Abstract
Background & Aims
Bariatric surgery has been shown to reverse type 2 diabetes, however the mechanisms by which this occurs remain undefined. Ileal interposition (IT) is a surgical model that isolates the effects of increasing the delivery of unabsorbed nutrients to the lower gastrointestinal tract. In this study we investigated the effects of IT surgery on glucose tolerance and diabetes onset in UCD-T2DM rats, a polygenic obese animal model of type 2 diabetes.
Methods
IT or sham surgery was performed on 4 month old male UCD-T2DM rats. All animals underwent an oral glucose tolerance test (OGTT). A subset was euthanized 2 months after surgery for tissue analyses. The remainder was followed until diabetes onset and underwent an oral fat tolerance test (OFTT).
Results
IT surgery delayed diabetes onset by 120 ± 49 days compared with sham surgery (P< 0.05) without a difference in body weight. During OGTT, IT-operated animals exhibited lower plasma glucose excursions (P< 0.05), improved early insulin secretion (P< 0.01) and 3-fold larger plasma GLP-17–36 excursions (P< 0.001) and no difference in GIP responses compared with sham-operated animals. Total plasma PYY excursions during the OFTT were 3-fold larger in IT-operated animals (P< 0.01). IT-operated animals exhibited lower adiposity (P< 0.05), smaller adipocyte size (P< 0.05), 25% less ectopic lipid deposition, lower circulating lipids and greater pancreatic insulin content compared with sham-operated animals (P< 0.05).
Conclusions
IT surgery delays the onset of diabetes in UCD-T2DM rats which may be related to increased nutrient-stimulated secretion of GLP-17–36 and PYY and improvements of insulin sensitivity, β-cell function and lipid metabolism.
Keywords: Bariatric surgery, diabetes prevention, glucagon-like peptide-1, peptide-YY
Background and Aims
Bariatric surgery, such as Roux-en-Y Gastric Bypass (RYGP), is currently the most effective long-term treatment for obesity 1, 2 and has been demonstrated to markedly improve glucose homeostasis 3–5, however the mechanisms by which this occurs remain undefined. The improvement of glucose homeostasis following bariatric surgery has been attributed to weight loss resulting from a reduction in gastric volume and/or reduced nutrient absorption, depending on the type of surgery. However, observations made in a number of clinical studies support a key role for endocrine changes in the reversal of type 2 diabetes after bariatric surgery. First, in patients with type 2 diabetes undergoing bariatric surgery, such as RYGB, glucose normalization often occurs prior to substantial weight loss 5, 6. Secondly, bariatric surgeries involving bypass of the proximal small intestine and/or biliopancreatic diversion are often more effective at improving obesity and reversing type 2 diabetes than bariatric surgeries involving only gastric restriction 5, 7.
These observations have lead to the development of the “hindgut” hypothesis which postulates that increased flux of unabsorbed nutrients in the distal small intestine results in the activation of a neuroendocrine negative feedback mechanism, often termed the “ileal brake,” which involves increased secretion of peptides, including glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) from L-cells located in the distal gastrointestinal tract 8. Increased secretion of these hormones may contribute to weight loss and improved glucose metabolism 8. GLP-17–36 (active form) acts to potentiate glucose-induced insulin secretion, inhibit glucagon secretion, decrease food intake, improve insulin sensitivity and may also promote β-cell proliferation 9. PYY3–36 (active form) acts to inhibit food intake and slow gastric motility and thus maintains weight loss 10, 11. Furthermore, elevations of these hormones after bariatric surgery have been reported in a number of clinical studies 7, 12.
RYGB surgery results in increased delivery of unabsorbed nutrients to the distal small intestine, but also involves gastric restriction and bypass of the duodenum. Ileal interposition (IT) is a surgical procedure in which a segment of ileum is inserted into the proximal small intestine and provides a surgical model whereby the effect of RYGB surgery to increase the flux of unabsorbed nutrients to the distal gastrointestinal tract can be isolated from gastric restriction and duodenal bypass. IT surgery has been shown to induce weight loss and improve insulin signaling in obese and diabetic animal models 13–16. The only diabetic animal models tested to date are the Goto-kakizaki rat and streptozotocin-treated Long-Evans rats, both of which demonstrate diabetes with a pathophysiology unlike that observed in clinical type 2 diabetes. Goto-kakizaki rats are not obese and insulin resistant 14. Thus, diabetes in this model is dependent on impaired islet function making the pathophysiology of diabetes in these animals more similar to type 1 diabetes. Similarly, streptozotocin treated rats are considered to be a model of type 1 diabetes as hyperglycemia is the result of chemically induced β-cell destruction. In this study we investigated the effects of IT surgery on diabetes onset, glucose tolerance, and several biochemical parameters in University of California at Davis Type 2 Diabetes Mellitus (UCD-T2DM) rats, a model of type 2 diabetes combining polygenic adult-onset obesity and insulin resistance with β-cell dysfunction 17.
Methods
Diets and Animals
Male UCD-T2DM rats were individually housed in hanging wire cages in the animal facility in the Department of Nutrition at the University of California, Davis and maintained on a 12:12 hour light-dark cycle. At 4 months of age rats underwent sham or IT surgery. Animals were followed until at least one year of age to determine the time to diabetes onset (long-term group) or were euthanized two months after surgery for tissue collection (short-term group). All animals enrolled in the study following successful surgeries completed the study. Baseline body weights, measured on the day of surgery, in the long-term groups were 585 ± 11 g and 594 ± 18 g in sham (n=9) and IT-operated animals (n=8), respectively. Baseline body weights in the short-term groups were 598 ± 13 g and 601 ± 13 g in sham (n=10) and IT-operated animals (n=10), respectively. All animals received ground chow (no. 5012, Ralston Purina, Belmont, CA). Food intake and body weight were measured three times a week. Non-fasting blood glucose was measured weekly with a glucose meter (One-Touch Ultra, LifeScan, Milpitas, CA) at 14:00–15:00 h. Diabetes onset was defined as a non-fasted blood glucose value above 11.1 mmol/l (200mg/dl) on two consecutive weeks. The experimental protocols were approved by the UC Davis Institutional Animal Care and Use Committee.
Ileal Interposition Surgery
IT surgery was performed as described by Koopmans et al. 18. Rats were placed on a liquid diet (Boost®, Novartis, Minneapolis, MN) three days prior to surgery and for 5–7 days post-surgery and received enrofloxacin (10 mg/kg, s.c.) before and after surgery. Anesthesia was induced and maintained with isoflurane (2–5%). A midline abdominal incision was made and a 10 cm segment of ileum 5–10 cm proximal to the ileocecal valve was isolated and transected. An anastomosis was made with the remaining ends of the ileum using 7-0 silk suture (Ethicon®). Next, a transection was made 5–10 cm distal to the ligament of Treitz. The isolated ileal segment was then inserted isoperistaltically. The transposed segment remained innervated and with its vasculature intact.
