Abstract
For ~3 decades, urethane has been (partially or solely) used as a successful anesthetic in numerous respiratory long-term facilitation (LTF) studies, which were performed on anesthetized, paralyzed, vagotomized and artificially ventilated animals of several different species. However, things become complicated when LTF of muscle activity is studied in un-paralyzed animals. For example, a commonly used acute intermittent hypoxia (AIH) protocol failed to induce muscle LTF in anesthetized, spontaneously breathing rats. But muscle LTF could be induced when hypoxic episode number was increased and/or anesthetics other than urethane were used. In these studies however, neither anesthetic nor paralysis was mentioned as a potential factor influencing AIH-induced muscle LTF. This study tested whether urethane inhibits AIH-induced genioglossal LTF (gLTF) in un-paralyzed ventilated rats, and if so, determined whether reducing urethane dose reverses this inhibition. Three groups of adult male Sprague-Dawley rats were anesthetized (Group 1: ~1.6 g·kg−1 urethane; Group 2: 50 mg·kg−1 α-chloralose + 0.9–1.2 g·kg−1 urethane; Group 3: 0.9 g·kg−1 urethane + 200–400 µg·kg−1·min−1 alphaxalone), vagotomized and mechanically ventilated. Integrated genioglossus activity was measured before, during and after AIH (5 episodes of 3-min isocapnic 12% O2, separated by 3-min hyperoxic intervals). The AIH-induced gLTF was absent in Group 1 rats (success rate was only ~1/7), but was present in Group 2 (in 10/12 rats) and Group 3 (in 11/11 rats) rats. The genioglossal response to hypoxia was not significantly different among the 3 groups. Collectively, these data suggest that urethane dose-dependently inhibits gLTF in un-paralyzed anesthetized rats.
Keywords: Urethane, Genioglossus muscle, Long-term facilitation, Respiratory control, Paralysis, Intermittent hypoxia
Acute intermittent hypoxia (AIH) or repeated carotid sinus nerve (CSN) stimulation induces a persistent increase in respiratory activity, known as long-term facilitation (LTF). LTF has been identified in many animal species [9, 20]. As AIH-induced LTF was also observed in snorers and obstructive sleep apnea (OSA) patients during sleep [1, 2], some have speculated that LTF may be a protective mechanism, promoting upper airway stability in OSA patients after repeated apneas/hypopneas [1, 3, 4, 10, 12].
Since LTF was first reported in 1980 [19], considerable progress has been made in understanding where and how LTF is generated and maintained. An overwhelming majority of the LTF studies were conducted in urethane-anesthetized, vagotomized, paralyzed and mechanically ventilated animals. Although AIH occasionally fails to induce LTF (~1 in 10–15), urethane as a reliable and successful anesthetic has never been questioned. However, things are different when LTF of respiratory muscle activity is studied. For example, a commonly used AIH protocol (3 episodes of short-term hypoxia) failed to induce LTF in un-paralyzed spontaneously breathing rats, which was attributed mainly to a higher level of baseline PaCO2 [8] or a smaller number of hypoxia episodes [21]. On the other hand, muscle LTF could be induced by AIH (or elicited by repeated carotid sinus nerve stimulation) in spontaneously breathing animals when hypoxic episode number was increased [21] or other anesthetics were used [13, 18, 21, 22]. In all these muscle LTF studies, however, neither anesthetic nor paralysis was mentioned as a potential factor determining whether LTF of muscle activity can be induced.
The objective of the present study was to assess the effect of urethane on the genioglossal electromyogram activity (EMGgg) LTF (gLTF) in anesthetized, un-paralyzed, ventilated (to achieve normocapnia) rats. Genioglossus is the principal tongue protruder muscle and important for the control of upper airway patency [3, 13, 18, 21]. Because so far no successful LTF study has ever been reported in solely urethane-anesthetized un-paralyzed animals and all successful LTF studies in un-paralyzed animals used other anesthetics totally or partially, we hypothesized that urethane would inhibit AIH-induced gLTF in un-paralyzed rats and this inhibition could be reversed by reducing the urethane dose.
