Abstract
Background & Aims
Surgical intervention produces sustainable weight loss and metabolic improvement in obese individuals. Vertical sleeve gastrectomy (VSG) produces dramatic, sustained weight loss; we investigated whether these changes result from improved sensitivity to leptin.
Methods
VSG was performed in Long-Evans rats with diet-induced obesity. Naïve or sham-operated rats, fed either ad libitum or pair-fed with the VSG group, were used as controls. Following surgery, body weights and food intake were monitored. We investigated energy expenditure, meal patterns, leptin sensitivity, and expression of pro-opiomelanocortin (POMC)/agouti-related peptide (AgRP)/neuropeptide Y (NPY) in the hypothalamus of the rats.
Results
We observed sustained losses in weight and body fat in male and female rats after VSG. Weight loss persisted after the disappearance of a transient, post-surgical food intake reduction. Resting energy expenditure was similar between control and VSG rats. VSG rats maintained their reduced body weights. However, they responded to a chronic food restriction challenge by overeating, which resulted in pre-restriction, rather than pre-VSG, body weights. Consistent with lower adiposity, VSG decreased plasma leptin levels. Although VSG slightly improved leptin’s anorectic action, the response was comparable to that observed in controls matched for adiposity by caloric restriction. Changes in hypothalamic neuropeptide expression were consistent with the lower body weight and lower leptin levels but cannot account for the sustained weight loss.
Conclusions
VSG causes sustained reduction in body weight, which results from loss of fat mass. The maintenance of weight loss observed did not result from changes in sensitivity to leptin.
Keywords: bariatric surgery, hypothalamus, arcuate, stomach
Introduction
Body fat is regulated by a complex neuroendocrine system, making it difficult to maintain weight loss achieved via caloric restriction. A key component of this regulatory system is the adipocyte hormone leptin. Leptin is secreted from white adipose tissue and the stomach, and it reduces food intake and body weight through its actions at the long leptin receptor (ObRb) in the central nervous system (CNS). In the arcuate nucleus of the hypothalamus (ARC), a major CNS energy balance control area, leptin exerts its catabolic action by stimulating pro-opiomelanocortin (POMC) neurons while inhibiting the expression of the endogenous MC3/4R antagonist Agouti-related peptide (AgRP) and the potent orexigen Neuropeptide Y (NPY)1. As a result, when injected directly into the 3rd-cerebral ventricle adjacent to the ARC, leptin reduces food intake and body weight2.
However, in most obese individuals, leptin levels are elevated3, 4 in direct proportion to body fat, and exogenous leptin treatment produces little or no weight loss5, 6. This failure of leptin to produce the same effects in obese individuals as it can in lean individuals is termed leptin resistance. Thus a key question for any weight loss regimen is whether it acts to reverse leptin resistance as a part of how weight loss is maintained.
Bariatric surgery produces greater weight loss and weight loss that is more durable than caloric restriction, and therefore is currently the most effective therapy for obesity7. Vertical sleeve gastrectomy (VSG) is one such bariatric surgical procedure that involves the creation of a reduced stomach lumen along the lesser curvature of the stomach through the removal of gastric tissues along the greater curvature from the fundus to the antrum. Stomach capacity is typically reduced 80% or more, and the intestine remains intact. This procedure produces dramatic weight loss in humans8–10 and in rodents11, 12. In fact, recent reports indicate that its efficacy is close to that of the more common Roux-en Y gastric bypass13, 14.
Although VSG is typically referred to as a “restrictive” procedure, evidence suggests that stomach volume reductions alone are unlikely to account for the profound efficacy of the procedure 15. We hypothesized that VSG-treated rats would actively defend their new lower body weights and would do so via changes in the leptin-hypothalamic axis. Such changes could occur either with increased leptin secretion, increased leptin action or direct changes on the key targets of leptin action in the hypothalamus. These mechanistic issues are difficult if not impossible to address solely in human subjects. Consequently, we developed a rat model of VSG used for the present studies.
Methods
Animals
Male and female Long-Evans rats (Harlan Laboratories, Indianapolis, IN; 250–300 g) were fed either a high-fat butter oil-based diet (HFD, Research Diets, New Brunswick, NJ, D12451; 45% fat; 4.73 kcal/g) or standard chow (Harlan-Teklad, Indianapolis, IN) for 8 weeks prior to surgery. Rats were housed at the University of Cincinnati at the Metabolic Diseases Institute under controlled conditions (12:12-h light-dark cycle, 50–60% humidity, 25 °C with free access to water and food except where noted). All procedures for animal use were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Experimental groups are outlined in Supplemental Table 1. Four cohorts labeled A, B, C and D and contained 24–67 rats each. Cohort A contained NAÏVE (N=8 male, N=8 female), SHAM (N=10 male, N=10 female) and VSG (N=9 male, N=9 female) rats. Cohorts B and D consisted of CHOW (N=10 in cohort D, N=20 in cohort B), SHAM (N=10 in cohort D, N=14 in cohort B), PF (N=8 in cohort D, N=13–15 in cohort B) and VSG (N=7 in cohort D, N=18 in cohort B). Cohort C included SHAM (N=8), PF (N=7) and VSG (N=9) rats. Rats fed HFD or chow diet prior to surgery were maintained on their respective diets after surgery. Where indicated, sham-operated subgroup was pair-fed to match the intake of the VSG group. To do this, the amount of food eaten by the VSG rats during the previous 24 h was provided in small portions at random times during the light/dark cycle to the PF rats. Fat and lean tissue mass was measured using nuclear magnetic resonance (NMR, Echo MRI: Echo Medical Systems, Houston, TX).
Blood was taken from the tip of the tail just before the onset of dark after 4 h of fasting for quantification of plasma leptin (postsurgical day 50) and after 2 h of fasting for quantification of plasma insulin and glucose (postsurgical day 125). At the end of each study, animals were placed briefly in a CO2 chamber and then sacrificed by decapitation during the light phase.
Surgical Procedures
For VSG, a laparotomy incision was made in abdominal wall, allowing the stomach to be isolated outside the abdominal cavity and placed on saline-moistened gauze pads. Loose gastric connections to the spleen and liver were released along the greater curvature and the suspensory ligament supporting the upper fundus was severed, thus widening the angle between lower esophagus and the fundus. The lateral 80% of the stomach was excised using an ETS 35-mm staple gun, leaving a tubular gastric remnant in continuity with the esophagus superiorly and the pylorus and duodenum inferiorly. This gastric sleeve was then reintegrated into the abdominal cavity. Finally, the abdominal wall was closed in layers. Sham surgery involved abdominal laparotomy incision and placement of the stomach out of the abdominal cavity followed by manually applying pressure with blunt forceps along a vertical line between the esophageal sphincter and the pylorus of the stomach. Following surgery, rats received intensive post-operative care for 3 days, consisting of twice-daily subcutaneous injections of 10 mL saline and 0.3 mL Buprenex. Rats were fasted 24 h prior to surgery and had post-surgical access only to Osmolite OneCal liquid diet until food was returned 3 days after surgery.
Energy expenditure and meal patterns
A continuous monitoring system (TSE Systems, Inc., Chesterfield, MO) was used to determine energy expenditure and meal patterns 28–30 days after surgery. Rats from each group (N = 14–18/surgical condition) were placed in the system for 96 h. The first 24 h were considered adaptation and the data from the next 72 h were analyzed. Data for indirect calorimetry analysis were sampled every 45 min. Data for food intake and meal pattern analysis were sampled every 15 min, and accumulated in 6-h blocks.
Fecal lipid content
Dietary lipid absorption was assessed using the Behenate method (N = 7–8/group) as described previously16. Briefly, rats were temporarily placed on a diet containing 5% sucrose polybehenate (behenic acid). After 24 h of acclimation to the diet, cages were changed and fecal pellets were collected after another 24 h. Fecal samples of about 10 mg were collected and fecal lipid content was assayed by gas chromatography of fatty acid methyl esters. Fat absorption was calculated from the ratio of behenic acid to other fatty acids in the diet and feces. During this time, PF rats were also fed the Behenate diet.
Food restriction study
On post-operative Day 50, rats within each dietary group (N = 14–20/group) were divided into two groups (N = 7–10) balanced on the basis of body weight and fat mass. One group received ad libitum access to food, while the other was food restricted by 73% for a period of 22 days to induce weight loss. This was followed by a recovery period in which all rats had ad libitum feeding
Leptin sensitivity test
Sensitivity to exogenous leptin was assessed at 20–22 days following surgery, using subgroups of N = 7–10 animals per surgical group. Rats received 3 consecutive intraperitoneal (i.p.) injections of either saline (vehicle) or leptin (PeproTech, Inc., Rocky Hill, NJ) at 24-h intervals. Leptin was administered at a dose of 1.46 mg/kg lean mass, as determined by NMR, in a volume of 0.5 mL water per 100 g total body mass. This dose is based on average lean body mass for a sham-operated, HFD-fed control rat and on a dose of 1 mg/kg leptin. Food was removed on the evening prior to the study, 2 h after the onset of the dark, and the injection was made 4 h prior to the onset of the dark cycle on each study day. Food was returned at the onset of the dark on each day, and food intake and body weight were monitored at 24-h intervals.
Gene Expression Studies
Neuropeptide expression was measured in the MBH by dissecting a wedge bounded rostrally by the optic chiasm, caudally by the mammillary bodies, and laterally by a cut connecting the optic tract to the 3rd ventricle. Tissue was homogenized in RLT buffer using a tissue lyser (QIAGEN, Inc., Valencia, CA) and RNA was extracted using a QIAGEN miniprep RNA extraction kit. An iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) was used, and quantitative PCR was performed using SYBR Green detection. 6–9 rats per group were studied in each experiment. Primers used in these studies are listed in Supplemental Table 2.
