Abstract
Background
Cystatin C has been proposed to better estimate renal function and predict cardiovascular disease (CVD) than serum creatinine. To expand on our previous report, we investigated whether the relationship of cystatin C to progression of coronary artery atherosclerosis (CA) differed between individuals with type 1 diabetes (T1D) and persons without diabetes.
Methods
Coronary artery calcium was measured twice over 2.4 ± 0.4 years (n = 1,123, age = 39 ± 9 years, 47% male, 45% T1D). Significant CA progression was defined as a ≥2.5 increase in square root calcium volume score or development of clinical coronary artery disease. Stepwise multiple logistic regression was performed to investigate whether the association of cystatin C to CA progression differed by T1D status.
Results
The main finding and novelty of this article is that while the univariate association of cystatin C to CA progression was similar in T1D patients and persons without diabetes mellitus and in the expected direction (increased cystatin C as a biomarker of worsening renal function associated with CA progression), the association of cystatin C to progression of CA differed by T1D status (P = 0.01) after adjustment for other CVD risk factors. Unexpectedly, in persons without diabetes mellitus having relatively normal renal function, increased cystatin C was associated with decreased CA progression (odd ratio [OR] = 0.65, 95% confidence interval 0.44–0.96, P = 0.029) after adjustment, primarily due to adjustment for body mass index (BMI). Removal of BMI from this model resulted in a 49% change in the OR.
Conclusions
Our hypothesis-generating data suggest a complex relationship among cystatin C, BMI, and CA progression that requires further study.
Introduction
Extensive literature exists on renal disease as a cardiovascular disease (CVD) risk factor in persons with and without type 1 diabetes (T1D).1–5 Data suggest that cystatin C may be a better estimator of renal function than serum creatinine.6–11 Cystatin C was less influenced by age, sex, and race than serum creatinine as an estimate of glomerular filtration rate (GFR) in a large cohort with chronic kidney disease12 and in longitudinal data in young adults with T1D and normal renal function at baseline.11 Cystatin C has been proposed to better estimate the slope in decline in GFR than serum creatinine and therefore to better detect trends in change in GFR to allow for clinical intervention.13,14
Numerous epidemiological investigations have reported that cystatin C as a biomarker of renal function better predicts CVD events and death than serum creatinine (or serum creatinine-based estimates of GFR).15–19 Consistent with these studies, we reported that in T1D subjects in the Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study, elevated cystatin C (indicating worsening GFR) was associated with an increased risk of progression of coronary artery atherosclerosis (CA).20
Therefore, our aim was to expand on our previous report in only individuals with T1D20 to investigate whether differences exist between T1D patients and subjects without diabetes mellitus (DM) (who had relatively normal renal function) in the relationship of cystatin C to progression of CA in the CACTI Study cohort.
Subjects and Methods
Study participants
The data presented in this report were collected as part of the baseline examination of 1,416 participants in the CACTI Study who were 19–56 years of age and included 652 participants with T1D and 764 participants without DM.21 All participants were asymptomatic for coronary artery disease (CAD) and had no history of coronary artery bypass graft, coronary angioplasty, or unstable angina. Participants with diabetes generally had been diagnosed when <30 years of age or had positive antibodies or a clinical course consistent with T1D. Of the 1,416 persons enrolled at baseline, 1,355 (93%) had available stored serum to measure cystatin C. Of these, 1,123 (83%) had data on progression of CA (mean follow-up time, 2.5 ± 0.4 years). Participants who did not have follow-up data were younger at baseline than those with follow-up data. All participants provided informed consent, and the study was approved by the Colorado Combined Institutional Review Board.
Examination and laboratory measurements
Participants completed the baseline examination between March 2000 and April 2002, and a more detailed description of the study and baseline characteristics of this cohort including collection of anthropometric, demographic, and laboratory data has been published.22 Insulin resistance was approximated as the inverse of the estimated glucose disposal rate.23 GFR (or creatinine clearance) was estimated by both the Cockcroft-Gault formula (GFRCG)24 and the Modification of Diet in Renal Disease equation (GFRMDRD),25 both based on serum creatinine. We also used the Mayo Clinic (GFRMC) equation.26
Laboratory analyses
After an overnight fast, blood was collected, centrifuged, and separated. Serum was stored at −70°C until assayed. Cystatin C was measured on stored serum samples in the clinical laboratory at University of Colorado Hospital, Denver, CO, using a commercially available particle enhanced immunonephelometric assay (Dade-Behring, Newark, DE). Stored samples from the subjects' baseline study visit had previously been thawed once. The coefficient of variation was 3.3%. Intra-assay precision is 2.3–4.1%, and inter-assay precision is 2.6–3.3%, per the package insert. Results are reported in mg/L with a sensitivity cutoff of 0.23 mg/L.
Imaging
All participants underwent two electron beam tomography scans using an Imatron (South San Francisco, CA) C-150 Ultrafast CT scanner within 5 min without contrast at baseline and two scans at follow-up as previously described22 using the standard acquisition protocol.27 The volume scores were calculated using the volumetric method, which is based on isotropic interpolation.28
Definition of CA progression and coronary artery calcification (CAC) progression
In this study, we defined CAC progression as reported by Hokanson,29 who noted that bias in the interscan variability of calcium volume score (CVS) values exists such that the variability increases as levels of coronary calcium increase; a difference between baseline and follow-up square root transformed CVS ≥2.5 is used to signify significant change in CVS because a change of this magnitude is <1% likely to be due to interscan variability. In addition, participants who had a CAD event (myocardial infarction, coronary artery bypass graft, angioplasty with stent, or death attributed to CAD as adjudicated by a three physician committee) were also considered as having CA progression based on having had a CAD event.