The Harvard Medical Area Standing Committee on Animals approved all experimental procedures. Experiments were mainly conducted on 3 groups of adult male Sprague-Dawley rats (250–350 g, colony CDIGS, Charles River, Wilmington, MA), which were anesthetized with urethane alone (Group 1: n=33), urethane + α-chloralose (Group 2: n=12) and urethane + alphaxalone (Group 3: n=11), respectively (all 3 anesthetics from Sigma-Aldrich, Natick, MA, USA).
Group 1 rats were anesthetized initially with isoflurane in a closed chamber and then injection of urethane (~1.6 g·kg−1, i.p.). The depth of anesthesia was adjusted so that the animal failed to show a reflex withdrawal of the hind paw to a strong pinch. The trachea was cannulated, and the rat was mechanically ventilated (Harvard Apparatus Inc, Holliston, MA, USA). The inspired gas mixture was 50% O2 (N2 balance) during baseline and other non-hypoxia periods. Bilateral vagotomy was performed at the mid-cervical level. End-tidal CO2 partial pressure (PETCO2) was monitored in the expired line of the ventilator circuit using a flow-through capnograph (Novametrix; Wallingford, CT, USA). PETCO2 values obtained with this method are close (usually within 1–2 mmHg) to arterial CO2 partial pressure (PaCO2). The CO2-apneic threshold was defined as the PETCO2 at which respiratory rhythmic activity resumed from hypocapnic apnea. PETCO2 was maintained at 3 mmHg above the threshold by manipulating the inspire CO2, and ventilator’s rate (its ratio to respiratory frequency was set at about 3:2) and/or tidal volume. Rectal temperature was maintained at 37.0–37.5 °C with a servo-controlled heat blanket and a heating lamp. Before AIH, a supplemental dose of urethane (0.16 g·kg−1) was given to provide stable anesthesia for the remainder of the experiment. At the end of the experiments, rats were sacrificed by a lethal dose of urethane (3.2 g·kg−1).
Group 2 rats were also anesthetized initially with isoflurane and then injection of α-chloralose (50 mg·kg−1, i.p.) plus urethane (0.9–1.2 g·kg−1, i.p.). Other preparation procedures were the same as those in Group 1 rats.
Group 3 rats were first anesthetized with isoflurane and then injected with urethane (0.9 g·kg−1, i.p.). This dose usually was not sufficient to have the rat fully anesthetized. Thus, isoflurane (1–2% in 30% O2; balance N2) was added via a facemask. After the trachea was cannulated, isoflurane was delivered via the tracheal cannula. Inspired gas mixture was 30% O2 (N2 balance) during baseline and other non-hypoxia periods. A femoral venous catheter was inserted for fluid administration. A femoral arterial catheter was placed to monitor arterial blood pressure (Statham Pressure Transducer, P23-id) and to withdraw arterial blood samples for blood gases and pH analysis (Opti CCA-TS, Osmetech, Roswell, Georgia) with correction for rectal temperature. PETCO2 was maintained at 4 mmHg above the CO2-apneic threshold by manipulating the ventilator’s rate and tidal volume. Before AIH, the anesthetic was slowly converted to alphaxalone (200–400 µg·kg−1·min−1 in DMSO solution, i.v.) and the isoflurane was gradually withdrawn in 10–20 min.
EMGgg activity was recorded by two insulated fine silver wires inserted into genioglossus muscle via the floor of the mouth. The EMGgg activity was filtered (300–10,000 Hz), amplified (2,000–10,000X, BMA-200 AC/DC Bioamplifier, CWE Inc., Ardmore, PA, USA), full-wave rectified and integrated (Paynter Filter, BAK Electronics, Mount Airy, MD; time constant: 100 ms). The integrated signals were digitized and acquired with computer software (LabView 8.0, National Instruments), and analyzed with a customized program developed in our laboratory. This program determined the amplitude and timing of integrated EMGgg activity, from which the minute EMGgg activity could be calculated.
