Abstract
Background
Research suggests that individuals who start drinking at an early age are more likely to subsequently develop alcohol dependence. Twin studies have demonstrated that the liability to age at 1st drink and to alcohol dependence are influenced by common genetic and environmental factors, however, age at 1st drink may also environmentally mediate increased risk for alcohol dependence. In this study, we examine whether age at 1st drink moderates genetic and environmental influences, via gene x environment interactions, on DSM-IV alcohol dependence symptoms.
Methods
Using data on 6,257 adult monozygotic and dizygotic male and female twins from Australia, we examined the extent to which age at 1st drink (a) increased mean alcohol dependence symptoms and (b) whether the magnitude of additive genetic, shared and non-shared environmental influences on alcohol dependence symptoms varied as a function of increasing age at 1st drink. Twin models were fitted in Mx.
Results
Risk for alcohol dependence symptoms increased with decreasing age at 1st drink. Heritable influences on alcohol dependence symptoms were considerably larger in those who reported an age at 1st drink prior to 13 years of age. In those with later onset of alcohol use, variance in alcohol dependence was largely attributable to non-shared environmental variance (and measurement error). This evidence for unmeasured gene x measured environment interaction persisted even when controlling for the genetic influences that overlapped between age at 1st drink and alcohol dependence symptoms.
Conclusions
Early age at 1st drink may facilitate the expression of genes associated with vulnerability to alcohol dependence symptoms. This is important to consider, not only from a public health standpoint, but also in future genomic studies of alcohol dependence.
INTRODUCTION
Alcohol dependence (AD) is a serious public health concern and contributes to 1.8 million deaths worldwide (WHO: Global Burden of Disease, 2009). In the U.S. alone, 11% of the adult population meets criteria for DSM-IV AD during their lifetime and 26% of those who drink alcohol endorse at least one dependence criterion during their lifetime (Grant et al., 2004; Saha et al., 2006). A consistently studied contributor to severity of alcohol dependence is age at first drink - according to the National Household Survey of Drug Use and Health (Substance Abuse and Mental Health Services Administration (SAMHSA), 2005) 86% of alcohol initiates in the U.S. are aged 20 or younger (mean age at 1st drink of 16.8 years). A wealth of epidemiological research suggests that underage drinking, particularly initiation of alcohol use at a young age, is associated with increased alcohol involvement, including heavier drinking, DUIs (Hingson et al., 2001; Hingson et al., 2002; Hingson et al., 2000; Hingson et al., 2004; Hingson et al., 2006; Hingson et al., 2008; Lynskey et al., 2007) and importantly, alcohol dependence (Chou and Pickering, 1992; Dawson et al., 2008; Grant and Dawson, 1997; Hingson et al., 2006; McGue et al., 2001b) as well as other disinhibitory behaviors (Hingson et al., 2008;McGue et al., 2001b) and drug use. Results emerging from two large scale epidemiological studies representative of the U.S. population show a 1.3–1.6 times increased risk for AD in those who initiate alcohol use at 15 years of age or younger and a corresponding reduction in risk for alcohol dependence (by 14%) with each one-year delay in alcohol initiation (Dawson et al., 2008; Grant and Dawson, 1997). These findings were in keeping with findings from a Canadian sample, where the increased risk for AD in early-onset drinkers persisted even after controlling for exposure to childhood trauma and psychopathology (DeWit et al., 2000).
A small number of genetically informative studies have investigated the association between age at 1st drink and AD. Using a sample of adult Virginia twins, Prescott & Kendler (1999) found that the prevalence of AD declined with increasing age at 1st drink, that one twin’s age at 1st drink was predictive of their co-twin’s AD and importantly, that the relationship between age at 1st drink and AD was attributable to a substantial degree to common genetic influences (18–29% genetic overlap). Grant and colleagues (Grant et al., 2005) used a similar strategy to assess age at first drink and AD in a sample of adult male Vietnam Era twins and reported that early (prior to age 17) regular alcohol use in one twin led to a 2.7 times increased likelihood of AD in the identical co-twin. These authors also reported a substantial correlation (0.59–0.64) between early regular drinking and AD. In a third study, Sartor and colleagues, using an offspring-of-twins design, found considerable evidence for increased risk of AD in those with an early age at 1st drink, but, importantly, did not find evidence that this risk varied by (paternally transmitted) genetic risk for AD (Sartor et al., 2007).
There is now overwhelming evidence suggesting that heritable influences play a substantial role in AD diagnoses and symptomatology (Heath, 2007; Kendler et al., 1992; McGue, 1999; Prescott et al., 1994). Additionally, there is support for familial influences on age at 1st drink (McGue et al., 2001a) and, as described above, there appears to be considerable genetic overlap between age at 1st drink and AD (Grant et al., 2005; Prescott and Kendler, 1999). However, age at 1st alcohol use can also be viewed as an environmental measure. In fact, neither Prescott and Kendler (1999) nor Grant et al. (2005) could unequivocally rule out the environmental impact of age at 1st (or regular) drink on AD. If early exposure to alcoholic beverages is an environmental measure, then it can be related to the highly heritable AD phenotypes (i.e., diagnosis and symptomatology) in multiple ways. First, its relationship with AD may be viewed in the context of unmeasured gene-measured environment correlation (or rGE) where the same latent/unmeasured genetic factors that induce individual differences in AD phenotypes also increase the likelihood of exposure to alcohol at an early age (Scarr, 1992; Scarr and McCartney, 1983). This could occur passively, through parental drinking behaviors or actively, through seeking out (like) peers who engage in problem behaviors that include early substance use.
Two independent studies have found evidence for rGE and for the possibility that common genetic etiology underlies age at 1st drink and AD (Grant et al., 2005; Prescott and Kendler, 1999). An alternative putative relationship between age at 1st drink and AD phenotypes, specifically, whether there is an unmeasured gene x measured environment (GxE) interaction, however, has not been previously examined. That is, after controlling for the main effects of rGE (i.e., those with an early age at 1st drink will have a greater likelihood of AD), to what extent does early exposure to alcohol moderate the role of genetic (and environmental) influences on later AD symptomatology? A GxE interaction implies that genetic influences on AD symptoms are moderated by early exposure to alcohol such that these genetic influences gain prominence (i.e., high heritability) only when there is exposure to the high risk environment, in this case, initiation of alcohol use at an early age (Gunzerath and Goldman, 2003; Heath et al., 2002; Heath and Nelson, 2002; Martin et al., 1987). Viewed another way, a GxE interaction may be interpreted as follows: individuals with genetic influences predisposing them to AD will be more likely to develop AD symptomatology if they are exposed to alcohol at an early age. A GxE interaction would therefore imply that, even in the presence of genetic vulnerability, delaying exposure to alcohol use will reduce the emergence of AD symptomatology.
In the current study, we use data on 6,257 adult male and female Australian twins to examine whether age at 1st drink modifies the extent to which genetic and environmental factors influence individual differences in DSM-IV AD symptoms.
