Abstract
Background and Aims
Integrin contact with basement membrane is a major determinant of epithelial cell polarity. β1 integrin heterodimers are the primary receptors for basement membrane in pancreatic acinar cells, which function to synthesize and directionally secrete digestive enzymes into a central lumen. Aberrant acinar secretion and exposure of the parenchyma to digestive enzyme activity leads to organ damage and pancreatitis.
Methods
β1 integrin conditional knockout mice were crossed to Ptf1a-Cre mice to ablate β1 integrin in the pancreas. Histopathology of aged and caerulein-treated mice were assessed by histology and immunocytochemistry. Directional secretion was determined in vitro by FM1-43 loading with caerulein stimulation.
Results
Pancreas-specific ablation of β1 integrin led to progressive organ degeneration, associated with focal acinar cell necrosis and ductal metaplasia along with widespread inflammation and collagen deposition. β1 integrin-null pancreata were highly susceptible to caerulein-induced acute pancreatitis, displaying an enhanced level of damage with no loss in regeneration. Degenerating β1-integrin null pancreata were marked by disruption of acinar cell polarity. PKCε, normally localized apically, was found in the cytoplasm where it can lead to intracellular digestive enzyme activation. β1 integrin-null acinar cells displayed indiscriminate secretion to all membrane surfaces, consistent with an observed loss of basolateral membrane localization of Munc18c, which normally prevents basal secretion of digestive enzymes.
Conclusion
Ablation of β1 integrin induces organ atrophy by disrupting acinar cell polarity and exposing the pancreatic parenchyma to digestive enzymes.
Keywords: Pancreatitis, necrosis, laminin, basement membrane
Introduction
Pancreatic acinar cells synthesize and secrete digestive enzymes into a central lumen connected to an intercalated ductal network. If the pancreatic parenchyma is aberrantly exposed to digestive enzymes, the tissue can be damaged, potentially resulting in acute pancreatitis (AP). Inappropriate exposure to digestive enzyme activity has been linked to intracellular enzyme activation, loss of inhibitor interactions and misdirected secretion1. AP is marked by necrotic cell death, inflammation and fibrosis followed by tissue regeneration. Repeated bouts of AP can lead to chronic pancreatitis (CP), with persistent inflammation and ductal metaplasia, which is associated with an increased risk of tumorigenesis2. Because dysregulation of acinar cell function leads to painful and sometimes lethal diseases, understanding acinar cell homeostasis is of critical importance.
Acinar cell viability and regeneration are regulated by interaction with the surrounding basement membrane (BM). The importance of BM in pancreas regeneration was recognized in a model of necrotizing pancreatitis, in which destruction of 90% of exocrine cells without disrupting the BM framework allows for complete organ regeneration3. In contrast, partial pancreatectomy results only in hyperplasia with compensatory organ enlargement4. As with most epithelial cells, acinar cells contact BM mainly through integrins5, 6, the largest family of cell-matrix adhesion molecules. Integrin/matrix interactions are important for epithelial cell polarity, maintenance of tissue integrity and activation of intracellular signaling molecules7, 8.
Integrins are heterodimers of α and β subunits. 18 α and 8 β subunits are known to generate 24 different heterodimers, the largest group being β1-containing integrins. Besides serving a structural role, β1 integrins integrate environmental cues translating them into intracellular responses7, including cell differentiation, survival and migration9, 10. In some tissues, β1 integrin is required for appropriate tissue architecture and cell polarity11. β1 integrin is involved in maintenance of stem cell populations, although its precise role is highly tissue specific. For instance, ablation of β1 integrin in intestine leads to stem cell expansion12, whereas in mammary gland it leads to stem cell depletion13, 14. In still other organs, β1 integrin maintains planar cell polarity of the stem cells15. Effects on cell survival and proliferation are also tissue-dependent16. A common theme that arises from each of these examples is that β1 integrin is a major regulator of tissue homeostasis.
Pancreatic acinar cells express α3β1 and α6β1 integrin heterodimers, which act as receptors for nonfibrillar collagens and laminins5 in the acinar cell BM6. To test the role of β1 integrin in exocrine pancreas, we generated a pancreas-specific β1 integrin-null mouse. β1 integrin-null pancreata were consistently smaller, but appeared to develop and function normally. Over the course of two years, there was a progressive loss of pancreatic mass correlating with the onset of a pancreatitis-like phenotype. β1 integrin-null pancreata also showed an enhanced level of tissue damage in a caerulein-induced model of pancreatitis. Concurrent with tissue degeneration, acini became disorganized with a general mislocalization of luminal structures and cells detached from the BM. Consistent with the disruption of cellular polarity, β1 integrin-null acinar cells secreted indiscriminately to all surfaces, resulting in cellular disintegration.
PKCε and Munc18c are two molecules requiring proper cellular localization to constrain acinar cell exposure to destructive digestive enzyme activity. PKCε mobilizes from the apical membrane to cytoplasmic structures upon caerulein stimulation, where it is involved in aberrant intracellular activation of digestive enzymes17. Munc18c is normally associated with the acinar cell basal plasma membrane where it binds Syntaxin4, preventing basal secretion of digestive enzymes18. Upon caerulein stimulation, Munc18c translocates to the cytoplasm, allowing secretion from the basal surface18. Both PKCε and Munc18c were mislocalized to the cytoplasm in β1 integrin null pancreata, suggesting that organ degeneration in these mice is due to inappropriate activation and mislocalized secretion of digestive enzymes into the pancreatic parenchyma.
Experimental procedures
Mice
Mice were maintained in a C57Bl/6J background. Protocols were approved by the Stony Brook University Institutional Animal Care and Use Committee.
Genotyping and detection of recombination
Cre-mediated recombination was detected using primers P1-GCCGCCACAGCTTTCTGCTGTAGG and P3- TGCCGCTCATCCGCCACA yielding an 800 bp PCR product indicating excision of the intervening DNA. Primers P1 and P2 (CTGATCAATCCAATCCAGGAAACC) were used to genotype.
