Abstract
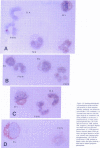
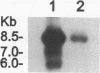
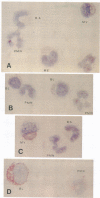
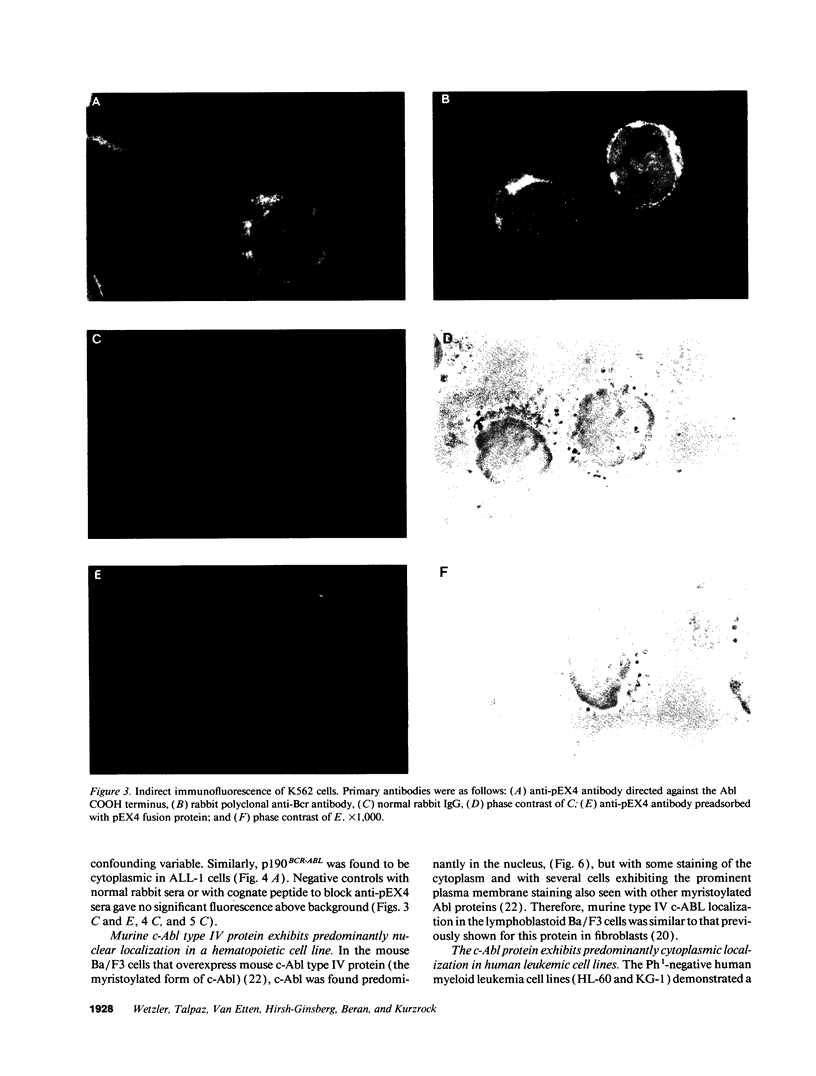
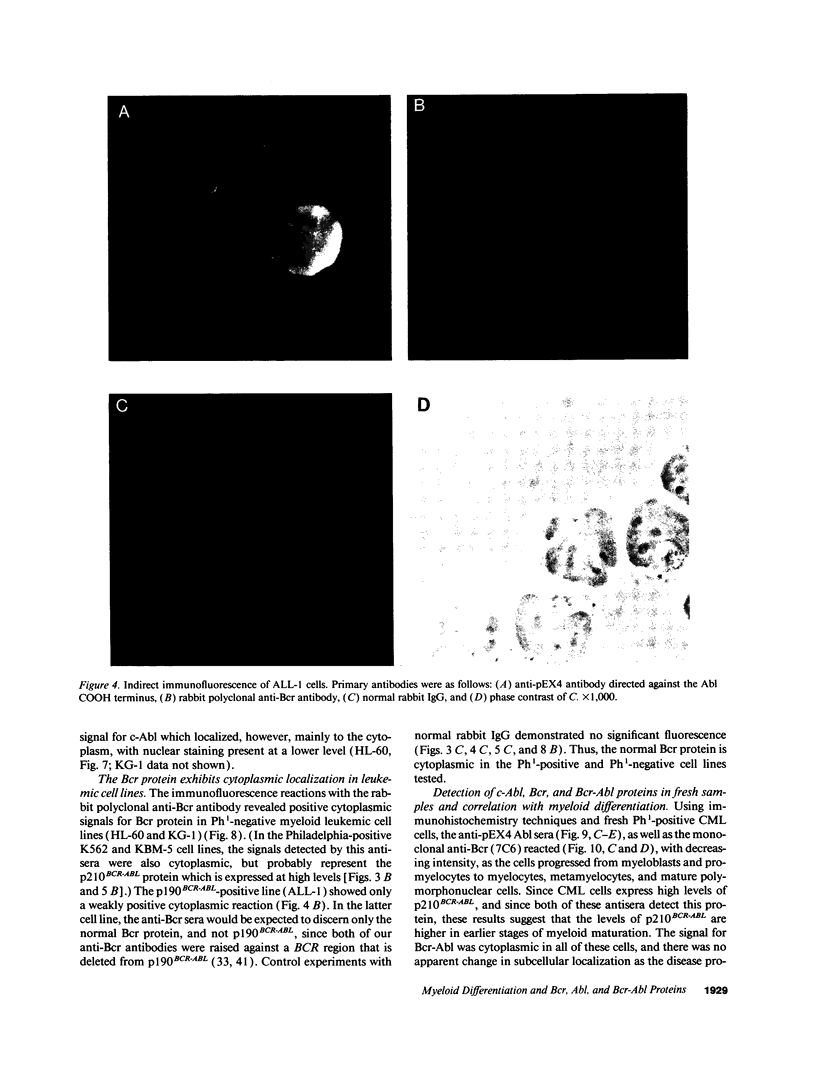
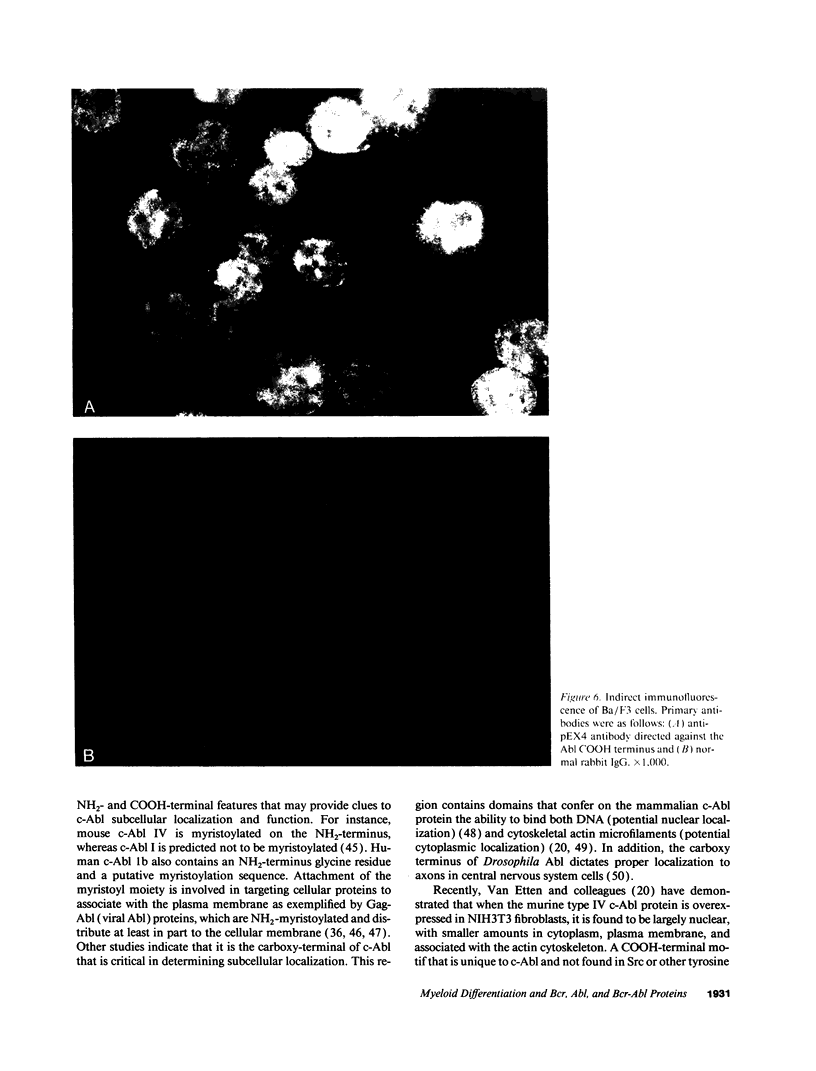
We used specific antisera and immunohistochemical methods to investigate the subcellular localization and expression of Bcr, Abl, and Bcr-Abl proteins in leukemic cell lines and in fresh human leukemic and normal samples at various stages of myeloid differentiation. Earlier studies of the subcellular localization of transfected murine type IV c-Abl protein in fibroblasts have shown that this molecule resides largely in the nucleus, whereas transforming deletion variants are localized exclusively in the cytoplasm. Here, we demonstrate that the murine type IV c-Abl protein is also found in the nucleus when overexpressed in a mouse hematopoietic cell line. However, in both normal and leukemic human hematopoietic cells, c-Abl is discerned predominantly in the cytoplasm, with nuclear staining present, albeit at a lower level. In contrast, normal endogenous Bcr protein, as well as the aberrant p210BCR-ABL and p190BCR-ABL proteins consistently localize to the cytoplasm in both cell lines and fresh cells. The results with p210BCR-ABL were confirmed in a unique Ph1-positive chronic myelogenous leukemia (CML) cell line, KBM5, which lacks the normal chromosome 9 and hence the normal c-Abl product. Because the p210BCR-ABL protein appears cytoplasmic in both chronic phase and blast crisis CML cells, as does the p190BCR-ABL in Ph1-positive acute leukemia, a change in subcellular location of Bcr-Abl proteins between cytoplasm and nucleus cannot explain the different spectrum of leukemias associated with p210 and p190, nor the transition from the chronic to the acute leukemia phenotype seen in CML. Further analysis of fresh CML and normal hematopoietic bone marrow cells reveals that p210BCR-ABL, as well as the normal Bcr and Abl proteins, are expressed primarily in the early stages of myeloid maturation, and that levels of expression are reduced significantly as the cells mature to polymorphonuclear leukocytes. Similarly, a decrease in Bcr and Abl levels occurs in HL-60 cells induced by DMSO to undergo granulocytic differentiation. The action of p210BCR-ABL and its normal counterparts may, therefore, take place during the earlier stages of myeloid development.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold L. J., Jr, Hammond P. W., Wiese W. A., Nelson N. C. Assay formats involving acridinium-ester-labeled DNA probes. Clin Chem. 1989 Aug;35(8):1588–1594. [PubMed] [Google Scholar]