METHODS
Sample
A sample of 6,257 adult Australian male and female twins that included monozygotic (MZ) and dizygotic (DZ) pairs as well as twins from incomplete pairs, were used. The full paired sample consisted of 494 MZ and 395 DZ same-sex male pairs and of 698 MZ and 513 DZ same-sex female pairs. Data on 661 DZ opposite sex pairs and 736 twins from pairs where a co-twin did not participate was also available – all twins were used for analyses. Twins were aged 24–36 (mean 30 [2.5] years) years at the time of interview, which were the source of data for the analyses presented here. All twins were born between 1964 and 1971 and were initially recruited through the Australian school systems and via mass media appeals. Parents initially registered the twins when they were children in 1980–1982 and the twins themselves were interviewed via telephone in 1996–2000, after informed consent was obtained from all participants, as approved by the Institutional Review Boards of the Washington University in St. Louis, USA and the Queensland Institute of Medical Research, Australia,. Further details regarding sample ascertainment and data collection are presented in related publications (Heath et al., 2001; Knopik et al., 2004; Lynskey et al., 2003; Nelson et al., 2002).
Measures
Diagnostic interviews were based on the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994; Hesselbrock et al., 1999), which was updated for DSM-IV and adapted for telephone use in the Australian sample (Heath et al., 1997).
Age at first drink was reported by participants in response to the question ‘How old were you the first time you had more than a sip of beer, wine or spirits’. To reduce the effects of outliers, those reporting ages below 5 years (30 individuals) were equated to 5 years and those reporting greater than 23 years of age at 1st drink (51 individuals) were equated to 23 years. Mean age for the 1st drink was 16.2 years [SD 2.5y] and 15.3 years [SD 2.6y] in women and men respectively.
DSM-IV alcohol dependence symptoms were ascertained using items from the SSAGA. These questions were only asked of individuals who reported either having drunk at least once a month for 6 months or longer or drinking to intoxication (exclusion of 354 individuals). Mean number of DSM-IV symptoms (range 0–7) were 0.95 (SD 1.3) and 1.52 (SD 1.6) in women and men respectively. We opted to use AD symptoms (log-transformed) instead of the diagnosis of DSM-IV AD because (a) using AD symptom count allows us to incorporate the severity of the AD diagnosis which may be quantitatively associated with age at first drink (i.e. early onset contributes to more symptoms), (b) the diagnosis of DSM-IV AD is reliant on a threshold (3+ criteria) which is currently under psychometric scrutiny, (c) power to detect G x E is enhanced when using a quantitative measure and (d) flexibility afforded by quantitative measures in twin modeling.
Conduct disorder may be viewed as potent mediator of any relationship between early-onset substance use, including age at 1st drink and later dependence symptomatology. Hence, we created residuals by regressing out the effects of a diagnosis of DSM-IV conduct disorder (p < 0.0001) from the log-transformed alcohol dependence (AD) symptoms score. This residualized score was subsequently used in the moderator models.
Survival Modeling
To examine the phenotypic association between age at 1st drink and AD symptoms, Cox proportional hazards models were used in STATA. Kaplan-Meier curves were computed for each zygosity group while adjusting standard errors for familial clustering using the Huber-White robust variance estimator.
Twin Modeling
Twin models, in Mx (Neale, 2004), were used to estimate the influence of latent risk factors on AD symptoms. These latent factors include: A or additive genetic risk factors, C or shared environmental risk factors and E or non-shared environmental risk factors (Fisher, 1918; Jinks and Fulker, 1970; Neale and Cardon, 1992). ‘A’ refers to the extent to which genetic factors influence the total population variation in a trait. MZ twins who share all their genes have a genetic correlation of 1.0 whereas DZ twins who, on average, share only half their segregating genes, have a genetic correlation of 0.5. Similarly, ‘C’ refers to the latent environmental risk factors that make members of a twin pair that are reared together similar to each other – C is correlated 1.0 across MZ and DZ twins (Eaves et al., 2003; Kendler and Gardner, Jr., 1998).
For some observed measures, ‘D’ or non-additive genetic effects (correlated 1.0 in MZ and 0.25 in DZ twin pairs), which is defined as the influence of latent non-additive genetic influences on the observed variable, may be of importance. Due to available degrees of freedom (Neale and Cardon, 1992; Sham, 1998), either D or C, but not both, may be estimated in a model where information from only twin pairs is used. Comparing twin-cotwin correlations allows for a straightforward test for the presence or absence of D. If the DZ correlation is significantly less than half of the observed MZ correlation, then the modeling of D is preferred over C.
Both A and C (or D) contribute to the familial sources of resemblance for an observed variable (e.g. alcohol consumption). E or the non-shared environmental influence, on the other hand, refers to the latent environmental risk factors that are unique to each member of the twin pair and could include any experience or influence that one twin does not share with their co-twin. In the twin design, E also includes an estimate of measurement error.
The classical twin model can also be extended to examine the role of environmental influences on estimates A, C and E. This is done using a set of 4 parameters:
B, which represents the influence of age at 1st drink on mean alcohol dependence symptoms (i.e. controlling for gene-environment correlation, or the possibility that earlier age at 1st drink is correlated with increasing AD symptoms) - if the mean for AD symptoms is μ, and M is the moderator (in our case, age at 1st drink), then the mean may be modified from μ to (μ+B*M). B represents control for gene-environment correlation – its magnitude and statistical significance also denote the extent to which a change in age at 1st drink is associated with phenotypic increase or decrease in AD symptomatology (i.e. do individuals with earlier age at 1st drink report greater AD symptomatology?).
3 parameters, X, Y and Z, which represent the change in A, C and E respectively as a function of changing age at 1st drink. If the effect of X is significant (represented by a Δχ2 greater than 3.84 for 1 degree of freedom), then there is evidence for gene x environment interaction (i.e. heritability of AD symptoms varies as function of age at 1st drink).
To model the effects of continuous moderators in Mx, Purcell (http://statgen.iop.kcl.ac.uk/gxe/) has made available a series of moderation models, which are based on previous models by Martin et al.(Martin et al., 1987; Purcell, 2002). In these models, the total variance V= [A+X*M]2 + [C+Y*M]2 + [E+Z*M]2 where A, C, E, X, Y and Z are freely estimated and X, Y and Z denote the moderation effects.
Sex differences were also examined. A, C, E, X, Y, Z and B were allowed to vary across male and female twins and change in the overall fit of the models when these parameters were equated across sexes was tested.
Moderation of a bivariate twin model: Whereas the univariate models effectively control for rGE so that no spurious GxE effects are detected, they do not explicitly model the inter-relationship between genetic influences on age at 1st drink and those on AD symptoms. In order to address this hypothesis, we fit a triangular decomposition (i.e. Cholesky) model (Commandant Benoit, 1924; Neale & Cardon, 1992) that examined the moderating influence of age at 1st drink while modeling the genetic correlation between age at 1st drink and AD symptoms. The model serves two important purposes: first, it models the extent to which common etiologic factors (genetic, shared and non-shared environment) contribute to the co-variation between age at 1st drink and AD symptoms – for instance, measuring common genetic influences on age at 1st drink and AD symptoms. This is the standard bivariate model (without moderation). Second, with the addition of moderation, the model allows an estimation of whether these common genetic influences, or those genes that influence the liability to AD symptoms specifically, are moderated by age at 1st drink – in other words, after accounting for common genetic factors, we can examine whether age at 1st drink also acts as a moderator of genetic influences on AD symptoms and the genetic ‘route’ that it moderates. We tested both the standard bivariate model and extended it to include moderation while controlling, jointly, for overlapping genetic and environmental factors.