Plasma leptin, insulin, and glucose quantification
Plasma leptin levels after 4 hours of fasting were quantified 50 days after surgery, using a rat leptin ELISA (Crystal Chem, Downers Grove, IL). Groups of 14–19 rats were analyzed. Plasma insulin levels were quantified 125 days after surgery, using a rat insulin ELISA (Crystal Chem, Downers Grove, IL). Plasma glucose levels on postsurgical day 125 were quantified using a plasma glucose analyzer (Analox Instruments, USA, Lunenberg, MA). Groups of 13–18 rats were analyzed for plasma insulin and glucose measurements. Plasma insulin and glucose were measured during the light phase of the light-dark cycle, after 2 hours of fasting.
Statistical Analysis
All data are expressed as mean ± SEM. Body weight, food intake, NMR, and TSE data were analyzed via 2-way ANOVA (Variables: treatment & time) with a Bonferroni post hoc test where appropriate. Pair-fed controls were excluded from meal pattern analysis because meal patterns were imposed by feeding schedule. Plasma leptin levels and hypothalamic gene expression profiles were analyzed using 1-way ANOVA followed by a Tukey post-hoc test where appropriate.
Results
Weight loss and fat mass loss following VSG persists despite transient reductions in food intake
Following surgery, VSG rats lost about 20% of initial weight (Figures 1A–B). After the initial period of weight loss (10 days), VSG animals gained an average of 1.03 ± 0.11 g/day for males and 1.63 ± 0.14 g/day for females. This is comparable to weight gain in both NAÏVE (females: 1.12 ± 0.05 g/day, males: 1.54 ± 0.14 g/day) and SHAM animals (females: 1.43 ± 0.10 g/day, males: 1.57 ± 0.11 g/day). Body weight remained reduced in VSG rats compared to SHAM and NAÏVE throughout the duration of the study. VSG induced a significant loss of fat mass, as assessed on Day 122 (Figure 1C). Lean mass did not change in VSG rats relative to SHAM or NAIVE (Figure 1D). VSG induced a significant, initial reduction in daily food intake (Figures 1E–F). This anorexia was transient and daily food intake for VSG rats and SHAM rats was no longer significantly different after Day 16 for males (Figure 1E). Females with VSG had similar changes with early significant reductions in body mass but a similar rate of regain (Figure 1B), reductions in fat mass (Figure 1C), maintenance of lean mass (Figure 1D), and transient anorexia (Figure 1F) compared to SHAM and NAÏVE animals.
FIGURE 1.
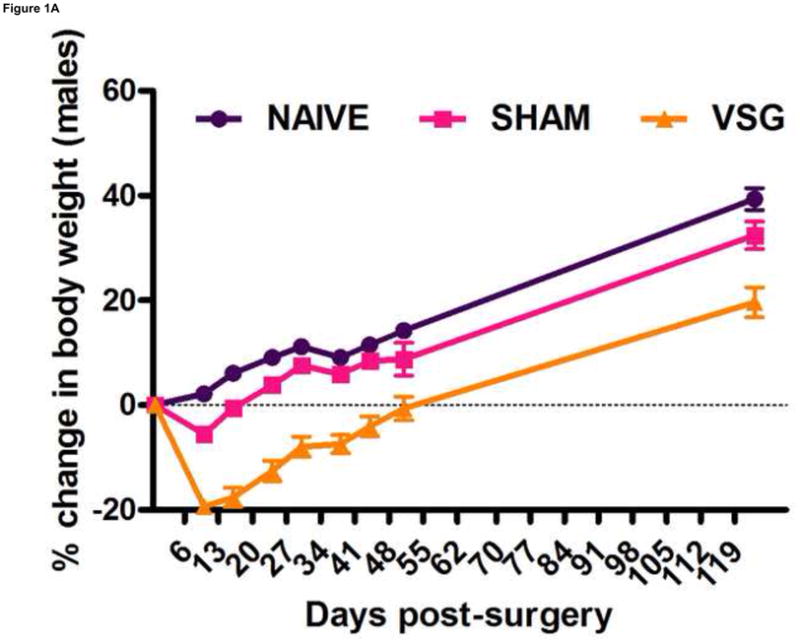
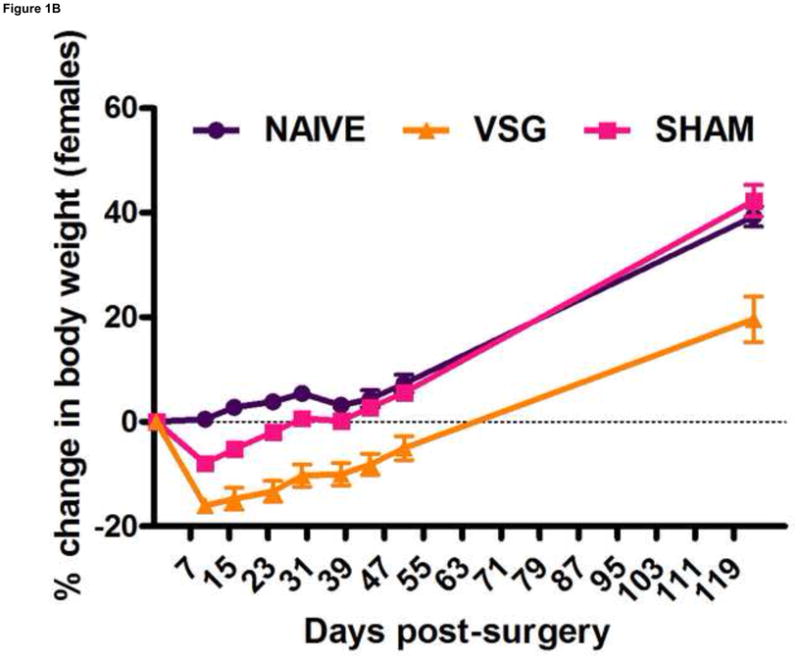
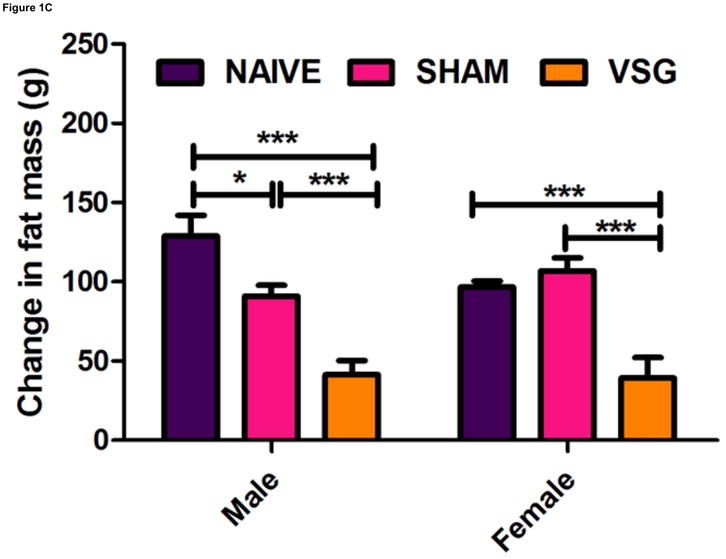
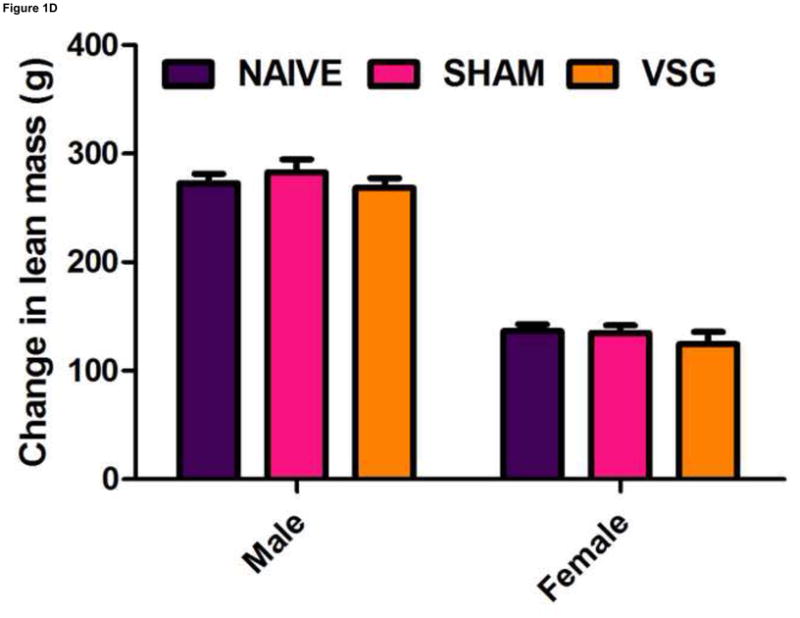
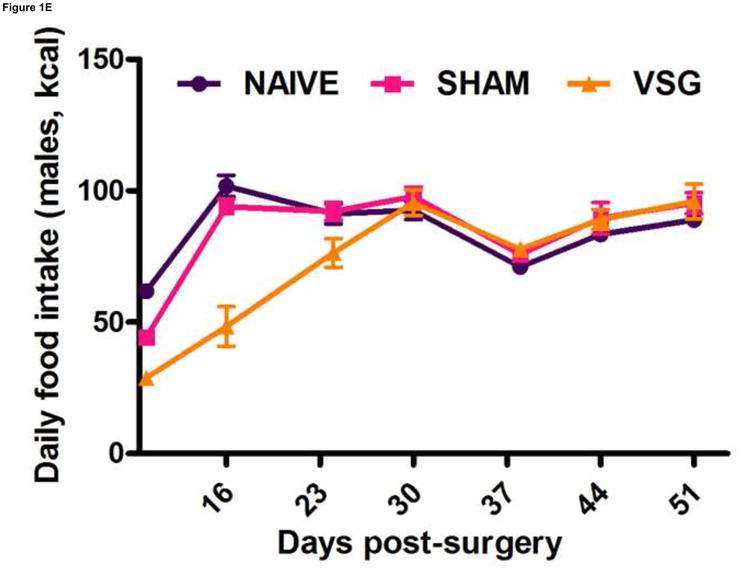
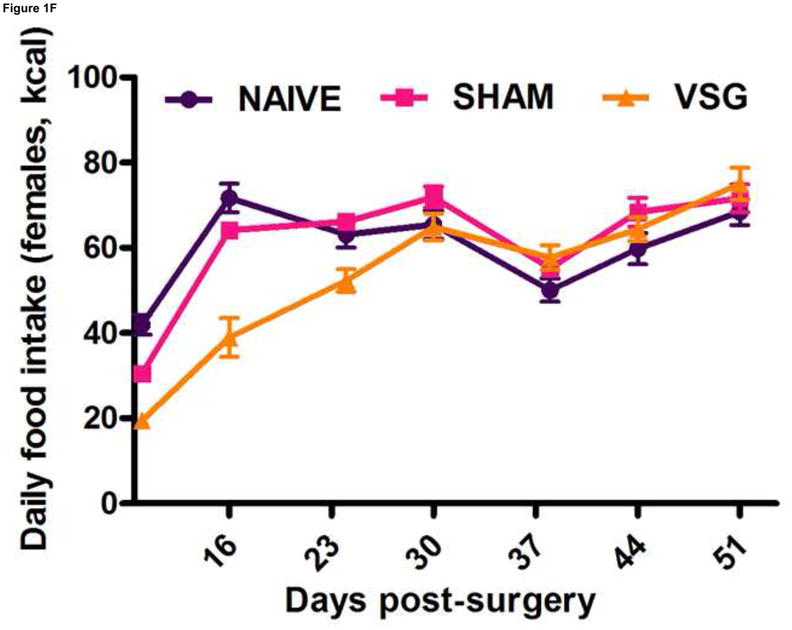