- Ben-Neriah Y., Bernards A., Paskind M., Daley G. Q., Baltimore D. Alternative 5' exons in c-abl mRNA. Cell. 1986 Feb 28;44(4):577–586. doi: 10.1016/0092-8674(86)90267-9. [DOI] [PubMed] [Google Scholar]
- Ben-Neriah Y., Daley G. Q., Mes-Masson A. M., Witte O. N., Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986 Jul 11;233(4760):212–214. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- Blick M., Romero P., Talpaz M., Kurzrock R., Shtalrid M., Andersson B., Trujillo J., Beran M., Gutterman J. Molecular characteristics of chronic myelogenous leukemia in blast crisis. Cancer Genet Cytogenet. 1987 Aug;27(2):349–356. doi: 10.1016/0165-4608(87)90018-5. [DOI] [PubMed] [Google Scholar]
- Caracciolo D., Valtieri M., Venturelli D., Peschle C., Gewirtz A. M., Calabretta B. Lineage-specific requirement of c-abl function in normal hematopoiesis. Science. 1989 Sep 8;245(4922):1107–1110. doi: 10.1126/science.2672339. [DOI] [PubMed] [Google Scholar]
- Chan L. C., Karhi K. K., Rayter S. I., Heisterkamp N., Eridani S., Powles R., Lawler S. D., Groffen J., Foulkes J. G., Greaves M. F. A novel abl protein expressed in Philadelphia chromosome positive acute lymphoblastic leukaemia. Nature. 1987 Feb 12;325(6105):635–637. doi: 10.1038/325635a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clarke M. F., Kukowska-Latallo J. F., Westin E., Smith M., Prochownik E. V. Constitutive expression of a c-myb cDNA blocks Friend murine erythroleukemia cell differentiation. Mol Cell Biol. 1988 Feb;8(2):884–892. doi: 10.1128/mcb.8.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Baltimore D. Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210bcr/abl protein. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9312–9316. doi: 10.1073/pnas.85.23.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Jackson P. K., Bernards A., Baltimore D. Nonmyristoylated Abl proteins transform a factor-dependent hematopoietic cell line. Mol Cell Biol. 1992 Apr;12(4):1864–1871. doi: 10.1128/mcb.12.4.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra K., Talpaz M., Riggs M. G., Eastman P. S., Zipf T., Ku S., Kurzrock R. Hybridization protection assay: a rapid, sensitive, and specific method for detection of Philadelphia chromosome-positive leukemias. Blood. 1991 Jan 15;77(2):238–242. [PubMed] [Google Scholar]
- Dhut S., Chaplin T., Young B. D. BCR-ABL and BCR proteins: biochemical characterization and localization. Leukemia. 1990 Nov;4(11):745–750. [PubMed] [Google Scholar]