Sham-operated animals were treated in the same manner as the IT group. Sham surgeries were performed by making transections in the same locations as in the IT-operated animals, but bowel segments were reattached by anastomosis in their original position. The intestines remained their original length in both surgeries, but were reorganized with respect to which intestinal segments were exposed to incoming nutrients only with the IT surgery.
Oral Glucose and Oral Fat Tolerance Tests
One month following surgery, an OGTT was performed on animals from the short-term and long-term groups. Animals were fasted overnight and then received a 50% dextrose solution (1 g/kg BW) by oral gavage. Blood was collected from the tail for measurement of glucose and insulin concentrations. A second aliquot of blood was placed in tubes containing EDTA, aprotinin and a DPP-IV inhibitor and analyzed for GLP-17–36 and total glucose-dependent insulinotropic polypeptide (GIP). Serum glucose was measured using an enzymatic colorimetric assay for glucose (Thermo DMA Louisville, CO). Serum insulin, plasma GLP-17–36 and total GIP were measured by rodent/rat specific ELISAs (Millipore, St. Charles, MO). The three sham-operated animals that developed diabetes by one month after surgery were excluded from this data set to avoid potential confounding effects of hyperglycemia (no IT-operated animals had developed diabetes by this time point).
At 3.5 months following surgery, a subset of animals in the long-term study groups were fasted overnight and received Intralipid (Fresenius Kabi; Uppsala, Sweden) by oral gavage (1.5 g/kg BW of a 20% solution). Blood was collected from the tail into tubes containing EDTA and aprotinin. Total PYY was measured by rat/mouse specific RIA (Millipore, St. Charles, MO).
Monthly Fasted Hormone and Metabolite Profiles
Baseline and monthly blood samples were collected after an 8 hour fast from rats in the long term groups into EDTA treated tubes. Plasma was assayed for glucose, insulin, triglycerides (TG), cholesterol, leptin, adiponectin and ghrelin. Fasting plasma samples were also collected from animals in the short term groups on the day of euthanasia and bile acids were measured using the Total Bile Acids Assay (Enzyme Cycling Method) kit (Diazyme, San Diego, CA). Plasma glucose, cholesterol and TG were measured using enzymatic colorimetric assays (Thermo DMA Louisville, CO; L-type TG H kit, Wako Chemicals USA, Inc., Richmond, VA). Insulin, leptin and adiponectin were measured with rodent/rat specific RIAs (rat leptin, mouse adiponectin, Millipore, St. Charles, MO).
Tissue Collection, Tissue Triglyceride Content and Adipocyte Size Determination
Two months after surgery, animals in the short-term groups were euthanized with an overdose of pentobarbital (200 mg/kg i.p.) after an overnight fast. Tissues were weighed and flash frozen in liquid nitrogen and stored at −80 °C. Liver, skeletal muscle, and adipose TG content were measured using the Folch method 19 for lipid extraction followed by spectrophotmetric measurement of TG content (Thermo Electron, Louisville, CO).
Mesenteric adipocytes were isolated according to the method of Rodbell 20 as modified by Mueller 21. 25 µl of packed adipocytes were added to an Accuvette containing 20 ml of Isoton (Beckman Coulter). The Accuvette was quickly placed on the sampling platform of the MultiSizer III and a 0.5 ml aliquot was counted and sized through a 280 micron aperture. Using the Multisizer 3.51 software, cells were counted and sorted into 300 size bins in a range of 12–1200 pl. The percent of total adipocyte volume was calculated for each bin and the maximum value was reported as the peak value.
Ileal Preproglucagon and Peptide YY mRNA expression
Ileal (~100 mg) samples were taken from the center of the transected segment and placed in a stabilization solution (1XTransPrep, nucleic acid purification lysis buffer; Applied Biosystems, Foster City, CA). Proteinase K and beads (SpexCertiprep, Metuchen, NJ) were added and samples were homogenized in a GenoGrinder 2000 (SpexCertiprep). Total RNA was extracted using a 6100 Nucleic Acid PrepStation (Applied Biosystems). The Quantitect reverse transcriptase kit (Qiagen) was used to DNase treat samples and generate cDNA. The probe and primers for preproglucagon (NM_012707, Rn01460420_g1) and PYY (NM_001034080, Rn 00562293_m1) and Beta-2 microglobulin (B2M) (NM_012512, Rn00560865_m1) were purchased from Applied Biosystems. Each PCR reaction contained a final concentration of 400 nM for each primer and 80 nM for the TaqMan ® probe and commercially available PCR mastermix (TaqMan ® Universal PCR Mastermix, Applied Biosystems). The samples were amplified in an automated fluorometer (7900 HT FAST Real Time PCR System, ABI). Final quantification was done using the comparative Ct method (User Bulletin #2, Applied Biosystems), and is reported as relative transcription to the sham-operated group after using B2M to normalize target genes’ Ct values.
Nodose Ganglion GLP-1R and Y2R protein expression
Nodose ganglia were homogenized on ice in lysis buffer and cocktail inhibitors (3.03g Tris base and 12.7ml 0.5M EDTA dissolved in 500ml ddH2O at pH 7.5; 1% Triton X-100, 1% anti-phosphatase, 1% protease inhibitor, 2.87ul/0.5ml PMSF), centrifuged and supernatant collected. Protein concentration was determined using the Bradford method (BioRad, Hercules, CA). 65µg of protein was separated by electrophoresis and transferred to nitrocellulose membranes. Membranes were probed for GLP-1 Receptor (GLP-1R) and neuropeptide Y receptor Y2 (NPY2R) using rabbit polyclonal anti-GLP-1R (Abcam, Cambridge, MA), goat anti-mouse NPY2R (United States Biological, Swampscott, MA) and rabbit anti-GAPDH (Cell Signaling Technology, Danvers, MA). Anti-biotin horse-radish peroxidase and goat anti-rabbit horse-radish peroxidase (Cell Signaling Technology, Danvers, MA) were used as secondary antibodies. Immunoblotted proteins were detected by chemiluminescence using 20X LumiGLO and 20X Peroxide reagents (Cell Signaling Technology, Danvers, MA). Optical densitometry of immunoreactive bands was measured using Image Quant version 5.1 (Molecular Dynamics, Piscataway, NJ).
Islet Immunohistochemistry and Pancreatic Insulin Content
Pancreas samples were collected and immunostained as previously described 17. Pancreatic insulin was extracted using a combination of methods by Dixit, Karam and Davidson 22–24. Insulin was extracted from pre-weighed pancreas samples by mincing, sonicating and then incubating samples overnight at 4°C in acid alcohol with aprotinin. Samples were centrifuged and the supernatant was collected and the pellet was washed once more with the same solution. An alcohol diethyl ether solution (38% alcohol, 62% ether) was added to the supernatants and incubated overnight at 4°C. Samples were centrifuged and the pellet was reconstituted in 0.01N hydrochloric acid and assayed for insulin content by RIA (Millipore, St. Charles, MO).