VSG resulted in loss of body weight in males (A, P < 0.01 vs. SHAM and NAÏVE) and females (B, P < 0.05 vs. SHAM and NAÏVE) at all time points measured. Body weight change was equivalent for SHAM and NAÏVE rats at all time points (P > 0.05). (Overall interaction of time vs. surgical treatment: P = 0.0042 for males, P < 0.0001 for females.) Weight loss after VSG was due to loss of fat tissue (C, P < 0.001 vs. SHAM or NAÏVE for both male and female rats; overall effect of surgical treatment, P < 0.0001). Lean tissue was not affected by surgical treatment (D, P > 0.05 for overall effect of treatment and for all male-male or female-female comparisons). Daily food intake was suppressed immediately following VSG in both males (E, P < 0.001 vs. SHAM and NAÏVE until Day 16) and females (F, P < 0.001 vs. SHAM and NAÏVE until Day 16, P < 0.05 vs. SHAM on Day 24). After Day 16 for males and Day 24 for females, daily caloric intake did not differ between groups (P > 0.05 for all group comparisons).
Weight loss following VSG is not due to altered energy expenditure or malabsorption
Anorexia following VSG is transient whereas the weight loss is persistent. Two possible explanations for this are altered energy expenditure or intestinal malabsorption following VSG. Using the Behenate method 16 for analysis of lipid absorption, VSG rats have normal lipid absorption and actually had significantly less lipid in the feces than rats in the SHAM group (Figure 2A). This strongly implies that fat mass loss after VSG is not due to intestinal malabsorption. We used indirect calorimetry to determine oxygen consumption on days ranging from 29 to 42 after surgery. At this time, VSG rats weighed less than SHAM (Figure 2B, P<0.01). VSG and SHAM animals also had equivalent daily caloric intake at this time, and so the PF group serves as a weight-matched control. There were no differences in energy expenditure (Figure 2C) or locomotor activity (Figure 2D) among the three tested groups. In fact, there was a trend toward decreased energy expenditure in both VSG and sham-operated pair-fed (PF) rats. Respiratory quotient (RQ) was significantly reduced (P<0.01) during the light phase in VSG and PF compared with SHAM (overall interaction, P<0.001, Figure 2E), indicating increased fat utilization. Interestingly, during the dark phase, the RQ reduction was unique to PF rats (Figure 2E). Because both weight reduced groups exhibited similar reductions in RQ during the light phase, we attribute this increase to reduced body mass.
FIGURE 2.
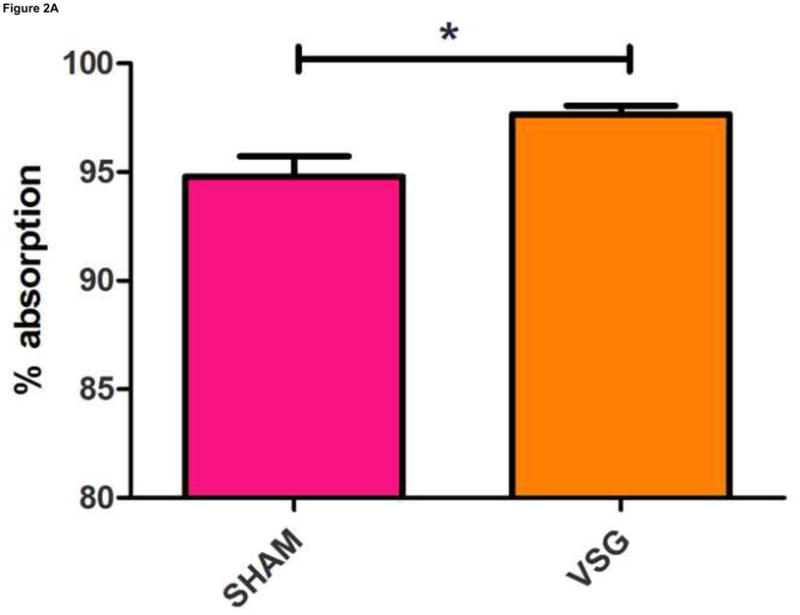
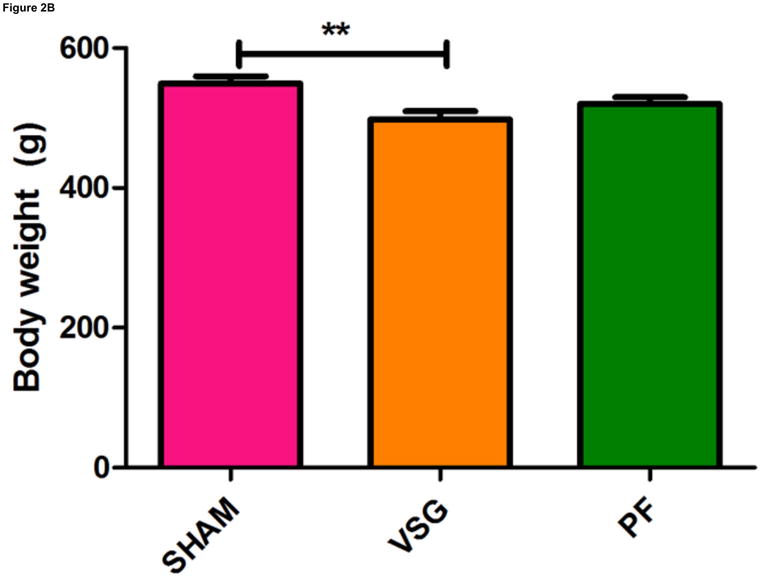
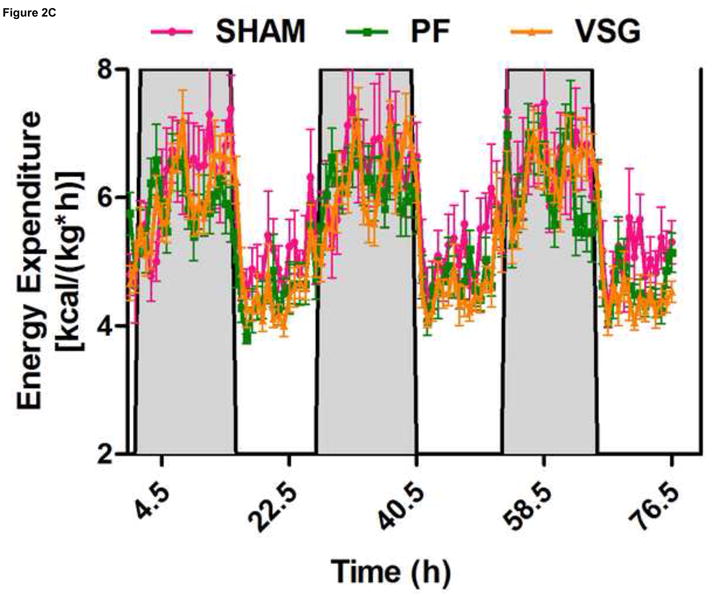
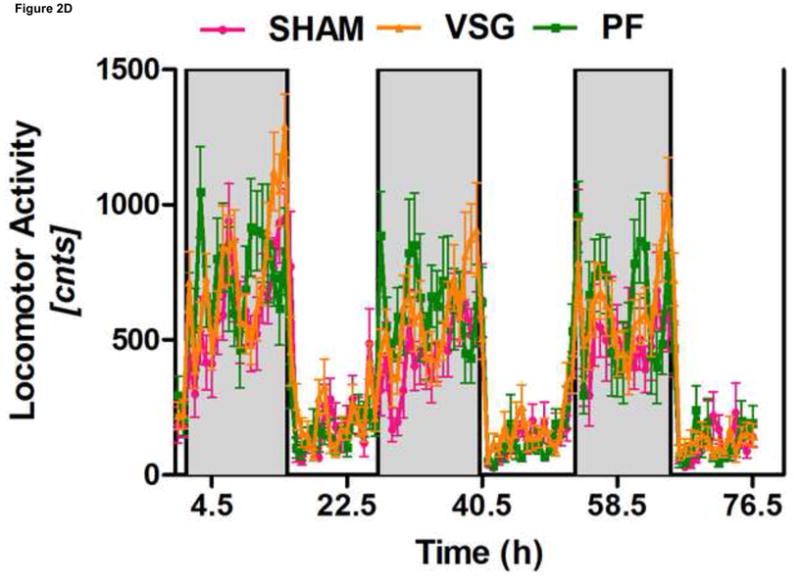
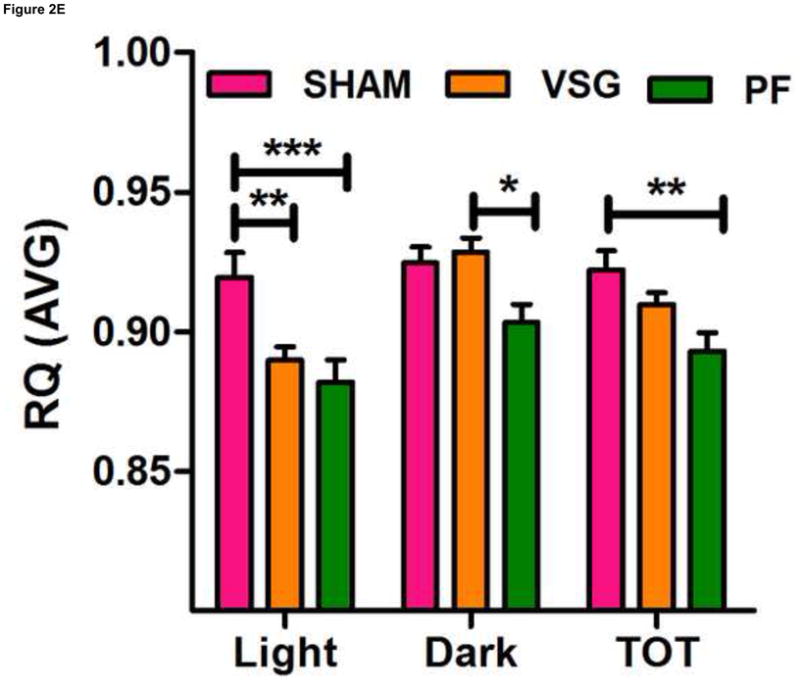
VSG is not a malabsorptive procedure as indicated by fecal lipid analysis (A, P = 0.0193). Energy expenditure was measured when VSG rats weighed significantly less than SHAM (B, P < 0.01). Energy expenditure was unchanged after VSG (C, interaction, P = 0.9699). Locomotor activity was increased during the dark phase in VSG vs. SHAM (D, P < 0.05) but did not differ for VSG vs. PF (P > 0.05). Light-phase RQ was depressed for VSG rats compared to SHAM (E, P < 0.01), but was unchanged relative to PF (P > 0.05). During the dark phase, RQ resembled SHAM but no significant differences between groups were found (P > 0.05 for VSG vs. SHAM or PF, P < 0.01 for SHAM vs. PF).
