Abstract
Kinetochore function is mediated through its interaction with microtubule plus ends embedded in the kinetochore outer plate. Here, we compare and evaluate current models for kinetochore microtubule attachment, beginning with a brief review of the molecular, biochemical, cellular, and structural studies upon which these models are based. The majority of these studies strongly support a model in which the kinetochore outer plate is a network of fibers that form multiple weak attachments to each microtubule, chiefly through the Ndc80 complex. Multiple weak attachments enable kinetochores to remain attached to microtubule plus ends that are continually growing and shrinking. It is unlikely that rings or “kinetochore fibrils” have a significant role in kinetochore microtubule attachment, but such entities could have a role in stabilizing attachment, modifying microtubule dynamics, and harnessing the energy released from microtubule disassembly. It is currently unclear whether kinetochores control and coordinate the dynamics of individual kinetochore microtubules.
Keywords: Kinetochore, Centromere, Mitosis, Microtubules, Microtubule dynamics, Hec1 complex, KMN network, Electron tomography
Introduction
The mammalian kinetochore is a small, transiently assembled structure that functions to attach chromosomes to microtubules (MTs), generate force for chromosome movements, and produce a signal that delays anaphase onset until all chromosomes are attached to the spindle [1–5]. These vital functions depend upon the kinetochore’s unique property of maintaining stable attachment to MT tips that continually alternate between growing and shrinking phases [1, 2, 6]. Furthermore, since vertebrate kinetochores are attached by bundles of 10–30 MTs, known as kinetochore fibers (K-fibers), kinetochores must have mechanisms to coordinate growth and shrinkage cycles of individual MTs, and of the K-fiber as a whole, with the direction of chromosome motion [1, 6, 7]. Disruption of any of these crucial kinetochore functions causes errors in chromosome segregation during cell division, which in turn can be associated with tumorigenesis [8].
Here, we briefly review classical structural studies on the mammalian kinetochore and current understanding of its molecular composition. We then focus on current models for kinetochore MT (kMT) attachment and control of kMT plus-end dynamics. We give particular attention to the role of the KMN network, and the outer plate network and kinetochore fibril models for kMT attachment.
Classical trilaminar model of kinetochore structure
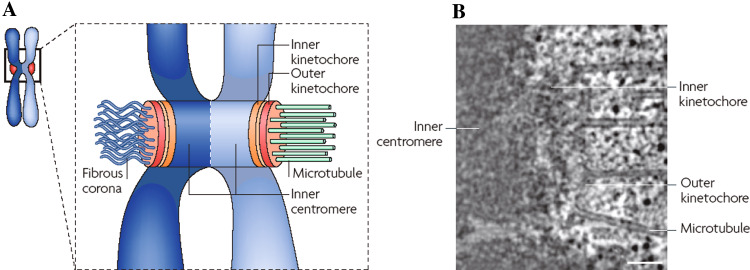
The classical trilaminar model of kinetochore structure is based upon numerous electron microscopy (EM) studies showing that vertebrate kinetochore structure consists of electron dense inner and outer plates, separated by an electron translucent middle layer (Fig. 1a) [9–12]. In addition, the unbound kinetochore was found to have an abundant fibrous corona radiating from the distal side of the outer plate. The corona disappears upon MT attachment, but reappears upon MT disassembly [13]. The plus ends of kMTs terminate in the 50–75 nm-thick outer plate [12, 14, 15]. Models based upon early EM studies postulated fibrous arrangements for the outer plate structure [9, 16]. However, the first electron tomographic analysis demonstrated that there is no regular pattern to the arrangement of the fibrous elements [17].
Fig. 1.
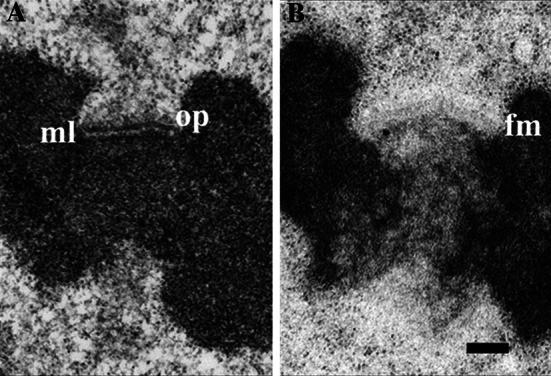
Electron micrographs of unbound kinetochores from PtK1 cells. a Specimen prepared by conventional methods. b Specimen prepared by high-pressure freezing and freeze-substitution. The outer plate (op) and electron translucent middle layer (ml) are indicated in a. The outer plate appears as a fibrous mat (fm) in b and the corona appears distal to the fibrous mat as a zone of ribosome-exclusion. In b, the translucent middle layer is absent or very small, and the cytoplasm is smooth and continuous without the empty spaces seen in a. Reprinted from [18] by permission from Springer Science and Business Media
EM studies on cells prepared by high-pressure freezing, combined with freeze-substitution, revealed that the outer plate is a 50 to 75-nm-wide fibrous mat, rather than a dense disk, and that the translucent middle zone is simply a space of variable width between the chromatin and the fibrous mat, rather than a distinct zone (Fig. 1b) [18]. In addition, fibers of the corona region in unbound kinetochores are so fine that the region appears as a 100 to 150-nm-wide ribosome-exclusion zone. Although using high-pressure freezing and freeze-substitution has a dramatic effect on the overall appearance of the kinetochore, the fibrous nature of the kinetochore is still strongly evident. Hence, the consistent feature seen in kinetochore outer plates in over 40 years of EM studies is a largely fibrous composition.
Molecular composition of mammalian kinetochores
The three-tired physical structure of the kinetochore is reflected in its molecular composition, which consists of more than 80 proteins arranged into core, middle, and transient domains (Fig. 2) [2, 3, 19]. The core domain is localized on the kinetochore inner plate (Fig. 2) and consists mostly of the constitutive centromere association network (CCAN), which includes centromere proteins (CENPs) A, C, H, I, K–R, T, and W [20, 21]. CENP-A is a homologue of histone H3 and its association with heterochromatin specifies the location of the kinetochore on the chromosome [22–24]. CENP-C and CENP-W/T then interact with CENP-A, and form two distinct pathways for the localization of other CCAN components and the MT-binding proteins [21, 25]. Members of the CCAN are required to form the middle domain and the outer plate. Thus, the CCAN functions as a platform for the formation of the middle domain. The middle domain is located distal to the heterochromatin and CCAN and has a direct role in MT attachment (Fig. 2) [2, 19]. The transient domain proteins are largely distal to the middle domain on the corona of unbound kinetochores. The transient domain is composed of the checkpoint proteins and other dynamic kinetochore proteins that turn over quickly on the kinetochore, such as BubR1, Mad2, CENP-E, and dynein [2, 5, 26, 27].