Statistics and Data Analysis
Data are presented as mean ± SEM. All statistical analyses were performed using GraphPad Prism 4.00 for Windows (GraphPad Software, San Diego, CA) except for 3-factor ANOVAs which were preformed using SAS 9.1 (Cary, NC). OGTT and OFTT data were compared by two-factor (time × treatment) repeated measures (RM) ANOVA followed by post-hoc analysis with Bonferroni’s multiple comparison test. Absolute changes of body weight, food intake and monthly circulating hormone and metabolite data were analyzed by mixed procedures three-factor (time, treatment, disease free days) repeated measures ANOVA. Animals were divided into tertiles based on disease free days post-surgery: 0–205, 206–250, 251+ days. The incidence of diabetes was analyzed by log-rank testing of Kaplan-Meier survival curves. The age of diabetes onset, tissue weights, tissue TG content, ileal mRNA levels and nodose ganglia protein levels were analyzed by Student’s t-test. Differences were considered significant at P<0.05.
Results
IT Surgery Delays the Onset of Diabetes without Reducing Body Weight
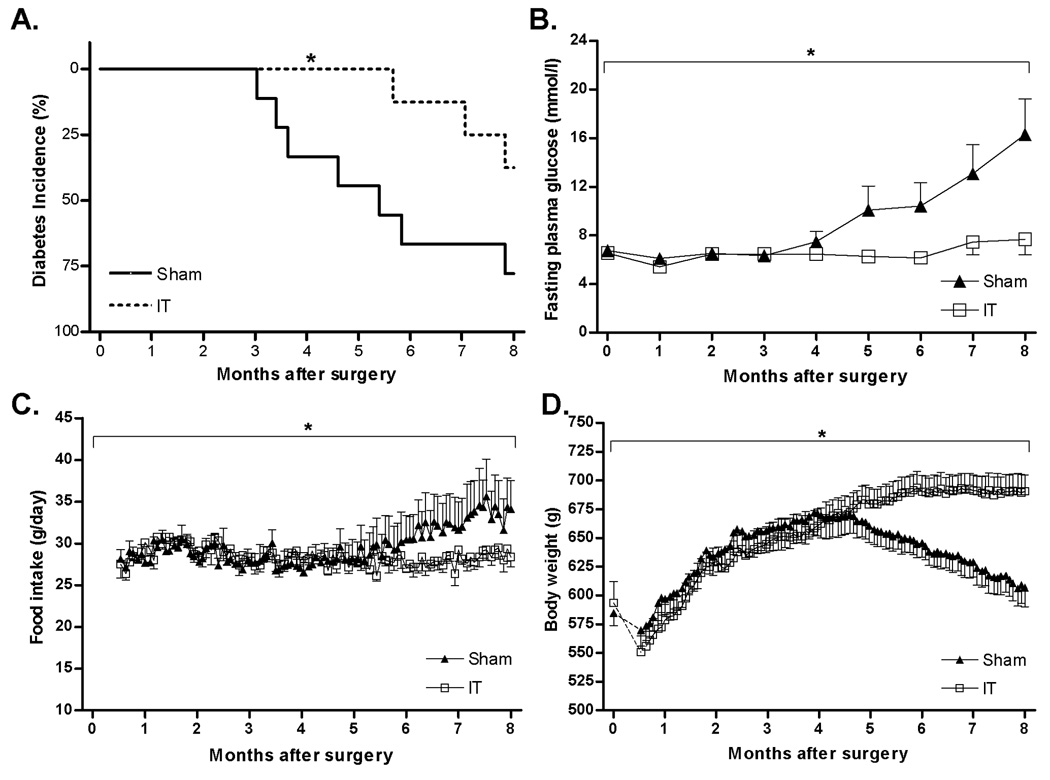
IT surgery delayed the onset of type 2 diabetes by 120 ± 49 days compared with sham-operated animals (Average age of onset: sham = 265 ± 19 days, IT = 385 ± 49 days; P< 0.05). IT surgery also reduced the incidence of diabetes such that by one year of age 78% of sham-operated animals were diabetic whereas only 38% of IT-operated animals were diabetic (Figure 1A) (P< 0.05). This delay in onset was clearly reflected in monthly fasting plasma glucose concentrations which were 54 ± 8% higher in sham-operated animals at 8 months after surgery (P< 0.05) (Figure 1B).
Figure 1.
Kaplan-Meier analysis of diabetes incidence in sham-operated (n= 9) and IT-operated animals (n= 8) (A). *P< 0.05 by log-rank test. Fasting plasma glucose concentrations (B), food intake (C) and body weight (D) in sham-operated (n= 9) and IT-operated animals (n= 8). *P< 0.05 disease × time by mixed procedures 3-factor (time, treatment and disease free days) repeated measures ANOVA. Values are expressed as mean ± SEM.
Food intake remained similar between sham and IT-operated animals during the first 5 months after surgery (Figure 1C) when diabetes incidence was low. Six months after surgery food intake began to increase in sham-operated animals due to increased incidence of diabetes and development of diabetic hyperphagia (disease free days × time, P< 0.05; 3-factor RM ANOVA) but not an effect of treatment (treatment × time, P= 0.20; 3-factor RM ANOVA). Body weight was similar between groups until 6 months after surgery when sham-operated animals began to lose weight due to increased incidence of diabetes (disease free days × time, P< 0.05; 3-factor RM ANOVA), but not an effect of treatment (treatment × time, P= 0.11; 3-factor RM ANOVA) (Figure 1D).