- Dhut S., Chaplin T., Young B. D. Normal c-abl gene protein--a nuclear component. Oncogene. 1991 Aug;6(8):1459–1464. [PubMed] [Google Scholar]
- Dhut S., Dorey E. L., Horton M. A., Ganesan T. S., Young B. D. Identification of two normal bcr gene products in the cytoplasm. Oncogene. 1988 Nov;3(5):561–566. [PubMed] [Google Scholar]
- Diekmann D., Brill S., Garrett M. D., Totty N., Hsuan J., Monfries C., Hall C., Lim L., Hall A. Bcr encodes a GTPase-activating protein for p21rac. Nature. 1991 May 30;351(6325):400–402. doi: 10.1038/351400a0. [DOI] [PubMed] [Google Scholar]
- Dikstein R., Heffetz D., Ben-Neriah Y., Shaul Y. c-abl has a sequence-specific enhancer binding activity. Cell. 1992 May 29;69(5):751–757. doi: 10.1016/0092-8674(92)90287-m. [DOI] [PubMed] [Google Scholar]
- Eisbruch A., Blick M., Evinger-Hodges M. J., Beran M., Andersson B., Gutterman J. U., Kurzrock R. Effect of differentiation-inducing agents on oncogene expression in a chronic myelogenous leukemia cell line. Cancer. 1988 Sep 15;62(6):1171–1178. doi: 10.1002/1097-0142(19880915)62:6<1171::aid-cncr2820620621>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Elefanty A. G., Hariharan I. K., Cory S. bcr-abl, the hallmark of chronic myeloid leukaemia in man, induces multiple haemopoietic neoplasms in mice. EMBO J. 1990 Apr;9(4):1069–1078. doi: 10.1002/j.1460-2075.1990.tb08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainstein E., Einat M., Gokkel E., Marcelle C., Croce C. M., Gale R. P., Canaani E. Nucleotide sequence analysis of human abl and bcr-abl cDNAs. Oncogene. 1989 Dec;4(12):1477–1481. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Ferguson B., Pritchard M. L., Feild J., Rieman D., Greig R. G., Poste G., Rosenberg M. Isolation and analysis of an Abelson murine leukemia virus-encoded tyrosine-specific kinase produced in Escherichia coli. J Biol Chem. 1985 Mar 25;260(6):3652–3657. [PubMed] [Google Scholar]
- Filmus J., Buick R. N. Relationship of c-myc expression to differentiation and proliferation of HL-60 cells. Cancer Res. 1985 Feb;45(2):822–825. [PubMed] [Google Scholar]
- Gewirtz A. M., Calabretta B. A c-myb antisense oligodeoxynucleotide inhibits normal human hematopoiesis in vitro. Science. 1988 Dec 2;242(4883):1303–1306. doi: 10.1126/science.2461588. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Hariharan I. K., Adams J. M., Cory S. bcr-abl oncogene renders myeloid cell line factor independent: potential autocrine mechanism in chronic myeloid leukemia. Oncogene Res. 1988;3(4):387–399. [PubMed] [Google Scholar]