Fig. 2.
Molecular domains of the kinetochore and their relation to the physical structure. a Schematic illustration of the major molecular domains of the kinetochore and centromere. b Approximate locations of the molecular domains in a single slice from an electron tomographic reconstruction. The CCAN proteins are located in the region marked inner kinetochore (equivalent to the inner plate) and the KMN network is located in the region marked outer kinetochore (equivalent to the outer plate). The inner kinetochore forms a platform for the outer kinetochore, while the outer kinetochore binds kMTs. Scale bar in b = 250 nm. Reprinted from [19] by permission from Macmillan Publishers Ltd
Genomic screens of C. elegans have identified an intricate kinetochore assembly pathway including a network of nine proteins that are required for attachment of kMTs [28–30]. The nine proteins, also known as KMN super complex, include the Mis12 and Ndc80 complexes, each containing four proteins, and KNL-1 (kinetochore null protein-1) (Ndc80 and the Ndc80 complex are also known as Hec1 and the Hec1 complex). A number of studies have established that these middle-domain proteins are required for chromosome attachment and proper genomic segregation during mitosis [29–38].
The direct role of the KMN network in binding kMTs was first indicated by sedimentation assays showing that two members of the KMN super complex, the Ndc80 complex and KNL-1, bind to MTs in vitro [30]. MT binding by both the Ndc80 complex and KNL-1 was enhanced by the presence of the other KMN components, indicating a synergistic effect from the complete network. Direct binding of the Ndc80/Nuf2 dimer to purified MTs has been observed in vitro at moderately high resolution (3 nm) by cryo-EM [39]. The finding of homologues for all nine KMN in yeast, frog oocytes, and human cells indicates the generality of the KMN network and its role in MT attachment [29, 36, 38, 40].
Structure of the Ndc80 complex and its interaction with MTs
The Ndc80 complex is the most thoroughly studied member of the KMN network. This hetero-tetramer is composed of Ndc80 (Hec1), Nuf2, Spc24, and Spc25 in a 1:1:1:1 stoichiometry [29, 30, 38, 41]. The Ndc80 complex is located on the kinetochore outer plate [37]. It is required for kMT attachment and maintenance of structural integrity of the outer plate [31–37]. Rotary shadow and immuno-EM studies have shown that the Ndc80 complex is a rod-shaped structure formed by association of the C-tail regions of the Ndc80/Nuf2 and Spc24/25 hetero-dimers [42]. High-resolution two-color LM, combined with SHREC analysis, indicates that when kMTs are attached to the kinetochore, the Ndc80 complex is oriented at a shallow but variable angle to the cylindrical axis of kMTs, and approximately perpendicular to the outer plate [43]. The Spec24/25 end of the Ndc80 complex points towards the inner kinetochore, and the Ndc80 end points towards the spindle pole and binds to the MT walls.
X-ray analyses of crystal structures of the Ndc80/Nuf2 dimer have found that both proteins contain a calponin homology domain, which is involved in MT attachment in other MT-associated proteins [44, 45]. However, MT attachment also requires the basic 80 N-terminal amino acid tail of Ndc80, which forms electrostatic interactions with the acidic C-terminal tail of tubulin [45–47]. Furthermore, the stability of kMT attachment is regulated by phosphorylation of the Ndc80 N-terminal tail, as evidenced by studies showing that blocking the phosphorylation sites with micro-injecting antibody or point mutations causes overly robust kMT binding and chromosome-segregation errors [46–48].
Kinetochore control of kMT stability and dynamics
The preceding section summarizes the molecular, biochemical, cellular, and structural evidence that establishes the Ndc80 complex as a major (if not the major) molecular component responsible for kMT attachment. Other members of the KMN are critical to Ndc80 function and KNL-1 probably contributes to kMT binding. However, identification of the kMT-binding components by itself does not resolve the long-standing question of how kinetochores remain attached to kMTs plus ends that are continuously switching between growing and shrinking states as the kinetochore switches between movement toward and away from its attached spindle pole [1, 2, 6, 7]. Since in vitro binding studies have indicated that both Ndc80 and KNL-1 form weak attachments to MTs, Cheeseman et al. [30] proposed that kinetochores form multiple weak attachments to each kMT plus end. This enables kinetochores to remain attached to growing and shrinking kMTs by forming and breaking multiple weak bonds at different sites along the MT lattice. As long as some attachments remain during each point in time, the kMT does not dissociate.
The multiple weak attachments model is consistent with the multiple connections per kMT seen in electron tomographic reconstructions [49, 50]. The model is also supported by biochemical analysis of Xenopus extracts and quantitative fluorescence light microscopy (LM) of yeast cells showing that both mammalian and yeast kinetochores have multiple copies of Ndc80 and KNL-1 per kMT [38, 41]. Furthermore, increasing the strength of kMT attachment by blocking the phosphorylation of the N-terminal tail of Ndc80 causes errors in mitotic chromosome segregation, implying that weak binding is required for proper function [46–48].
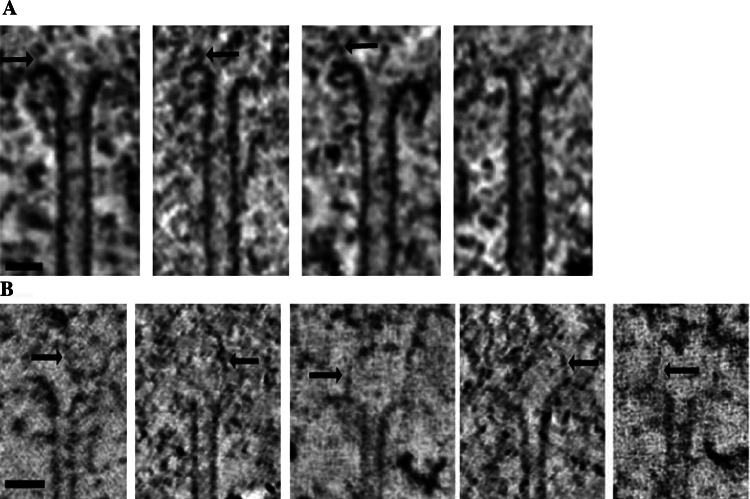
The multiple weak attachment model provides a mechanism by which kinetochores can remain attached to dynamic kMT plus ends, but it does not explain how kinetochores control kMT dynamics. It had long been assumed that kinetochores have a mechanism for controlling kMT dynamics because the K-fiber as a whole undergoes dynamic switching between growth and shrinkage as kinetochores move toward and away from their attached spindle poles during the various stages of chromosome alignment and segregation [1, 2]. However, recent results from electron tomography indicate that the assumption is at best an oversimplification because in situ two-thirds of the kMTs in metaphase PtK1 and Drosophila S2 cells have plus ends in the curved or flared conformation (Fig. 3a) [15]. Similar results have been obtained from an extensive analysis of protofilament curvature in kMT plus ends in electron tomographic reconstructions (Fig. 3b) [50]. The flared plus-end conformation is indicative of MT disassembly and thought to arise from hydrolysis of β-tubulin bound GTP to GDP [51, 52].