The time course of altered hypothalamic neuropeptide expression is not consistent with a causal role in VSG-induced weight loss
VSG induced a specific loss of fat mass which endured after the anorexia abated. Whereas weight loss due to caloric restriction is often followed by hyperphagia and weight regain, VSG rats were not hyperphagic and did not regain lost weight gain following the initial anorectic period. We asked whether improved central leptin sensitivity might prevent overeating in VSG rats. If central leptin sensitivity were improved, there should be changes in the expression of hypothalamic genes regulated by leptin (i.e. increased expression of the hypothalamic neuropeptide POMC and reduced expression of AgRP and NPY). However, there were no significant changes in the expression of NPY (Figure 3A, P = 0.7155), AgRP (Figure 3B, P = 0.8222), or POMC (Figure 3C, P = 0.5501) in MBH lysates among VSG, SHAM, and NAÏVE animals at 122 days post-surgery when body weights were relatively stable.
FIGURE 3.
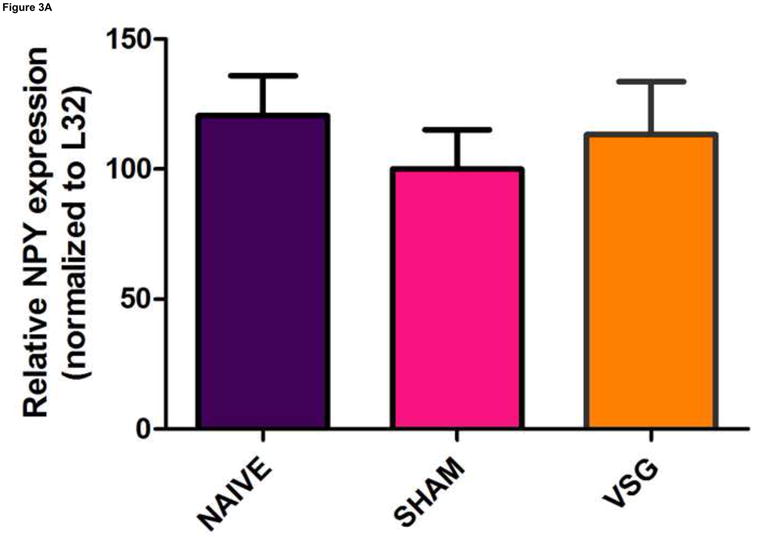
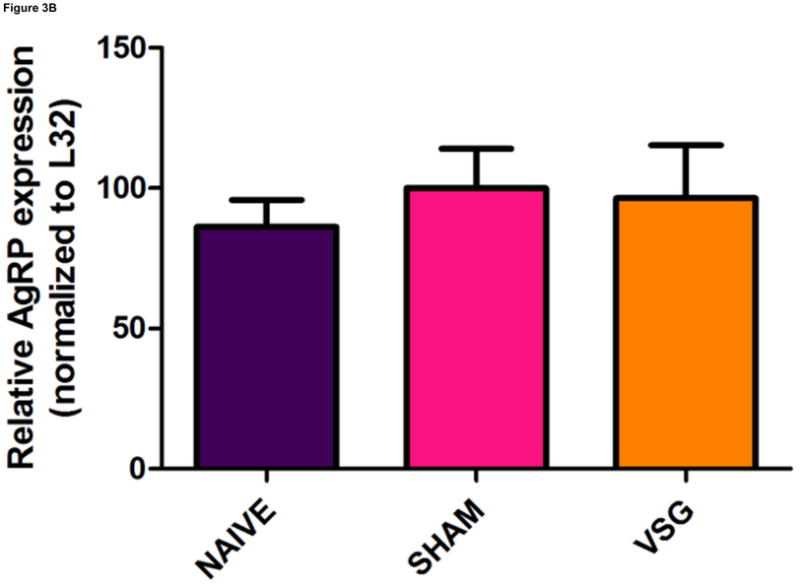
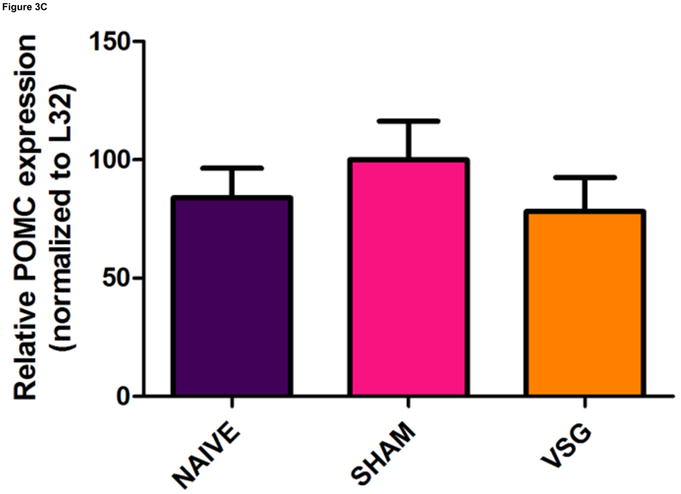
Expression of melanocortin sytem neuropeptides in MBH lysates was unaffected by VSG. Transcripts examined include NPY (A, P = 0.7155), AgRP (B, P = 0.8222), and POMC (C, P = 0.5501).
One shortcoming of this experiment is that we could not determine whether the gene expression pattern is comparable to that of an animal in a weight-reduced state produced by food restriction. To address this, we examined the expression of the same genes in a separate cohort of VSG rats and compared them to PF rats at earlier time post-surgical points. Both VSG and PF resulted in similar loss of fat mass by postsurgical Days 9 and 34 (Figure 4A, P < 0.001 vs. presurgical fat mass for each time point). Body weight and food intake data for this cohort are shown in Supplemental Figure 1. POMC (Figure 4C), NPY (Figure 4D), and AgRP (Figure 4E) expression in MBH lysates were unchanged (P > 0.05 for all group comparisons at each time point) at 10 and 35 days post-surgery. Although there was a trend toward increased AgRP and NPY expression in PF rats, this may have been secondary to the longer period of fasting in these animals as a result of the pair-feeding regimen. Taken together, these data do not support the hypothesis that these key targets of leptin action are crucial to the food intake reduction observed after VSG. Rather, these data point to the observed changes in hypothalamic expression as resulting from reduced intake, weight loss and lower leptin levels.
FIGURE 4.