- Hariharan I. K., Harris A. W., Crawford M., Abud H., Webb E., Cory S., Adams J. M. A bcr-v-abl oncogene induces lymphomas in transgenic mice. Mol Cell Biol. 1989 Jul;9(7):2798–2805. doi: 10.1128/mcb.9.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N., Jenster G., ten Hoeve J., Zovich D., Pattengale P. K., Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature. 1990 Mar 15;344(6263):251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M., West S. R., Gertler F. B., Hoffmann F. M. A novel tyrosine kinase-independent function of Drosophila abl correlates with proper subcellular localization. Cell. 1990 Nov 30;63(5):949–960. doi: 10.1016/0092-8674(90)90498-4. [DOI] [PubMed] [Google Scholar]
- Jackson P., Baltimore D. N-terminal mutations activate the leukemogenic potential of the myristoylated form of c-abl. EMBO J. 1989 Feb;8(2):449–456. doi: 10.1002/j.1460-2075.1989.tb03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki E. S., Clark S. S., Coyne M. Y., Smith S. D., Champlin R., Witte O. N., McCormick F. P. Diagnosis of chronic myeloid and acute lymphocytic leukemias by detection of leukemia-specific mRNA sequences amplified in vitro. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5698–5702. doi: 10.1073/pnas.85.15.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher M. A., McLaughlin J., Witte O. N., Rosenberg N. Induction of a chronic myelogenous leukemia-like syndrome in mice with v-abl and BCR/ABL. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6649–6653. doi: 10.1073/pnas.87.17.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipreos E. T., Wang J. Y. Cell cycle-regulated binding of c-Abl tyrosine kinase to DNA. Science. 1992 Apr 17;256(5055):382–385. doi: 10.1126/science.256.5055.382. [DOI] [PubMed] [Google Scholar]
- Kipreos E. T., Wang J. Y. Differential phosphorylation of c-Abl in cell cycle determined by cdc2 kinase and phosphatase activity. Science. 1990 Apr 13;248(4952):217–220. doi: 10.1126/science.2183353. [DOI] [PubMed] [Google Scholar]
- Kitazawa S., Maeda S., Sugiyama T. Immunocytochemical evaluation of abl-gene products in leukemic cell lines. Med Oncol Tumor Pharmacother. 1990;7(1):35–41. doi: 10.1007/BF03000489. [DOI] [PubMed] [Google Scholar]