IT Surgery Improves Glucose Tolerance and Insulin Secretion and Increases Nutrient Stimulated GLP-1 and PYY Secretion
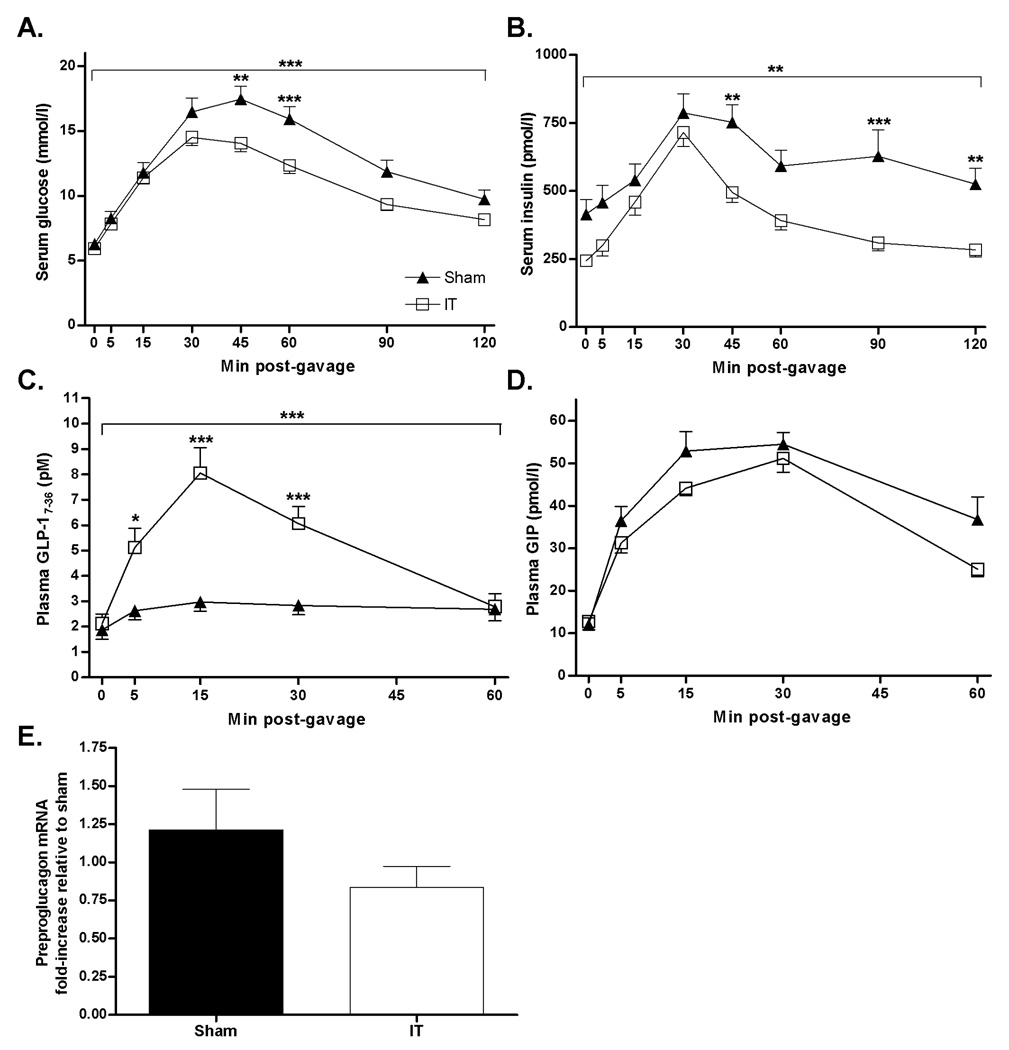
Glucose excursions in response to oral glucose administration were lower in pre-diabetic IT compared with pre-diabetic sham-operated animals (Glucose AUC: sham = 1587 ± 96, IT = 1324 ± 53 mmol/l × 180 min, P< 0.05) (Figure 2A). Fasting serum insulin concentrations were 45 ± 5% lower (P< 0.01) and the insulin AUC was 34 ± 5% lower in IT compared with sham-operated animals (Insulin AUC: sham = 74423 ± 7359, IT = 49128 ± 3352 pmol/l × 180 min, P< 0.01) (Figure 2B). Furthermore, the percent increase of plasma insulin concentrations from baseline to 30 min after glucose administration was 314 ± 26% in IT-operated animals and 201 ± 13% in sham-operated animals (P< 0.01), indicating improvement of glucose-stimulated insulin secretion with IT surgery. Peak GLP-17–36 secretion was 3-fold higher in IT compared with sham-operated animals (GLP-17–36 AUC: sham = 166±20, IT = 361 ± 40 pM × 60 min, P< 0.001) (Figure 2C). However, ileal preproglucagon mRNA levels at two months after surgery did not differ between groups (Figure 2E). Circulating GIP concentrations did not differ between groups (GIP AUC: sham = 2740 ± 148, IT = 2347 ± 105 pmol/l × 60 min) (Figure 2D).
Figure 2.
Serum glucose (A), serum insulin (B) plasma GLP-17–36 (C) and plasma GIP (D) concentrations following an oral glucose gavage (1g/kg body weight, 50% dextrose solution) in sham (n=15) and IT-operated animals (n=18) at 1 month after surgery. ***P< 0.0001, **P< 0.001 by two-factor (time × treatment) ANOVA; *P< 0.05, **P< 0.01, ***P< 0.001 by Bonferroni’s posttest. Ileal preproglucagon mRNA expression 2 months after surgery in sham (n=10) and IT (n=7) operated animals (E). Values are expressed as mean ± SEM.
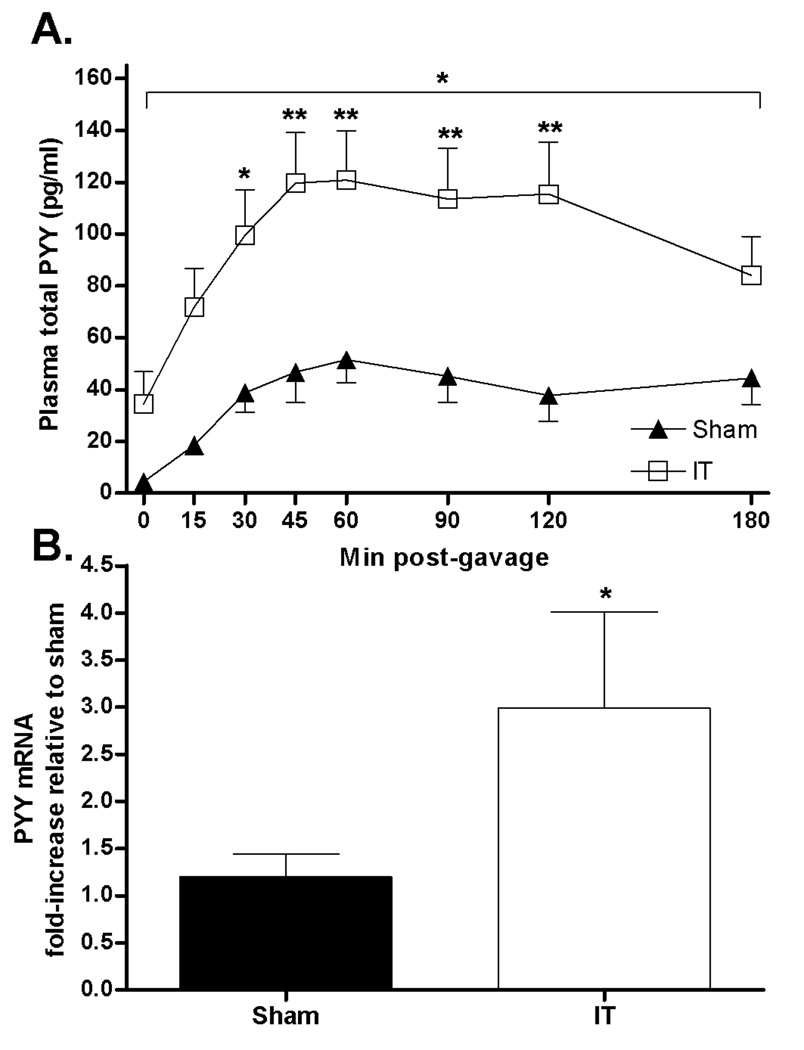
Peak PYY excursions in response to an oral lipid load were 3-fold higher in IT compared with sham-operated animals (PYY AUC: sham = 14264 ± 2572, IT = 36895 ± 6011 pg/ml × 180 min; P< 0.01) (Figure 3A). Furthermore, fasting plasma PYY concentrations were 8-fold higher in IT compared with sham-operated animals (P< 0.05). Ileal PYY mRNA content was 2-fold higher in IT compared with sham-operated animals (Figure 3B).