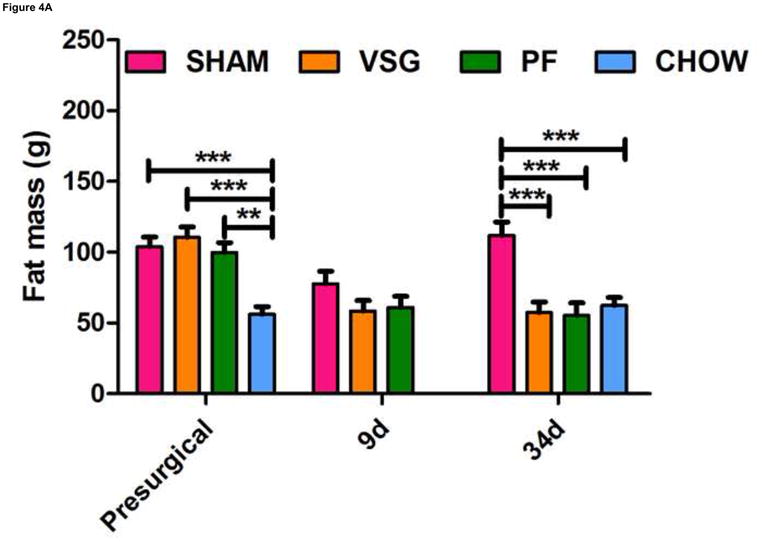
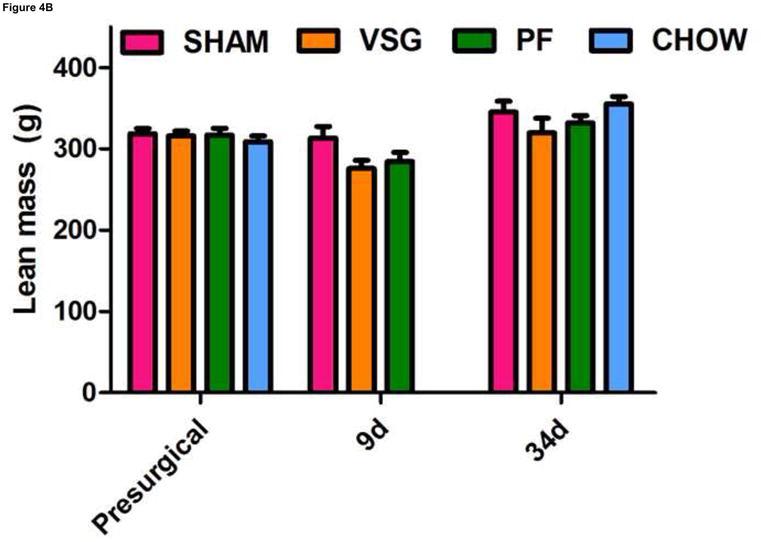
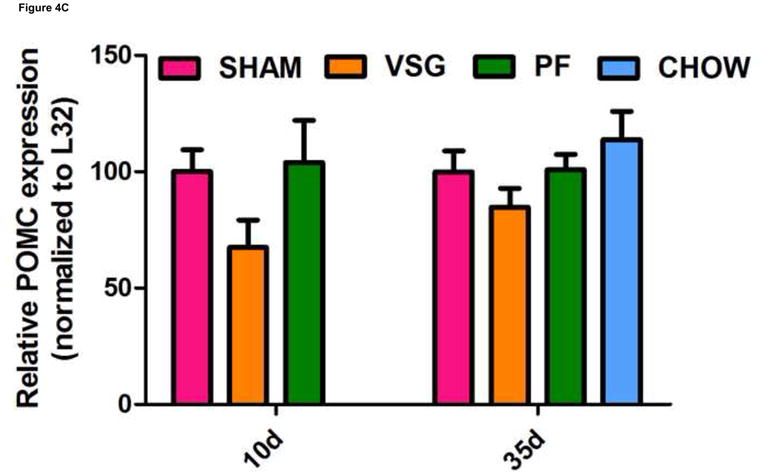
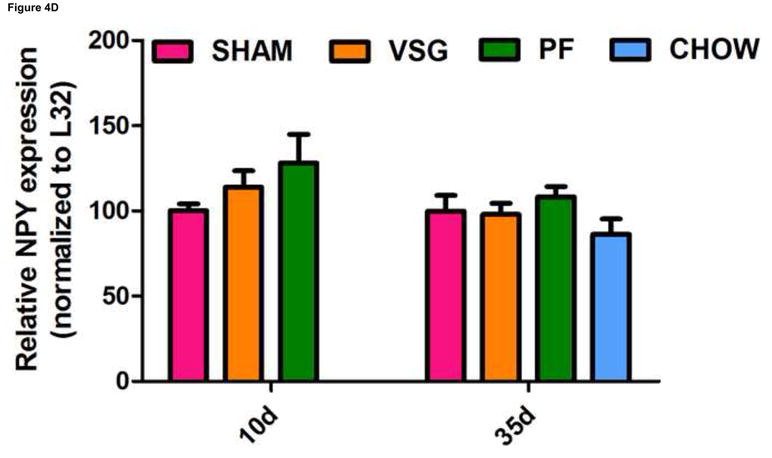
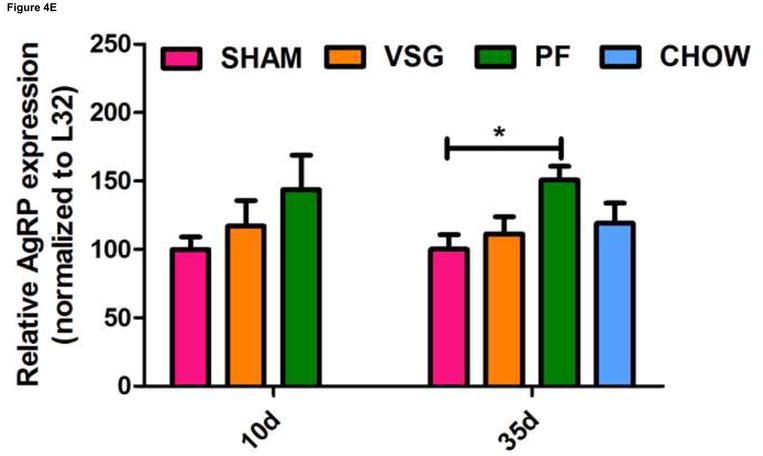
VSG-induced fat mass loss is due primarily to caloric restriction, a phenomenon not mediated by hypothalamic melanocortin signaling. Fat mass was significantly reduced in all groups at 9 days (A, P < 0.01 for PF and P < 0.001 for VSG vs. presurgical fat mass) and at 34 days (A, P < 0.01 for PF and P < 0.001 for VSG vs. presurgical fat mass). At 34 days, fat mass was reduced compared to SHAM for PF (P < 0.001), CHOW (P < 0.001), and VSG (P < 0.001) animals (one-way ANOVA for fat mass at 34 days, P < 0.0001). A small reduction in fat mass was observed at 9 days in SHAM rats, but this fat mass was regained by 34 days (A, P < 0.05, 9 day vs. 34 day fat mass). Lean mass is unchanged by 34 days for all animals (B, P > 0.05 presurgical vs. 34 day fat mass for all groups). Small reductions in lean mass were observed at 9 days for VSG (P < 0.01) and PF (P > 0.05) animals, but lean mass recovery was evident by Day 34 (VSG, P > 0.05 for all comparisons at each time point). Lean mass was not different (P > 0.05) among groups at each of the three measured time points. Expression of POMC did not differ among groups (C, P > 0.05 for all comparisons at each time point) and did not change differentially among the groups over time (C, interaction P = 0.5934, effect of treatment P = 0.0340, effect of time P = 0.5809). The same is true for NPY (D, P > 0.05 for all comparisons at each time point) and AgRP (E, P > 0.05 for all comparisons at each time point except P < 0.05 for SHAM vs. PF on Day 35).
VSG produces altered meal patterns
Using continuous monitoring to record meal patterns, we found that VSG rats display a unique pattern of meal ingestion. At the time of the assessment, daily food intake was equivalent for SHAM and VSG animals. While rats typically eat the majority of their calories during the dark, VSG rats ate small meals of consistent size throughout the light-dark cycle (Figure 5A). These meals were smaller (Figure 5A) and more frequent (Figure 5B) compared to SHAM. Meal duration (Figure 5C) and ingestive speed (Figure 6D) were consistent among groups across the light-dark cycle. VSG rats spent more time eating than SHAM (Figure 5E). Total 24-h food intake for VSG rats was different than that of other groups (Figure 6F).
FIGURE 5.