Experimental pancreatitis and tissue processing
Control (saline-injected) and experimental (caerulein-injected) groups consisted of age-matched (6-7 weeks) mice. Mice were injected intraperitoneally with 7 hourly injections of 50 μg/kg caerulein (Sigma-Aldrich, St. Louis, MO) followed by 1 hour, 24 hours or 1 week of recovery. Some mice were injected with 100 mg/kg BrdU (Sigma-Aldrich) 24 and 2 hours prior to sacrifice. Mice were sacrificed and blood collected by cardiac puncture for plasma preparation. A portion of pancreas was fixed in 10% formalin and paraffin embedded. 5 μm sections were stained with Masson’s trichrome stain (Sigma-Aldrich) or picrosirius red (Polysciences, Inc). Another tissue segment was fixed in paraformaldehyde for 3 hours, equilibrated in 30% sucrose and frozen at -80°C in OCT resin (Sakura Finetek, Torrance, CA).
Immunostaining and LacZ detection
Immunohistochemistry was performed as previously described19. Slides were counterstained in hematoxylin, dehydrated, mounted and photographed on an Olympus BX41 light microscope.
Immunofluorescence was performed on 8 μm cryosections. Sections were air-dried, washed in PBS and fixed 10 min in 4% paraformaldehyde. Slides were blocked 1 hour in 3% BSA/PBS and incubated with primary antibody (see Supplemental information) or Alexafluor488-labeled phalloidin. Slides were washed in PBS–Tween-20 and incubated with Alexafluor488 or Alexafluor594–conjugated secondary antibody (Invitrogen) and mounted in Vectashield containing DAPI (Vector Laboratories). Images were acquired on a Zeiss 510LS Meta confocal microscope.
LacZ reporter expression was detected on 8 μm frozen sections. Sections were washed in PBS, fixed in 1% formalin for 1 min and incubated 12-16 hours at 37°C in 40mM phosphate/citrate buffer (pH 7.5), 2 mM MgCl2, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, X-gal (Sigma-Aldrich) at a concentration of 1 mg/ml.
Primary acinar cells and live cell imaging
Primary acinar cells of 4-7 weeks old mice were prepared by modifying a published protocol18 (see Supplemental Information). For imaging, cells were incubated in Krebs Ringer Solution with 2 μM FM1-43 (Invitrogen, Carlsbad, CA) and imaged on a Zeiss LSM510 confocal microscope equipped with heated stage and objective. After obtaining stable fluorescent signal, caerulein was added to 1 nM.
Amylase activity assay and protein quantitation
Amylase levels were determined using Liquid Amylase Reagent (Pointe Scientific, Canton, MI). Plasma was diluted 1:800 and tissue lysates 1:4000 to 1:25000 in 200 μl of reagent. Amylase content was determined according to manufacturer’s instructions, compared to a standard curve using purified amylase (Sigma-Aldrich).
Protein lysates were prepared by sonication on ice of ~100 mg pancreatic tissue in 50 mM Tris, 0.5% NP-40, 150 mM NaCl with Complete protease inhibitor (Roche, Nutley, NJ). Protein concentration was measured by BCA kit (Pierce, Rockford, IL).
Statistical Analysis
Minitab software (Minitab, State College, PA) was used for statistical analysis. Group means were compared by unpaired, 2-tailed, Student’s T-test.
Results
Pancreas-specific β1 integrin ablation
To study the role of β1 integrin in the pancreas we crossed conditional β1 integrin-null mice20, which carry functional copies of the β1 integrin gene, with exons 2-7 flanked by LoxP sites (β1-Itgflox/flox), to Ptf1a-Cre mice21. Ptf1a-Cre mice carry a Cre recombinase transgene homologously recombined into the Ptf1a locus, resulting in Cre expression and recombination in pancreatic progenitor cells and thus in most adult exocrine and endocrine cells. Cre recombination excises exons 2-7, juxtaposing a promoterless β-galactosidase reporter gene with the endogenous β1 integrin promoter, allowing for confirmation of gene deletion at the cellular level.
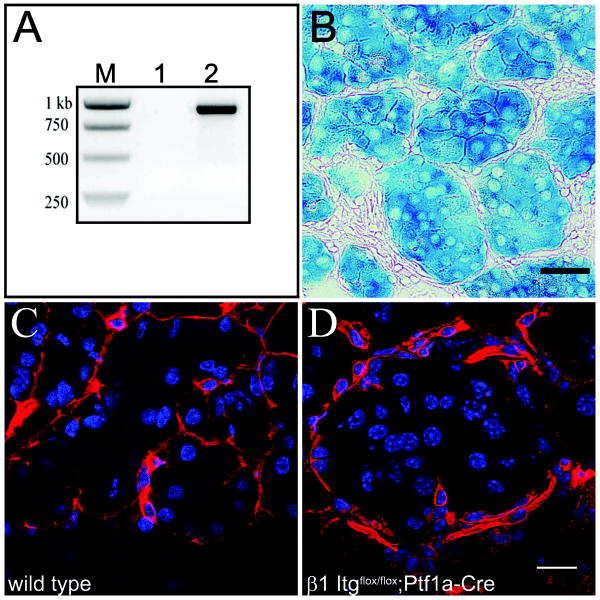
Cre recombination in β1Itgflox/flox;Ptf1a-Cre mice was determined by allele-specific PCR (Fig. 1A). LacZ reporter activity in β1-Itgflox/flox;Ptf1a-Cre mice, as determined by X-gal staining, was found in >95% of acinar, ductal and islet cells (Fig. 1B). β1 integrin protein loss was confirmed by immunofluorescence (Fig. 1 C&D). β1 integrin ablation in exocrine cells appeared to be near complete, while surrounding cells expressed robust levels of the protein.
Figure 1. β1 integrin in β1-Itgflox/flox;Ptf1a-Cre pancreata.
(A) Allele-specific primers give a recombination-dependent 800 bp product in pancreas (lane 2), but not tail DNA (lane 1) of a β1-Itg flox/flox;Ptf1a-cre mouse. (B) X-gal staining confirms recombination-dependent expression of LacZ reporter in acinar cells of β1Itg flox/flox;Ptf1a-Cre pancreata. Scale bar = 40 μm. (C&D) Immunofluorescence for β1 integrin (red) showing basolateral staining of acinar cells in wild type pancreas (C), but not in β1-Itg flox/flox;Ptf1a-Cre pancreas (D). Interstitial cells surrounding acini show immunoreactivity regardless of genotype. Scale bar = 20 μm. DAPI shown in blue.