Fig. 3.
Conformations of kMT plus ends in situ. a A gallery of 1.6-nm-thick slices from electron tomographic volumes of PtK1 kinetochores illustrating the range of bending observed in flared conformations of kMT plus ends. Putative kinetochore fibrils are indicated by arrows. b A similar gallery of 1.0-nm-thick slices with putative kinetochore fibrils indicated by arrows. Scale bars = 50 nm. a Modified from [15] with permission from Elsevier. b Reprinted from [50] with permission from Elsevier
The conclusion that two-thirds of the kMTs in metaphase kinetochores are in the disassembly state is inconsistent with the dynamic state of the K-fiber as a whole because chromosomes oscillate about the spindle equator without exhibiting net movement toward either spindle pole during metaphase. In fact, the K-fiber as a whole exhibits net kMT assembly at the plus during metaphase, in order to compensate for continual kMT disassembly at the minus-ends [53]. Hence, there is not a simple correlation between kMT plus-end conformation and the growth or shrinkage of the K-fiber as a whole.
Lack of a correlation between kMT plus-end conformation and K-fiber growth or shrinkage can be explained by kMT plus ends cycling between assembly and disassembly states without dissociating from the kinetochore outer plate [15]. Kinetochore attachment prevents kMTs in the disassembly state from rapidly depolymerizing. Growth or shrinkage of the K-fiber as a whole is postulated to occur by biasing kinetochores to favor assembly or disassembly conformations, rather than directly controlling the dynamic state of each kMT. An alternative model postulates that kMTs are able to assemble from curved conformations, implying that MTs can grow from the GDP-tubulin lattice under appropriate conditions [50, 54, 55]. Biased cycling and growth from the flared conformation are not mutually exclusive mechanisms. Indeed, both models point out the insufficiency of numerous published illustrations showing two distinct states for the kinetochore, one where all of the kMTs have straight plus ends, and the other where all of the kMTs have flared plus ends.
Ring and sleeve models for kMT attachment
There is substantial in vitro data demonstrating that the energy released from MT disassembly at the plus end can move objects, including isolated chromosomes, toward the MT minus-end [56–58]. Since minus-end-directed motors are dispensable for poleward motion of the kinetochore in yeast, it has been suggested that MT disassembly drives poleward motion of chromosomes [4, 59]. Early theoretical considerations led to the proposal that chromosome movement could be coupled to MT disassembly by the kinetochore-forming sleeves around the kMT plus ends [60].
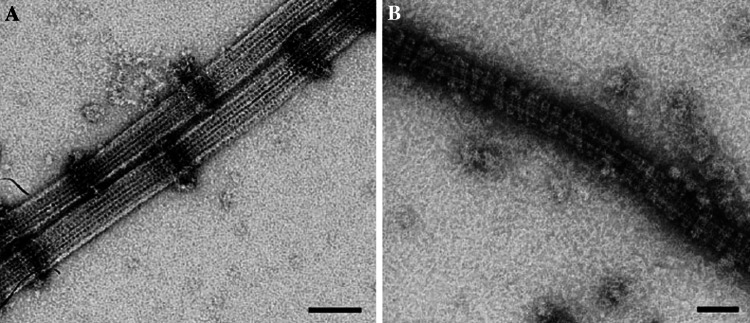
The sleeve model has remained popular for many years, particularly after it was found that purified Dam1 complex, which is required for chromosome alignment in Saccharomyces cerevisiae, forms rings around in vitro assembled MTs (Fig. 4a) [61–63]. In addition, when the MTs are transferred to conditions that disassemble MTs, the rings move along the MT lattice (Fig. 4b) [62, 63]. However, equivalent rings have not been found around kMTs in tomographic reconstructions of yeast or mammalian kinetochores [49, 50, 64]. Furthermore, there is barely enough Dam1 complex at each yeast kinetochore to form a single ring per kMT, and MT disassembly can be harnessed for movement by fewer Dam1 complexes than are needed to form a ring [65]. Finally, Dam1 is not required for chromosome segregation in most fungi species and sequence homologues have not been found in higher organisms [50, 64].
Fig. 4.
Assembly of Dam1 rings on MTs. a Purified Dam1 complex is able to form rings around in vitro assembled MTs. b Complete decoration of the MTs is observed at higher concentrations of the Dam1 complex. Scale bar = 50 nm. Reprinted from [62] with permission from Elsevier
Recent publications have shown that the Ska complex is required for stable kMT attachment in human cells, indicating that the Ndc80 complex alone is insufficient for stable kMT attachment [66–68]. This suggests that the Ska complex might be a functional homologue of the Dam1 complex. However, there is no evidence of ring formation in EM images of the Ska1 complex bound to in vitro-assembled MTs [68]. Hence, collectively, the current data fail to support a significant role for rings or sleeves in kMT attachment or coupling kMT disassembly to kinetochore motion towards the spindle pole, particularly in mammalian cells.