RESULTS
Survival Models
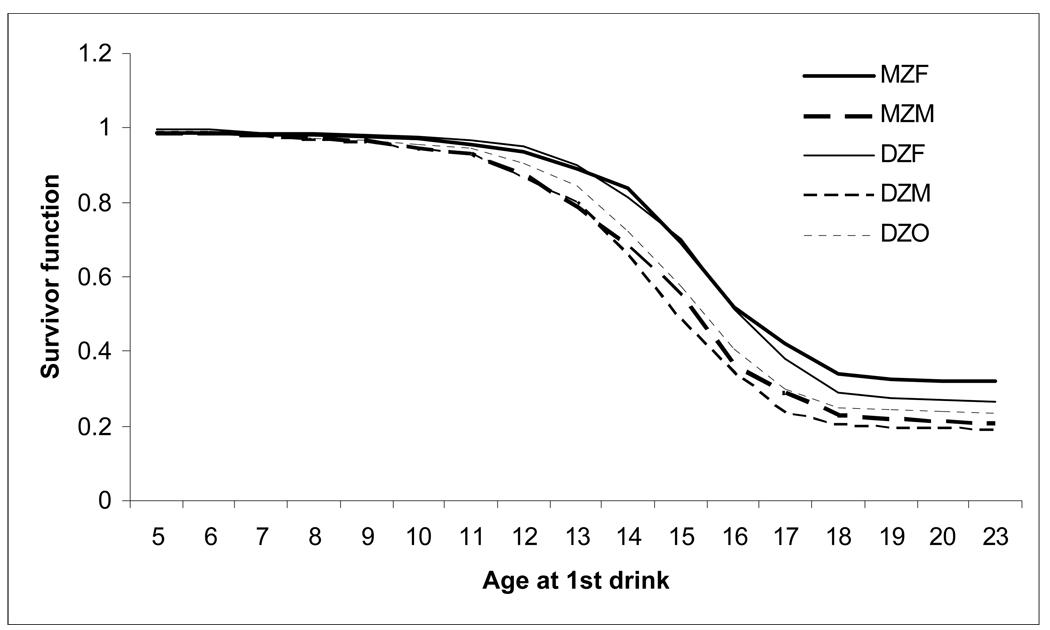
Kaplan-Meier curves for each of the five zygosity groups is shown in Figure 1. In all instances, there was a sharp decline in the likelihood of endorsing one or more AD symptoms (survivorship) with increasing age at 1st drink.
Figure 1.
Decreasing hazards of endorsing one or more DSM-IV alcohol dependence symptoms (survival = endorsed 1+ alcohol dependence symptom) with increasing age at 1st drink.
Twin correlations
With the exception of a somewhat lower DZ correlation in the opposite sex pairs (rDZO=0.08), DZ correlations (rDZM=0.24, rDZF=0.17) were no less than half the MZ correlations (rMZM=0.30, rMZF=0.34), suggesting the role of additive genetic, and possibly, shared environmental factors.
MZ and DZ correlations were also examined in twin pairs who were stratified by a binary measure representing early vs late age at 1st drink. The advantage of this method is that it allows a quick estimation of whether the magnitude of heritable influences on AD symptoms varies as a function of age at 1st drink. The disadvantage is that this method is highly underpowered to pick up modest to moderate G x E effects as it is reliant on data from complete pairs that are concordant (for early-onset or for late-onset) or discordant for age at 1st drink, which is arbitrarily dichotomized. In our sample, about 22% of the participants reported an age at 1st drink of 14 years or younger – the cut-off was chosen based on the distribution of the data, to allow for adequate number of subjects in the computation of correlations and from inspection of the change in heritability estimates when age of onset was viewed continuously (see Figure 2 and 3). Concordant early-onset twin pairs (both reported age at 1st drink at 14 years or younger) had an MZ and DZ (combined across sexes) correlation for AD symptoms of 0.36 and 0.12 respectively. MZ and DZ correlations in concordant late-onset (both twins reported age at 1st drink at 15 years of older) were 0.33 and 0.17 respectively while in discordant pairs, MZ and DZ correlations were 0.34 and 0.14 respectively. Therefore, heritability in the concordant early, concordant late and discordant pairs suggests greater heritable influences in those with early age at 1st drink.
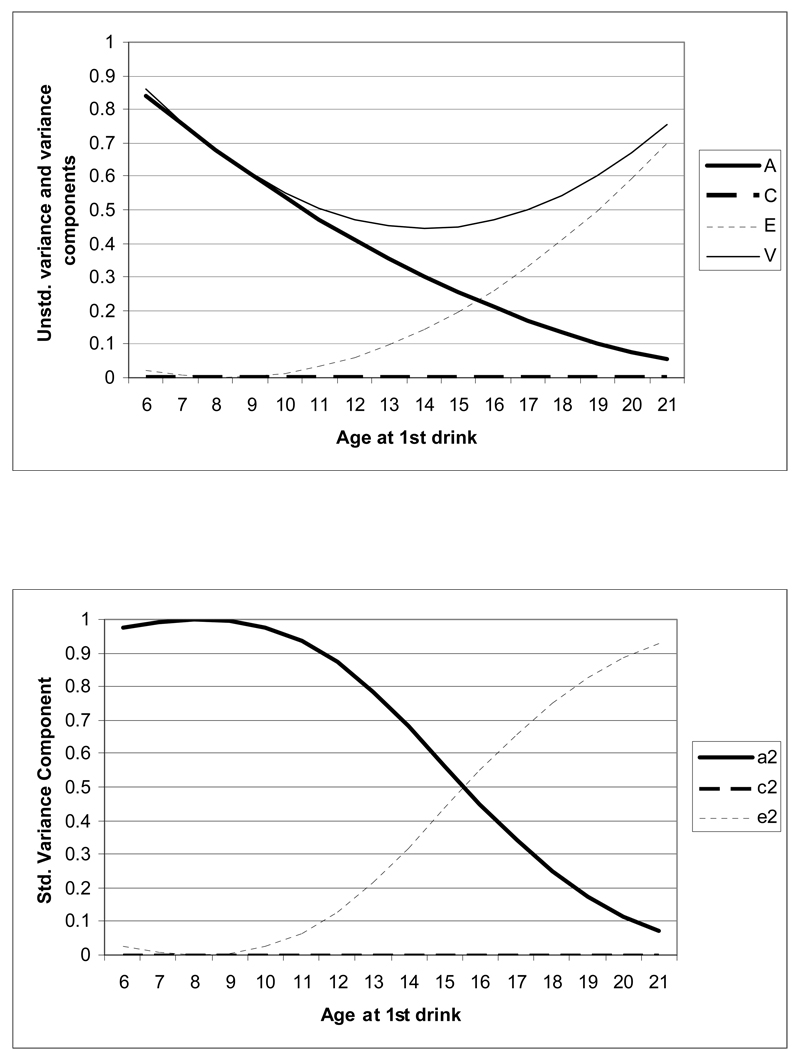
Figure 2.