Loss of β1 integrin causes pancreatic degeneration
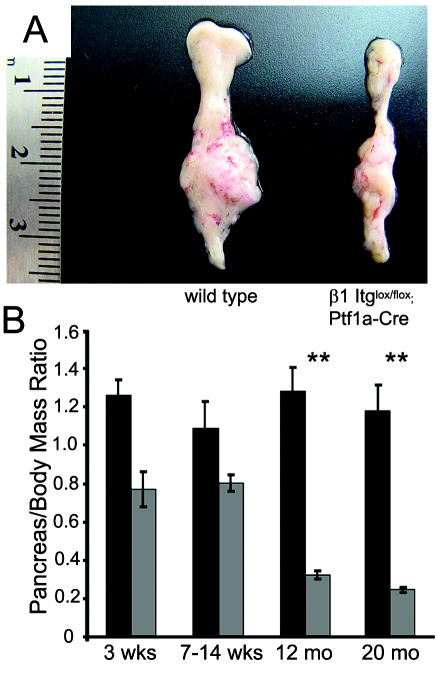
β1-Itgflox/flox;Ptf1a-Cre mice were born at the expected Mendelian ratio, had a normal lifespan (~2 years) and were grossly indistinguishable from β1-Itgflox/flox littermates. At the gross organ level, we observed an obvious and progressive reduction in pancreatic mass in β1-Itgflox/flox;Ptf1a-Cre mice (Fig 2A). For quantitation, we compared pancreas to body mass ratios of wild type and β1-Itgflox/flox;Ptf1a-Cre mice. In the four age groups examined (3 weeks, 7-14 weeks, 12 months and 20 months) wild type pancreata were consistently ~1.1% of total body mass, regardless of age. In β1-Itgflox/flox;Ptf1a-Cre mice, relative pancreatic mass was smaller (~0.8%) but stable up to 14 weeks of age. Relative pancreatic mass progressively decreased to 0.25% by 12 months, a relative size that was stable up to 20 months of age (Fig. 2B).
Figure 2. β1-Itgflox/flox;Ptf1a-Cre pancreata degenerate with age.
(A) Gross phenotype of 1 year-old wild type and β1-Itg flox/flox;Ptf1a-Cre pancreata. (B) Pancreas/body mass ratio in β1-Itg flox/flox;Ptf1a-Cre mice at 3 weeks (n=7, each group) and 7-14 weeks of age (n=4, each group) was ~70% that of wild type mice. By 12 months, relative pancreatic mass in β1-Itg flox/flox;Ptf1a-Cre mice decreased to ~25% of control (P<0.05, n=9 for wild type, n=15 for β1-null), which remains stable at 20 months (n=9, each group.
Histological examination revealed that up to 2 weeks of age, both wild type and β1-Itgflox/flox;Ptf1a-Cre mice developed a grossly normal exocrine pancreas (Suppl. Fig 1A&B). Wild type acinar cells, independent of age, had normal structural symmetry with nuclei basally localized (Suppl Fig. 1A,C&E, Suppl Fig. 2). By 3 weeks, β1-Itgflox/flox;Ptf1a-Cre pancreata were characterized by the appearance of larger acini, marked by cells with little cytoplasm centrally-located in acinar structures (Suppl Fig 1D&F, Suppl. Fig. 2). After 3 weeks of age, the percentage of abnormal acini increased from 1-2% of total to approximately 50% at 3 months and was the predominant type by one year of age.
With increasing age, some properties usually associated with pancreatitis were observed in β1-Itgflox/flox;Ptf1a-Cre pancreata. Histological signs of acinar cell necrosis and a mild stromal reaction were common in animals one year of age or older (Suppl. Fig 1F). To quantify necrosis, we performed HMGB-1 immunohistochemistry. In normal cells, HMGB-1 is nuclear, associated with chromatin. In necrotic cells, it is transported out of the nucleus and expelled into the extracellular space, where it acts as an inflammatory cytokine22, 23. We detected ~5 times the number of acinar cells that had lost nuclear and gained cytoplasmic HMGB1 staining in 4/6 12 month old β1-Itgflox/flox;Ptf1a-Cre mice, compared to wild type, with many necrotic cells focally localized (Fig 4B). Loss of β1 integrin function in other systems has been shown to enhance other forms of cell death, including apoptosis and senescence24, which could contribute to tissue degeneration. However, cleaved caspase-3 immunohistochemistry showed no apoptosis in β1-Itgflox/flox;Ptf1a-Cre pancreata (data not shown), similar to β1 integrin-null mammary13 and intestine12. We attempted to quantitate senescence-associated β-galactosidase activity but LacZ reporter activity from the targeting construct was too prominent at pH 6, as determined by staining β1-Itgflox/WT;Ptf1a-Cre pancreata.
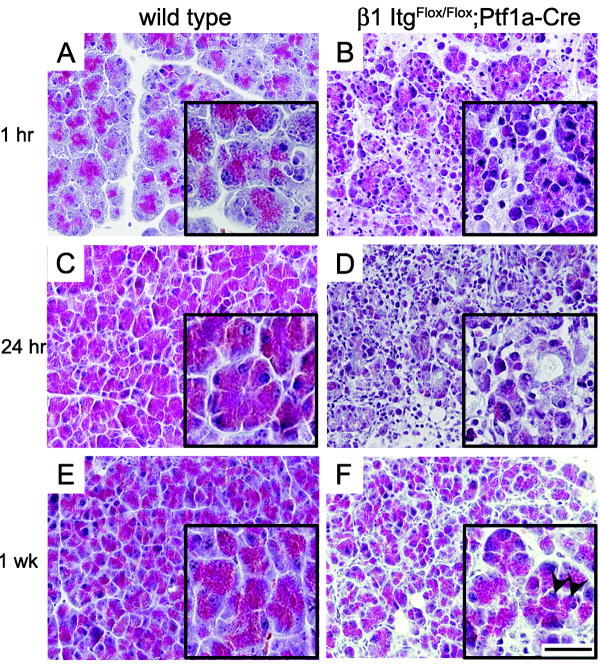
Figure 4. Enhanced caerulein-induced damage in β1-Itgflox/flox;Ptf1a-Cre pancreata.
10 week old mice were injected hourly with 50 μg/kg caerulein for 7 hours and sacrificed 1 hour (A,B), 24 hours (C,D) and 1 week (E,F) after the final injection. Masson’s trichrome stained sections from β1-Itgflox/flox;Ptf1a-Cre pancreata with one hour recovery (B) indicated extensive vacuolization and loss of membrane integrity compared to wild type controls (A). Wild type pancreata recovered after 24 hours (C), while β1-Itg flox/flox;Ptf1a-Cre pancreata showed poor tissue integrity and persistence of inflammatory cells (D). 1 week after injection both wild type (E) and β1-Itg flox/flox;Ptf1a-Cre (F) pancreata had largely recovered but with aberrant acini commonly present in caerulein-treated β1-null pancreata. Arrows indicate centrally localized nuclei (F, inset). (Scale bar main picture= 40μm; inset= 80μm).