Fibrous network model of kMT attachment
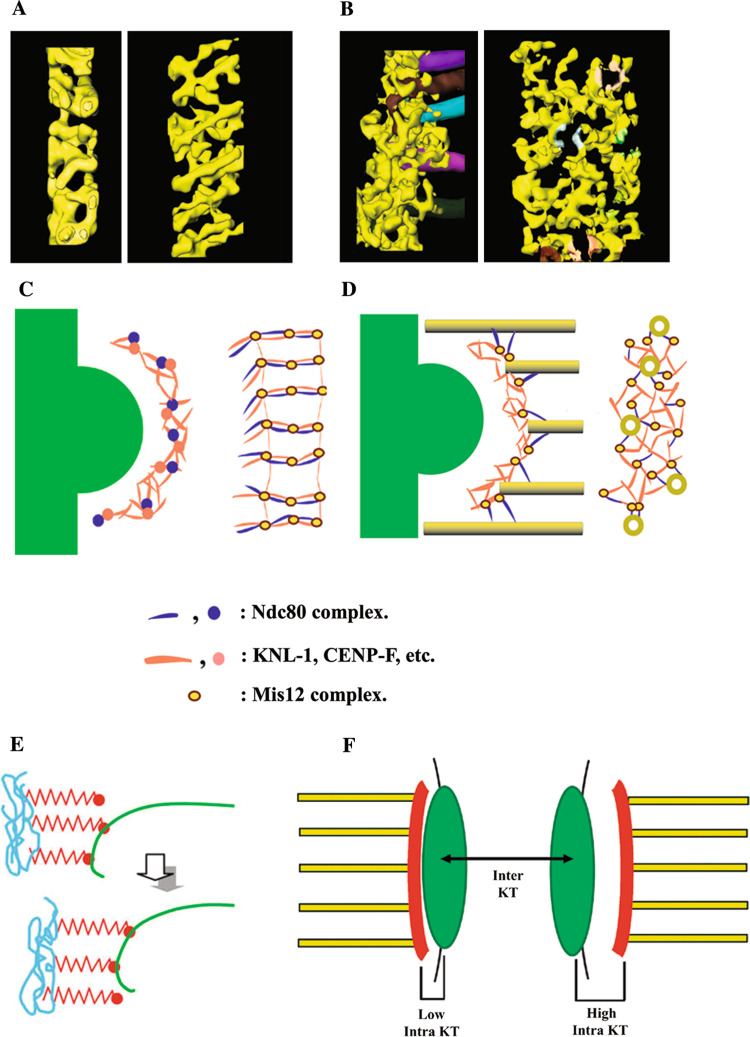
Electron tomography studies of high-pressure frozen/freeze-substituted kinetochores in PtK1 cells revealed that in the absence of MT attachment, the outer plate is a network of long fibers oriented in the plane of the plate (Fig. 5a, c) [49]. This agrees with biochemical and structural studies that the many of the key components of the MT-binding domain are fibrous [29, 42, 69]. Upon MT attachment, the outer plate rearranges into an irregular network of short fibers that form two distinct kinds of attachments to the plus ends of kMTs (Fig. 5b, d) [49]. One type of attachment is made by fibers that remain oriented in the plane of the outer plate and bind radially to the tips of kMT plus ends. The other type of attachment is formed by fibers that extend distally from the outer plate and bind to the sides of kMTs, roughly 50 nm from the tip of the plus ends. Kinetochores also form lateral attachments to the walls of MTs whose plus ends are not embedded in the kinetochore. These data suggest that the kinetochore outer plate in vertebrate cells is a flexible fibrous network that functions like a spider web, rather than a series of discrete MT-binding sties [49]. When the outer plate encounters MT-plus ends, or the lateral surface of MTs in the vicinity, the network undergoes a conformational change that enables MT-binding proteins to capture the MT by forming attachments to the tips and walls of MT plus ends, much as a spider web entangles prey that come in contact with it.
Fig. 5.
Illustrations of the outer plate network and kinetochore fibril models for kMT attachment. a, b Three-dimensional surface renderings of the outer plate in PtK1 kinetochores, based on the tracing of the outer plate fibrous components in kinetochores without (a) and with (b) kMTs. In each case, end-on views are shown on the left and en face views on the right with the outer plate network shown in yellow. Tracings of kMTs are shown in various colors in the edge view of the bound kinetochore in (b). The kMTs have been digitally removed from the en face view in (b), but shaded colors on the network fibers show where the corresponding kMTs insert into the outer plate. Note the dramatic reorganization of the outer plate upon kMT attachment. d Schematic illustrations corresponding to the surface renderings in a and b. A plausible arrangement for the KMN components is indicated by an assignment of shapes and colors in the key. e Illustration of the kinetochore fibril model. Kinetochore fibrils (shown in red) are thought to arise from chromatin in the inner kinetochore (shown in blue) and bind to the inner lumen of individual protofilaments of kMT plus ends (shown in green). In this way, the fibrils could restrict the curvature of the GDP lattice and harness some of the energy released from kMT disassembly for chromosome movement. f Illustration of how movement of the outer plate network relative to the underlying inner kinetochore could account for the intra-kinetochore stretch that is correlated with release of the spindle assembly checkpoint [71, 72]. Intra-kinetochore stretch is measured by labeling an inner kinetochore component (CENP-A) with GFP (location indicated by the green area in f), and an outer kinetochore component (Ndc80 or Mis12) with m-Cherry Red (location indicated by the red area in f). The hypothesized location of the outer plate (in red) in low and high intra-kinetochore stretch is indicated by “Low Intra KT” and “High intra KT” on the left and right sides of the figure. Inter-KT indicates the stretch between sister kinetochores, which is not correlated with checkpoint release. a–d Adapted from [49] with permission from Macmillan Publishers Ltd. e Reprinted from [50] with permission from Elsevier. f Reprinted from [73] with permission from Rockefeller University Press
Technical limitations prevent molecular identification of the structural features detected in electron tomographic reconstructions, but it is likely that at least some of the lateral attachments to MT walls correspond to the Ndc80 complex because these features occur at similar orientations and distances from MT ends as observed for Ndc80/Nuf2 dimers binding to in vitro assembled MTs [39, 49]. In addition, two-color fluorescence LM indicates that the Ndc80 head is about 50 nm distal from kMT plus ends and other members of the KMN super complex [43]. Hence, taken together, the data from electron tomography, in vitro EM, biochemical studies, and two-color fluorescence LM suggest that KNL-1 and the Mis12 complex are part of the network of outer plate fibers, with KNL-1 forming attachments to the tips of kMT plus ends and the rod-like Ndc80 complex extending out from the outer plate and attaches to walls of MT lattice (Fig. 5b, d) [30, 39, 43, 49]. While other proteins could also be directly involved in kMT attachment or perform a stabilizing role, current evidence indicates that the Ndc80 complex and KNL-1 are the major players. Electron tomographic reconstructions also predict that in unbound kinetochores the Ndc80 head is located near or in the outer plate (Fig. 5a, c). This hypothesis is supported by immuno-EM images showing that the Ndc80 head is on the distal surface of the outer plate in unbound kinetochores [37].