- Kloetzer W., Kurzrock R., Smith L., Talpaz M., Spiller M., Gutterman J., Arlinghaus R. The human cellular abl gene product in the chronic myelogenous leukemia cell line K562 has an associated tyrosine protein kinase activity. Virology. 1985 Jan 30;140(2):230–238. doi: 10.1016/0042-6822(85)90361-7. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Davis R. L., Watanabe S. M., Ponticelli A. S., Schiff-Maker L., Rosenberg N., Witte O. N. Only site-directed antibodies reactive with the highly conserved src-homologous region of the v-abl protein neutralize kinase activity. J Virol. 1984 Jul;51(1):223–232. doi: 10.1128/jvi.51.1.223-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Kurzrock R., Gutterman J. U., Talpaz M. The molecular genetics of Philadelphia chromosome-positive leukemias. N Engl J Med. 1988 Oct 13;319(15):990–998. doi: 10.1056/NEJM198810133191506. [DOI] [PubMed] [Google Scholar]
- Kurzrock R., Kloetzer W. S., Talpaz M., Blick M., Walters R., Arlinghaus R. B., Gutterman J. U. Identification of molecular variants of p210bcr-abl in chronic myelogenous leukemia. Blood. 1987 Jul;70(1):233–236. [PubMed] [Google Scholar]
- Kurzrock R., Shtalrid M., Romero P., Kloetzer W. S., Talpas M., Trujillo J. M., Blick M., Beran M., Gutterman J. U. A novel c-abl protein product in Philadelphia-positive acute lymphoblastic leukaemia. Nature. 1987 Feb 12;325(6105):631–635. doi: 10.1038/325631a0. [DOI] [PubMed] [Google Scholar]
- Kurzrock R., Shtalrid M., Talpaz M., Kloetzer W. S., Gutterman J. U. Expression of c-abl in Philadelphia-positive acute myelogenous leukemia. Blood. 1987 Nov;70(5):1584–1588. [PubMed] [Google Scholar]
- Lugo T. G., Pendergast A. M., Muller A. J., Witte O. N. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990 Mar 2;247(4946):1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Chianese E., Witte O. N. In vitro transformation of immature hematopoietic cells by the P210 BCR/ABL oncogene product of the Philadelphia chromosome. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6558–6562. doi: 10.1073/pnas.84.18.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter J. R., Wang J. Y. Activation of tyrosinase kinase and microfilament-binding functions of c-abl by bcr sequences in bcr/abl fusion proteins. Mol Cell Biol. 1991 Mar;11(3):1553–1565. doi: 10.1128/mcb.11.3.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Richardson J. M., Morla A. O., Wang J. Y. Reduction in protein tyrosine phosphorylation during differentiation of human leukemia cell line K-562. Cancer Res. 1987 Aug 1;47(15):4066–4070. [PubMed] [Google Scholar]