The stromal reaction evident in aged β1-Itgflox/flox;Ptf1a-Cre mice was composed primarily of fibroblast specific protein positive, α-smooth muscle actin negative cells (data not shown), suggesting an immature fibrotic response. Consistent with this, a thin layer of collagen deposition, determined by picrosirius red staining, was found sporadically in relatively young β1-Itgflox/flox;Ptf1a-Cre mice (6-12 months), but was widespread in β1-Itgflox/flox;Ptf1a-Cre mice 20 months of age and older (Fig 3B). Most of the remaining stromal cells were macrophage with rare neutrophils also present (Fig. 3D). Active acinar cell necrosis in older β1-Itgflox/flox;Ptf1a-Cre mice (Fig 3A) and macrophage/neutrophil infiltration was suggestive of AP. However, examination of sections through the entire depth of pancreata older than one year, we found multiple focal lesions resembling CP, including epithelial metaplasia (Fig 3C), in 20/24 β Itgflox/flox;Ptf1a-Cre mice, including 9/9 20 month old mice, compared to 0/15 in wild type age matched controls. Together, our data support a model where β1 integrin ablation leads to an age dependent pancreatitis-like phenotype.
Figure 3. β1-Itgflox/flox;Ptf1a-Cre pancreas degeneration.
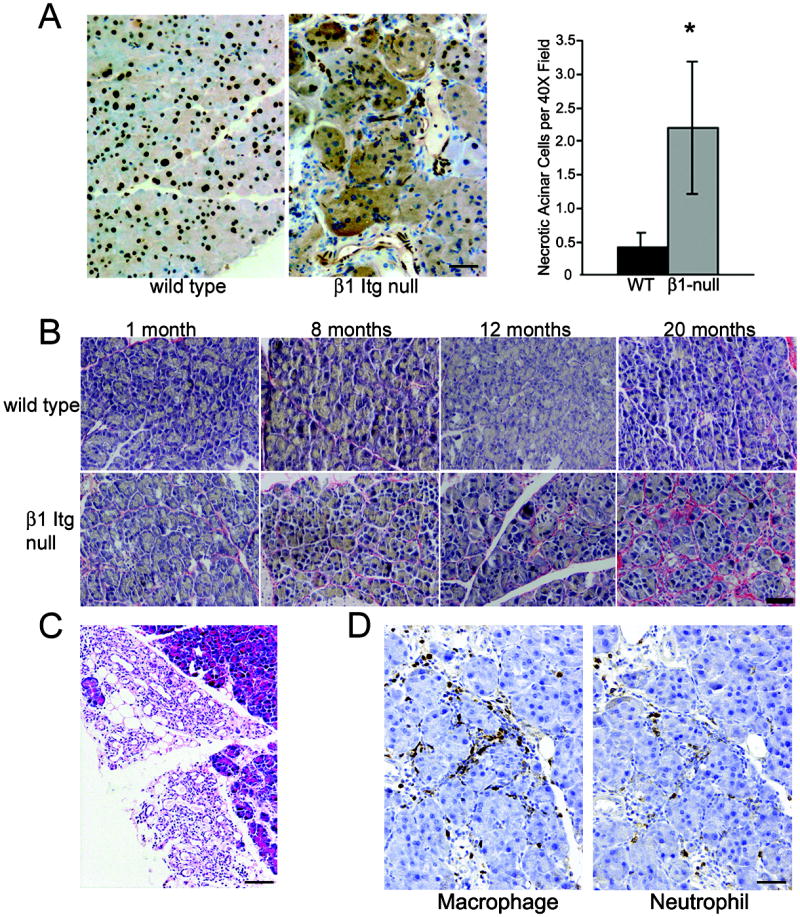
(A) HMGB1 staining shows nuclear localization in acinar cells of 1.3 year old wild type pancreata. Age-matched β1-Itg flox/flox;Ptf1a-Cre pancreata consistently show areas of loss of nuclear and gain of cytoplasmic HMGB-1 staining, indicating necrosis (n=6, each group). Graph shows quantitation of acinar cells with cytoplasmic HMGB1 per 40x field indicates ~5 times the number of necrotic cells in β1-Itg flox/flox;Ptf1a-Cre compared to controls (P<0.05). (Averages of 3 mice/genotype, 10 random 40X fields/mouse; error bars ± SEM). Scale bar = 50 μm) (B) Picrosirius red stain showing increasing collagen deposition specifically in β1-Itg flox/flox;Ptf1a-Cre pancreata. Scale bar = 50 μm (C) Focal regions resembling CP, including inflammatory infiltrates, fibrosis and acinar-to-ductal metaplasia found exclusively in β1-Itg flox/flox;Ptf1a-Cre mice older than 1 year. Scale bar = 100 μm. (D) Immunohistochemistry showing macrophage and neutrophil infiltration found in 12 month old β1-Itg flox/flox;Ptf1a-Cre pancreas. Scale bar =100 μm
Caerulein-induced damage is more profound in βItgflox/flox;Ptf1a-Cre mice
Tissue degeneration in β1-Itgflox/flox;Ptf1a-Cre pancreata suggested an abnormally high level of constant tissue damage, defective tissue regeneration or both. In order to address these possibilities, we employed a model of caerulein-induced pancreatitis1. Supramaximal doses of caerulein induce a discharge of acinar enzymes, resulting in tissue damage and a mild, reversible episode of AP.
Eight week old wild type and β1-Itgflox/flox;Ptf1a-Cre mice received 7 hourly 50 μg/kg caerulein injections and were sacrificed at 1 hour, 24 hours or 1 week after the last injection. At 1 hour, wild type pancreata showed a mild AP phenotype (Fig. 4A). In contrast, β1-Itgflox/flox;Ptf1a-Cre acinar cells showed extensive vacuolization and discharge of membrane-containing bodies and loss of acinar structure (Fig 4B). At 24 hours, wild type mice had largely recovered and appeared phenotypically normal (Fig. 4C), but β1-Itgflox/flox;Ptf1a-Cre mice continued to display large areas of acinar necrosis, little regeneration of acinar tissue and a persistence of inflammatory cells (Fig.4D). With one week of recovery, the majority of pancreata in both genotypes had largely healed (Fig. 4 E & F), but many acini in β1-Itgflox/flox;Ptf1a-Cre mice showed an enlarged, disorganized architecture and persistent stromal reaction (Fig. 5F) reminiscent of >1 year old untreated mutant mice (Suppl Fig. 1F).