Statistical methods
Data are presented as arithmetic means and SDs for continuous variables (geometric means and ranges for log-transformed variables) and percentages for categorical variables stratified by T1D and CA progression status (see Table 1). To investigate whether the association of cystatin C to CA progression differed by T1D status, stepwise multiple logistic regression in a combined model was performed including a cystatin C × T1D interaction term with a value of P < 0.1 as the criteria for entry and removal from the model. Age, baseline CVS, and sex were forced into all models. All variables in Table 1 were considered for inclusion in the stepwise model predicting CA progression. This model is presented with variables added sequentially: first, demographics (age, sex) and baseline CVS; second, body mass index (BMI), systolic blood pressure (SBP), and high-density lipoprotein (HDL), all CVD risk factors that entered the stepwise model; and, finally, to account for possible differences by T1D status, T1D interactions with all variables if their P values were <0.1 (see Table 2). Next, stepwise multiple logistic regression was performed in a model in the subjects with DM only, and each variable was removed to assess its role as a confounder (see Table 3). Cystatin C was also examined by quartiles, and its association to CA progression was approximately linear. Odds ratio (OR) values are presented per SD change in the predictor variables.
Table 1.
Baseline Characteristics of Subjects with Cystatin C Measurements (n = 1,123), Stratified by T1D and CA Progression Status
| |
T1D |
Non-DM |
|
|
||||
|---|---|---|---|---|---|---|---|---|
| Variable | Progressor (n = 131) | Nonprogressor (n = 377) | P valuea | Progressor (n = 64) | Nonprogressor (n = 551) | P valuea | P valueb | P valuec |
| Age (years) | 43.4 ± 7.8 | 34.8 ± 8.4 | <0.0001 | 46.7 ± 7.4 | 39.1 ± 8.6 | <0.0001 | 0.006 | <0.0001 |
| Female/male (%) | 42%/58% | 58%/42% | 0.002 | 19%/81% | 53%/47% | <0.0001 | 0.0005 | 0.25 |
| Race (non-Hispanic white %) | 94% | 95% | 0.55 | 92% | 85% | 0.10 | 0.66 | <0.0001 |
| Duration of diabetes (years) | 29.3 ± 8.5 | 21.2 ± 8.2 | <0.0001 | NA | NA | NA | NA | NA |
| Baseline square root CVS | 7.7 ± 10.0 | 1.0 ± 3.0 | <0.0001 | 5.9 ± 8.3 | 0.5 ± 1.6 | <0.0001 | 0.20 | 0.008 |
| Cystatin C (mg/L) | 1.01 ± 0.64 | 0.78 ± 0.16 | <0.0001 | 0.81 ± 0.11 | 0.78 ± 0.10 | 0.02 | 0.0006 | 0.81 |
| Serum creatinine (mg/dL) | 1.2 (1.1–1.4) | 1.1 (1.0–1.3) | <0.0001 | 1.2 (1.1–1.4) | 1.2 (1.1–1.3) | 0.009 | 0.03 | 0.16 |
| AER (μg/min) | 10.2 (5.3–31.3) | 5.6 (3.9–11.0) | <0.0001 | 4.2 (3.1–5.6) | 4.1 (3.1–5.7) | 0.63 | <0.0001 | <0.0001 |
| GFRCG (mL/min/1.73 m2) | 80.6 ± 33.3 | 88.8 ± 23.9 | 0.003 | 96.3 ± 22.1 | 83.3 ± 21.0 | <0.0001 | 0.0001 | 0.0004 |
| GFRMDRD (mL/min/1.73 m2) | 59.2 ± 17.9 | 66.5 ± 12.8 | <0.0001 | 65.0 ± 9.6 | 64.6 ± 10.0 | 0.76 | 0.003 | 0.02 |
| GFRMC (mL/min/1.73 m2) | 84.3 ± 28.4 | 102.1 ± 21.1 | <0.0001 | 86.9 ± 17.3 | 96.9 ± 18.0 | <0.0001 | 0.43 | 0.0001 |
| HbA1c (%) | 8.1 ± 1.2 | 7.7 ± 1.3 | 0.10 | 5.7 ± 0.5 | 5.4 ± 0.4 | 0.0001 | <0.0001 | <0.0001 |
| Insulin dose (Units/kg/day) | 0.57 ± 0.27 | 0.61 ± 0.26 | 0.12 | NA | NA | NA | NA | NA |
| BMI (kg/m2) | 26.7 ± 4.6 | 25.9 ± 4.1 | 0.07 | 29.9 ± 5.8 | 25.6 ± 4.5 | <0.0001 | 0.002 | 0.26 |
| Average waist (cm) | 88.6 ± 12.3 | 83.7 ± 11.9 | <0.0001 | 100 ± 13.8 | 84.3 ± 13.5 | <0.0001 | <0.0001 | 0.42 |
| Fat at L4–5 (cm2) | ||||||||
| Visceral | 10.5 ± 0.6 | 10.3 ± 0.6 | 0.0001 | 11.1 ± 0.6 | 10.6 ± 0.6 | <0.0001 | <0.0001 | <0.0001 |
| Subcutaneous | 11.7 ± 0.7 | 11.7 ± 0.6 | 0.74 | 12.0 ± 0.6 | 11.7 ± 0.6 | <0.0001 | 0.004 | 0.69 |
| 1/EGDR | 0.15 ± 0.05 | 0.12 ± 0.05 | <0.0001 | 0.12 ± 0.03 | 0.10 ± 0.02 | <0.0001 | <0.0001 | <0.0001 |
| SBP (mm Hg) | 125 ± 14 | 114 ± 12 | 0.0001 | 120 ± 11 | 114 ± 12 | 0.0001 | 0.04 | 0.36 |
| DBP (mm Hg) | 79 ± 9 | 77 ± 8 | 0.009 | 84 ± 8 | 79 ± 8 | 0.0001 | 0.0005 | 0.0005 |