Figure 3.
Plasma total PYY concentrations following an oral intralipid gavage (1.5g/kg body weight, 20% lipid solution) at 3.5 months after surgery in sham-operated (n=7) and IT-operated (n=7) animals (A). *P< 0.05 by two-factor (time × treatment) ANOVA; *P< 0.05, **P< 0.01 by Bonferroni’s posttest. Ileal PYY mRNA expression 2 months after surgery in sham (n=10) and IT (n=7) operated animals (B). *P< 0.05 by Student’s t-test. Values are expressed as mean ± SEM.
In contrast to the observed changes in postprandial GLP-17–36 and PYY secretion, GLP-1R and NPY2R protein content in the nodose ganglion did not differ between sham and IT-operated animals (Supplemental Figure 1).
IT Surgery Improves Islet morphology and Increases Pancreatic Insulin Content
Figure 4 shows representative images of pancreas sections from prediabetic IT and sham-operated animals two months after surgery. Islets from IT-operated animals (Figure 4B, D, F) appeared more densely stained for insulin with better preservation of islet architecture than islets from sham-operated animals (Figure 4A, C, E). Furthermore, pancreatic insulin content was 2.5-fold higher in IT compared with sham-operated animals (P< 0.05) (Table 1).
Figure 4.
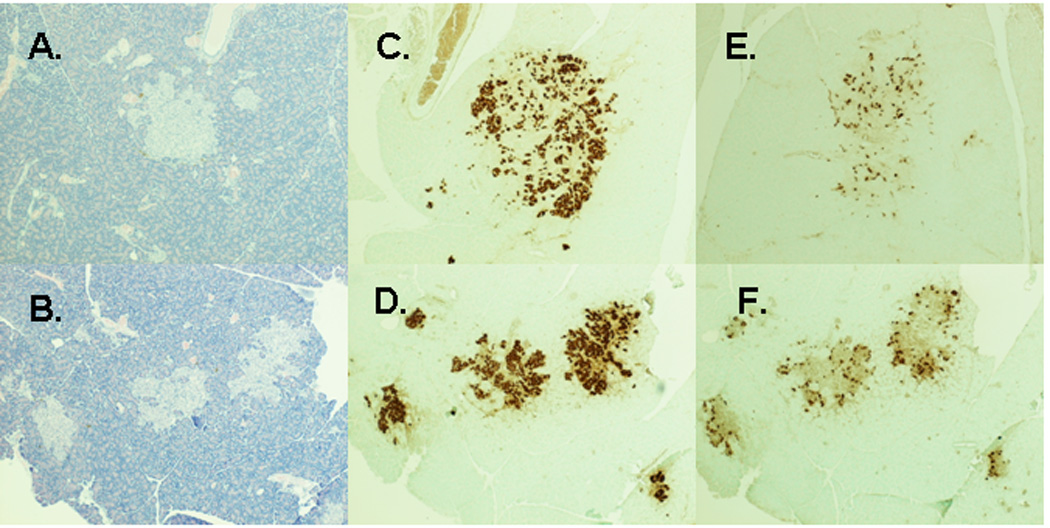
Representative images of pancreas sections from prediabetic sham and IT-operated animals at 2 months after surgery. Hematoxylin and eosin stain of pancreas sections from sham-operated (A) and IT-operated animals (B). Anti-insulin immunostaining of pancreas sections from sham-operated (C) and IT-operated (D) animals. Anti-glucagon immunostaining of pancreas sections from sham-operated (E) and IT-operated (F) animals.
Table 1.
Tissue weights, Tissue TG content and pancreatic insulin content
| Sham | IT | |
|---|---|---|
| BW (g) | 626 ± 19 | 599 ± 19 |
| Epididymal fat depots (g) | 8.9 ± 0.5 | 6.7 ± 0.8 * |
| Retroperitoneal fat depots (g) | 12.0 ± 0.8 | 8.8 ± 1.2* |
| Subcutaneous depot (g) | 45 ± 4 | 38 ± 4 |
| Mesenteric depot (g) | 9.9 ± 0.5 | 8.6 ± 0.8 |
| Total white adipose tissue (g) | 76 ± 5 | 62 ± 7 |
| Heart (g) | 1.5 ± 0.1 | 1.5 ± 0.1 |
| Kidney (g) | 1.8 ± 0.1 | 1.7 ± 0.1 |
| Liver (g) | 21 ± 1 | 20 ± 1 |
| Liver TG content (mg/g) | 28 ± 2 | 21± 3* |
| Skeletal muscle TG content (mg/g) | 5.0 ± 0.7 | 3.7 ± 0.3* |
| Inguinal TG content (%) | 67 ± 2 | 66 ± 3 |
| Mesenteric TG content (%) | 66 ± 3 | 54 ± 5* |
| Pancreatic insulin content (µg/g) | 5.8 ± 1.7 | 14.2 ± 4.1* |
Values are mean ± SEM.
P<0.05 compared to sham by Student's t-test. Sham n=10, IT n=10.
IT Surgery Improves Circulating Lipids and Insulin, but does not Affect Adiponectin
Fasting plasma TG concentrations were significantly lower in IT compared with sham-operated animals due to increased diabetes incidence in the sham-operated group (disease free days × time, P< 0.01; 3-factor RM ANOVA), and possibly due to an effect of treatment (treatment × time, P= 0.08; 3-factor RM ANOVA) (Figure 5A). Fasting plasma cholesterol concentrations were significantly lower in IT compared with sham-operated animals due to an effect of treatment (treatment × time, P< 0.05; 3-factor RM ANOVA) and not an effect of diabetes incidence (disease free days × time, P= 0.17; 3-factor RM ANOVA) (Figure 5B). Fasting plasma bile acid concentrations were 2 times higher in IT compared with sham-operated animals at two months after surgery (P< 0.05) (Figure 5C).
Figure 5.