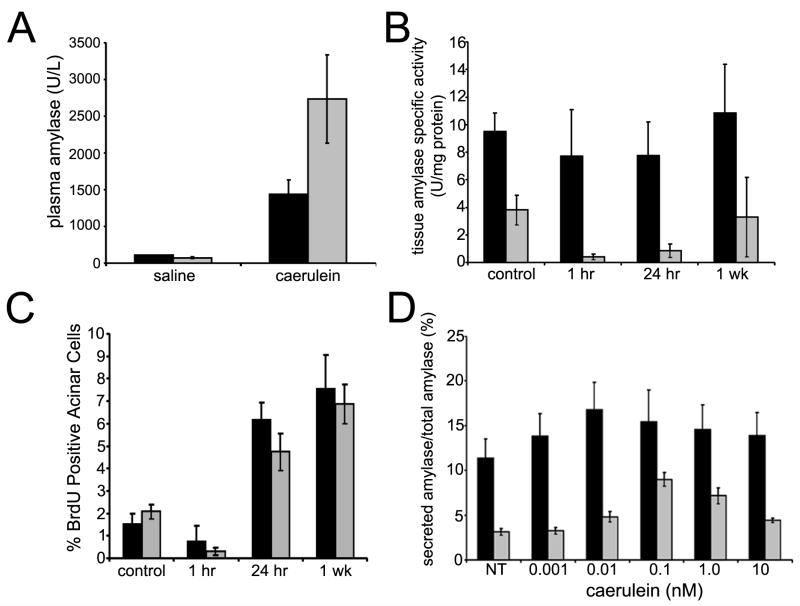
Figure 5. Caerulein response in β1-Itgflox/flox;Ptf1a-Cre mice.
(A) Plasma amylase levels from caerulein-treated mice 1 hour after recovery. Normalized to saline-injected controls, the relative increase after caerulein treatment in β1-Itg flox/flox;Ptf1a-Cre mice (grey bars) was >2 fold greater than wild type (black bars). Averages of 3 or more mice/group; error bars ± SEM. (B) Wild type pancreatic lysates did not show significant changes in tissue amylase after caerulein injection. β1-Itgflox/flox;Ptf1a-Cre (grey bars) saline-injected controls had significantly less tissue amylase activity than wild type. Normalized to saline-injected controls, β1-Itgflox/flox;Ptf1a-Cre pancreatic lysates showed a decrease (P<0.05) at 1 and 24 hours post-treatment. (C) Acinar cell proliferation was measured by immunohistochemistry for BrdU incorporation. Relative incorporation was similar at 1 hr, 24 hrs and 1 week after caerulein treatment in wild type and β1-Itg flox/flox;Ptf1a-Cre mice. Each bar represents blind counts of BrdU positive nuclei divided by total acinar cells from of 3 randomly selected 40X fields from each of 5 slides/mouse, 3 mice/time point and treatment condition. Error bars ± SEM. (D) Primary acinar cell explants were treated with caerulein at various concentrations. Conditioned media and lysates were collected after 30 minutes and amylase activity determined. Media amylase relative to total amylase is depicted. Maximal amylase levels were found at 10 pM (wild type) and 100 pM (β1-null) caerulein. (Averages from triplicate assays for 3 or more mice; error bars ± SEM).
Plasma amylase activity, a marker of acute pancreatic injury25, was elevated after caerulein injections, but was higher in β1-Itgflox/flox;Ptf1a-Cre mice compared to wild type (Fig 5A, P <0.05). To further examine caerulein response, we measured amylase specific activity in tissue lysates as an indicator of functional exocrine tissue. Interestingly, saline-injected wild type controls had a 3-fold higher specific activity compared to age-matched, β1-Itgflox/flox;Ptf1a-Cre control mice (Fig 5B), indicating less zymogen production or storage in β1 integrin-null pancreata. Relative to untreated mice, amylase activity in wild type pancreata dropped only by ~20% 1 hour post treatment compared to >90% in β1-Itgflox/flox;Ptf1a-Cre lysates. At 24 hours, amylase activity in both mice recovered to their 1 hr levels and returned to control levels after 1 week, consistent with the degree of recovery seen histologically.
Tissue regeneration can be dependent on maintenance of an adult progenitor population, which has been shown to be a function of β1 integrin in other organs14, 26. However, recent work suggests that regeneration of exocrine pancreas is from the division of adult acinar cells27. With these observations in mind, we tested the regenerative capacity of caerulein-damaged pancreata by measuring BrdU incorporation in acinar cells as a measure of proliferation. The percent of BrdU positive acinar cells rose to similar levels starting at 24 hrs after the final injection in both genotypes and remained high 1 week after treatment (Fig 5C). These data suggested that β1-Itgflox/flox;Ptf1a-Cre mice were comparable to wild type in their proliferative response, supporting the hypothesis that enhanced tissue damage was primarily responsible for the dramatic phenotypic difference between wild type and mutant pancreata after caerulein treatment.
To test if the enhanced degree of damage was due to an exaggerated sensitivity to caerulein in β1-Itgflox/flox;Ptf1a-Cre acinar cells, we isolated acinar explants from wild type and β1-Itgflox/flox;Ptf1a-Cre mice and measured amylase secretion in response to a variety of caerulein concentrations. β1-Itgflox/flox;Ptf1a-Cre acinar cells showed a peak response at 0.1 nM caerulein, a concentration 10-fold higher than the optimal concentration for wild type acinar cell and resulted in approximately 50% less enzyme released. Thus, β1-Itgflox/flox;Ptf1a-Cre acinar cells did not show increased sensitivity or responsiveness to caerulein. In summary, our data suggest that β1-Itgflox/flox;Ptf1a-Cre mice are prone to an abnormal degree of pancreatic damage when stimulated to secrete digestive enzymes.