| Hypertension (yes/no) | 64% | 33% | <0.0001 | 32% | 13% | <0.0001 | 0.002 | |
| Total cholesterol (mg/dL) | 176 ± 32 | 173 ± 33 | 0.28 | 200 ± 40 | 190 ± 36 | 0.048 | <0.0001 | <0.0001 |
| LDL-cholesterol (mg/dL) | 102 ± 26 | 98 ± 29 | 0.28 | 125 ± 35 | 114 ± 33 | 0.03 | <0.0001 | <0.0001 |
| HDL-cholesterol (mg/dL) | 55 ± 17 | 57 ± 16 | 0.40 | 44 ± 12 | 52 ± 15 | <0.0001 | <0.0001 | <0.0001 |
| Triglycerides (mg/dL) | 83 (68–119) | 75 (58–102) | 0.007 | 142 (89–193) | 100 (73–144) | 0.0001 | <0.0001 | <0.0001 |
| CRP (μg/mL) | 2.2 ± 3.0 | 1.9 ± 2.0 | 0.28 | 1.7 ± 1.1 | 1.8 ± 1.9 | 0.61 | 0.10 | 0.45 |
| PAI-1 (ng/mL) | 19.1 ± 25.1 | 16.1 ± 22.0 | 0.19 | 42.0 ± 39.4 | 24.9 ± 24.8 | 0.0003 | <0.0001 | <0.0001 |
| Fibrinogen (mg/dL) | 275 ± 63 | 257 ± 62 | 0.005 | 273 ± 58 | 261 ± 61 | 0.10 | 0.80 | 0.50 |
| Homocysteine (μmol/L) | 8.6 (6.9–10.6) | 7.2 (6.2–8.73) | <0.0001 | 8.6 (7.6–10.3) | 7.9 (6.7–9.6) | 0.004 | 0.68 | <0.0001 |
| Alcohol (drinks/month) | 13.2 ± 24.2 | 13.7 ± 25.2 | 0.83 | 25.3 ± 38.2 | 18.1 ± 27.9 | 0.16 | 0.02 | 0.01 |
| Smoking | ||||||||
| Current | 15% | 9% | 0.10 | 8% | 9% | 0.70 | 0.14 | 0.94 |
| Ever | 24% | 18% | 0.09 | 22% | 22% | 0.98 | 0.68 | 0.11 |
Data are mean ± SD values, percentages, or median (interquartile range). CRP, C-reactive protein; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; LDL, low-density lipoprotein; NA, not applicable.
P value comparing progression status within T1D or non-DM strata.
P value comparing T1D progressors to non-DM progressors.
P value comparing T1D nonprogressors to non-DM nonprogressors.
Table 2.
OR for Progression of CA per SD Increase in Cystatin C in Stepwise Multiple Logistic Regression, Combined Models with a Cystatin C × T1D Interaction Term Included
| |
OR (95% CI); P value |
|
|
|---|---|---|---|
| Model | T1D | Non-T1D | Cystatin C × T1D P value |
| Model 1: Cystatin C, T1D, cystatin C × T1D status | 1.26 (1.15–1.38); <0.0001 | 1.33 (1.04–1.69); 0.029 | 0.70 |
| Model 2: Model 1 + age, baseline CVS, sex | 1.16 (1.06–1.28); 0.002 | 1.03 (0.78–1.38); 0.82 | 0.44 |
| Model 3: Model 2 + BMI, SBP, HDL | 1.14 (1.04–1.27); 0.007 | 0.81 (0.59–1.10); 0.17 | 0.03 |
| Model 4: Model 3 + BMI × T1D, SBP × T1D, sex × T1D | 1.13 (1.03–1.26); 0.01 | 0.73 (0.52–1.02); 0.067 | 0.01 |
Table 3.
Predictors of CA Progression in Subjects Without DM, Best Model from Stepwise Multiple Logistic Regression Analysis (n = 553) in the Subset with AER Data
| Variable | OR, 95% CIa | P value |
|---|---|---|
| Age | 2.31 (1.49–3.56) | 0.0002 |
| Baseline CVS | 2.21 (1.56–3.15) | <0.001 |
| Sex | 3.57 (1.61–7.94) | 0.002 |
| Cystatin C | 0.65 (0.44–0.96) | 0.029 |
| BMI | 2.88 (1.98–4.20) | <0.0001 |
| AER | 0.68 (0.45–1.02) | 0.059 |
OR and 95% CI are per SD of each variable: cystatin C = 0.10 mg/L, age = 8.8 years, CVS = 3.6 Agatston units (square root transformed), sex = male, BMI = 4.9 kg/m2, AER = 1.9 μg/min (log transformed).
Because covariate adjustment had different effects in the non-DM model versus the T1D model, we next examined the correlation between cystatin C and each of the covariates stratified by diabetes status. Fisher Z transformations were used to test for significant differences between correlation coefficients for T1D patients versus subjects without DM (see Table 4). Covariates with a correlation coefficient value of P < 0.1 for either T1D patients or subjects without DM were entered into backward elimination linear regression models stratified by T1D status. Variables with P < 0.05 in either stratified model were then forced into both models, and Wald tests were used to compare estimates from the two models (i.e., to test for interaction). We used stratified models for these analyses to allow the residual variance to differ by T1D status. Beta coefficients for each covariate standardized to the entire cohort and their respective P values were calculated for the stratified models (see Table 5).
Table 4.