Monthly measurements of fasting plasma TG (A), cholesterol (B), insulin (D), adiponectin (E) and leptin (F) concentrations in sham (n=9) and IT (n=8) operated animals. +P< 0.05, +++P< 0.001 treatment × time; *P< 0.05, **P< 0.01, ***P< 0.001 disease × time by mixed procedures 3-factor (time, treatment and disease free days) repeated measures ANOVA. Fasting plasma bile acids at two months after surgery in sham (n=10) and IT (n=10) operated animals (C). *P<0.05 by Student’s t-test. Values are expressed as mean ± SEM.
Fasting plasma insulin concentrations remained stable in the IT-operated group, whereas fasting plasma insulin concentrations decreased starting at 5 months after surgery in the sham-operated animals due to both an effect of treatment (treatment × time, P< 0.001; 3-factor RM ANOVA) and diabetes incidence (disease free days × time, P< 0.001; 3-factor RM ANOVA) (Figure 5D). Fasting plasma adiponectin concentrations did not differ between groups (Figure 5E). Plasma leptin concentrations were significantly lower in IT compared with sham-operated animals due to an effect of diabetes incidence (disease free days × time, P< 0.05; 3-factor RM ANOVA), but not an effect of treatment (treatment × time, P= 0.41; 3-factor RM ANOVA) (Figure 5F).
IT Surgery Decreases Adipocyte Size and Tissue TG Content
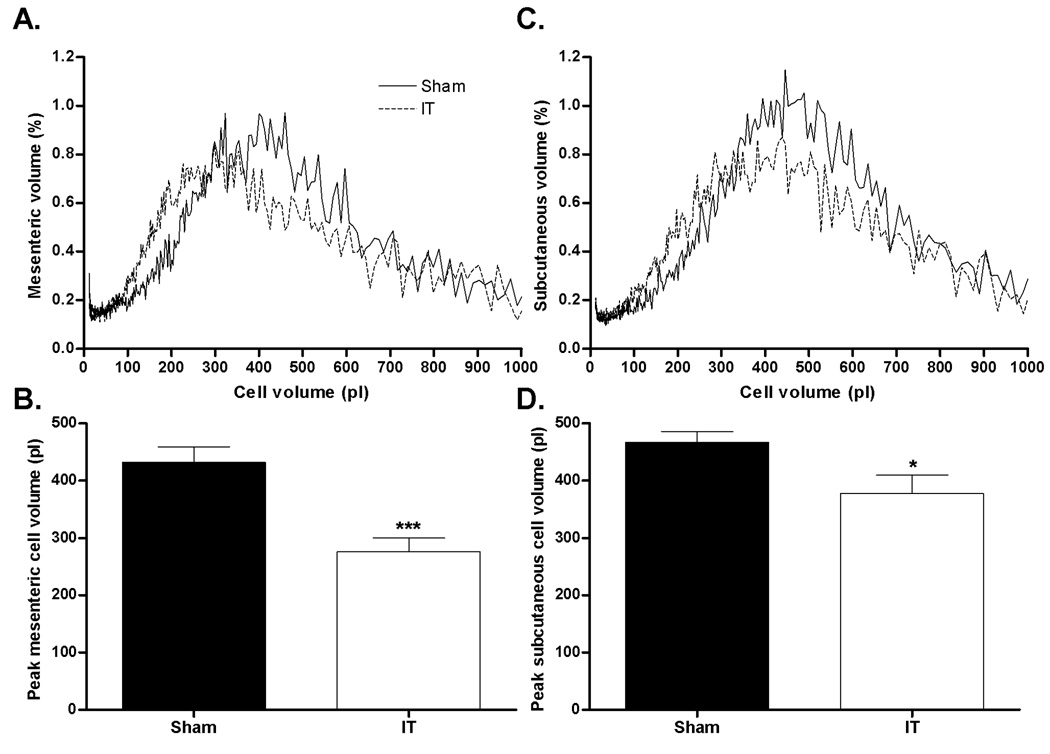
Despite comparable body weight at two months after surgery, IT-operated animals had smaller epididymal and retroperitoneal adipose depots compared with sham-operated animals (P< 0.05) (Table 1). Peak mesenteric (Figure 6A and B) and subcutaneous (Figure 6C and D) adipocyte volumes were ~35% smaller in IT compared with sham-operated animals (P< 0.05). Mesenteric fat depot, liver and skeletal muscle TG content were all ~25% lower in IT compared with sham-operated animals (P< 0.05) (Table 1).
Figure 6.
Mesenteric (A) and subcutaneous cell (C) volume distribution and mesenteric (B) and subcutaneous (D) peak cell volume. *P< 0.05, ***P< 0.001 by Student’s t-test. Values are expressed as mean ± SEM.
Conclusions
In the present study we investigated the effects of IT surgery to delay the onset of type 2 diabetes in UCD-T2DM rats. This is the first study to investigate the efficacy of IT surgery to delay the development of type 2 diabetes. Given the reproducible effects of bariatric surgery to normalize glucose homeostasis, and given the increasing prevalence of type 2 diabetes, the effects and mechanisms of different types of bariatric surgery to delay or prevent the development of type 2 diabetes should be investigated for the potential identification of new therapies for diabetes. In this study, IT surgery markedly delayed the onset of type 2 diabetes compared with sham surgery. The similar body weights in sham and IT-operated animals provides support for the hypothesis that surgery-induced changes of endocrine function are likely to have an important role in the delay of diabetes onset, independent of differences in body weight. The observation that increases of nutrient-stimulated GLP-17–36 and PYY secretion in IT-operated animals did not result in decreased food intake brings into question the role of endogenous GLP-1 and PYY in the regulation of food intake and suggests that post-operative increases of these hormones may not be responsible for the weight loss observed after bariatric surgeries, such as RYGB. However, the increases of nutrient-stimulated GLP-17–36 secretion may have contributed to the observed improvements of islet function, insulin sensitivity and lipid metabolism in IT compared with sham-operated rats.
Increases of GLP-1 may have improved islet function by increasing islet glucose sensitivity and insulin secretory capacity. GLP-1 has been shown to not only potentiate glucose stimulated insulin secretion, but also to increase insulin synthesis, stimulate β-cell proliferation and prevent β-cell apoptosis 9, 25. Improvement of glucose-stimulated insulin secretion was clearly demonstrated at one month after surgery during the OGTT in which IT-operated animals exhibited 3-fold greater glucose-stimulated insulin secretion compared with sham-operated animals. Furthermore, IT-operated animals had 2.5-fold greater pancreatic insulin content compared with sham-operated animals.