Moderation of variance and of additive genetic (a2), shared environmental (c2) and non-shared environmental (e2) influences in women. The upper panel shows change in total variance and in the A, C and E components by age at first drink – in this upper panel, the variance curve, at any point, reflects the sum of the unstandardized A, C an E.
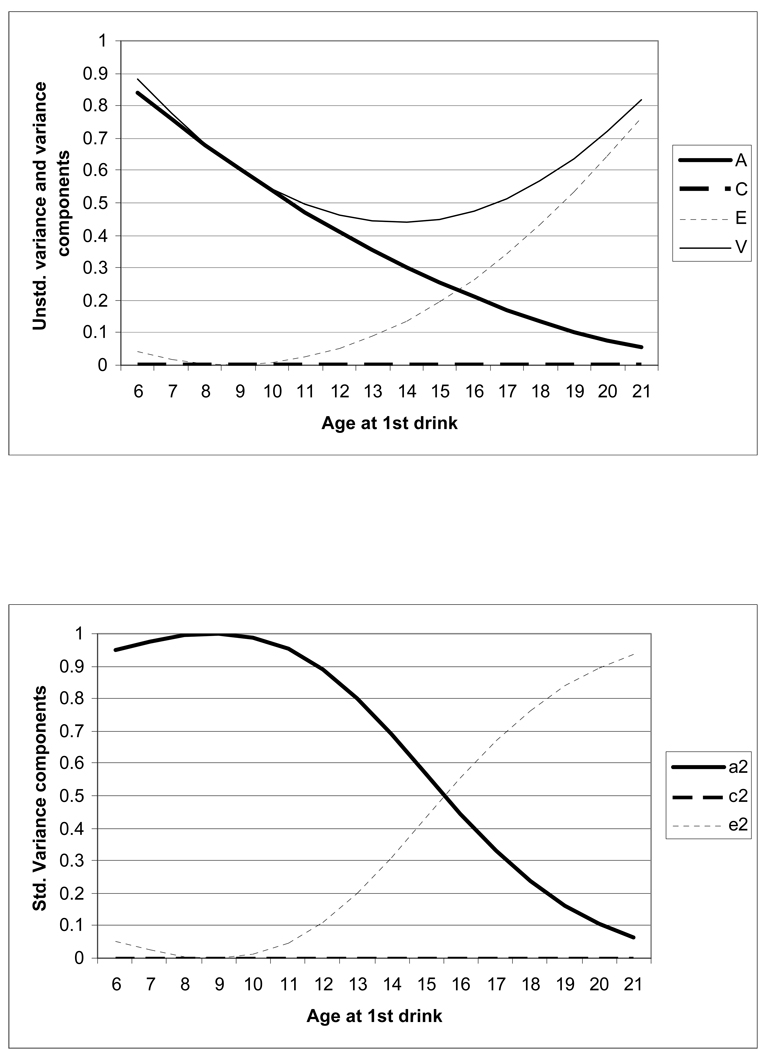
Figure 3.
Moderation of variance and of additive genetic (a2), shared environmental (c2) and non-shared environmental (e2) influences in men. The upper panel shows change in total variance and in the A, C and E components by age at first drink – in this upper panel, the variance curve, at any point, reflects the sum of the unstandardized A, C an E. The lower panel shows change in A, C and E when they are standardized such that: (a+x*moderator)2+(c+y*moderator)2+(e+z*moderator)2=1.0, i.e. the variance is set to 1.0. Now, the change in A reflects moderation of heritable factors.
Twin modeling
Table 1 shows the change in model fit (calculated by subtracting the −2 loglikelihood of the full model, with all parameters specified, from the −2 loglikelihood of a model where a parameter is constrained). The table also includes raw (un-standardized and un-squared) parameter estimates – estimates were not bounded and hence, can be positive or negative, however those estimates that include zero within their 95% confidence limits were not statistically significant. In both sexes, lower age at 1st drink was associated with increasing AD symptoms (bmen = −0.0293; bwomen= −0.0412) – this B parameter serves two purposes – it controls for rGE and its magnitude and statistical significance represent the extent to which age at 1st drink is phenotypically associated with AD symptoms. After accounting for rGE, there was statistical evidence for moderation of A and E. As shown in Table 1, when X, the path representing moderation of genetic influences on AD symptoms by age at 1st drink, was set to zero, there was a substantial deterioration of model fit. This statistically significant change in model fit was also noted when Z (path denoting moderation of non-shared environmental influences) was set to zero. However, both the shared environmental path (C) and its moderation (Y) could be constrained to zero. Thus, there was support for age of 1st drink modifying the magnitude of additive genetic and non-shared environmental influences on AD symptoms. These first sets of analyses were conducted jointly in men and women, allowing parameters to vary across sexes (hence, the 2 degree of freedom change in Table 1). Next, we tested for sex differences and some evidence was found. While the main effects of age at 1st drink on AD symptoms (via B) and the path denoting genetic factors and their moderation (A, via X) could be equated across sexes, the path denoting non-shared environment and its moderation (E, via Z) could not. Thus, our best-fitting model allowed for the main effects of age at 1st drink on AD symptoms and moderation of additive genetic and non-shared environmental factors influencing AD symptoms, where the former two estimates were sex invariant while the latter demonstrated sex differences.
Table 1.
Un-standardized path estimates (un-squared) and change in chi-square for univariate twin models examining the moderation and mediation effects of age at 1st drink on AD symptoms in male and female twins.
| Description of parameter tested | Parameter in full model [95% C.I.] | Degrees of freedom |
Δχ2 | |
|---|---|---|---|---|
| Female | Male | |||
| Raw additive genetic influences | 1.49 [0.47, 1.68] |
1.07 [0.78, 1.29] |
- | - |
| Raw shared environmental influences | 0.001 [−0.69, 1.56]ns |
0.34 [−0.23, 0.83]ns |
- | - |
| Raw non-shared environmental influences | 0.54 [0.52, 0.57] |
0.64 [0.60, 0.67] |
- | - |
| Does age at 1st drink modify mean AD symptoms endorsed in both sexes? | −0.04 [−0.05, −0.02] |
−0.03 [−0.04, −0.02] |
2 | 240.77 |
| Does age at 1st drink moderate the magnitude of genetic influences on AD symptoms in both sexes? |
−0.09 [−0.10, −0.01] |
−0.05 [−0.07, −0.04] |
2 | 72.55 |
| Does age at 1st drink moderate the magnitude of shared environmental influences on AD symptoms in both sexes?* |
−0.02 [−0.10, 0.03]ns |
−0.02 [−0.05, 0.02]ns |
2 | 0.36 |
| Does age at 1st drink moderate the magnitude of non-shared environmental influences on AD symptoms in both sexes? |
−0.07 [−0.07, −0.06] |
−0.07 [−0.08, −0.07] |
2 | 40.65 |
| Is moderation of mean AD symptoms the same across sexes? | −0.03 [−0.04, −0.02] |
1 | 0.69 | |
| Is moderation of genetic influences the same across sexes? | Genetic Influence: 1.19 [0.99, 1.37] Moderation: −0.05 [−0.06, −0.04] |
2 | 4.87 | |
| Is moderation of shared environmental influences the same across sexes?* | Shared environmental Influence: 0.24 [−0.25, 0.72]ns Moderation: −0.009 [−0.03, 0.04]ns |
2 | 1.43 | |
| Is moderation of non-shared environmental influences the same across sexes? | Non-shared environmental Influence: 0.97 [0.88, 1.04] Moderation: −0.06 [−0.06, −0.06] |
2 | 44.32 | |
|
*Can all shared environmental influences (main effect and moderator) be constrained to zero? |
N/A | 4 | 1.48 | |
NOTE: For df=1, Δχ2 values exceeding 3.84 are significant at p<0.05; For df=2, Δχ2 values exceeding 5.99 are significant at p< 0.05; For df=4, Δχ2 values exceeding 9.49 are significant at p<0.05.