Correlation Coefficients (R) and P Values for Cystatin C, Stratified by Diabetes Status
| |
T1D |
Non-DM |
|
||
|---|---|---|---|---|---|
| Variable | R | P value | R | P value | T1D interaction |
| Age | 0.19 | <0.0001 | 0.14 | 0.0007 | 0.39 |
| Gender | 0.11 | 0.02 | 0.22 | <0.0001 | 0.046a |
| Non-Hispanic white race | −0.04 | 0.39 | −0.08 | 0.048 | 0.49 |
| Diabetes' duration | 0.21 | <0.001 | NA | NA | NA |
| HbA1c | 0.04 | 0.32 | 0.14 | 0.0008 | 0.13 |
| Insulin dose | −0.11 | 0.02 | NA | NA | NA |
| BMI | −0.06 | 0.17 | 0.25 | <0.0001 | <0.0001 |
| Average waist | 0.03 | 0.51 | 0.33 | <0.0001 | <0.0001 |
| Fat | |||||
| Visceral | 0.04 | 0.32 | 0.33 | <0.0001 | <0.0001 |
| Subcutaneous | −0.10 | 0.03 | 0.15 | 0.0003 | <0.0001 |
| 1/EGDR | 0.18 | <0.0001 | 0.23 | <0.0001 | 0.35 |
| SBP | 0.33 | <0.0001 | 0.22 | <0.0001 | 0.04a |
| DBP | 0.14 | 0.002 | 0.25 | <0.0001 | 0.06 |
| Total cholesterol | 0.04 | 0.35 | −0.02 | 0.62 | 0.30 |
| LDL | 0.02 | 0.64 | −0.03 | 0.52 | 0.44 |
| HDL | −0.05 | 0.30 | −0.25 | <0.0001 | 0.0005 |
| Triglyceridesb | 0.18 | <0.0001 | 0.25 | <0.0001 | 0.20 |
| CRP | 0.07 | 0.13 | 0.07 | 0.06 | 0.89 |
| PAI-1 | 0.04 | 0.34 | 0.24 | <0.0001 | 0.0006 |
| Fibrinogen | 0.18 | <0.0001 | 0.10 | 0.01 | 0.046a |
| Serum creatinineb | 0.83 | <0.0001 | 0.45 | <0.0001 | <0.0001 |
| AERb | 0.48 | <0.0001 | −0.002 | 0.96 | <0.0001 |
| GFRCG | −0.39 | <0.0001 | −0.06 | 0.13 | < 0.0001 |
| GFRMDRD | −0.58 | <0.0001 | −0.31 | <0.0001 | <0.0001 |
| GFRMC | −0.64 | <0.0001 | −0.45 | <0.0001 | <0.0001 |
| Homocysteineb | 0.51 | <0.0001 | 0.31 | <0.0001 | 0.0001 |
| Physical activity | −0.04 | 0.44 | −0.07 | 0.11 | 0.26 |
| Alcohol (drinks/month) | −0.10 | 0.03 | 0.03 | 0.53 | 0.86 |
| Smoking | |||||
| Current | 0.03 | 0.46 | 0.07 | 0.09 | 0.57 |
| Ever | 0.07 | 0.10 | 0.02 | 0.68 | 0.35 |
CRP, C-reactive protein; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; LDL, low-density lipoprotein.
Not significant when corrected for multiple comparisons (Bonferroni corrected α = 0.00185).
Log-transformed, significant T1D interactions (P < 0.05) bold.
Table 5.
Determinants of Cystatin C in Multiple Linear Regression
| |
T1D |
Non-DM |
|
||
|---|---|---|---|---|---|
| Variable | B estimate | P value | B estimate | P value | Interaction P value |
| Gender (female vs. male) | −0.120 | <0.0001 | −0.038 | 0.0001 | 0.0003 |
| HDLa | −0.021 | 0.02 | −0.012 | 0.008 | 0.35 |
| BMIa | −0.025 | 0.02 | 0.009 | 0.02 | 0.003 |
| PAI-1a | −0.001 | 0.90 | 0.013 | 0.0007 | 0.20 |
| Fibrinogena | 0.031 | 0.002 | 0.009 | 0.01 | 0.04 |
| Serum creatinineb | 0.243 | <0.0001 | 0.058 | <0.0001 | <0.0001 |
| Homocysteineb | 0.017 | 0.11 | 0.022 | <0.0001 | 0.64 |
| AERb | 0.020 | 0.01 | −0.013 | 0.04 | 0.001 |
SD: BMI = 4.7 kg/m2; HDL = 15.7 mg/dL; PAI-1 = 25.1 ng/mL; fibrinogen = 63.6 mg/dL; serum creatinine = 1.2 mg/dL; homocysteine = 1.3 nmol/L; AER = 3.1 μg/min.
Estimate per SD increase.
Estimate per SD increase of the log-transformed variable.
Results
Baseline characteristics are presented in Table 1 with differences between the T1D and non-DM groups as previously reported.30 Within the T1D and non-DM groups, differences existed by progression status with those who progressed having elevated CVD risk factors. Cystatin C was higher and had greater variance in T1D patients than in persons without DM (0.84 ± 0.37 mg/L vs. 0.78 ± 0.10 mg/L, P = 0.0006; P < 0.0001 for equality of variance). Of note is that cystatin C was only minimally (but statistically significantly) different by progression status in subjects without DM (0.81 ± 0.10 mg/L vs. 0.78 ± 0.10 mg/L; P = 0.02), but albumin excretion rate (AER) did not differ. In contrast, renal function markers were significantly different by progression status in the T1D subjects. Also, BMI was similar in T1D progressors and non-progressors but much higher in non-DM progressors than in non-progressors adjusted for sex (data not shown).