IT-operated animals also exhibited improved insulin sensitivity compared with sham-operated animals based on decreased fasting plasma insulin concentrations and the lower glucose and insulin excursions during the OGTT. Increases of GLP-1 may have improved insulin sensitivity by reducing glucotoxicity and lipotoxicity 26. GLP-1 reduces glucotoxicity by improving islet function, insulin sensitivity and reducing hepatic gluconeogenesis 9, 27. GLP-1 may contribute to reductions in lipotoxicity by stimulating fat oxidation during meals 28. Thus, increases of nutrient-stimulated GLP-17–36 release may have contributed to a reduction in adipocyte size in IT compared with sham-operated animals by increasing lipolysis in fat cells during meals 29. This decrease of adipocyte size may have contributed to lower ectopic and circulating TG concentrations in IT-operated animals, thereby contributing to improved insulin sensitivity. Larger adipocytes, especially in the mesenteric adipose depot, have been shown to be more insulin resistant 30 and therefore less sensitive to the anti-lipolytic actions of insulin, resulting in greater FFA secretion into the portal vein and TG accumulation in the liver 31. TG deposition in the liver is considered a major contributor to hepatic insulin resistance 32, 33 and also promotes increased circulating TG resulting in greater TG deposition in peripheral tissues 34, further exacerbating systemic insulin resistance 32, and possibly islet lipotoxicity 35.
Changes of bile acids, which are increased after RYGB in humans 36 and IT surgery in rodents 15, 37, may represent another mechanism by which bariatric surgery leads to improved insulin sensitivity and resolution of diabetes. Interposition of the ileum proximally may increase absorptive capacity of the intestinal epithelium resulting in greater bile acid reabsorption. Bile acids have been shown to increase energy expenditure, improve circulating lipid profiles and glucose homeostasis and stimulate GLP-1 and PYY secretion 38–41. Furthermore, simply diverting the common bile duct to the distal small intestine in streptozotocin-treated rats has been reported to completely resolve diabetes 42. Thus, post-operative increases of bile acids may have contributed to the increases of circulating GLP-17–36 and PYY concentrations and decreases of circulating TG and cholesterol concentrations.
Unlike some previous studies of IT surgery in rats 13, 16, ileal preproglucagon mRNA levels were not elevated in IT-operated animals, suggesting that increases of circulating GLP-17–36 concentrations following IT surgery in UCD-T2DM rats are primarily due to increased secretion. Similar to previously reported data 16, the increase of nutrient-stimulated PYY secretion was accompanied by an increase of ileal PYY mRNA expression. Thus, increases of plasma PYY concentrations were likely due to increased synthesis and secretion.
As expected, GIP secretion did not differ between IT and sham-operated animals since the position of the duodenum remained unchanged after both surgeries. This is in contrast to results obtained after RYGB, in which GIP secretion is often decreased, likely due to the surgical exclusion of the duodenum from contact with ingested nutrients 7, 12. Decreases of GIP have been proposed to contribute to the glucose lowering effects of RYGB 43. However, in this study, improvements of glucose homeostasis and a delay in diabetes onset were observed without reductions of GIP responses.
In conclusion we have demonstrated for the first time that IT surgery can delay the onset of diabetes in an animal model of type 2 diabetes. Factors that may be involved in delaying diabetes onset include increases of plasma bile acid concentrations and nutrient-stimulated GLP-17–36 secretion. Increases of circulating bile acid concentrations may have contributed to the increases of GLP-17–36 secretion and improvements of circulating lipids. The increases of GLP-17–36 secretion likely contributed to improvements of insulin sensitivity, islet function, and lipid metabolism. It would be of interest to investigate why the onset of diabetes was only delayed and not prevented all together after IT surgery. Adaptation (e.g., downregulation of GLP-1 production and secretion) in the transposed segment of distal intestine could account for this. Measurements of stimulated GLP-1 secretion and GLP-1 expression in the transposed segment at later times after surgery would be informative in this regard. Further studies of the effects of IT and other bariatric surgical procedures in the UCD-T2DM rat model will provide additional insight into the surgically induced improvements of metabolism and identify new strategies for the prevention and treatment of type 2 diabetes.
Supplementary Material
Acknowledgments
The immunohistochemistry studies were supported by the facilities at the VA Puget Sound Health Care System, Seattle, Washington. Dr. Baskin is Senior Research Career Scientist, Research and Development Service, Department of Veterans Affairs Puget Sound Health Care System, Seattle, WA. We thank Sunhye Kim and Riva Dill for their extensive help with animal care, monitoring and data collection, Joyce Murphy for her wonderful technical support with pancreatic immunohistochemistry, and Tammi Olineka and the Lucy Whittier Molecular and Diagnostic Core Facility for rtPCR work. We thank Susan Bennett, Cheryl Phillips and the Meyer Hall Animal Facility staff for providing excellent animal care.
Grant Support: This research was supported in part by the University of California, Davis Veterinary Scientist Training Program and NIH grant AT-002993 and DK-087307. Dr. Havel’s laboratory also receives or received funding during the project period from the National Institutes of Health Grants R01 HL-075675, R01 HL-09333, AT-003545, and the American Diabetes Association. Immunohistochemistry and islet analysis work was supported by NIH NIDDK grant P30 DK-17047 through the Cellular and Molecular Imaging Core of the University of Washington Diabetes Endocrinology Research Center. Nodose ganglion work was supported by NIH grant DK-58558 (H.E.R.).
Abbreviations
- (AUC)
Area under the curve
- (GIP)
glucose-dependent insulinotropic polypeptide
- (GLP-1)
Glucagon-like peptide-1
- (GLP-1R)
Glucagon-like peptide-1 receptor
- (IT)
Ileal interposition
- (NPY2R)
neuropeptide Y receptor Y2
- (OFTT)
Oral fat tolerance test
- (OGTT)
Oral glucose tolerance test
- (RM ANOVA)
Repeated Measures ANOVA
- (RYGP)
Roux-en-Y Gastric Bypass
- (UCD-T2DM)
University of California at Davis Type 2 Diabetes Mellitus Rat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosoures: The authors have no conflicts of interest.
Author Contributions: B.P.C. designed study, preformed the surgical procedures, acquired and interpreted data, wrote the first draft and revised all subsequent drafts; A.D.S. contributed to study concept and design, provided surgical training and provided critical revision of the manuscript for intellectual content; K.L.S. assisted with statistical analysis and provided critical revision of the manuscript for intellectual content; J.L.G. contributed to study design and acquired and interpreted data; J.L. acquired and interpreted data; H.E.R.’s laboratory performed gene expression analyses, and she interpreted data and provided critical revision of the manuscript for intellectual content; D.G.B.’ laboratory performed immunohistochemistry and he interpreted data and provided critical revision of the manuscript for intellectual content; P.J.H. obtained funding and contributed to study design, data interpretation, was overall director of the project and provided critical revision of the manuscript for intellectual content.