denotes models used to converge upon a test for constraining all shared environmental influences to zero; ‘ns’ = not significant at p < 0.05.
Change in the magnitude of both un-standardized and standardized variance components as a function of age at 1st drink are shown in Figures 2 and 3 for women and men, respectively. In women and men, change in the total variance (shown by the light solid line) in AD symptoms followed a U-shaped distribution with decreasing variance until age 14 followed by an increase in variance. However, variance in AD symptoms in those with an early age at 1st drink was largely explained by heritable factors whereas the variance in AD symptoms in those who had their 1st drink later in life, particularly after age 18, was largely attributable to unique environmental factors (and/or measurement error). The reduction in the relative magnitude of genetic factors as age at 1st drink increases is shown by the heavy solid line. The corresponding increase in non-shared environmental influences with increasing age at 1st drink is represented by the dotted line. The heavy solid line (representing genetic factors) and the dotted line (representing non-shared environment) intersect, in both men and women, at 13–14 years suggesting differences in the architecture of AD symptoms in those with ages at 1st drink prior and subsequent to this cut-off.
Overlapping influences
Age at 1st drink was only modestly heritable (9–14%) with a large proportion of variance (76–80%) due to shared environmental influences. First, to estimate the extent to which covariation in age at 1st drink and AD symptoms could be attributed to overlapping genetic and environmental influences, a Cholesky/standard bivariate decomposition model, without moderation, was fit, separately to male and female data. The model revealed significant overlap of genetic (RG=0.50–0.95) influences on age at 1st drink and AD symptoms. Shared environmental factors on AD symptoms were completely overlapping with age at 1st drink in women (RC=1.00), which is in agreement with findings from the univariate moderation models described above where no evidence for C was detected. Non-shared environmental factors were important for both age at 1st drink (10–11% of variance) and AD symptoms (54–60% of variance) but were un-correlated (RE=0)
Moderation of common and specific genetic influences
Next, we combined the moderation component with the standard Cholesky. Results from this bivariate moderation model are shown in Table 2. The full model for men and women (excluding DZ opposite sex twins, allowing for full sex differences) is presented. Path estimates shown are raw – those estimates where 95% confidence intervals encompass zero could be dropped from the model without a significant of model fit. These non-significant paths included all shared environmental influences on AD symptoms (common and specific) and moderation of the genetic influences specific to AD symptoms in male twins. Therefore, merging information from the moderation model and the Cholesky, the standard bivariate model revealed that genetic influences on age at 1st drink and AD symptoms were partially overlapping and that even after accounting for this overlap, there was evidence for early age at 1st drink increasing variance attributable to heritable factors on AD symptoms – in females, both the overlapping and specific genetic influences were moderated while in males, only those genetic influences that were overlapping between age at 1st drink and AD symptoms were moderated by age at 1st drink. In the bivariate moderation model, however, overlapping non-shared environmental factors could not be constrained to zero.
Table 2.
Raw parameter estimates with 95% confidence limits for the bivariate moderation model where genetic influences on AD symptoms are moderated age at 1st drink.
| Description of parameter tested | Raw parameter estimate [95% C.I.] | |
|---|---|---|
| Female | Male | |
| Raw additive genetic influences on age at 1st drink | −0.96 [−1.37, −0.51] | −1.36 [−1.78, −0.89] |
| Raw additive genetic influences overlapping between age at 1st drink and AD symptoms |
−0.24 [−0.64, −0.04] | −0.18 [−0.47, −0.03] |
| Raw additive genetic influences specific to AD symptoms | −0.61 [−0.85, −0.29] | −0.29 [−0.66, −0.18] |
| Raw shared environmental influences on age at 1st drink | 1.23 [0.80, 1.48] | 1.11 [0.23, 1.49] |
| Raw shared environmental influences overlapping between age at 1st drink and AD symptoms |
−0.09 [−0.20, 0.04]ns | −0.02 [−0.16, 0.24]ns |
| Raw shared environmental influences specific to AD symptoms | 0.00 [−0.21, 0.24]ns | −0.23 [−0.29, 0.25]ns |
| Raw non-shared environmental influences on age at 1st drink | 1.85 [1.77, 1.94] | 1.88 [1.79, 1.99] |
| Raw non-shared environmental influences overlapping between age at 1st drink and AD symptoms |
−0.05 [−0.09, −0.01] | −0.05 [−0.09, −0.01] |
| Raw non-shared environmental influences specific to AD symptoms |
0.45 [0.43, 0.48] | 0.45 [0.42, 0.47] |
| Moderation of additive influences overlapping between age at 1st drink and AD symptoms |
0.02 [0.01, 0.04] | 0.02 [0.01, 0.04] |
| Moderation of additive genetic influences specific to AD symptoms |
0.02 [0.002, 0.04] | 0.01 [−0.04, 0.03]ns |
NOTE: Parameters are un-standardized/un-squared (i.e. when squared and summed, they do not represent the total variance). Estimates are, therefore, not bounded between 0 and 1 – they are statistically significant if they are positive or negative. Estimates where the 95% C.I. encompasses zero are not statistically significant and may be constrained to 0 without a statistical deterioration in model fit – these are denoted by the superscript ‘ns’ (not significant)
DISCUSSION
We sought to examine the relationship between age at 1st drink and DSM-IV AD symptomatology. Our analyses revealed that even after controlling for the main effects of age at 1st drink on AD symptomatology (i.e. more symptoms in those with earlier age at 1st drink) and for gene-environment correlation (i.e. common genetic influences on age at 1st drink and AD symptoms), heritable influences on AD symptoms decreased with increasing age at 1st drink. In contrast, in individuals who initiated alcohol use after the age of 18 years, nearly all variance on AD symptoms could be attributed to unique environmental factors and/or measurement error.