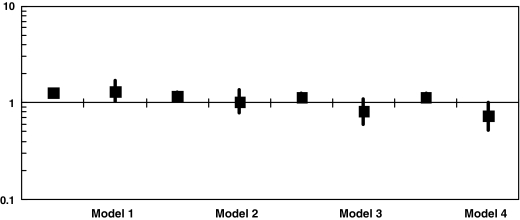
The relationship between cystatin C and CA progression was explored in stepwise multiple logistic regression in the full cohort (Table 2 and Fig. 1). Results of this analysis demonstrated the expected association of increasing cystatin C to CA progression in T1D participants, even with sequential adjustment for additional CVD risk factors as previously reported in an analysis including only T1D subjects.20 In subjects without DM, cystatin C had the expected association to CA progression in univariate analysis (Model 1, OR = 1.33, 95% confidence interval [CI] 1.04–1.69, P = 0.029), but after adjustment for age, sex, and baseline CVS this association approached the null and was not significant (Model 2, OR = 1.03, 95% CI 0.78–1.38, P = 0.82). With further adjustment for covariates that entered the stepwise model (Model 3) and then with significant interaction terms added (Model 4), the association between cystatin C and CA progression differed significantly by T1D status (interaction P values: P = 0.03 for Model 3, P = 0.01 for Model 4). In the final adjusted model (Model 4), cystatin C was positively associated with CA progression in individuals with T1D (OR = 1.13, 95% CI 1.03–1.26, P = 0.01) and inversely associated in persons without DM (OR = 0.73, 95% CI 0.52–1.02, P = 0.067), although only reaching borderline significance in the non-DM model.
FIG. 1.
OR for progression of CA per SD increase in cystatin C in stepwise multiple logistic regression, combined models with a cystatin C × T1D interaction term included: Model 1, cystatin C, T1D, cystatin C × T1D status; Model 2, Model 1 + age, baseline CVS, sex; Model 3, Model 2 + BMI, SBP, HDL; and Model 4, Model 3 + BMI × T1D, SBP × T1D, sex × T1D.
Given these unexpected results and to further investigate the relationship of cystatin C to CA progression in participants without DM, the relationship of cystatin C to CA progression was explored in additional stepwise multiple logistic regression analysis including the subset of subjects without DM with AER data (n = 553). In participants without DM, cystatin C was significantly associated with less CA progression (OR = 0.65, 95% CI 0.44–0.96, P = 0.029) while adjusting for other covariates that entered the model (BMI, which entered the model first, then cystatin C, then AER) in addition to covariates forced into the model (age, sex, and baseline CVS) (Table 3). Each SD increase of cystatin C (0.10 mg/L) was associated with a 35% decrease in the odds of CA progression. Similarly, for each SD decrease in AER the odds of CA progression increased by 32%. AER appeared to be missing at random in our dataset (missingness was not related to any of the variables of interest in our study), and repeating the model with a missing value indicator showed the same results. Next, confounders of the relationship of cystatin C to CA progression were investigated, and removal of BMI from the model presented in Table 3 resulted in the largest change toward the null in the OR for cystatin C (49%) and AER (32%), respectively, indicating BMI is the primary confounder of the association of cystatin C (and AER) to CA progression. Similar results were obtained with waist circumference and visceral fat in place of BMI. (Removal of other covariates resulted in changes between 2% and 17% [data not shown].) Additionally, a cystatin C × BMI interaction term was entered into the model in Table 3, but it was not significant (P = 0.89).
To further investigate why covariate adjustment may have had different effects in the non-DM model versus the T1D model, correlation coefficients between cystatin C and other variables, stratified by T1D status, were determined (Table 4). In T1D patients and subjects without DM, renal-related measures were correlated with cystatin C, whereas obesity-related measures were significantly correlated in persons without DM. Homocysteine was correlated with cystatin C in both T1D patients and those without DM. Variables with significantly different correlation (P < 0.05) in T1D as compared to persons without DM included gender, BMI, waist circumference, visceral fat, subcutaneous fat, SBP, HDL, plasminogen activator inhibitor-1 (PAI-1), fibrinogen, serum creatinine, AER, GFRCG, GFRMDRD, and homocysteine.
Correlates of cystatin C stratified by T1D status were evaluated with multiple linear regression analysis with a backward elimination approach (Table 5). Stratified models were then fit to the data including variables significant in either group, and Wald tests were used to compare estimates from the two models (i.e., test for interaction). In both T1D patients and persons without DM, gender, HDL, BMI, fibrinogen, serum creatinine, and AER were significantly associated with cystatin C. Additionally, PAI-1 and homocysteine were significantly associated with cystatin C in persons without DM. Significant interactions by T1D status existed for gender, BMI, fibrinogen, serum creatinine, and AER with stronger correlations in patients with T1D.
Conclusions
The main finding and novelty of this article is that although the univariate association of cystatin C to CA progression was similar in T1D patients and persons without DM and in the expected direction (increased cystatin C as a biomarker of worsening renal function associated with CA progression), the association of cystatin C to progression of CA differed by T1D status after adjustment for other CVD risk factors in multivariate analysis (P = 0.01 for the T1D × cystatin C interaction). Unexpectedly, in persons without DM increased cystatin C was associated with decreased CA progression after adjustment, primarily driven by adjustment for BMI. In T1D patients, the measured risk factors did not explain all of the association between cystatin C and progression of CA, and the association remained significant after adjustment.