Kinetochore fibril model
Recently, the kinetochore fibril model was proposed as an alternative for kMT attachment (Figs. 3b, 5e) [50]. Like the outer plate network model, the fibril model is based upon electron tomography of a large number of kinetochores from PtK1 cells prepared by high-pressure freezing and freeze-substitution. However, instead of analyzing individual kMT plus ends as a unit, McIntosh and colleagues traced 4–8 protofilaments at the plus end of each kMT. This was accomplished by examining each kMT rotated about its cylindrical axis in 28° increments to find successive protofilaments. Traces of kMT plus ends drawn at the different rotation angles were used to measure protofilament curvatures. About half of the kMT protofilaments in metaphase cells had curvatures intermediate between the curvatures measured for assembling and disassembling MTs in published images of in vitro assembled MTs. The mean and range of protofilament curvatures did not change significantly from metaphase to anaphase and the variation of protofilament curvature on each individual kMT was approximately the same as in the pooled sample.
The authors postulated that intermediate protofilament curvatures are caused by slender fibrils that come from the inner kinetochore and attach to the lumen-facing surface of individual protofilaments (Figs. 3b, 5e). Attachment of the slender fibrils is envisioned as constraining the curvature of the GDP lattice and harnessing the resulting tension to create a poleward-directed force at the kinetochore. Hence, McIntosh and colleagues proposed that in mammalian cells “kinetochore fibrils”, rather than rings, are used to harness the energy of MT disassembly for moving chromosomes towards the attached spindle pole.
Comparison of the fibril and network models
On the surface, it is surprising that two different labs working with the same cell type, using essentially the same methods to optimize structural preservation and compute electron tomographic reconstructions, come up with completely different models for kMT attachment. McIntosh and colleagues attributed this partially to minor technical differences in the procedures, but this is unlikely because the advantages the McIntosh group gained using higher accelerating voltage and a finer tilt-angle increment while collecting tilt images for electron tomography are negated by examining thicker sections than the McEwen group [15, 49, 50]. Furthermore, the quality of images published by McEwen and colleagues is obviously sufficient to detect the fibrils proposed by the McIntosh group (i.e., compare Fig. 3a, b). In our view, the major difference in the models arises from the different emphasis and interest of the two research groups. Thus, while the McEwen group did report fibers coming from the inner kinetochore to the vicinity of kMT plus ends, these were interpreted as attaching the outer plate to the inner kinetochore rather than harnessing the energy of kMT disassembly for poleward motion [49]. Similarly, the McIntosh group detected the outer plate network but discounted its importance because it was not detected in all slices of their tomograms [50]. However, the outer plate becomes clear in 3D representations, as opposed to slice-by-slice analysis (Fig. 5a, b). The outer plate is also more prominent and continuous in unbound kinetochores, which the McIntosh group did not study.
The strength of the study by McIntosh and colleagues is the quantitative analysis of protofilament curvature. An earlier study had noted a variation in protofilament curvature at kMT plus ends, but the analysis was qualitative [15]. An important conclusion that can be drawn from studies of kMTs and non-kMTs is that in the intracellular environment MT assembly probably occurs from flared plus ends [50, 55]. Thus, it appears that the GDP lattice can support MT growth in certain environments and that control of MT dynamics is more complex than previously envisioned.
However, it is unlikely that kinetochore fibrils, as described by McIntosh et al. [50], play a major role in kMT capture and attachment because growing MTs have to penetrate the outer plate network and adopt a flared conformation to be assessable to the fibrils (Figs. 3b, 5e). Kinetochore encounters with growing MTs are relatively infrequent [70]. Therefore, it is more likely that kinetochores display crucial MT-binding components on the distal surface in order to maximize chances of capturing nearby MTs, as predicted by the network model, rather than burying the binding components in the heterochromatin, behind a network of other kinetochore proteins [43, 49]. Furthermore, it appears that not all kMTs have fibril attachments, meaning something else must maintain kMT attachment [50].
Another major weakness of the kinetochore fibril model is that currently there are no plausible molecular candidates for such a molecule. The authors did demonstrate that Ndc80 and CENP-E could couple movement of microbeads to MT depolymerization in vivo. However, immuno-electron microscopy and immuno-fluorescence studies place these molecules distal to kMT-plus ends in the kinetochore [26, 27, 37, 43]. Furthermore, both Ndc80 and CENP-E are known to bind to the walls of MTs, not the lumen side [39].
The network model, on the other hand, fits well with a wide range of molecular and structural studies. First, the outer plate has been observed by electron microscopy for over 40 years and electron tomographic studies of the unbound kinetochore from specimens prepared by conventional methods and high-pressure/freeze-substitution show that the basic arrangement of fibers is the same except that the structure is more collapsed in conventional preparations [17, 49]. Second, the network model fits well with the three-domain model for the arrangement of kinetochore molecular components that was derived from numerous immuno-fluorescence, and biochemistry data. Third, the KMN supercomplex is a highly plausible molecular candidate for the core of the outer plate network because it contains three proteins that are known to bind kMTs (Ndc80, Nuf2, KNL-1), it is located in the right place, depletion of components such as Ndc80 severely disrupt kMT attachment and the structure of the outer plate, and features in the electron tomograms resemble those of the Ndc80/Nuf2 dimer bound to in vitro assembled MTs [29, 30, 37, 39, 43, 48, 49].
The network model explains variation in protofilament curvature as easily as the fibril model by postulating that the protofilament curvature of the GDP lattice is constrained by being enmeshed in a fibrous network that pushes against the sides of the kMT tip, rather than pulling on the lumen side of individual kMTs (Figs. 2d, 5b, d) [15, 49]. The network model also readily explains recent reports that intra-kinetochore stretching is linked to satisfying the spindle assembly checkpoint [71, 72]. This intra-kinetochore stretching is predicted by unpublished observations that the outer plate can be located at variable distances from the underlying chromatin, as illustrated in Fig. 5f [73]. Such movements could also stabilize kMTs by moving Ndc80 away from the major site of Aurora B phosphorylation [74]. Finally, rearrangements in the spacing between putative outer-plate components detected by high-resolution two-color LM are similar to those predicted from the differences in the electron tomographic reconstructions of attached and unattached kinetochore outer plates (Fig. 5a–d) [43, 49].