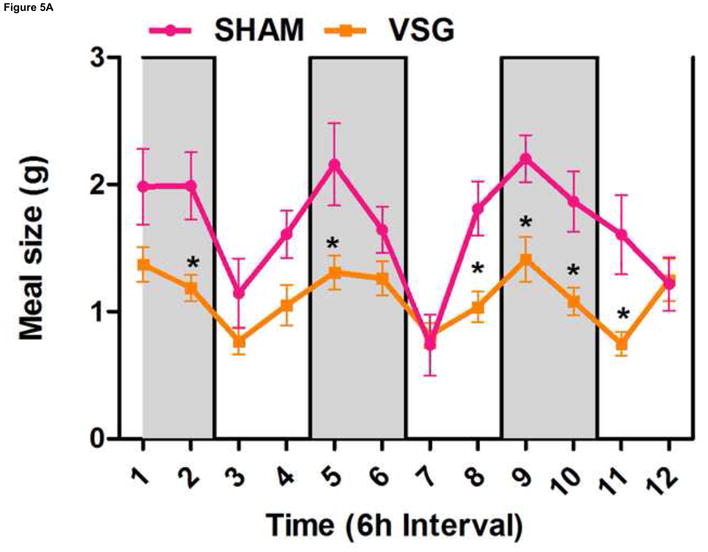
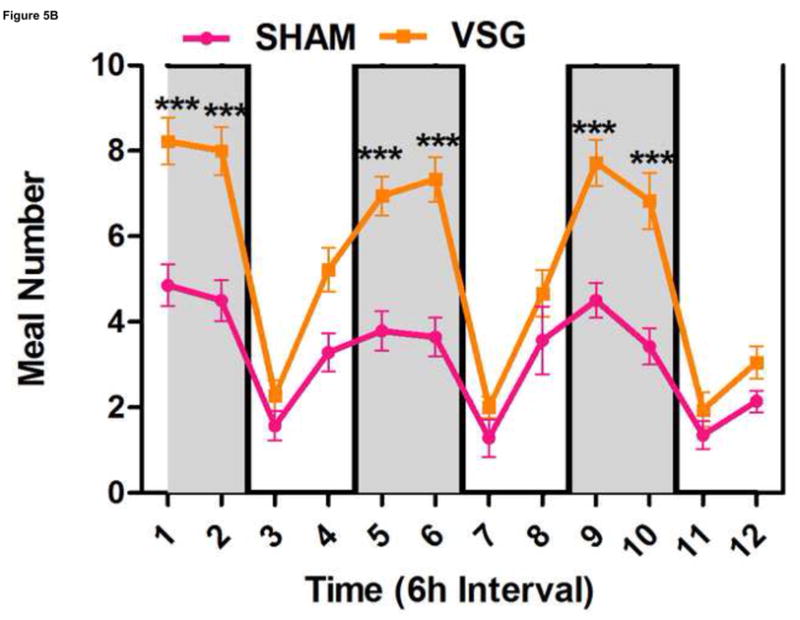
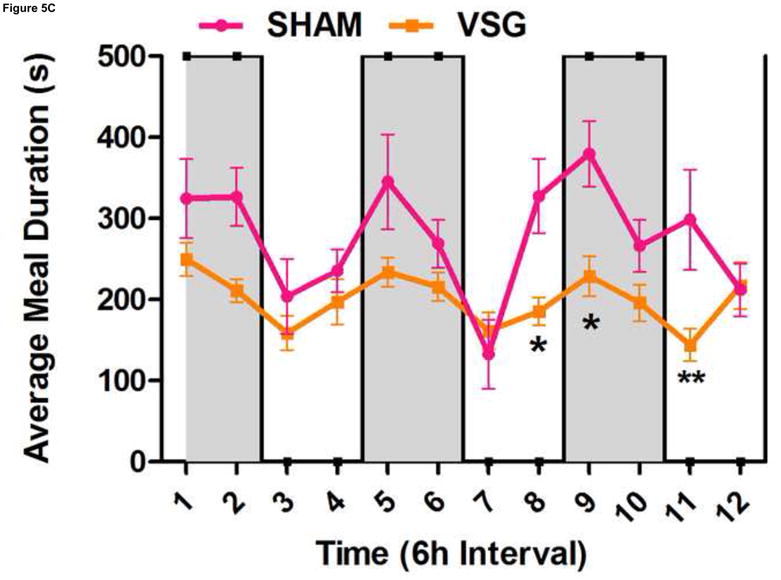
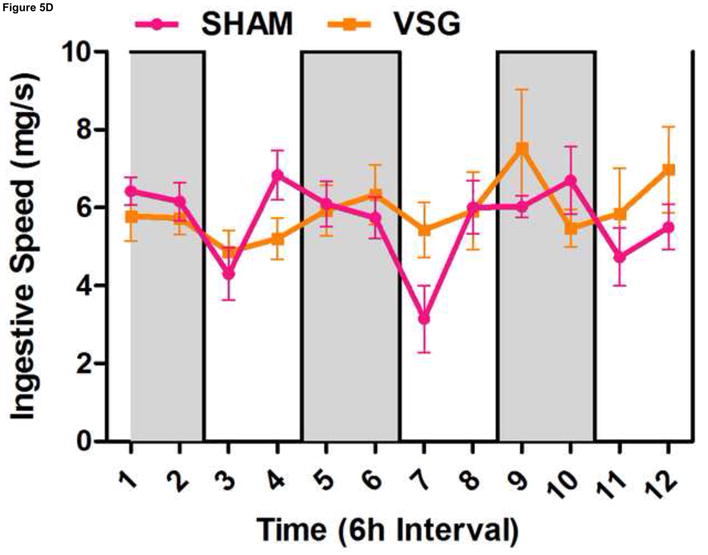
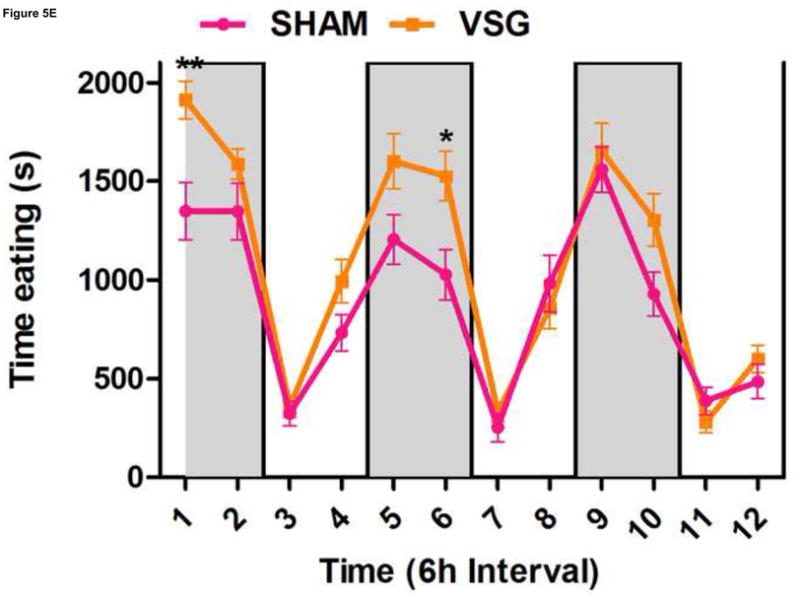
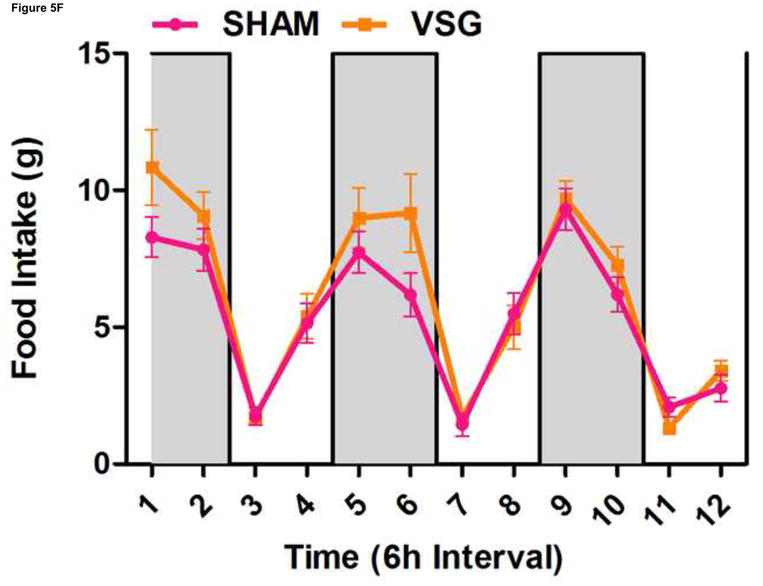
VSG alters meal patterns. After VSG, rats consume smaller meals than SHAM (A, effect of treatment P = 0.0004). Dark phase meal number was increased following VSG (B, effect of treatment P < 0.0001, interaction between treatment and time, P < 0.0001). These meals were shorter in duration than those consumed by SHAM rats (C, effect of treatment, P = 0.0003). Ingestive speed for meals in VSG rats was unchanged (D, P = 0.0237), but overall time spent eating was greater for VSG rats (E, effect of treatment, P < 0.0001, interaction between treatment and time, P = 0.0064). Total food intake did not differ for VSG and SHAM rats (F, P = 0.2758).
FIGURE 6.
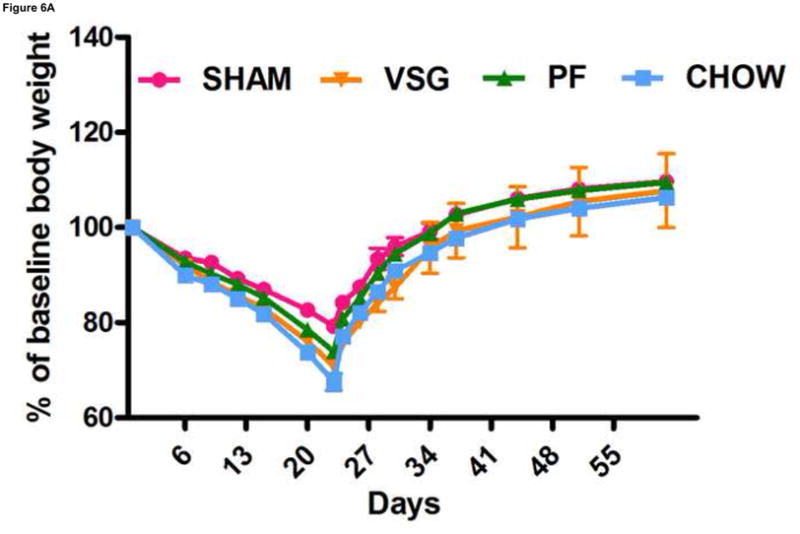
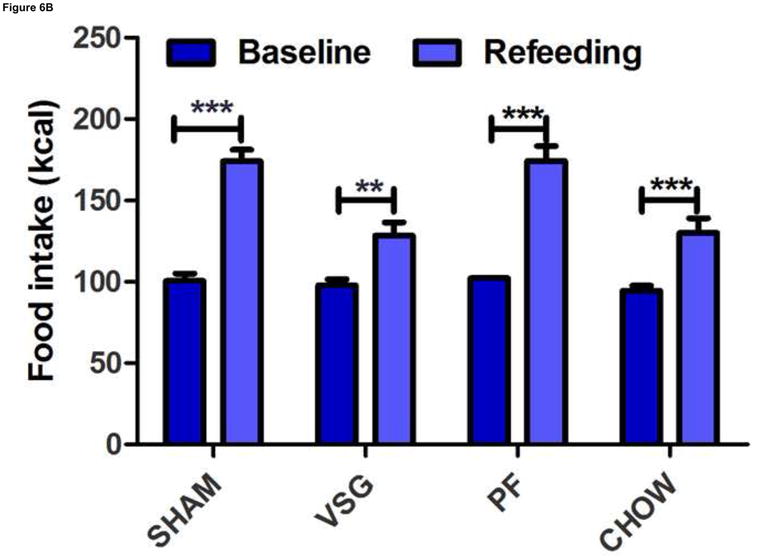
VSG does not impair the ability to overeat or to regain body weight after caloric restriction. After 22 days of caloric restriction, all rats regained body weight to exceed pre-restriction weight and did so along a similar trajectory (A, interaction P = 0.9937). During the first 24 h of ad libitum feeding after the period of caloric restriction, all rats consumed significantly more calories than pre-restriction baseline (B, P < 0.001 for SHAM, PF, CHOW, P < 0.01 for VSG).