As previously discussed by Prescott & Kendler (1999), two prevailing hypotheses have dominated our understanding of this relationship: the non-causal/common vulnerability (genetic and environmental) and the causal/environmental hypotheses. Our study sought to examine a third possibility – one that explores whether the genetic (and environmental) architecture of AD is different in those who begin their drinking careers early in life – this may be referred to as the Moderation/Interaction hypothesis.
Non-Causal/Common vulnerability hypothesis
According to the non-causal/common vulnerability hypothesis, age at 1st drink and subsequent AD symptomatology are both influenced by overlapping genetic and environmental factors. These factors may predispose individuals to initiation of alcohol use at a young age and to later development of AD symptomatology (and potentially, to a host of other problem behaviors) (Jessor, 1987; Jessor and Jessor, 1977; Kendler et al., 2003; Krueger et al., 2002; McGue et al., 2006; McGue and Iacono, 2005; Young et al., 2006). If there was evidence for the common vulnerability hypothesis, we would, first, identify significant correlations between the genetic and environmental influences on age at 1st drink and AD symptoms and, second, after accounting for these shared predispositions, there would be no additional relationship between age at 1st drink and AD symptoms. As shown in our study (and in prior work by Prescott & Kendler (1999)), there is considerable evidence for this. Particularly in our study, we found that significant proportions of the genetic influences on age at 1st drink and AD symptoms were shared, particularly in men. Work by Sartor and colleagues (in press), using dichotomous measures of early-onset drinking and DSM-IV AD in the same sample have found no evidence for sex differences but reported genetic overlap across the measures – differences across that study and the present may be attributable to our use of quantitative indices (and exclusion of the effects of conduct disorder). The public health implication of these common etiologic underpinnings is that initiatives targeted at increasing the age at 1st drink may not have a direct causal impact on reduced AD symptoms as individuals already bear the predisposing influences for AD symptoms. However, we do not find support for the second assumption of the correlated vulnerabilities model – in our study, even after controlling for shared predispositions, there continues to be a moderating influence of age at 1st drink on AD symptoms.
Causal/Environmental Hypothesis
According to this hypothesis, the relationship between age at 1st drink and AD symptomatology extends beyond shared predispositions. The most stringent form of this hypothesis posits that early age at 1st drink leads to onset of AD symptomatology. Less stringent forms suggest that even after accounting for common predispositions (or common vulnerabilities), age at 1st drink exerts a causal influences on AD symptoms. Discordant twin studies (Grant et al., 2005; Prescott and Kendler, 1999) have been widely used to test this hypothesis. By selecting pairs of twins who are discordant for early onset drinking and examining their corresponding risk for AD symptoms, these studies show that even in genetically matched individuals, those who start drinking at an early age are at increased risk for developing AD. This study design has also demonstrated that this increased risk, which may be construed as causal, is in part accounted for by the individual-specific environmental milieu in which age at 1st drink and subsequent AD develop. We do not test for this hypothesis, but if this hypothesis were supported, then prevention efforts targeted at increasing the age at 1st drink would have an impact on the likelihood of developing AD via causal pathways or via reduction of those environmental exposures (e.g. childhood traumas) that jointly increase the likelihood of early drinking and AD.
The Moderation/Interaction Hypothesis
This study tested a third mechanism via which age at 1st drink may be related to AD symptoms – according to this hypothesis, the extent to which genetic and environmental factors influence AD symptoms differs in those with earlier versus later age at 1st drink – in other words, age at 1st drink moderates/interacts with the extent to which genetic and environmental factors influence liability to AD symptoms. Evidence supporting this hypothesis was obtained by showing that heritable factors for AD symptoms are more pronounced in those who start drinking at an early age. However, in those who initiated their drinking careers later in life, especially during early adulthood, individual differences in their AD symptomatology was largely due to those environmental factors that are unique to each individual (and measurement error). The implication of this model, from a public health standpoint, is that encouraging adolescents to delay their initiation of alcohol consumption may serve to buffer the expression of familial predisposition to later alcohol-related problems.
Common vulnerabilities & Moderation
It is unlikely that a single hypothesis adequately explains the relationship between age at 1st drink and AD symptomatology. For instance, we find evidence, both for common vulnerabilities (i.e. common genetic factors) and for moderation (i.e. increased role of genetic influences on AD symptoms in those with early age at 1st drink). Therefore, the current study also tested whether after accounting for the common vulnerability underlying the timing of 1st drink and AD symptomatology, earlier age at 1st drink had a moderating influence on individual differences in AD symptomatology. We found that the relationship between age at 1st drink and AD symptoms could be attributed to (a) shared genetic and familial environmental factors and also to (b) moderation of these shared and specific genetic influences by age at 1st drink.
Gene x Environment Interaction
Moderation of the heritable component in individual differences in AD symptoms by age at 1st drink represents a G x E interaction. If earlier age at 1st drink is assumed to be representative of higher environmental risk, then our finding of increasing heritability with increasing environmental risk is consistent with prior studies which have shown that genetic influences on alcoholism risk tend to be manifest in the absence of protective environmental influences. For instance, Heath et al (Heath et al., 1989) found the heritability of alcohol consumption to be significantly higher in unmarried vs. married women and Koopmans and colleagues (Koopmans et al., 1999) found higher heritability of alcohol initiation in those with a non-religious upbringing. Our study suggests that, likewise, later age at 1st drink may serve to buffer against a genetic predisposition to AD symptoms. This finding has considerable implications. From a public health perspective, delaying the initiation of drinking in adolescents may dampen the influence of inherited and familial predisposition to AD, even though some of these predispositions are overlapping with the timing of 1st drink. Future genomic efforts targeted at identifying genes for AD symptomatology may also wish to consider their findings in the context of age at 1st drink. First, our findings suggest that individuals with adult onset of drinking may not be genetically informative in the study of AD symptomatology as variation in their AD symptoms is largely non-genetic in origin. Second, gene expression for AD symptoms is maximal in those with earlier age at 1st drink. When power permits, this G x E interaction should be modeled as it may increase our ability to isolate genes for AD. Third, our study underscores the importance of genetic influences on a putative environmental measure. As reviewed by Kendler and Baker (Kendler and Baker, 2007), a majority of known environmental factors have been found to have a heritable component – and these genetic underpinnings, when shared with the outcome of interest, may represent gene-environment correlations (rGE) (Plomin et al., 1977; Rutter et al., 2006; Scarr and McCartney, 1983).When not controlled for, these correlations can produce spurious GxE effects. In the context of gene-finding efforts, controls for rGE will be necessary prior to examining GxE. This may be accomplished by isolating those genes that jointly influence risk for AD and increase likelihood of early initiation of alcohol use.