This is the first article, to our knowledge, investigating the association of cystatin C to CA progression in a relatively healthy cohort without DM with normal renal function and the first to examine wither the association differs by T1D status. Because the findings from this study were unexpected and counterintuitive, it should be considered a hypothesis-generating study requiring confirmation in additional datasets. Cystatin C has been proposed to be a superior marker of GFR than serum creatinine or serum creatinine-based estimates of GFR14,31 and to be a better predictor of CVD deaths15 and progression of CA.20 Given these reports in the literature, cystatin C may be used more commonly both in large epidemiological studies and clinically. Therefore, our observation on the differing relationship in multivariate analysis between T1D patients and persons without DM of cystatin C to CA progression is important for future applications and merits further explanation and investigation.
A number of possible explanations exist to explain these data. Once adjusted for BMI, elevated cystatin C has a protective effect on CA progression (P = 0.029) in those persons without DM with relatively normal renal function and less variability in cystatin C than the T1D subjects. Of note is that the association of AER and serum creatinine (common markers of renal function) to cystatin C differed by T1D status, with serum creatinine less positively and AER inversely associated with cystatin C in individuals without DM compared to T1D patients. An intriguing possibility is that cystatin C could have a protective effect on the progress of atherosclerosis as has been noted in mouse and human models.32–38 In the CACTI cohort only cystatin C, and not serum creatinine, GFRCG, or GFRMDRD, was associated with CA progression. Although cystatin C has been used as a biomarker of GFR in epidemiological studies, basic science investigations have demonstrated that cystatin C inhibits elastin-degrading cysteine proteases, thereby diminishing their degradation of extracellular matrix proteins which is central in the atherosclerotic process.33 Furthermore, cystatin C gene polymorphisms were related to cystatin C concentrations39,40 and associated with more coronary artery stenoses39 but not with secondary CVD events.40 The possibility exists that although cystatin C serves as a reliable biomarker of GFR, it also certainly has a biologic function, and in persons with normal renal function higher concentrations of cystatin C could be protective in the atherosclerotic process, whereas in persons with impaired renal function these proposed beneficial biologic functions of cystatin C could be overwhelmed by the deleterious effects of kidney disease on CVD. Another possibility could be the complexity of the relationship of cystatin C to renal function and renal function to CAC. The Multi-Ethnic Study of Atherosclerosis has reported that cystatin C was not associated with cross-sectional CAC severity after adjustment for other risk factors in a population with mild to moderate kidney dysfunction.41
BMI has been reported to be a stronger predictor of CAC progression in individuals without DM than T1D persons in the CACTI study.42 In persons without DM cystatin C, as a marker of early renal dysfunction, could be on the causal pathway between increased BMI and CAD.43 In T1D persons higher BMI has been associated with CAC, but its relationship with CAC severity was inverse or nonexistent.44 In T1D patients, the relationship of glycemia to BMI (and other CVD risk factors) may not be as strong as the association of glycemia to CAD. In these data cystatin C was associated with renal function in both T1D patients and subjects without DM (although more strongly in T1D patients than in those without DM), whereas BMI (and other obesity measures) was associated with cystatin C in those without DM but not T1D patients.
Alternately, residual confounding may have been induced with adjustment for BMI. Other variables not measured or known to be measured imprecisely (e.g., physical activity and diet variables) may be causally associated with both BMI and CA progression. Failure to adjust for these additional risk factors could result in a model that overestimates the effect on BMI on CA progression, thus underestimating the cystatin C to CA progression association. There may also be other confounders (not necessarily associated with BMI) that were not measured or adequately controlled for in the current analysis.
Some limitations in our data need to be acknowledged and addressed in future studies. First, we do not compare cystatin C values to gold standard measures of GFR. However, recent data suggest cystatin C better estimates GFR than serum creatinine in subjects with chronic kidney disease,12 as do longitudinal data in young adults with T1D.11 Also, we use a surrogate marker of CAD instead of health outcomes such as CAD events or death as the CACTI cohort is relatively young and asymptomatic for CAD at enrollment, and the few CAD events as of this writing is a limitation (n = 15 for the cohort [11 with T1D and four without DM] in this analysis; a sensitivity analysis performed after removing these subjects with CAD events did not change the results); data on patient outcomes are being collected prospectively. However, extensive methodologic detail has been taken to carefully define CAC progression in the CACTI cohort,29 and this methodology has been used in other datasets.45,46 Additionally, CAC has been used as a surrogate marker of CAD, with higher CAC indicating higher CVD risk in populations with a wide range of renal function.47
In conclusion, our data suggest a complex relationship between cystatin C and CA progression in subjects without DM having normal renal function with BMI as a significant confounder of the observed univariate association. In these data cystatin C was associated with renal function markers in both T1D patients and subjects without DM (although more strongly in T1D patients than in subjects without DM), whereas BMI (and other obesity measures) was associated with cystatin C in those without DM, but not T1D patients. As cystatin C is being more commonly used as a biomarker of renal function and a predictor of CVD, future studies should be aware of this relationship and interpret results accordingly. Longitudinal and genetic data on cystatin C as well as the relationship of cystatin C to CVD outcomes in additional cohorts will confirm or reject these relationships.