- Rohrschneider L. R., Najita L. M. Detection of the v-abl gene product at cell-substratum contact sites in Abelson murine leukemia virus-transformed fibroblasts. J Virol. 1984 Aug;51(2):547–552. doi: 10.1128/jvi.51.2.547-552.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Scherle P. A., Dorshkind K., Witte O. N. Clonal lymphoid progenitor cell lines expressing the BCR/ABL oncogene retain full differentiative function. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1908–1912. doi: 10.1073/pnas.87.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Roe B. A., Canaani E. Alternative splicing of RNAs transcribed from the human abl gene and from the bcr-abl fused gene. Cell. 1986 Oct 24;47(2):277–284. doi: 10.1016/0092-8674(86)90450-2. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strife A., Clarkson B. Biology of chronic myelogenous leukemia: is discordant maturation the primary defect? Semin Hematol. 1988 Jan;25(1):1–19. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonini G. P., Radzioch D., Gronberg A., Clayton M., Blasi E., Benetton G., Varesio L. Erythroid differentiation and modulation of c-myc expression induced by antineoplastic drugs in the human leukemic cell line K562. Cancer Res. 1987 Sep 1;47(17):4544–4547. [PubMed] [Google Scholar]
- Van Etten R. A., Jackson P., Baltimore D. The mouse type IV c-abl gene product is a nuclear protein, and activation of transforming ability is associated with cytoplasmic localization. Cell. 1989 Aug 25;58(4):669–678. doi: 10.1016/0092-8674(89)90102-5. [DOI] [PubMed] [Google Scholar]
- Watanabe S. M., Rosenberg N. E., Witte O. N. A membrane-associated, carbohydrate-modified form of the v-abl protein that cannot be phosphorylated in vivo or in vitro. J Virol. 1984 Sep;51(3):620–627. doi: 10.1128/jvi.51.3.620-627.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Reitz M. S., Jr, Gallo R. C. Retrovirus sequences in a leukemic gibbon and its contact: evidence for partial provirus in the nonleukemic gibbon. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2032–2036. doi: 10.1073/pnas.76.4.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. C., Witte O. N. Selective transformation of primitive lymphoid cells by the BCR/ABL oncogene expressed in long-term lymphoid or myeloid cultures. Mol Cell Biol. 1988 Oct;8(10):4079–4087. doi: 10.1128/mcb.8.10.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ar-Rushdi A., Negrini M., Kurzrock R., Huebner K., Croce C. M. Fusion of the bcr and the c-abl genes in Ph'-positive acute lymphocytic leukemia with no rearrangement in the breakpoint cluster region. Oncogene. 1988 Apr;2(4):353–357. [PubMed] [Google Scholar]
- de Klein A., van Kessel A. G., Grosveld G., Bartram C. R., Hagemeijer A., Bootsma D., Spurr N. K., Heisterkamp N., Groffen J., Stephenson J. R. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982 Dec 23;300(5894):765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]