Summary and conclusion
The bulk of biochemical, molecular, light and electron microscopy, and electron tomography data strongly support a model of the kinetochore outer plate as an interconnected network of proteins that functions as the primary site for kMT attachment and checkpoint signaling. At most, rings and sleeves have a minor role in chromosome congression in budding yeast. The kinetochore fibril model is accompanied by theoretical considerations useful for studying force generation, and it is possible that kinetochore fibrils have a role in modifying kMT dynamics. It is unlikely, however, that kinetochore fibrils have a significant role in kMT capture or attachment. Both the outer plate network and kinetochore fibril models agree that binding to the kinetochore constrains the curvature of the GDP-tubulin lattice in kMT plus ends and that kMTs are stabilized by kinetochore attachment preventing the rapid disassembly of kMTs whose plus ends are in the disassembly conformation. At this point it is not clear how (or even if) the kinetochore controls and coordinates the dynamics of individual kMTs.
While most of the differences in the outer plate network and kinetochore fibril models are matters of focus and interpretation, the correlation of MT plus-end conformation with growth and shrinkage of the K-fiber as a whole, and the mechanism by which the kinetochore influences protofilament shape, still need to be reconciled. Resolution of these and other remaining questions concerning the correlation between kinetochore structure and function will require further studies to determine the structures of individual kinetochore components, and correlations of LM and EM immuno-localizations with high-resolution structural studies of the kinetochore in different functional states. The fact that there are still fundamental questions concerning kMT attachment and the control of kMT dynamics illustrates that kinetochore structure and function are far more complex and subtle than originally anticipated.
Acknowledgments
We are grateful to Dr. Alexey Khodjakov for discussion and helpful comments while preparing this manuscript. BFM and YD are supported by National Institutes of Health R01GM06627 to BFM and P41 RR01219 to C. Mannella. We also acknowledge technical support from Wadsworth Center’s Core for Electron Microscopy.
References
- 1.Rieder CL, Salmon ED. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 1998;8:310–318. doi: 10.1016/S0962-8924(98)01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maiato H, Deluca J, Salmon ED, Earnshaw WC. The dynamic kinetochore–microtubule interface. J Cell Sci. 2004;117:5461–5477. doi: 10.1242/jcs.01536. [DOI] [PubMed] [Google Scholar]
- 3.Chan GK, Liu ST, Yen TJ. Kinetochore structure and function. Trends Cell Biol. 2005;15:589–598. doi: 10.1016/j.tcb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka K, Kitamura E, Kitamura Y, Tanaka TU. Molecular mechanisms of microtubule-dependent kinetochore transport toward spindle poles. J Cell Biol. 2007;178:269–281. doi: 10.1083/jcb.200702141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 6.Kline-Smith SL, Walczak CE. Mitotic spindle assembly and chromosome segregation: refocusing on microtubule dynamics. Mol Cell. 2004;15:317–327. doi: 10.1016/j.molcel.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 7.McIntosh JR, Grishchuk EL, West RR. Chromosome–microtubule interactions during mitosis. Annu Rev Cell Dev Biol. 2002;18:193–219. doi: 10.1146/annurev.cellbio.18.032002.132412. [DOI] [PubMed] [Google Scholar]
- 8.Holland AJ, Cleveland DW. Boveri revisited: chromosomal instability, aneuploidy, and tumorigenesis. Nat Rev Mol Cell Biol. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinkley BR, Stubblefield E. The fine structure of the kinetochore of a mammalian cell in vitro. Chromosoma. 1966;19:28–43. doi: 10.1007/BF00332792. [DOI] [PubMed] [Google Scholar]
- 10.Jokelainen PT. The ultrastructure and spatial organization of the metaphase kinetochore in mitotic rat cells. J Ultrastruct Res. 1967;19:19–44. doi: 10.1016/S0022-5320(67)80058-3. [DOI] [PubMed] [Google Scholar]
- 11.Roos UP. Light and electron microscopy of rat kangaroo cells in mitosis. II. Kinetochore structure and function. Chromosoma. 1973;41:195–220. doi: 10.1007/BF00319696. [DOI] [PubMed] [Google Scholar]
- 12.Rieder CL. The structure of the cold-stable kinetochore fiber in metaphase PtK1 cells. Chromosoma. 1981;84:145–158. doi: 10.1007/BF00293368. [DOI] [PubMed] [Google Scholar]
- 13.Cassimeris L, Rieder CL, Rupp G, Salmon ED. Stability of microtubule attachment to metaphase kinetochores in PtK1 cells. J Cell Sci. 1990;96:9–15. doi: 10.1242/jcs.96.1.9. [DOI] [PubMed] [Google Scholar]
- 14.Euteneuer U, McIntosh JR. Structural polarity of kinetochore microtubules in PtK1 cells. J Cell Biol. 1981;89:338–345. doi: 10.1083/jcb.89.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.VandenBeldt KJ, Barnard RM, Hergert PJ, Meng X, Maiato H, McEwen BF. Kinetochores use a novel mechanism for coordinating the dynamics of individual microtubules. Curr Biol. 2006;16:1217–1223. doi: 10.1016/j.cub.2006.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ris H, Witt PL. Structure of the mammalian kinetochore. Chromosoma. 1981;82:153–170. doi: 10.1007/BF00286101. [DOI] [PubMed] [Google Scholar]
- 17.McEwen BF, Arena JT, Frank J, Rieder CL. Structure of the colcemid-treated PtK1 kinetochore outer plate as determined by high voltage electron microscopic tomography. J Cell Biol. 1993;120:301–312. doi: 10.1083/jcb.120.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen BF, Hsieh C-E, Mattheyses AL, Rieder CL. A new look at kinetochore structure in vertebrate somatic cells using high-pressure freezing and freeze substitution. Chromosoma. 1998;107:366–375. doi: 10.1007/s004120050320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheeseman IM, Desai A. Molecular architecture of the kinetochore–microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- 20.Okada M, Cheeseman IM, Hori T, Okawa K, McLeod IX, Yates JR, 3rd, Desai A, Fukagawa T. The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat Cell Biol. 2006;8:446–457. doi: 10.1038/ncb1396. [DOI] [PubMed] [Google Scholar]