Acinar polarity is disrupted in β1 integrin-null acini
Since β1 integrin null cells were not inherently more sensitive to caerulein, we hypothesized that the enhanced damage observed in the mutants was related to the aberrant acini within the degenerating pancreata (Suppl Fig 1F). To further characterize this phenotype, we performed co-immunofluorescence for laminin α2 and ZO-1 to demarcate BM and acinar lumen, respectively. 3 week old mice showed no significant architectural differences between the two genotypes, but older mice displayed a clearly larger diameter encompassed by BM (Fig. 6A, Suppl. Fig 3). Differences in diameter in β1-Itgflox/flox;Ptf1a-Cre acini became apparent at 7 weeks of age (data not shown) and this difference was pronounced in mice 1 year or older. Consistent with tissue histology, β1-Itgflox/flox;Ptf1a-Cre acini frequently showed a number of nuclei that lost their close apposition with BM, suggesting a disruption of cell-BM contact (Fig 6A, Suppl. Fig. 2).
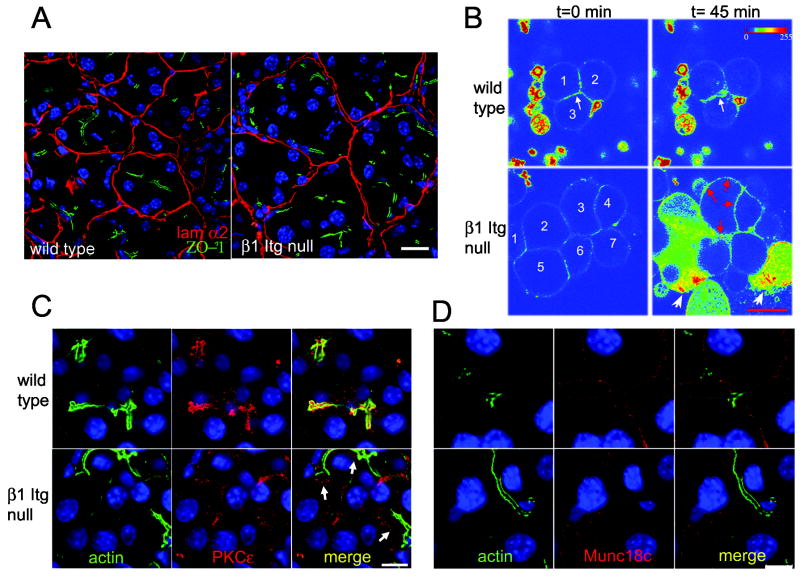
Figure 6. β1-Itgflox/flox;Ptf1a-Cre acini display defective cell polarity.
(A) 12 month old wild type and β1-Itgflox/flox;Ptf1a-Cre pancreata were stained for laminin α2 (red) and ZO-1 (green) to highlight BM and lumen, respectively. DAPI in blue. (B) Isolated wild type and β1-Itgflox/flox;Ptf1a-Cre acini were loaded with FM1-43 fluorescent dye and monitored by live cell fluorescent imaging. When fluorescence stabilized, 1nM caerulein was added. Shown is an intensity map pseudocolor (Zeiss LSM Image Examiner software). At time 0, fluorescent signal in wild type and β1-Itgflox/flox;Ptf1a-Cre acini were comparable. After 45 min, wild type acini showed increased apical fluorescence (white arrow). β1-Itgflox/flox;Ptf1a-Cre acini showed increased fluorescence at all cell surfaces (red arrows), displayed a discharge of membrane bodies, loss of cell integrity and release of intracellular content (white arrowheads). Numbers identify cells; unnumbered fluorescent bodies are membrane fragments not filtered out during preparation. Scale bar = 20 μm. Data are representative of 3 mice/genotype, 6-7 weeks of age. (C,D) 12 month old wild type and β1-Itgflox/flox;Ptf1a-Cre pancreata were co-stained for F-actin with phalloidin (green) and either PKCε(C, red) or Munc-18c (D, red) and DAPI (blue). Arrows in (C) indicate subluminal staining of PKCε in β1-Itgflox/flox;Ptf1a-Cre acinar cells.
β1-Itgflox/flox;Ptf1a-Cre pancreata also showed an alteration in acinar organization revealed by ZO-1 immunofluorescence. While wild type acini showed a lumen closely aligned with the geometric center of a given acinus, β1-Itgflox/flox;Ptf1a-Cre acini had multiple ZO-1 -positive regions dissociated from the acinar center (Fig 6A, Suppl Fig 3), with the average distance between lumen and acinus geometric center being significantly larger (Suppl Fig. 3 p<0.01). Consistent with a proper acinar structure, amylase was concentrated mostly apically in young and old wild type and young β1-Itgflox/flox;Ptf1a-Cre mice (Suppl. Fig. 2A&B). In contrast, in older β1-Itgflox/flox;Ptf1a-Cre mice, amylase staining was frequently adjacent to BM or surrounding the centrally-localized cells (Suppl. Fig 2). This aberrant organization suggested that directional secretion into a defined central lumen may be compromised.
To test if β1-Itgflox/flox;Ptf1a-Cre acinar cells lacked appropriate directional secretion, we employed time-course fluorescent imaging of living cells loaded with FM1-43, a cell impermeable dye that fluoresces when membrane-bound. Fusion of exocytic vesicles with plasma membrane increases the dye-accessible surface, intensifying fluorescent signal at sites of intracellular membrane fusion28, 29. Isolated acini harvested from 4-7 week old wild type and β1-Itgflox/flox;Ptf1a-Cre mice were loaded with FM1-43. Stable fluorescence was achieved within 15 minutes and 1nM caerulein was added to stimulate secretion (Fig. 6B). Over a period of 45 minutes, wild type acini displayed the expected increase in fluorescent intensity at the apical surface28. β1-Itgflox/flox;Ptf1a-Cre acini, however, showed intense fluorescence localized indiscriminately to all membrane surfaces (Fig 6B), accompanied by a dramatic discharge of membrane bodies, an overall loss of cell integrity and release of intracellular content (Fig 6B, arrowheads), consistent with the enhanced damage response observed in vivo.
Disrupted polarity at the structural and secretory levels in β1-Itgflox/flox;Ptf1a-Cre acini, combined with the pancreatitis-like phenotype in vivo led us to examine PKCε and Munc18c, whose mislocalization have been associated with pancreatitis induction. PKCε which normally localizes apically in acinar cells17, translocates to the cytoplasm upon treatment with supramaximal doses of caerulein, where it is involved in intracellular activation of digestive enzymes17. Immunofluorescence for PKCε on 12 month old wild type control pancreata showed the expected colocalization with cortical actin at the luminal surface (Fig 6C). In contrast, in β1-Itgflox/flox;Ptf1a-Cre acini, PCKε was predominantly localized to the cytoplasm in a region just proximal to the apical surface, reminiscent of its localization in caerulein-treated acinar cells in vitro17.