Baseline EMGgg activity was measured at ~60 min after completion of the surgical preparation. Following the baseline measurement, rats were exposed to 5 episodes of 3-min isocapnic hypoxia (12% O2), separated by 3-min intervals of hyperoxia (50% O2 in Groups 1 and 2, 30% O2 in Group 3). The EMGgg activity was then measured during and up to 60 min after the AIH to determine hypoxic genioglossal responses (HGR) and gLTF, respectively. In some Group 3 rats, arterial blood samples (~0.3 ml) were drawn during baseline, hypoxic exposure and at 45 min post-hypoxia to ensure an isocapnic condition.
Integrated EMGgg activity was averaged in ~1 min bins at each target time point. HGR data were collected during the last minute of the 3-min hypoxia exposure when the amplitude of integrated EMGgg activity had reached a plateau, and were averaged over five hypoxia episodes since there was no significant difference among these episodes. Variables determined in each time point included peak amplitude of integrated EMGgg activity (arbitrary units), EMGgg burst frequency (bursts·min−1) and minute EMGgg activity (peak amplitude × burst frequency). Changes from baseline in the peak amplitude and minute activity were normalized as a percentage of the baseline (%baseline). Changes from baseline in the burst frequency used absolute units (bursts·min−1).
For LTF, the within-group increases from baseline and between-group differences in baseline and the post-hypoxia peak amplitude (also in the burst frequency and minute activity) were statistically analyzed by a two-way ANOVA with repeated measures, followed by the Student-Newman-Keuls post-hoc test (SigmaStat version 3.1, Jandel Corporation, San Rafael, CA, USA). For the apneic threshold and HGR, a one-way ANOVA was used to statistically analyze the differences between and within groups. P<0.05 was considered significant. All values are expressed as means ± SE.
Although the one-way ANOVA revealed a significant group effect (F2,53=3.789; P=0.03), the post-hoc tests showed no significant differences (all P>0.05) in the CO2-apneic threshold for EMGgg activity between any 2 of the 3 groups (Group 1: 41.6 ± 0.6 mmHg, n=33; Group 2: 44.7 ± 1.4, n=12; Group 3: 41.5 ± 0.8, n=11). The HGR was also not significantly different among the 3 groups in the peak amplitude (Fig. 1), burst frequency (Group 1: 11.6 ± 2.8 bursts·min−1; Group 2: 19.8 ± 1.8; Group 3: 14.8 ± 5.3) or minute activity (Group 1: 308.2 ± 39.8% above baseline; Group 2: 428.6 ± 89.6%; Group 3: 363.1 ± 70.1%), as the one-way ANOVA revealed an insignificant overall group effect in the peak amplitude (F2,50=0.428; P=0.654), burst frequency (F2,50=1.313; P=0.278) and minute activity data (F2,50=1.056; P=0.356).
Fig. 1.