- 21.Hori T, Amano M, Suzuki A, Backer CB, Welburn JP, Dong Y, McEwen BF, Shang W-H, Suzuki E, Okawa K, Cheeseman IM, Fukagawa T. The CCAN makes multiple contacts with centromeric DNA to provide distinct pathways to the outer kinetochore. Cell. 2008;135:1039–1052. doi: 10.1016/j.cell.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Marshall OW, Choo KHA. Three-dimensional localization of CENP-A suggests a complex higher order structure of centromeric chromatin. J Cell Biol. 2008;183:1193–1202. doi: 10.1083/jcb.200804078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foltz DR, Jansen LE, Bailey AO, Yates JR, 3rd, Bassett EA, Wood S, Black BE, Cleveland DW. Centromere-specific assembly of CENP-a nucleosomes is meditated by HJURP. Cell. 2009;137:472–484. doi: 10.1016/j.cell.2009.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada M, Okawa K, Isobe T, Fukagawa T. CENP-H-containing complex facilitates centromere deposition of CENP-A with FACT and CHD1. Mol Biol Cell. 2009;20:3986–3995. doi: 10.1091/mbc.E09-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amano M, Suzuki A, Hori T, Backer C, Oawa K, Cheeseman IM, Fukagawa T. The CENP-S complex is essential for the stable assembly of outer kinetochore structure. J Cell Biol. 2009;186:173–182. doi: 10.1083/jcb.200903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooke CA, Schaar B, Yen T, Earnshaw WC. Localization of CENP-E in fibrous corona and outer plate of mammalian kinetochores from prometaphase through anaphase. Chromosoma. 1997;106:446–455. doi: 10.1007/s004120050266. [DOI] [PubMed] [Google Scholar]
- 27.Yao X, Anderson KL, Cleveland DW. The microtubule-dependent motor centromere-associated protein E (CENP-E) is an integral component of kinetochore corona fibers that link centromeres to spindle microtubules. J Cell Biol. 1997;139:435–447. doi: 10.1083/jcb.139.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai A, Rybina S, Muller-Reichert T, Shevchenko A, Shevchenko A, Hyman A, Oegema K. KNL-1 directs assembly of the microtubule-binding interface of the kinetochore in C. elegans . Genes Dev. 2003;17:2421–2435. doi: 10.1101/gad.1126303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheeseman IM, Niessen S, Anderson S, Hyndman F, Yates JR, 3rd, Oegema K, Desai A. A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 2004;18:2255–2268. doi: 10.1101/gad.1234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 31.DeLuca JG, Howell BJ, Canman JC, Hickey JM, Fang G, Salmon ED. Nuf2 and Hec1 are required for retention of the checkpoint proteins Mad1 and Mad2 to kinetochores. Curr Biol. 2003;13:2103–2109. doi: 10.1016/j.cub.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 32.Hori T, Haraguchi T, Hiraoka Y, Kimura H, Fukagawa T. Dynamic behavior of Nuf2-Hec1 complex that localizes to the centrosome and centromere and is essential for mitotic progression in vertebrate cells. J Cell Sci. 2003;116:3347–3362. doi: 10.1242/jcs.00645. [DOI] [PubMed] [Google Scholar]
- 33.McCleland ML, Gardner RD, Kallio MJ, Daum JR, Gorbsky GJ, Burke DJ, Stukenberg PT. The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev. 2003;17:101–114. doi: 10.1101/gad.1040903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bharadwaj R, Qi W, Yu HT. Identification of two novel components of the human NDC80 kinetochore complex. J Biol Chem. 2004;279:13076–13085. doi: 10.1074/jbc.M310224200. [DOI] [PubMed] [Google Scholar]
- 35.McCleland ML, Kallio MJ, Barrett-Wilt GA, Kestner CA, Shabanowitz J, Hunt DF, Gorbsky GJ, Stukenberg PT. The vertebrate Ndc80 complex contains Spc24 and Spc25 homologs, which are required to establish and maintain kinetochore–microtubule attachment. Curr Biol. 2004;14:131–137. doi: 10.1016/j.cub.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 36.Obuse C, Iwasaki O, Kiyomitsu T, Goshima G, Toyoda Y, Yanagida M. A conserved Mis12 centromere complex is linked to heterochromatic HP1 and outer kinetochore protein Zwint-1. Nat Cell Biol. 2004;6:1135–1141. doi: 10.1038/ncb1187. [DOI] [PubMed] [Google Scholar]
- 37.Deluca JG, Dong Y, Hergert P, Strauss J, Hickey JM, Salmon ED, McEwen BF. Hec1 and Nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol Biol Cell. 2005;16:519–531. doi: 10.1091/mbc.E04-09-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emanuele MJ, McCleland ML, Satinover DL, Stukenberg PT. Measuring the stoichiometry and physical interactions between components elucidates the architecture of the vertebrate kinetochore. Mol Biol Cell. 2005;16:4882–4892. doi: 10.1091/mbc.E05-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson-Kubalek E, Cheeseman IM, Yoshioka C, Desai A, Milligan RA. Orientation and structure of the Ndc80 complex on the microtubule lattice. J Cell Biol. 2008;182:1055–1061. doi: 10.1083/jcb.200804170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kline SL, Cheeseman IM, Hori T, Fukagawa T, Desai A. The human Mis12 complex is required for kinetochore assembly and proper chromosome segregation. J Cell Biol. 2006;173:9–17. doi: 10.1083/jcb.200509158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joglekar AP, Bouck DC, Molk JN, Bloom KS, Salmon ED. Molecular architecture of a kinetochore–microtubule attachment site. Nat Cell Biol. 2006;8:581–585. doi: 10.1038/ncb1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei RR, Sorger PK, Harrison SC. Molecular organization of the Ndc80 complex, an essential kinetochore component. Proc Natl Acad Sci USA. 2005;102:5363–5367. doi: 10.1073/pnas.0501168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan X, O’Quinn RP, Pierce HL, Joglekar AP, Gall WE, Deluca JD, Carroll CW, Song-Tao L, Yen TJ, McEwen BF, Stukenberg PT, Desai A, Salmon ED. Protein architecture of the human kinetochore microtubule attachment site. Cell. 2009;137:672–684. doi: 10.1016/j.cell.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei RR, Al-Bassam J, Harrison SC. The Ndc80/HEC1 complex is a contact point for kinetochore–microtubule attachment. Nat Struct Mol Biol. 2007;14:54–59. doi: 10.1038/nsmb1186. [DOI] [PubMed] [Google Scholar]