Sex differences
A test of sex differences within the context of interaction models is challenging. In our study, there was little evidence for sex differences, which is consistent with the literature on diagnostic AD and other measures of alcohol-related problems (Pickens et al., 1991; Heath et al., 1997; Prescott et al., 1999). Shared environmental factors, when detected, may induce sex differences. In our study, we were able to constrain shared environmental influences to zero – however, in the models where age at 1st drink was jointly modeled with AD symptoms, evidence for shared environmental influences on AD symptoms were detected in some instances but found to be completely overlapping with those influences that make members of a twin pair similar for age at 1st drink. Importantly, these shared environmental factors were only detected in female twins, which supports prior findings of McGue and colleagues (1992) demonstrating that shared environmental influences on alcohol-related symptomatology may be more prominent in women while genetic influences are more pronounced in men, particularly those with early onset of AD symptoms and with work by Prescott and colleagues (1994a, 1994b) that found no evidence for shared environment on alcohol-problems but reported significant shared environmental influences on lifetime abstinence (which is correlated with lifetime ever drinking) as well as on past year alcohol consumption, where shared environmental influences on the latter were only significant in women. It is notable that, in the present study, sex differences were attributable to non-shared environmental factors, which were somewhat higher in male twins – to what extent this sex difference refers to reduced measurement precision in males reporting on their AD symptoms, particularly when they initiate drinking later in life, or to important individual-specific environmental incidents (e.g. trauma) that impact male drinking more distinctly than they do female, drinking warrants further exploration. Furthermore, while parameters from the bivariate moderation model appeared largely consistent across the sexes, AD symptom-specific genetic factors in males were not moderated by age at 1st drink – this may have been due to the high genetic overlap between age at 1st drink and AD symptoms in males, reduced power or exclusion of the DZ opposite sex twins that augment our ability to detect sex differences.
Comparison with diagnostic alcohol dependence
To examine whether our GxE effects would persist when diagnostic DSM-IV AD was used, we computed MZ and DZ correlations for a diagnosis of AD in those with early versus later onset of drinking (using 14 years and younger to denote early drinking). While there was insufficient power to detect sex differences, we found MZ correlations to range between 0.50–0.52 and DZ correlations to range between 0.14–0.24 with somewhat lower DZ correlations in twin pairs concordant for onset of drinking prior to age 15. These findings suggest that either our increased heritability estimates in those with early age at 1st drink are due to the inclusion of non-diagnostic individuals in our measure of AD symptomatology (i.e. moderation occurs at sub-threshold levels) or due to increased power to detect GxE when using continuous indices of both outcome (AD symptoms) and moderator (age at 1st drink). Additionally, the secondary correlations also imply that shared environmental influences on AD symptomatology, if any, may be attributable to the inclusion of sub-threshold drinkers.
Limitations
Some limitations of our study are noteworthy: First, our sample consisted of Caucasian young adult Australians. To the extent that heritability of age at 1st drink and AD symptomatology vary by race or ethnicity, our results may not extrapolate to populations with other demographic characteristics. Second, our results from secondary analyses examining the effect of age at 1st drink and models examining the moderation of common and specific genetic influences on AD symptoms (e.g. bivariate moderation) may have been somewhat limited by power. As a result, we restricted ourselves to only testing for moderation of genetic influences in our secondary analyses of bivariate moderation (i.e. C and E were modeled as unmoderated variables). The models for these analyses are fairly complex, and as a consequence, we also did not include the modeling of opposite sex pairs for these models. Finally, while the normality of the distribution of AD symptoms improved upon log-transformation, there was some skewness in the measure when conduct disorder was not regressed out. We reran our analyses with the raw log-transformed AD symptoms measure and while the overall findings of significant moderation remained unchanged, we view those results (not shown) with some caution.
Conclusions
Most psychiatric disorders are likely a consequence of complex gene x gene and gene x environment interactions (Heath et al., 2002; Rutter et al., 2006). Our study presents age at 1st drink as a potential risk factor in the etiology of AD symptoms. From a gene-finding perspective, investigators may be able to enhance their ability to isolate genes for AD by considering them in the context of early age at 1st drink. From a public health standpoint, later age at 1st drink may serve to protect against a familial predisposition to subsequent AD symptomatology. Even though this environmental measure may share genetic vulnerability with AD, it is a modifiable environmental factor that needs further consideration.
Acknowledgments
Funding: Research reported here is supported by AA07728 (PI Heath), AA11998 (PI Heath) and AA13221 (PI Heath), DA018660 (PI Lynskey) and funds to Dr. Agrawal from ABMRF/Foundation for Alcohol Research.
Reference List
- Bucholz KK, Cadoret RJ, Cloninger RC, Dinwiddie SH, Hesselbrock V, Nurnberger JI, Reich T, Schmidt I, Schuckit MA. A New, Semi-Structured Psychiatric Interview For Use In Genetic Linkage Studies. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Chou SP, Pickering RP. Early onset of drinking as a risk factor for lifetime alcohol-related problems. Br J Addict. 1992;87:1199–1204. doi: 10.1111/j.1360-0443.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Commandant Benoit. Note sur une méthode de résolution des équations normales provenant de l'application de la méthode des moindres carrés à un système d'équations linéaires en nombre inférieur à celui des inconnues (Procédé du Commandant Cholesky) Bulletin géodésique. 1924;2:67–77. [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Eaves L, Foley D, Silberg J. Has the "Equal Environments" assumption been tested in twin studies? Twin Res. 2003;6:486–489. doi: 10.1375/136905203322686473. [DOI] [PubMed] [Google Scholar]
- Fisher RA. The correlation between relatives on the supposition of Mendelian inheritance. Transactions of the Royal Society of Edinburgh. 1918;52:399–433. [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant JD, Scherrer JF, Lynskey M, Lyons MJ, Eisen S, Tsuang MT, True W, Bucholz K. Adolescent Alcohol Use is a Risk Factor for Adult Alcohol and Drug Dependence: Evidence from a Twin Design. Psychol Med in review. 2005 doi: 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- Gunzerath L, Goldman D. G x E: a NIAAA workshop on gene-environment interactions. Alcohol Clin Exp Res. 2003;27:540–562. doi: 10.1097/01.ALC.0000057944.57330.65. [DOI] [PubMed] [Google Scholar]
- Heath AC. Genetic influences on alcoholism risk. A review on adoption and twin studies. Alc Health Res World. 2007;19:166–171. [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heath AC, Howells W, Kirk KM, Madden PA, Bucholz KK, Nelson EC, Slutske WS, Statham DJ, Martin NG. Predictors of non-response to a questionnaire survey of a volunteer twin panel: findings from the Australian 1989 twin cohort. Twin Res. 2001;4:73–80. doi: 10.1375/1369052012182. [DOI] [PubMed] [Google Scholar]
- Heath AC, Jardine R, Martin NG. Interactive Effects of Genotype and Social Environment on Alcohol Consumption in Female Twins. Journal of Studies on Alcohol. 1989;50:38–47. doi: 10.15288/jsa.1989.50.38. [DOI] [PubMed] [Google Scholar]