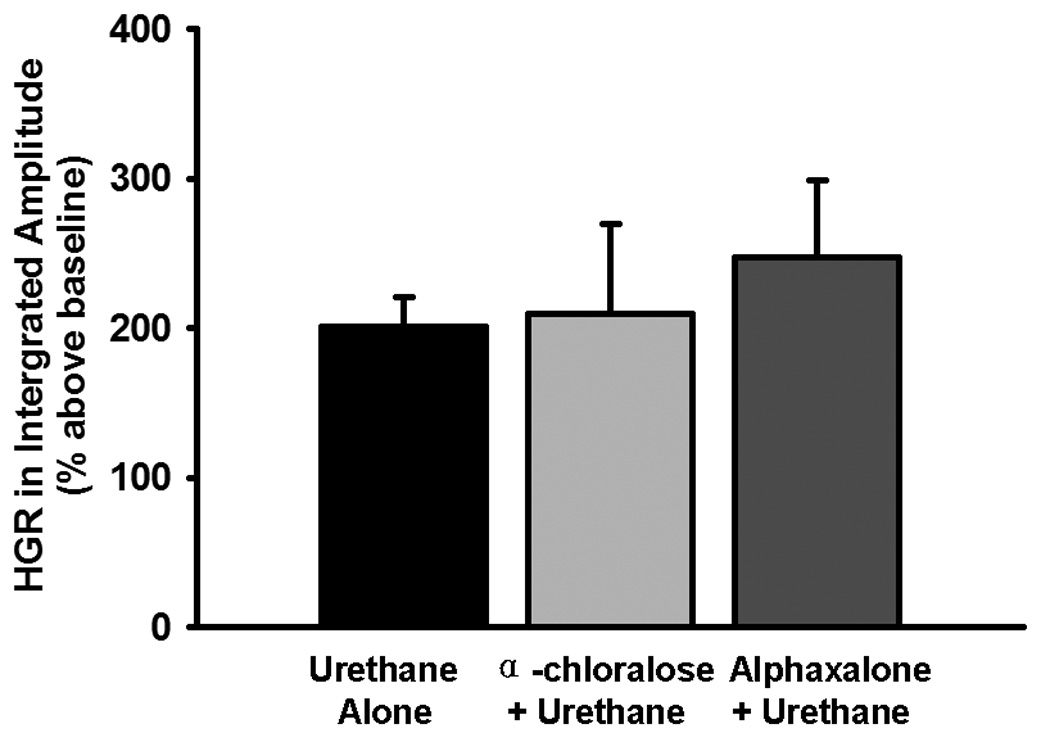
The effect of different urethane doses on the hypoxic genioglossal response (HGR) in peak amplitude of integrated genioglossal electromyogram activity. The HGR data were obtained from Group 1 (urethane alone, n=33), Group 2 (α-chloralose + urethane, n=12) and Group 3 (alphaxalone + urethane, n=11) rats. These data were the average HGR to 5 episodes of 3-min isocapnic hypoxia (12% O2), calculated as a percentage increase above the baseline (% above baseline) and expressed as means ± SE. There were no significant differences between any 2 of the 3 groups (all P>0.393).
The AIH induced a persistent increase in the peak amplitude of integrated EMGgg activity in both Group 2 and Group 3 rats but not in Group 1 rats (Figs. 2 and 3A). The two-way ANOVA revealed a significant interaction effect (F8,212=11.656; P<10−6) between the group factor (three levels: Group 1, Group 2 and Group 3) and time factor (5 levels: baseline, 15, 30, 45 and 60 min post-hypoxia), and a significant overall group effect (F2,212=20.461; P<10−6) in peak amplitude data. The post-hypoxia peak amplitude was increased from baseline at all time points in Group 3 rats and at 30 and 60 time points in Group 2 rats (all P<0.05), but was not significantly different from baseline at any time points in Group 1 rats (all P>0.06; Fig. 3A). The peak amplitude was also smaller at all time points in Group 1 rats vs. Group 2 or Group 3 rats (all P<0.05), except that at 45 min, the peak amplitude was larger in Group 3 rats vs. Group 1 or Group 2 rats (all P<0.05; Fig. 3A).
Fig. 2.