VSG does not impair regulatory responses to food restriction
Because VSG is considered a restrictive procedure, sustained weight loss has been hypothesized to be the result of an inability to consume sufficient calories to regain presurgical body mass. To test this hypothesis, we asked whether VSG rats are able to consume additional calories in response to a period of food restriction and additional weight loss. Twenty-two days of chronic food restriction produced significant weight loss in all groups (P < 0.0001, Figure 6A). After 11 days, rats from all groups had regained the majority of the lost weight and did so by consuming more calories than the rats that were not restricted, and there were no differences among groups in this regard (Figure 6B). Thus, the VSG rats have the capacity to ingest increased numbers of calories when challenged to do so but choose not to do so to regain the weight lost after the VSG procedure.
VSG does not improve leptin sensitivity beyond the expected consequence of weight loss
As indicated above, although VSG rats are capable of overeating, they do not do so to return their reduced weight to normal following surgery. We hypothesized that improved leptin sensitivity might suppress hyperphagic behavior after surgery and limit weight regain at later time points. This was assessed directly by administering exogenous leptin. After 3 daily i.p. injections of leptin, VSG and PF rats exhibited significant and comparable reductions in food intake (P < 0.01 for VSG leptin vs. vehicle; p < 0.05 for PF leptin vs. vehicle, Figure 7A). SHAM rats, as expected, were leptin resistant (p > 0.05 leptin vs. vehicle, Figure 7A).
FIGURE 7.
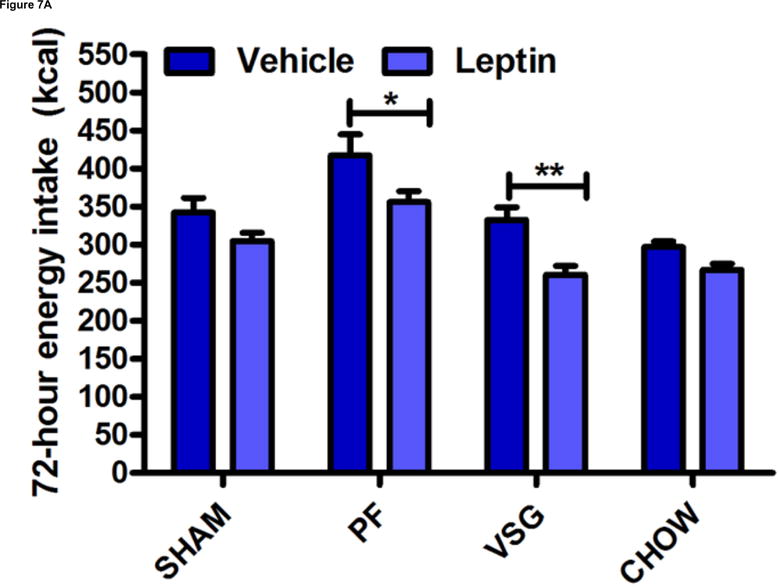
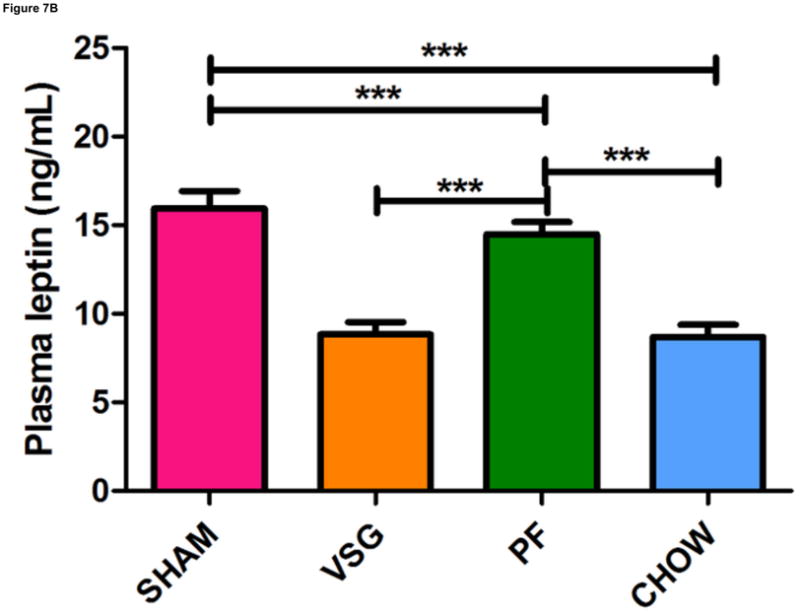
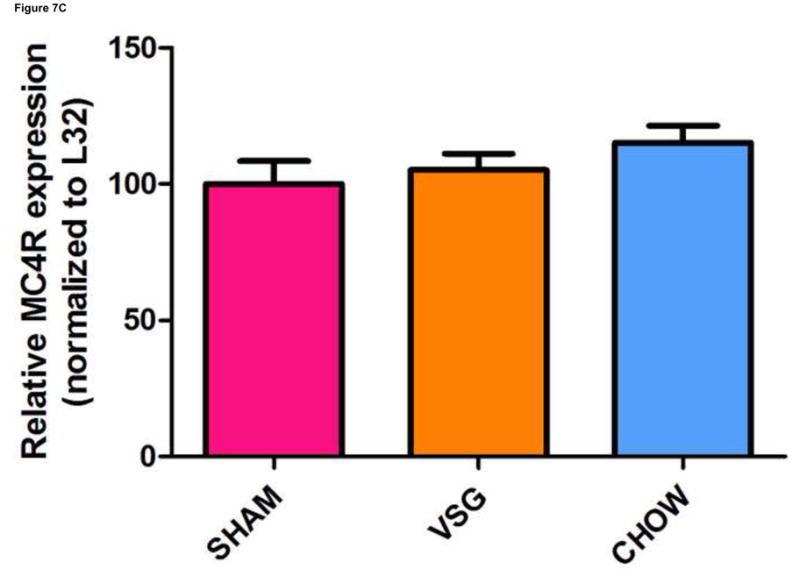
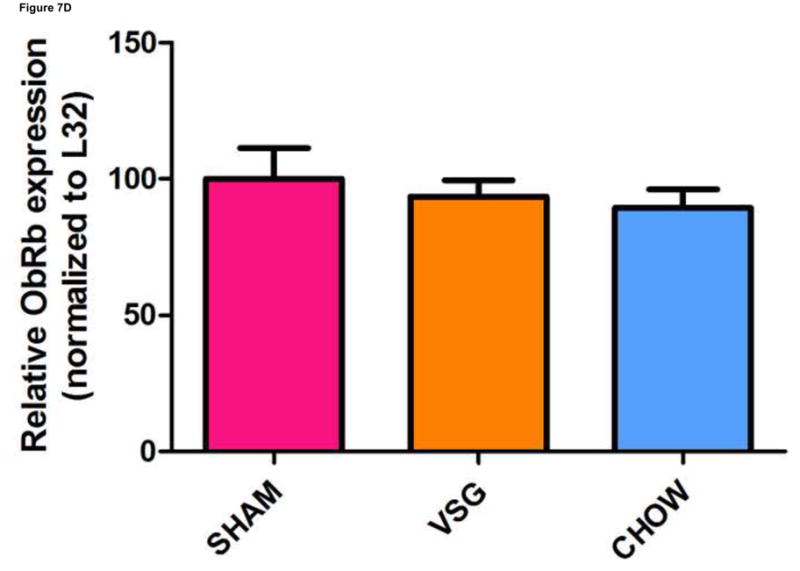
VSG improves leptin sensitivity secondary to weight loss. After VSG, rats exhibited an enhanced anorectic response to exogenous leptin as compared to SHAM (A, P < 0.01 for VSG, P > 0.05 for SHAM or CHOW). However, a similar response was observed after leptin injection in PF rats (A, P < 0.05 for PF). An overall interaction of drug and treatment (surgery and diet) was observed (P = 0.3938). Plasma leptin levels (B) were reduced after VSG as compared to PF (P < 0.0001), SHAM (P<0.001), or CHOW (P < 0.001). Effect of treatment, P = 0.0001. VSG affected the expression of neither MC4R (C, P = 0.28) nor ObRb (D, P = 0.64).