Munc18c, whose basal localization in acinar cells is important for its inhibition of basal secretion in vitro29, is also translocated to the cytoplasm with supramaximal cerulein. In wild type pancreata, Munc18c was invariably concentrated at the basolateral membrane (Fig 6D), colocalizing with E-cadherin (Supp. Fig. 4). In 7/7 β1-Itgflox/flox;Ptf1a-Cre pancreata, between 8-20 months of age, Munc18c was frequently found in the cytoplasm with no apparent association with the basolateral surface (Fig 6D, Supp. Fig. 4), a localization never observed in age matched wild type controls. However, many acinar cells in β1-Itgflox/flox;Ptf1a-Cre pancreata maintained basolateral staining of Munc18c (Suppl. Fig. 4). Altogether, the β1-Itgflox/flox;Ptf1a-Cre pancreata displayed disrupted acinar cell polarity, including mislocalization of molecules critical for preventing exposure of the pancreatic parenchyma to destructive digestive enzyme activity.
Discussion
β1 integrin is known to contribute to cell polarity, multiple signal transduction pathways and maintenance of stem cells in many tissues. We found that pancreas-specific ablation of β1 integrin leads to progressive organ degeneration, marked by a phenotype similar to pancreatitis. β1-Itgflox/flox;Ptf1a-Cre acini in older mice were larger, distinguished by acinar cells no longer in contact with BM. β1-Itgflox/flox;Ptf1a-Cre acinar explants, when stimulated by caerulein, did not directionally secrete to the luminal surface and quickly lost cellular integrity.
Surprisingly, apart from age-dependent organ degeneration, the pancreas develops and functions essentially normally in β1-Itgflox/flox;Ptf1a-Cre mice. Organization of acini in 1-2 week-old mutants was indistinguishable from that in wild type mice. At this age, the only distinctive feature in β1-Itgflox/flox;Ptf1a-Cre pancreata was a breakdown in the boundary separating islet and acinar cell compartments (Supp. Fig 5). It was only at 3 weeks of age that abnormal acinar structures began to appear. Interestingly, β cell viability and production of insulin in response to glucose have been shown to be β1 integrin dependent in vitro30-32. However, β1-Itgflox/flox;Ptf1a-Cre mice, which have ablated β1 integrin from most islet cells, showed no increase in β cell apoptosis, had normal fasting blood glucose levels, weight gain and lifespan. This dichotomy may be due to functional compensation by other matrix receptors in pancreatic development that is maintained in adult islets or failure of in vitro systems to fully recapitulate the in vivo environment. Whichever the case, we conclude that β1 integrin is not essential for basic pancreatic function or development.
Given the diverse roles of β1 integrin in stem/progenitor cells of other tissues,14, 26, 33 it was surprising to find no definitive impairment of progenitor cell function in β1-Itgflox/flox;Ptf1a-Cre mice. The best evidence supporting such a role is the smaller pancreas size found even in young mutant mice (Fig 2). Adult organ mass has been hypothesized to be dependent on the number of progenitor cells available during development34, suggesting that the progenitor cell population may be depleted in β1-Itgflox/flox;Ptf1a-Cre mice. In contrast, we found no significant impairment in the recovery of β1-Itgflox/flox;Ptf1a-Cre pancreata from caerulein-induced tissue damage. However, progenitor cells may not be required for this process as acinar regeneration after caerulein treatment has been shown to be primarily through division of existing acinar cells27. Notably, examination of β1-Itgflox/flox;Ptf1a-Cre acini by electron microscopy revealed no obvious differences in number or morphology of centroacinar cells, the proposed pancreatic progenitor (data not shown).
Pancreatic degeneration in β1-Itgflox/flox;Ptf1a-Cre mice correlated to overall larger acini and loss of cell/matrix contact by a number of acinar cells. Similar to β1 integrin-null mammary epithelia14, cells abandoned in the acinar center did not appear to undergo anoikis and thus are unlikely to be a major contributor to overall tissue atrophy. We propose that intracellular activation and basolateral secretion of digestive enzymes are the primary causes of the degenerative phenotype, although these cells lacking a defined basal surface are likely to contribute to and be victims of aberrant enzyme activity. It is tempting to suggest that β1 integrin has a direct role in organizing molecules that control enzyme activation and secretion, but most evidence contradicts this possibility. β1 integrin itself is localized basolaterally in acinar cells (Fig 1), inconsistent with a direct role in maintaining apical PKCε. While Munc18c is normally located basolaterally, β1-Itgflox/flox;Ptf1a-Cre pancreata have virtually no acinar β1 integrin. The presence of some basolateral Munc18c makes a direct role for β1 integrin in Munc18c localization unlikely. Interestingly, Munc18c translocation to the cytoplasm has been shown to be dependent on PKCα phosphorylation29, which directly interacts with β1 integrin35. When examined by immunofluorescence, we found a consistent loss of PKCα at the lateral, but not basal, acinar cell membrane compared to wild type pancreata, where it was associated with all surfaces (data not shown). Whether this change in PKCα localization is responsible for Munc18c translocation is unknown. We propose instead that the loss of β1 integrin causes a more global distortion of acinar cell polarity, affecting the localization of these molecules, among others. Attempts to visualize other structural polarity proteins, such as Par3 and Par6, reflected what we observed with ZO-1: an organized localization to an aberrantly-positioned apical surface, suggesting that the signals that establish polarity are intact, but polarity is corrupted sufficiently to lead to inappropriate exposure of the parenchyma to destructive enzymes.
Supplementary Material
Acknowledgments
We thank Drs. Fred Gorelick and James Jamieson (Yale University) for helpful suggestions, evaluation of electron micrographs and tissue histology; Drs. Kay Washington (Vanderbilt University) and Kenneth Shroyer (Stony Brook University) for pathology consultations; Dr. Christopher Wright for Ptf1a-Cre mice; and Dr. Holly Colognato (Stony Brook University) for critical reading of the manuscript.