Acknowledgments
Support for this study was provided by NHLBI grant R01 HL61753, HL79611, and DERC Clinical Investigation Core P30 DK57516 from the National Institutes of Health. The study was performed at the Adult GCRC at the University of Colorado Denver Health Sciences Center supported by grant M01-RR00051 from the National Institutes of Health, at the Barbara Davis Center for Childhood Diabetes, and at Colorado Heart Imaging Center in Denver, CO. D.M.M. was supported by grant K23 DK075360. The results presented in this article have not been published previously in whole or part, except in abstract format.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sarnak MJ. Levey AS. Schoolwerth AC. Coresh J. Culleton B. Hamm LL. McCullough PA. Kasiske BL. Kelepouris E. Klag MJ. Parfrey P. Pfeffer M. Raij L. Spinosa DJ. Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 2.Go AS. Chertow GM. Fan D. McCulloch CE. Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Molitch ME. DeFronzo RA. Franz MJ. Keane WF. Mogensen CE. Parving HH. Steffes MW. Nephropathy in diabetes. Diabetes Care. 2004;27(Suppl 1):S79–S83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 4.Weiner DE. Tighiouart H. Amin MG. Stark PC. MacLeod B. Griffith JL. Salem DN. Levey AS. Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 5.Orchard TJ. Costacou T. Kretowski A. Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006;29:2528–2538. doi: 10.2337/dc06-1161. [DOI] [PubMed] [Google Scholar]
- 6.Laterza OF. Price CP. Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 7.Dharnidharka VR. Kwon C. Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 8.Buysschaert M. Joudi I. Wallemacq P. Hermans MP. Performance of serum cystatin-C versus serum creatinine in subjects with type 1 diabetes. Diabetes Care. 2003;26:1320. doi: 10.2337/diacare.26.4.1320. [DOI] [PubMed] [Google Scholar]
- 9.Filler G. Bokenkamp A. Hofmann W. Le BT. Martinez-Bru C. Grubb A. Cystatin C as a marker of GFR—history, indications, and future research. Clin Biochem. 2005;38:1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Tan GD. Lewis AV. James TJ. Altmann P. Taylor RP. Levy JC. Clinical usefulness of cystatin C for the estimation of glomerular filtration rate in type 1 diabetes: reproducibility and accuracy compared with standard measures and iohexol clearance. Diabetes Care. 2002;25:2004–2009. doi: 10.2337/diacare.25.11.2004. [DOI] [PubMed] [Google Scholar]
- 11.Premaratne E. Macisaac RJ. Finch S. Panagiotopoulos S. Ekinci E. Jerums G. Serial measurements of cystatin C are more accurate than creatinine-based methods in detecting declining renal function in type 1 diabetes. Diabetes Care. 2008;31:971–973. doi: 10.2337/dc07-1588. [DOI] [PubMed] [Google Scholar]
- 12.Stevens LA. Coresh J. Schmid CH. Feldman HI. Froissart M. Kusek J. Rossert J. Van LF. Bruce RD., III Zhang YL. Greene T. Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perkins BA. Nelson RG. Ostrander BE. Blouch KL. Krolewski AS. Myers BD. Warram JH. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005;16:1404–1412. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perkins BA. Krolewski AS. Early nephropathy in type 1 diabetes: a new perspective on who will and who will not progress. Curr Diabetes Rep. 2005;5:455–463. doi: 10.1007/s11892-005-0055-7. [DOI] [PubMed] [Google Scholar]
- 15.Shlipak MG. Sarnak MJ. Katz R. Fried LF. Seliger SL. Newman AB. Siscovick DS. Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 16.Shlipak MG. Katz R. Sarnak MJ. Fried LF. Newman AB. Stehman-Breen C. Seliger SL. Kestenbaum B. Psaty B. Tracy RP. Siscovick DS. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 17.Shlipak MG. Wassel Fyr CL. Chertow GM. Harris TB. Kritchevsky SB. Tylavsky FA. Satterfield S. Cummings SR. Newman AB. Fried LF. Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2006;17:254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 18.Koenig W. Twardella D. Brenner H. Rothenbacher D. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rate. Clin Chem. 2005;51:321–327. doi: 10.1373/clinchem.2004.041889. [DOI] [PubMed] [Google Scholar]
- 19.Luc G. Bard JM. Lesueur C. Arveiler D. Evans A. Amouyel P. Ferrieres J. Juhan-Vague I. Fruchart JC. Ducimetiere P. Plasma cystatin-C and development of coronary heart disease: the PRIME Study. Atherosclerosis. 2006;185:375–380. doi: 10.1016/j.atherosclerosis.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Maahs DM. Ogden LG. Kretowski A. Snell-Bergeon JK. Kinney GL. Berl T. Rewers M. Serum cystatin C predicts progression of subclinical coronary atherosclerosis in individuals with type 1 diabetes. Diabetes. 2007;56:2774–2779. doi: 10.2337/db07-0539. [DOI] [PubMed] [Google Scholar]
- 21.Maahs DM. Kinney GL. Wadwa P. Snell-Bergeon JK. Dabelea D. Hokanson J. Ehrlich J. Garg S. Eckel RH. Rewers MJ. Hypertension prevalence, awareness, treatment, and control in an adult type 1 diabetes population and a comparable general population. Diabetes Care. 2005;28:301–306. doi: 10.2337/diacare.28.2.301. [DOI] [PubMed] [Google Scholar]
- 22.Dabelea D. Kinney G. Snell-Bergeon JK. Hokanson JE. Eckel RH. Ehrlich J. Garg S. Hamman RF. Rewers M. Effect of type 1 diabetes on the Gender difference in coronary artery calcification: a role for insulin resistance?: the Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52:2833–2839. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 23.Williams KV. Erbey JR. Becker D. Arslanian S. Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49:626–632. doi: 10.2337/diabetes.49.4.626. [DOI] [PubMed] [Google Scholar]