- 45.Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, DeLuca JD, De Wulf P, Salek M, Rappsiber J, Moores CA, Salmon ED, Musacchio A. Implications for kinetochore–microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133:427–439. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guimaraes GJ, Dong Y, DeLuca KF, McEwen BF, DeLuca JG. Kinetochore–microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr Biol. 2008;18:1778–1784. doi: 10.1016/j.cub.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller SA, Johnson ML, Stukenberg PT. Kinetochore attachments require an interaction between unstructured tails on microtubules and Ndc80 (Hec1) Curr Biol. 2008;18:1785–1791. doi: 10.1016/j.cub.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 49.Dong Y, VandenBeldt KJ, Meng X, Khodjakov A, McEwen BF. The outer plate in vertebrate kinetochores is a flexible network with multiple microtubule interactions. Nat Cell Biol. 2007;9:516–522. doi: 10.1038/ncb1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McIntosh JR, Grishchuk EK, Morphew MK, Efremov AK, Zhudenkov K, Volkov VA, Cheeseman IM, Desai A, Mastronarde DN, Ataullakhanov FI. Fibrils connect microtubule tips with kinetochores: a mechanism to couple tubulin dynamics to chromosome motion. Cell. 2008;135:322–333. doi: 10.1016/j.cell.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandelkow EM, Mandelkow E, Milligan RA. Microtubule dynamics and microtubule caps: a time-resolved cryo-electron microscopy study. J Cell Biol. 1991;114:977–991. doi: 10.1083/jcb.114.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang HW, Nogales E. Nucleotide-dependent bending flexibility of tubulin regulates microtubule assembly. Nature. 2005;435:911–915. doi: 10.1038/nature03606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maiato H, Khodjakov A, Rieder CL. Drosophila CLASP is required for the incorporation of microtubule subunits into fluxing kinetochore fibres. Nat Cell Biol. 2005;7:42–47. doi: 10.1038/ncb1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.VanBuren V, Cassimeris L, Odde DJ. Mechanochemical model of microtubule structure and self-assembly kinetics. Biophys J. 2005;89:2911–2926. doi: 10.1529/biophysj.105.060913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoog JL, Schwartz C, Noon AT, O’Toole ET, Mastronarde DN, McIntosh JR, Antony C. Organization of interphase microtubules in fission yeast analyzed by electron tomography. Cell. 2007;12:349–361. doi: 10.1016/j.devcel.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 56.Koshland DE, Mitchison TJ, Kirschner MW. Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature. 1988;331:499–504. doi: 10.1038/331499a0. [DOI] [PubMed] [Google Scholar]
- 57.Coue M, Lombilo VA, McIntosh JR. Microtubule depolymerization promotes particle movement in vitro. J Cell Biol. 1991;112:1165–1175. doi: 10.1083/jcb.112.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lombillo VA, Nislow C, Yen TJ, Gelfand VI, McIntosh JR. Antibodies to the kinesin motor domain and CENP-E inhibit microtubule depolymerization-dependent motion of chromosomes in vitro. J Cell Biol. 1995;128:107–115. doi: 10.1083/jcb.128.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grishchuk EL, McIntosh JR. Microtubule depolymerization can drive poleward chromosome motion in fission yeast. EMBO J. 2006;25:4888–4896. doi: 10.1038/sj.emboj.7601353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hill T. Theoretical problems related to the attachment of microtubules to kinetochores. Proc Natl Acad Sci USA. 1985;82:4404–4408. doi: 10.1073/pnas.82.13.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miranda JJ, De Wulf P, Sorger PK, Harrison SC. The Yeast DASH complex forms closed rings on microtubules. Nat Struct Mol Biol. 2005;12:138–143. doi: 10.1038/nsmb896. [DOI] [PubMed] [Google Scholar]
- 62.Westermann S, Avila-Sakar A, Wang HW, Niederstrasser H, Wong J, Drubin DG, Nogales E, Barnes G. Formation of a dynamic kinetochore- microtubule interface through assembly of the Dam1 ring complex. Mol Cell. 2005;17:277–290. doi: 10.1016/j.molcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 63.Westermann S, Wang HW, Avila-Sakar A, Drubin DG, Nogales E, Barnes G. The Dam1 kinetochore ring complex moves processively on depolymerizing microtubule ends. Nature. 2006;440:565–569. doi: 10.1038/nature04409. [DOI] [PubMed] [Google Scholar]
- 64.McIntosh JR. Rings around kinetochore microtubules in yeast. Nat Struct Mol Biol. 2005;12:210–212. doi: 10.1038/nsmb0305-210. [DOI] [PubMed] [Google Scholar]
- 65.Gestaut DR, Graczyk B, Cooper J, Widlund PO, Zelter A, Wordeman L, Asbury CL, Davis TN. Phosphoregulation and depolymerization-driven movement of the Dam1 complex do not require ring formation. Nat Cell Biol. 2008;10:379–381. doi: 10.1038/ncb1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaitanos TN, Santamaria A, Jeyaprakash AA, Wang B, Conti E, Nigg EA. Stable kinetochore–microtubule interactions depend on the Ska complex and its new component Ska3/C13Orf3. EMBO J. 2009;28:1442–1452. doi: 10.1038/emboj.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Theis M, Slabicki M, Junqueira M, Paszkowski-Rogacz M, Sontheimer J, Kittler R, Heninger A-K, Glatter T, Kruusmaa K, Poser I, Hyman AA, Pisabarro MT, Gstaiger M, Aebersold R, Shevchenko A, Buchholz F. Comparative profiling identifies C13orf3 as a component of the Ska complex required for mammalian cell division. EMBO J. 2009;28:1453–1465. doi: 10.1038/emboj.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Welburn JP, Grishchuk EL, Backer CB, Wilson-Kubalek EM, Yates JR, III, Cheeseman IM. The human kinetochore Ska1 complex facilitates microtubule depolymerization-coupled motility. Dev Cell. 2009;16:374–385. doi: 10.1016/j.devcel.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McAinsh AD, Tytell JD, Sorger PK. Structure, function, and regulation of budding yeast kinetochores. Annu Rev Cell Dev Biol. 2003;19:519–539. doi: 10.1146/annurev.cellbio.19.111301.155607. [DOI] [PubMed] [Google Scholar]
- 70.Wollman R, Cytrynbaum EN, Jones JT, Meyer T, Scholey JM, Mogilner A. Efficient chromosome capture requires a bias in the search-and-capture process during mitotic-spindle assembly. Curr Biol. 2005;15:828–832. doi: 10.1016/j.cub.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 71.Maresca TJ, Salmon ED. Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J Cell Biol. 2009;184:373–381. doi: 10.1083/jcb.200808130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uchida KSK, Takagaki K, Kumada K, Hirayama Y, Noda T, Hirota T. Kinetochore stretching inactivates the spindle assembly checkpoint. J Cell Biol. 2009;184:383–390. doi: 10.1083/jcb.200811028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McEwen BF, Dong Y. Releasing the spindle assembly checkpoint without tension. J Cell Biol. 2009;184:355–356. doi: 10.1083/jcb.200812016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu D, Vader G, Vromans MJM, Lampson MA, Lens SMA. Sensing chromosome bi-orientation by spatial separation of Aurora B kinase from kinetochore substrates. Science. 2009;323:1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]