Grant Support: NIH R01CA100126, VA Merit Award and the Knapp Chair for Pancreatic Cancer Research to HCC
Footnotes
Bombardelli- study concept and design; acquisition, analysis and interpretation of data; drafting of the manuscript; statistical analysis
Carpenter- acquisition, analyis and interpretation of data, drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis
Wu- acquisition and analysis of data, drafting of the manuscript; technical support
Alston- acquisition of data; technical support
DelGiorno- acquisition of data; technical support
Crawford- study concept and design; acquisition, analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; study supervision
No conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nagar AB, Gorelick FS. Acute pancreatitis. Curr Opin Gastroenterol. 2002;18:552–7. doi: 10.1097/00001574-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Hassan MM, Bondy ML, Wolff RA, Abbruzzese JL, Vauthey JN, Pisters PW, Evans DB, Khan R, Chou TH, Lenzi R, Jiao L, Li D. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol. 2007;102:2696–707. doi: 10.1111/j.1572-0241.2007.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald PJ, Carol BM, Rosenstock L. Pancreatic acinar cell regeneration. Nature. 1966;212:594–6. doi: 10.1038/212594a0. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald PJ, Herman L, Carol B, Roque A, Marsh WH, Rosenstock L, Richards C, Perl D. Pancreatic acinar cell regeneration. Am J Pathol. 1968;52:983–1011. [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang FX, Naselli G, Harrison LC. Distinct distribution of laminin and its integrin receptors in the pancreas. J Histochem Cytochem. 2002;50:1625–32. doi: 10.1177/002215540205001206. [DOI] [PubMed] [Google Scholar]
- 6.Miner JH, Li C, Patton BL. Laminins alpha2 and alpha4 in pancreatic acinar basement membranes are required for basal receptor localization. J Histochem Cytochem. 2004;52:153–6. doi: 10.1177/002215540405200202. [DOI] [PubMed] [Google Scholar]
- 7.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 8.Calderwood DA. Integrin activation. J Cell Sci. 2004;117:657–66. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- 9.Brakebusch C, Fassler R. beta 1 integrin function in vivo: adhesion, migration and more. Cancer Metastasis Rev. 2005;24:403–11. doi: 10.1007/s10555-005-5132-5. [DOI] [PubMed] [Google Scholar]
- 10.Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J Pathol. 2003;201:632–41. doi: 10.1002/path.1472. [DOI] [PubMed] [Google Scholar]
- 11.Yu W, Datta A, Leroy P, O’Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16:433–45. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones RG, Li X, Gray PD, Kuang J, Clayton F, Samowitz WS, Madison BB, Gumucio DL, Kuwada SK. Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J Cell Biol. 2006;175:505–14. doi: 10.1083/jcb.200602160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, Schuetz G, Mueller U, Streuli CH, Hynes NE. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO J. 2005;24:1942–53. doi: 10.1038/sj.emboj.7600674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, Fassler R, Thiery JP, Glukhova MA. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10:716–22. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–80. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stupack DG, Cheresh DA. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci. 2002;115:3729–38. doi: 10.1242/jcs.00071. [DOI] [PubMed] [Google Scholar]
- 17.Thrower EC, Osgood S, Shugrue CA, Kolodecik TR, Chaudhuri AM, Reeve JR, Jr, Pandol SJ, Gorelick FS. The novel protein kinase C isoforms -delta and -epsilon modulate caerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1344–53. doi: 10.1152/ajpgi.00020.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaisano HY, Lutz MP, Leser J, Sheu L, Lynch G, Tang L, Tamori Y, Trimble WS, Salapatek AM. Supramaximal cholecystokinin displaces Munc18c from the pancreatic acinar basal surface, redirecting apical exocytosis to the basal membrane. J Clin Invest. 2001;108:1597–611. doi: 10.1172/JCI9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crawford HC, Scoggins CR, Washington MK, Matrisian LM, Leach SD. Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J Clin Invest. 2002;109:1437–44. doi: 10.1172/JCI15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, Werner S, Fassler R. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–34. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 23.Parrish W, Ulloa L. High-mobility group box-1 isoforms as potential therapeutic targets in sepsis. Methods Mol Biol. 2007;361:145–62. doi: 10.1385/1-59745-208-4:145. [DOI] [PubMed] [Google Scholar]
- 24.Kren A, Baeriswyl V, Lehembre F, Wunderlin C, Strittmatter K, Antoniadis H, Fassler R, Cavallaro U, Christofori G. Increased tumor cell dissemination and cellular senescence in the absence of beta1-integrin function. Embo J. 2007;26:2832–42. doi: 10.1038/sj.emboj.7601738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- 26.Campos LS, Leone DP, Relvas JB, Brakebusch C, Fassler R, Suter U, ffrench-Constant C. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 2004;131:3433–44. doi: 10.1242/dev.01199. [DOI] [PubMed] [Google Scholar]
- 27.Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Castillo CF, Warshaw AL, Thayer SP. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007;133:1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cochilla AJ, Angleson JK, Betz WJ. Monitoring secretory membrane with FM1-43 fluorescence. Annu Rev Neurosci. 1999;22:1–10. doi: 10.1146/annurev.neuro.22.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Lam PP, Cosen Binker LI, Lugea A, Pandol SJ, Gaisano HY. Alcohol redirects CCK-mediated apical exocytosis to the acinar basolateral membrane in alcoholic pancreatitis. Traffic. 2007;8:605–17. doi: 10.1111/j.1600-0854.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang R, Li J, Lyte K, Yashpal NK, Fellows F, Goodyer CG. Role for beta1 integrin and its associated alpha3, alpha5, and alpha6 subunits in development of the human fetal pancreas. Diabetes. 2005;54:2080–9. doi: 10.2337/diabetes.54.7.2080. [DOI] [PubMed] [Google Scholar]
- 31.Parnaud G, Hammar E, Rouiller DG, Armanet M, Halban PA, Bosco D. Blockade of beta1 integrin-laminin-5 interaction affects spreading and insulin secretion of rat beta-cells attached on extracellular matrix. Diabetes. 2006;55:1413–20. doi: 10.2337/db05-1388. [DOI] [PubMed] [Google Scholar]
- 32.Hammar E, Parnaud G, Bosco D, Perriraz N, Maedler K, Donath M, Rouiller DG, Halban PA. Extracellular matrix protects pancreatic beta-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes. 2004;53:2034–41. doi: 10.2337/diabetes.53.8.2034. [DOI] [PubMed] [Google Scholar]
- 33.Zhu AJ, Haase I, Watt FM. Signaling via beta1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci U S A. 1999;96:6728–33. doi: 10.1073/pnas.96.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–91. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 35.Ng T, Shima D, Squire A, Bastiaens PI, Gschmeissner S, Humphries MJ, Parker PJ. PKCalpha regulates beta1 integrin-dependent cell motility through association and control of integrin traffic. Embo J. 1999;18:3909–23. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.