- 24.Cockcroft DW. Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS. Bosch JP. Lewis JB. Greene T. Rogers N. Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 26.Rule AD. Larson TS. Bergstralh EJ. Slezak JM. Jacobsen SJ. Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 27.Agatston AS. Janowitz WR. Hildner FJ. Zusmer NR. Viamonte M., Jr Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 28.Callister TQ. Cooil B. Raya SP. Lippolis NJ. Russo DJ. Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology. 1998;208:807–814. doi: 10.1148/radiology.208.3.9722864. [DOI] [PubMed] [Google Scholar]
- 29.Hokanson JE. MacKenzie T. Kinney G. Snell-Bergeon JK. Dabelea D. Ehrlich J. Eckel RH. Rewers M. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol. 2004;182:1327–1332. doi: 10.2214/ajr.182.5.1821327. [DOI] [PubMed] [Google Scholar]
- 30.Maahs DM. Ogden LG. Snell-Bergeon JK. Kinney GL. Wadwa RP. Hokanson JE. Dabelea D. Kretowski A. Eckel RH. Rewers M. Determinants of serum adiponectin in persons with and without type 1 diabetes. Am J Epidemiol. 2007;166:731–740. doi: 10.1093/aje/kwm125. [DOI] [PubMed] [Google Scholar]
- 31.Shlipak MG. Cystatin C: research priorities targeted to clinical decision making. Am J Kidney Dis. 2008;51:358–361. doi: 10.1053/j.ajkd.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Bengtsson E. To F. Grubb A. Hakansson K. Wittgren L. Nilsson J. Jovinge S. Absence of the protease inhibitor cystatin C in inflammatory cells results in larger plaque area in plaque regression of apoE-deficient mice. Atherosclerosis. 2005;180:45–53. doi: 10.1016/j.atherosclerosis.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 33.Bengtsson E. To F. Hakansson K. Grubb A. Branen L. Nilsson J. Jovinge S. Lack of the cysteine protease inhibitor cystatin C promotes atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:2151–2156. doi: 10.1161/01.ATV.0000179600.34086.7d. [DOI] [PubMed] [Google Scholar]
- 34.Liu J. Sukhova GK. Sun JS. Xu WH. Libby P. Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1359–1366. doi: 10.1161/01.ATV.0000134530.27208.41. [DOI] [PubMed] [Google Scholar]
- 35.Shi GP. Sukhova GK. Grubb A. Ducharme A. Rhode LH. Lee RT. Ridker PM. Libby P. Chapman HA. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999;104:1191–1197. doi: 10.1172/JCI7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sukhova GK. Shi GP. Do cathepsins play a role in abdominal aortic aneurysm pathogenesis? Ann N Y Acad Sci. 2006;1085:161–169. doi: 10.1196/annals.1383.028. [DOI] [PubMed] [Google Scholar]
- 37.Sukhova GK. Wang B. Libby P. Pan JH. Zhang Y. Grubb A. Fang K. Chapman HA. Shi GP. Cystatin C deficiency increases elastic lamina degradation and aortic dilatation in apolipoprotein E-null mice. Circ Res. 2005;96:368–375. doi: 10.1161/01.RES.0000155964.34150.F7. [DOI] [PubMed] [Google Scholar]
- 38.Sukhova GK. Zhang Y. Pan JH. Wada Y. Yamamoto T. Naito M. Kodama T. Tsimikas S. Witztum JL. Lu ML. Sakara Y. Chin MT. Libby P. Shi GP. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eriksson P. Deguchi H. Samnegard A. Lundman P. Boquist S. Tornvall P. Ericsson CG. Bergstrand L. Hansson LO. Ye S. Hamsten A. Human evidence that the cystatin C gene is implicated in focal progression of coronary artery disease. Arterioscler Thromb Vasc Biol. 2004;24:551–55. doi: 10.1161/01.ATV.0000117180.57731.36. [DOI] [PubMed] [Google Scholar]
- 40.Loew M. Hoffmann MM. Koenig W. Brenner H. Rothenbacher D. Genotype and plasma concentration of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events. Arterioscler Thromb Vasc Biol. 2005;25:1470–1474. doi: 10.1161/01.ATV.0000168416.74206.62. [DOI] [PubMed] [Google Scholar]
- 41.Ix JH. Katz R. Kestenbaum B. Fried LF. Kramer H. Stehman-Breen C. Shlipak MG. Association of mild to moderate kidney dysfunction and coronary calcification. J Am Soc Nephrol. 2008;19:579–585. doi: 10.1681/ASN.2007070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snell-Bergeon J. Kinney GL. Maahs DM. Rewers M. The association between obesity, coronary artery calcification in type 1 diabetes. Conference Proceedings; Stellenbosch, South Africa. International Diabetes Epidemiology Group; 2006. [Google Scholar]
- 43.Retnakaran R. Connelly PW. Harris SB. Zinman B. Hanley AJ. Cystatin C is associated with cardiovascular risk factors and metabolic syndrome in Aboriginal youth. Pediatr Nephrol. 2007;22:1007–1013. doi: 10.1007/s00467-007-0471-9. [DOI] [PubMed] [Google Scholar]
- 44.Conway B. Miller RG. Costacou T. Fried L. Kelsey S. Evans RW. Edmundowicz D. Orchard TJ. Double-edged relationship between adiposity and coronary artery calcification in type 1 diabetes. Diabetes Vasc Dis Res. 2007;4:332–339. doi: 10.3132/dvdr.2007.061. [DOI] [PubMed] [Google Scholar]
- 45.Mehrotra R. Budoff M. Hokanson JE. Ipp E. Takasu J. Adler S. Progression of coronary artery calcification in diabetics with and without chronic kidney disease. Kidney Int. 2005;68:1258–1266. doi: 10.1111/j.1523-1755.2005.00522.x. [DOI] [PubMed] [Google Scholar]
- 46.Jung HH. Kim SW. Han H. Inflammation, mineral metabolism and progressive coronary artery calcification in patients on haemodialysis. Nephrol Dial Transplant. 2006;21:1915–1920. doi: 10.1093/ndt/gfl118. [DOI] [PubMed] [Google Scholar]
- 47.Budoff MJ. Achenbach S. Blumenthal RS. Carr JJ. Goldin JG. Greenland P. Guerci AD. Lima JA. Rader DJ. Rubin GD. Shaw LJ. Wiegers SE. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]