References
- 1.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793–2796. doi: 10.1001/jama.288.22.2793. [DOI] [PubMed] [Google Scholar]
- 2.Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004;350:1075–1079. doi: 10.1056/NEJMp048029. [DOI] [PubMed] [Google Scholar]
- 3.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 4.Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–2525. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 5.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15:474–481. doi: 10.1381/0960892053723402. [DOI] [PubMed] [Google Scholar]
- 6.Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E, Rao RH, Kuller L, Kelley D. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004;70:1–4. discussion 4–5. [PubMed] [Google Scholar]
- 8.Strader AD. Ileal transposition provides insight into the effectiveness of gastric bypass surgery. Physiol Behav. 2006;88:277–282. doi: 10.1016/j.physbeh.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 10.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 11.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–2130. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 12.Rodieux F, Giusti V, D'Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16:298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 13.Patriti A, Aisa MC, Annetti C, Sidoni A, Galli F, Ferri I, Gulla N, Donini A. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced Proglucagon gene expression and L-cell number. Surgery. 2007;142:74–85. doi: 10.1016/j.surg.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Patriti A, Facchiano E, Annetti C, Aisa MC, Galli F, Fanelli C, Donini A. Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg. 2005;15:1258–1264. doi: 10.1381/096089205774512573. [DOI] [PubMed] [Google Scholar]
- 15.Strader AD, Clausen TR, Goodin SZ, Wendt D. Ileal Interposition Improves Glucose Tolerance in Low Dose Streptozotocin-treated Diabetic and Euglycemic Rats. Obes Surg. 2009;19:96–104. doi: 10.1007/s11695-008-9754-x. [DOI] [PubMed] [Google Scholar]
- 16.Strader AD, Vahl TP, Jandacek RJ, Woods SC, D'Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288:E447–E453. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 17.Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, Reed BJ, Sweet IR, Griffen SC, Havel PJ. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1782–R1793. doi: 10.1152/ajpregu.90635.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koopmans HS, Sclafani A. Control of body weight by lower gut signals. Int J Obes. 1981;5:491–495. [PubMed] [Google Scholar]
- 19.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 20.Rodbell M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- 21.Mueller WM, Gregoire FM, Stanhope KL, Mobbs CV, Mizuno TM, Warden CH, Stern JS, Havel PJ. Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology. 1998;139:551–558. doi: 10.1210/endo.139.2.5716. [DOI] [PubMed] [Google Scholar]
- 22.Davidson JK, Haist RE. A Study of the Levels of Extractable Insulin of Guinea Pig Pancreas. Can J Physiol Pharmacol. 1964;42:315–317. doi: 10.1139/y64-037. [DOI] [PubMed] [Google Scholar]
- 23.Dixit PK, Lowe IP, Heggestad CB, Lazarow A. Insulin Content of Microdissected Fetal Islets Obtained from Diabetic and Normal Rats. Diabetes. 1964;13:71–77. doi: 10.2337/diab.13.1.71. [DOI] [PubMed] [Google Scholar]
- 24.Karam JH, Grodsky GM. Insulin content of pancreas after sodium fluoroacetate-induce hyperglycemia. Proc Soc Exp Biol Med. 1962;109:451–454. doi: 10.3181/00379727-109-27233. [DOI] [PubMed] [Google Scholar]
- 25.Salehi M, Aulinger BA, D'Alessio DA. Targeting beta-cell mass in type 2 diabetes: promise and limitations of new drugs based on incretins. Endocr Rev. 2008;29:367–379. doi: 10.1210/er.2007-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–2659. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- 27.Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Andersen DK, Elahi D. Clinical review: The extrapancreatic effects of glucagon-like peptide-1 and related peptides. J Clin Endocrinol Metab. 2009;94:1843–1852. doi: 10.1210/jc.2008-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boschmann M, Engeli S, Dobberstein K, Budziarek P, Strauss A, Boehnke J, Sweep FC, Luft FC, He Y, Foley JE, Jordan J. Dipeptidyl-peptidase-IV inhibition augments postprandial lipid mobilization and oxidation in type 2 diabetic patients. J Clin Endocrinol Metab. 2009;94:846–852. doi: 10.1210/jc.2008-1400. [DOI] [PubMed] [Google Scholar]
- 29.Sancho V, Trigo MV, Martin-Duce A, Gonz Lez N, Acitores A, Arnes L, Valverde I, Malaisse WJ, Villanueva-Penacarrillo ML. Effect of GLP-1 on D-glucose transport, lipolysis and lipogenesis in adipocytes of obese subjects. Int J Mol Med. 2006;17:1133–1137. [PubMed] [Google Scholar]
- 30.Foley JE, Laursen AL, Sonne O, Gliemann J. Insulin binding and hexose transport in rat adipocytes. Relation to cell size. Diabetologia. 1980;19:234–241. doi: 10.1007/BF00275275. [DOI] [PubMed] [Google Scholar]
- 31.Olefsky JM. Insensitivity of large rat adipocytes to the antilipolytic effects of insulin. J Lipid Res. 1977;18:459–464. [PubMed] [Google Scholar]
- 32.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55 Suppl 2:S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 34.Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–1617. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- 35.Unger RH. Longevity, lipotoxicity and leptin: the adipocyte defense against feasting and famine. Biochimie. 2005;87:57–64. doi: 10.1016/j.biochi.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, Auwerx J, Goldfine AB. Serum Bile Acids Are Higher in Humans With Prior Gastric Bypass: Potential Contribution to Improved Glucose and Lipid Metabolism. Obesity (Silver Spring) 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuchiya T, Kalogeris TJ, Tso P. Ileal transposition into the upper jejunum affects lipid and bile salt absorption in rats. Am J Physiol. 1996;271:G681–G691. doi: 10.1152/ajpgi.1996.271.4.G681. [DOI] [PubMed] [Google Scholar]
- 38.Adrian TE, Ballantyne GH, Longo WE, Bilchik AJ, Graham S, Basson MD, Tierney RP, Modlin IM. Deoxycholate is an important releaser of peptide YY and enteroglucagon from the human colon. Gut. 1993;34:1219–1224. doi: 10.1136/gut.34.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corradini SG, Eramo A, Lubrano C, Spera G, Cornoldi A, Grossi A, Liguori F, Siciliano M, Pisanelli MC, Salen G, Batta AK, Attili AF, Badiali M. Comparison of changes in lipid profile after bilio-intestinal bypass and gastric banding in patients with morbid obesity. Obes Surg. 2005;15:367–377. doi: 10.1381/0960892053576839. [DOI] [PubMed] [Google Scholar]
- 40.Modica S, Moschetta A. Nuclear bile acid receptor FXR as pharmacological target: are we there yet? FEBS Lett. 2006;580:5492–5499. doi: 10.1016/j.febslet.2006.07.082. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 42.Ermini M, Iaconis E, Mori A. The effects of bilio-jejunal diversion on streptozotocin diabetes in the rat. Acta Diabetol Lat. 1991;28:79–89. doi: 10.1007/BF02732117. [DOI] [PubMed] [Google Scholar]
- 43.Flatt PR. Effective surgical treatment of obesity may be mediated by ablation of the lipogenic gut hormone gastric inhibitory polypeptide (GIP): evidence and clinical opportunity for development of new obesity-diabetes drugs? Diab Vasc Dis Res. 2007;4:151–153. doi: 10.3132/dvdr.2007.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.