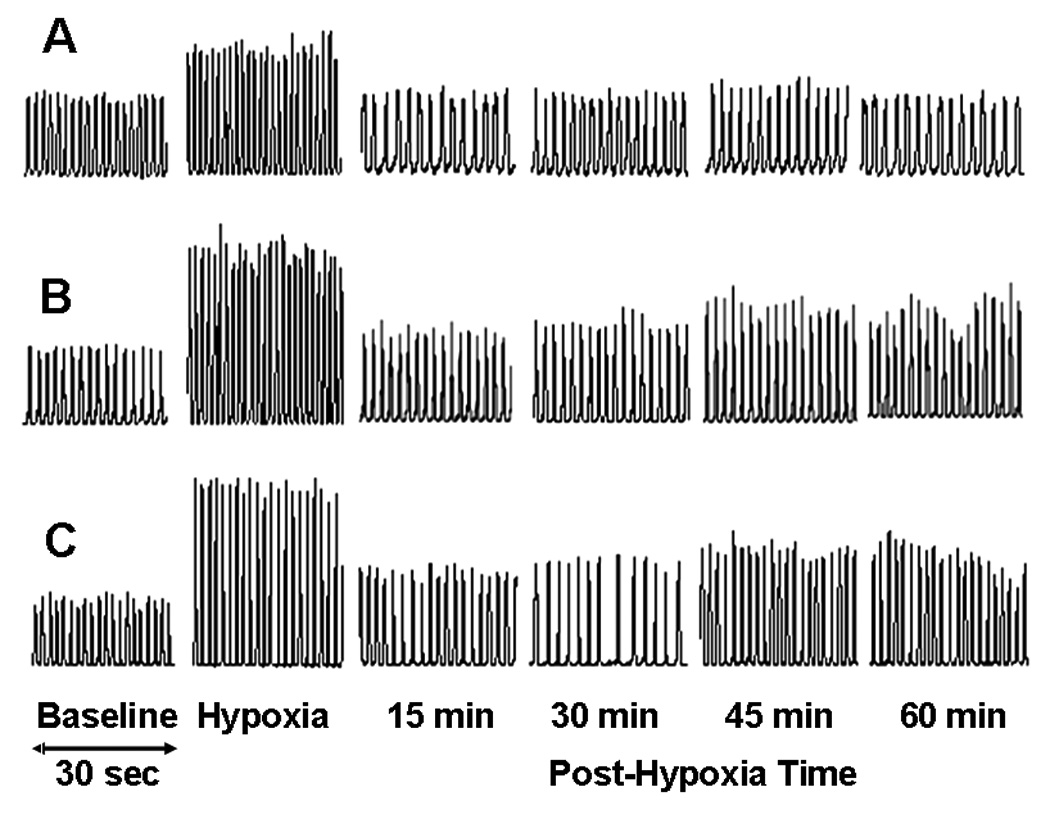
Integrated genioglossal electromyogram (EMGgg) activity recorded before (baseline), during and after AIH (5 episodes of 3-min isocapnic 12% O2 with 3-min intervals of hyperoxia) in one Group 1 (urethane alone, A), one Group 2 (α-chloralose + urethane, B) and one Group 3 (alphaxalone + urethane, C) rats.
Fig. 3.