- Heath AC, Nelson EC. Effects of the interaction between genotype and environment. Research into the genetic epidemiology of alcohol dependence. Alcohol Res Health. 2002;26:193–201. [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Todorov AA, Nelson EC, Madden PA, Bucholz KK, Martin NG. Gene-environment interaction effects on behavioral variation and risk of complex disorders: the example of alcoholism and other psychiatric disorders. Twin Res. 2002;5:30–37. doi: 10.1375/1369052022875. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Levenson S, Jamanka A, Voas R. Age of drinking onset, driving after drinking, and involvement in alcohol related motor-vehicle crashes. Accid Anal Prev. 2002;34:85–92. doi: 10.1016/s0001-4575(01)00002-1. [DOI] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Zakocs R. Age of drinking onset and involvement in physical fights after drinking. Pediatrics. 2001;108:872–877. doi: 10.1542/peds.108.4.872. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Assailly JP, Williams AF. Underage drinking: frequency, consequences, and interventions. Traffic Inj Prev. 2004;5:228–236. doi: 10.1080/15389580490465256. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Edwards EM. Age at drinking onset, alcohol dependence, and their relation to drug use and dependence, driving under the influence of drugs, and motor-vehicle crash involvement because of drugs. J Stud Alcohol Drugs. 2008;69:192–201. doi: 10.15288/jsad.2008.69.192. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Jamanka A, Howland J. Age of drinking onset and unintentional injury involvement after drinking. JAMA. 2000;284:1527–1533. doi: 10.1001/jama.284.12.1527. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Jessor R. Problem-behavior theory, psychosocial development, and adolescent problem drinking. Br J Addict. 1987;82:331–342. doi: 10.1111/j.1360-0443.1987.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Jessor R, Jessor SL. Problem Behavior and Psychosocial Development: A Longitudinal Study. New York: New York Academic Press; 1977. [Google Scholar]
- Jinks J, Fulker DW. Comparison of the Biometrical Genetical, MAVA and Classical Approaches to the Analysis of Human Behavior. Psychological Bulletin. 1970;73:311–349. doi: 10.1037/h0029135. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Baker JH. Genetic influences on measures of the environment: a systematic review. Psychol Med. 2007;37:615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO., Jr Twin studies of adult psychiatric and substance dependence disorders: are they biased by differences in the environmental experiences of monozygotic and dizygotic twins in childhood and adolescence? Psychol Med. 1998;28:625–633. doi: 10.1017/s0033291798006643. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. A population-based twin study of alcoholism in women. JAMA. 1992;268:1877–1882. [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PA, Bucholz KK, Slutske WS, Nelson EC, Statham D, Whitfield JB, Martin NG. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychol Med. 2004;34:1519–1530. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Slutske WS, van Baal GC, Boomsma DI. The influence of religion on alcohol use initiation: evidence for genotype X environment interaction. Behav Genet. 1999;29:445–453. doi: 10.1023/a:1021679005623. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Lynskey MT, Bucholz KK, Madden PA, Heath AC. Early-onset alcohol-use behaviors and subsequent alcohol-related driving risks in young women: a twin study. J Stud Alcohol Drugs. 2007;68:798–804. doi: 10.15288/jsad.2007.68.798. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, Statham DJ, Martin NG. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Martin NG, Eaves LJ, Heath AC. Prospects for detecting genotype X environment interactions in twins with breast cancer. Acta Genet Med Gemellol (Roma ) 1987;36:5–20. doi: 10.1017/s0001566000004542. [DOI] [PubMed] [Google Scholar]
- McGue M. The behavioral genetics of alcoholism. Current Directions in Psychological Science. 1999;8:109–115. [Google Scholar]
- McGue M, Iacono WG. The association of early adolescent problem behavior with adult psychopathology. Am J Psychiatry. 2005;162:1118–1124. doi: 10.1176/appi.ajp.162.6.1118. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Krueger R. The Association of Early Adolescent Problem Behavior and Adult Psychopathology: A Multivariate Behavioral Genetic Perspective. Behav Genet. 2006 doi: 10.1007/s10519-006-9061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Elkins I. Origins and consequences of age at first drink. II. Familial risk and heritability. Alcohol Clin Exp Res. 2001a;25:1166–1173. [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001b;25:1156–1165. [PubMed] [Google Scholar]
- McGue M, Pickens RW, Svikis DS. Sex and age effects on the inheritance of alcohol problems: a twin study. J Abnorm Psychol. 1992;101:3–17. doi: 10.1037//0021-843x.101.1.3. [DOI] [PubMed] [Google Scholar]
- Neale MC. Dept of Psychiatry, Box # 980710. Richmond VA: 2004. Statistical Modeling with Mx; p. 23298. [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic studies of Twins and Families. Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- Nelson EC, Heath AC, Madden PA, Cooper ML, Dinwiddie SH, Bucholz KK, Glowinski A, McLaughlin T, Dunne MP, Statham DJ, Martin NG. Association between self-reported childhood sexual abuse and adverse psychosocial outcomes: results from a twin study. Arch Gen Psychiatry. 2002;59:139–145. doi: 10.1001/archpsyc.59.2.139. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychol Bull. 1977;84:309–322. [PubMed] [Google Scholar]
- Pickens RW, Svikis DS, McGue M, Lykken DT, Heston LL, Clayton PJ. Heterogeneity in the inheritance of alcoholism. A study of male and female twins. Arch Gen Psychiatry. 1991;48:19–28. doi: 10.1001/archpsyc.1991.01810250021002. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population-based sample of U.S. twins. Alcohol Clin Exp Res. 1999;23:1136–1144. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Hewitt JK, Truett KR, Heath AC, Neale MC, Eaves LJ. Genetic and environmental influences on lifetime alcohol-related problems in a volunteer sample of older twins. J Stud Alcohol. 1994a;55:184–202. doi: 10.15288/jsa.1994.55.184. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Hewitt JK, Heath AC, Truett KR, Neale MC, Eaves LJ. Environmental and genetic influences on alcohol use in a volunteer sample of older twins. J Stud Alcohol. 1994b;55:18–33. doi: 10.15288/jsa.1994.55.18. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at first drink and risk for alcoholism: a noncausal association. Alcohol Clin Exp Res. 1999;23:101–107. [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Saha TD, Chou SP, Grant BF. Toward an alcohol use disorder continuum using item response theory: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2006;36:931–941. doi: 10.1017/S003329170600746X. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Heath AC, Jacob T, True W. The role of childhood risk factors in initiation of alcohol use and progression to alcohol dependence. Addiction. 2007;102:216–225. doi: 10.1111/j.1360-0443.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Bucholz KK, Madden PAF, Martin NG, Heath AC. Timing of First Alcohol Use and Alcohol Dependence: Evidence of Common Genetic Influences. Addiction. doi: 10.1111/j.1360-0443.2009.02648.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr S. Developmental theories for the 1990s: development and individual differences. Child Dev. 1992;63:1–19. [PubMed] [Google Scholar]
- Scarr S, McCartney J. How people make their own environment: A theory of genotype greater than environmental effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Sham P. Statistics in Human Genetics. London, UK: John Wiley and Sons; 1998. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) 2004 National Survey on Drug Use and Health. 2005 [Google Scholar]
- WHO. Global Burden of Disease. Burden of Disease atrributable to: Alcohol. 2009
- Young SE, Rhee SH, Stallings MC, Corley RP, Hewitt JK. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use: general or specific? Behav Genet. 2006;36:603–615. doi: 10.1007/s10519-006-9066-7. [DOI] [PubMed] [Google Scholar]