F440. doi: 10.1152/ajprenal.1986.250.3.F430. [DOI] [PubMed] [Google Scholar]
- Kikeri D., Azar S., Sun A., Zeidel M. L., Hebert S. C. Na(+)-H+ antiporter and Na(+)-(HCO3-)n symporter regulate intracellular pH in mouse medullary thick limbs of Henle. Am J Physiol. 1990 Mar;258(3 Pt 2):F445–F456. doi: 10.1152/ajprenal.1990.258.3.F445. [DOI] [PubMed] [Google Scholar]
- Kikeri D., Sun A., Zeidel M. L., Hebert S. C. Cell membranes impermeable to NH3. Nature. 1989 Jun 8;339(6224):478–480. doi: 10.1038/339478a0. [DOI] [PubMed] [Google Scholar]
- Kinne R., Kinne-Saffran E., Schütz H., Schölermann B. Ammonium transport in medullary thick ascending limb of rabbit kidney: involvement of the Na+,K+,Cl(-)-cotransporter. J Membr Biol. 1986;94(3):279–284. doi: 10.1007/BF01869723. [DOI] [PubMed] [Google Scholar]
- Knepper M. A. NH4+ transport in the kidney. Kidney Int Suppl. 1991 Jul;33:S95–102. [PubMed] [Google Scholar]
- Krapf R. Basolateral membrane H/OH/HCO3 transport in the rat cortical thick ascending limb. Evidence for an electrogenic Na/HCO3 cotransporter in parallel with a Na/H antiporter. J Clin Invest. 1988 Jul;82(1):234–241. doi: 10.1172/JCI113576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S., Ishibashi K., Nagai T., Marumo F. Regulation mechanisms of intracellular pH of Xenopus laevis oocyte. Biochim Biophys Acta. 1992 Oct 6;1137(1):45–51. doi: 10.1016/0167-4889(92)90098-v. [DOI] [PubMed] [Google Scholar]
- Völkl H., Lang F. Electrophysiology of ammonia transport in renal straight proximal tubules. Kidney Int. 1991 Dec;40(6):1082–1089. doi: 10.1038/ki.1991.318. [DOI] [PubMed] [Google Scholar]
- Wangemann P., Wittner M., Di Stefano A., Englert H. C., Lang H. J., Schlatter E., Greger R. Cl(-)-channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship. Pflugers Arch. 1986;407 (Suppl 2):S128–S141. doi: 10.1007/BF00584942. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Koseki C., Taniguchi J., Imai M. Functional heterogeneity in the hamster medullary thick ascending limb of Henle's loop. Pflugers Arch. 1987 May;408(6):600–608. doi: 10.1007/BF00581162. [DOI] [PubMed] [Google Scholar]