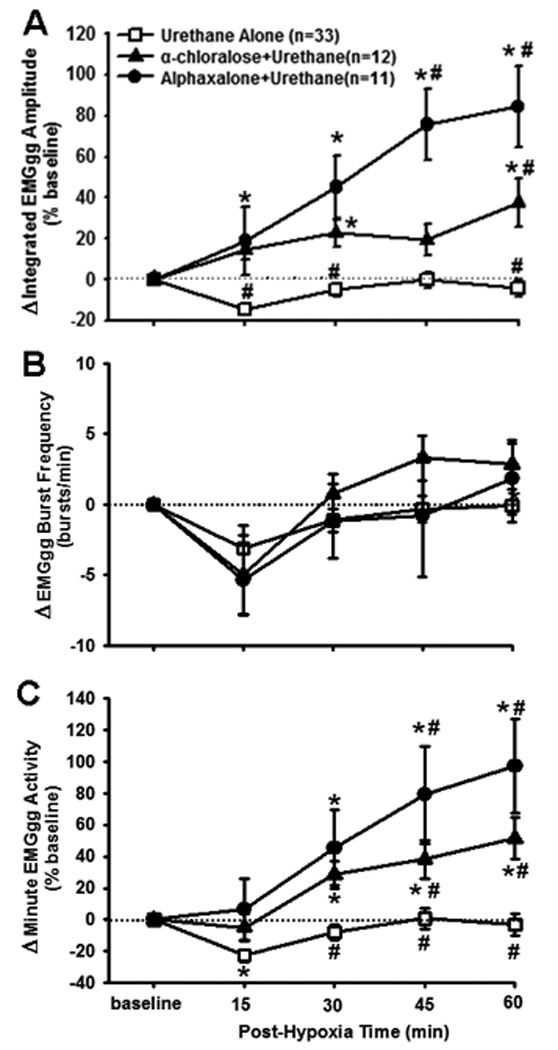
The effect of different urethane doses on genioglossal electromyogram activity (EMGgg) LTF. (A) Average changes from baseline in peak amplitude of integrated EMGgg activity, normalized as a percentage of the baseline (%baseline), (B) changes from baseline in EMGgg burst frequency (bursts·min−1), and (C) changes from baseline in minute EMGgg activity, normalized as a percentage of the baseline (%baseline). These data were obtained before and after AIH (5 episodes of 3-min isocapnic 12% O2 with 3-min intervals of hyperoxia) in Group 1 (urethane alone, n=33), Group 2 (α-chloralose + urethane, n=12) and Group 3 (alphaxalone + urethane, n=11) rats. Data are expressed as means ± SE. * Significant difference from the baseline; # Significant difference from the other 2 groups (P<0. 05).
Integrated EMGgg burst frequency was not significantly changed after AIH in any of the 3 groups (Fig. 3B), as both the interaction (F8,212=1.024; P=0.419) and group effect (F2,212=0.355; P=0.703) in burst frequency data were insignificant. Finally, minute EMGgg activity data after AIH (Fig. 3C) were very similar to the peak amplitude data (Fig. 3A), except that the minute activity was decreased from baseline at 15 min in Group 1 rats and was different from one another at 45 min (all P<0.05).
Although AIH failed to induce gLTF in Group 1 as a whole, it induced gLTF in a few Group 1 rats. If the criterion was set at 10% above baseline for the average gLTF magnitude between 30–60 min (i.e., averaging gLTF for the last 3 time points), AIH induced gLTF in 7 of the 33 Group 1 rats (actually, the same 7 rats would be selected if the criterion was set at 15% for the average gLTF magnitude between 45–60 min) and most of them look like a typical LTF in paralyzed rats. The success rate of inducing gLTF was actually lower than 7/33 if taking into account other succeeded and failed ones, in which intercostal muscle activity rather than EMGgg activity was recorded, or Fischer 344 (CDF) or female rats were used (data not shown). The overall success rate was only ~1/7 if all the urethane-alone rats were counted. According to the same criterion, AIH failed to induce gLTF in 2 of the 12 Group 2 rats, but successfully induced gLTF in each of the 11 Group 3 rats.
In six Group 3 rats, the average PaCO2 at 45 min post-hypoxia (44.0 ± 0.95 mmHg) was not significantly different from baseline (42.8 ± 1.32; P=0.303), indicating a consistent isocapnic condition throughout the experiments. The average PaO2 at 45 min post-hypoxia (96.0 ± 5.4) appeared to be decreased from baseline (100.8 ± 7.3) but the decrease was insignificant (P=0.443), thus indicating a consistent normoxic condition after AIH. The hypoxic PaCO2 and PaO2 were 40.0 ± 1.25 mmHg and 35.9 ± 1.2, respectively.
The present study demonstrated that AIH failed to induce gLTF in one group of solely urethane-anesthetized un-paralyzed rats, but successfully induced gLTF in the other 2 groups of rats, in which urethane had been partially replaced by α-chloralose and alphaxalone, respectively. The HGR was not significantly different among the 3 groups. These data suggest that urethane inhibits gLTF in un-paralyzed rats and this inhibition can be reversed by replacing about half of the urethane dose with some other anesthetics.