Plasma leptin levels on Day 50 were lower in VSG rats than in PF (p < 0.001) or CHOW (P <0.001) controls (Figure 7B). Similar trends persisted on day 125 (Supplemental Figure 2A). Plasma insulin (Supplemental Figure 2B) and glucose (Supplemental Figure 2C) were unaffected by VSG on day 125. When regression lines for fat mass vs. plasma leptin concentration on day 50 were compared, y-intercepts (P < 0.0001) but not slopes (P = 0.72) differed significantly between groups (Supplemental Figures 3A–E). We interpret this outcome to mean that basal leptin output by adipose tissue is lower for VSG and Sham-chow rats. Because PF and VSG rats had improved leptin sensitivity compared with both obese and chow-fed controls, we looked at hypothalamic leptin receptor (ObRb) and melanocortin 4 receptor (MC4R) expression. Expression of MC4R (P = 0.28, Figure 7C) and ObRb (P = 0.64, Figure 7D) in MBH lysates obtained on Day 125 after the surgery was similar between groups.
Discussion
The current rat model of VSG produces significant and durable weight loss in obese male and female rats. Importantly, this rodent model of VSG leads the rat to defend a new, lower body weight. Unlike what is seen following weight loss by caloric restriction, rats following VSG do not overeat to compensate for this reduced fat mass. Additionally, we provide strong evidence that the weight loss cannot be attributed to an improvement in leptin sensitivity.
Weight loss secondary to VSG was due to the selective loss of fat tissue, with lean tissue expanding along the rat’s normal growth curve. The loss of weight was almost entirely due to food restriction since there was no effect of VSG on overall energy expenditure or caloric absorption from the GI tract. Interestingly, food intake was reduced for only 3 weeks immediately following VSG and then the animals went back to eating the comparable calories as SHAM-treated rats. The VSG rats did this by eating smaller but more frequent meals. These data suggest that the VSG rats adapt their meal patterns to the reduced stomach volume caused by the surgery. Nevertheless, VSG rats are clearly capable of consuming even more calories when necessary as indicated by the hyperphagic response to the chronic caloric restriction (Figure 7). The VSG rats are capable of defending their body weight after chronic food restriction and do so by increasing their food intake (Figure 7). In fact, the VSG rats had comparable efficiency to regain lost weight after chronic food restriction as controls. After chronic caloric restriction, VSG rats overate relative to their own pre-restriction baseline intake and, perhaps more strikingly, ate more relative to SHAM and CHOW baseline intake. This experiment has two important implications. First, like the control groups, VSG rats appear to defend their body weight, albeit it at a lower level. Thus, instructing patients who have undergone this procedure to lose additional weight by dieting is likely to be no more successful than comparable advice to patients who have not had the procedure. Second, whatever the effect of the physical restriction in the VSG rats, they are willing to overcome such restriction to overeat when given the appropriate metabolic stimulus. Thus, it is unlikely that physical restriction per se is what prevents VSG rats from responding to the weight loss and anorexia that occur in the weeks following the procedure.
If restriction is not the primary reason why VSG rats consume less food, there remains a key question about the underlying mechanism. We hypothesized that the surgery may reverse the leptin resistance that is normally associated with exposure to HF diets. First, we determined that leptin levels are low in VSG rats. In fact, leptin levels at Day 50 after the surgery may be inappropriately low in VSG rats as compared to either SHAM or even PF rats (Supplemental Figure 3). This would be consistent with the hypothesis that it is not additional leptin but rather increased leptin sensitivity that drives the reduced body weight. However, if VSG rats are more sensitive to leptin, leptin-regulated genes should be expressed at a level consistent with those of an animal with greater leptin levels; i.e., hypothalamic POMC should be elevated and AgRP and NPY should be reduced. However, over the course of a number of experiments, the results indicated no change or increased AgRP and NPY gene expression while POMC was either not changed or decreased.
Such results are not consistent with increased leptin sensitivity. However there is also evidence that leptin actions are not mediated solely via MBH systems17–19, so we performed a more direct assessment of leptin’s ability to reduce food intake in VSG, SHAM and PF rats. As expected, leptin produced only a small, non-significant reduction of food intake over 3 days in SHAM rats. VSG rats significantly reduced their food intake in response to leptin, but PF rats had a similar reduction, implying that hypophagia as opposed to surgery per se is important. Collectively, the evidence does not support a conclusion of increased activity of the leptin-melanocortin axis following VSG. On the other hand, our findings are consistent with data from Lopez and colleagues20 demonstrating weight loss after VSG in obese, leptin-resistant Zucker rats. Thus, the ability of VSG surgery to result in weight loss is based on some other effect of the surgery, one that bypasses leptin resistance (and leptin deficiency) rather than reversing it. Nonetheless, it may be the case that increased leptin sensitivity in VSG rats contributes to the maintenance of the weight loss as they are losing weight.
VSG is often considered to be a restrictive procedure which produces weight loss by reducing caloric intake during meals21. The current results support part of that contention in that a temporary reduction in food intake appears responsible for the profound initial body fat loss observed and VSG does result in smaller meal size. However, the results are not consistent with the possibility that this is driven primarily by the restriction caused by the surgery. VSG rats defend their new, albeit lower, body weight and can increase their food intake to do so. Further, smaller meal sizes are eventually compensated by increased numbers of meals. Thus, VSG does not impair an animal’s ability to overeat, but suppresses the animal’s drive to overeat. Further, this change in the rat’s motivation to eat is not the result of important alterations in leptin production or the actions of the leptin-melanocortin axis. These insights into one bariatric surgery highlight both the need and the difficulty of understanding how these procedures produce such powerful effects. Answers to these key questions will provide important insights into how future treatments, both surgical and non-surgical, can be designed.
Supplementary Material
Acknowledgments
This work was supported by NIH grants DK54890 and DK083870 and Ethicon Endo-Surgery.
Footnotes
Disclosures- The following authors have conflicts to disclose:
Randy J. Seeley- Amylin Pharmaceuticals, Eli Lilly, Johnson & Johnson, Zafgen Merck
Matthias Tschöep- Marcadia Biotech, Ambrx Inc., Johnson & Johnson
Darleen A. Sandoval- Johnson & Johnson
Stephen C. Woods- Johnson & Johnson
The other authors have no potential conflicts (financial, professional, or personal) to disclose that are relevant to the manuscript
Author involvement: MAS- study concept, design, execution, analysis, and write-up. DPT- study design, execution, and analysis. APC, HWP, DAS, and M. Tschoep- study interpretation and discussion. JB and M. Toure- surgical design and execution. SCW and RJS- study concept, design, analysis, and writing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woods SC, Schwartz MW, Baskin DG, Seeley RJ. Food intake and the regulation of body weight. Annual Review of Psychology. 2000;51:255–277. doi: 10.1146/annurev.psych.51.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of hypothalmic targets of leptin action. Journal of Clinical Investigation. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 4.McGregor GP, Desaga JF, Ehlenz K, Fischer A, Heese F, Hegele A, Lammer C, Peiser C, Lang RE. Radiommunological Measurement of Leptin in Plasma of Obese and Diabetic Human Subjects. Endocrinology. 1996;137:1501–04. doi: 10.1210/endo.137.4.8625930. [DOI] [PubMed] [Google Scholar]
- 5.Scarpace PJ, Matheny M, Tumer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience. 2001;104:1111–7. doi: 10.1016/s0306-4522(01)00142-7. [DOI] [PubMed] [Google Scholar]
- 6.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. Journal of CLinical Investigation. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 8.Cottam D, Qureshi FG, Mattar SG, Sharma S, Holover S, Bonanomi G, Ramanathan R, Schauer P. Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc. 2006;20:859–63. doi: 10.1007/s00464-005-0134-5. [DOI] [PubMed] [Google Scholar]
- 9.Mognol P, Chosidow D, Marmuse JP. Laparoscopic sleeve gastrectomy (LSG): review of a new bariatric procedure and initial results. Surg Technol Int. 2006;15:47–52. [PubMed] [Google Scholar]
- 10.Mognol P, Chosidow D, Marmuse JP. Laparoscopic sleeve gastrectomy as an initial bariatric operation for high-risk patients: initial results in 10 patients. Obes Surg. 2005;15:1030–3. doi: 10.1381/0960892054621242. [DOI] [PubMed] [Google Scholar]
- 11.Pereferrer FS, Gonzalez MH, Rovira AF, Blasco SB, Rivas AM, del Castillo Dejardin D. Influence of sleeve gastrectomy on several experimental models of obesity: metabolic and hormonal implications. Obes Surg. 2008;18:97–108. doi: 10.1007/s11695-007-9351-4. [DOI] [PubMed] [Google Scholar]
- 12.de Bona Castelan J, Bettiol J, d’Acampora AJ, Castelan JV, de Souza JC, Bressiani V, Giroldi SB. Sleeve gastrectomy model in Wistar rats. Obes Surg. 2007;17:957–61. doi: 10.1007/s11695-007-9150-y. [DOI] [PubMed] [Google Scholar]
- 13.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–7. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 14.Peterli R, Wolnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flue M, Beglinger C. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–41. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 15.Melissas J, Koukouraki S, Askoxylakis J, Stathaki M, Daskalakis M, Perisinakis K, Karkavitsas N. Sleeve gastrectomy: a restrictive procedure? Obes Surg. 2007;17:57–62. doi: 10.1007/s11695-007-9006-5. [DOI] [PubMed] [Google Scholar]
- 16.Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology. 2004;127:139–44. doi: 10.1053/j.gastro.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- 18.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–46. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 20.Lopez PP, Nicholson SE, Burkhardt GE, Johnson RA, Johnson FK. Development of a Sleeve Gastrectomy Weight Loss Model in Obese Zucker Rats. J Surg Res. 2008 doi: 10.1016/j.jss.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baltasar A, Serra C, Perez N, Bou R, Bengochea M, Ferri L. Laparoscopic sleeve gastrectomy: a multi-purpose bariatric operation. Obes Surg. 2005;15:1124–8. doi: 10.1381/0960892055002248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