In un-paralyzed urethane-anesthetized spontaneously breathing rats, an AIH protocol (3 hypoxia episodes), commonly used in LTF studies using paralyzed animals, failed to induce gLTF, which might be attributed to a higher baseline PaCO2, ~7–9 mmHg above the CO2-apneic threshold [8]. In the present study, however, a more effective AIH protocol (5 hypoxia episodes; cf. Ref. [16]) still failed to induce gLTF when baseline PETCO2 was 3 mmHg above the threshold (Fig. 1), suggesting that the higher PaCO2 was not a major factor. In un-paralyzed Saffan-anesthetized spontaneously breathing rats, 8 but not 3 hypoxia episodes induced LTF [21]. However, we found that the same 8 hypoxia episodes failed to induce gLTF in 2 separate un-paralyzed urethane-anesthetized rats (the average LTF magnitude: −30.9%, −28.8%), suggesting that the small episode number is not the only factor. In addition, we noticed that respiratory entrainment to mechanical ventilation somewhat remained even after vagotomy when the ratio of respiratory frequency to ventilator rate was very close to 1:1 or 1:2. Thus the frequency mismatching might be a negative factor. However, we found that AIH still failed to induce gLTF in 2 separate rats (the average LTF magnitude: 0.1%, 6.5%), which were ventilated by a servo-controlled pump (SAR-830/AP, CWE Inc., Ardmore, PA, USA). On the other hand, LTF of upper airway and/or pump muscle activities could be induced in un-paralyzed animals when urethane was replaced by other anesthetics totally [18, 21] or partially [13, 22]. We also found that gLTF could be induced in one group of un-paralyzed solely pentobarbital-anesthetized rats (n=12). The gLTF at the 4 time points were 56.2 ± 52.6%, 79.8 ± 54.9%, 104.6 ± 68.3% and 164.1 ± 66.2%, respectively (1-way ANOVA: F4,44=3.312; P=0.019). These data were not included in our major results (Fig. 3) because EMGgg activity was substantially suppressed compared to the other groups. We thus argue that it was urethane that inhibited gLTF in a dose-dependent manner in un-paralyzed rats. Moreover, this inhibition was not limited to gLTF only, since intercostal muscle activity LTF was similarly inhibited.
All our experiments were conducted in anesthetized, un-paralyzed, vagotomized and ventilated rats, in which arterial blood gases and body temperature could be controlled. The ventilator rate was set in proportion to the rat’s respiratory frequency (approximate 3:2 ratio) by carefully adjusting the ventilator’s rate and/or tidal volume to eliminate periodic unstable breathings caused by the residual respiratory entrainment to ventilator. Rats were vagotomized to achieve more stable EMGgg signals, which were especially important for LTF study. But vagotomy was not necessary, as AIH induced even larger gLTF in 2 separate vagi-intact urethane+alphaxalone rats (the average gLTF magnitude: 61.8%, 150%), and vagotomy should facilitate gLTF manifestation [13] in Group 1 rats. Arterial blood samples were not collected from every rat. However, the blood gas data collected from the last 6 rats of Group 3 were reasonable and very much in keeping with previous results of this lab [17, 24]. In addition, maintenance of isocapnia by just monitoring and adjusting PETCO2 has been shown to be practical in our experimental setting. Adjustment of ventilator rate based simply on discrepancy between PETCO2 and PaCO2 almost never happened. In Group 3 rats, the baseline PETCO2 was set a little higher and the inspired O2 was set a little lower to make the respiratory rhythmic signal more evident and stable. These settings, however, were unlikely to undermine our overall goal because theoretically they should reduce the chance to induce gLTF and decrease gLTF magnitude, as gLTF is numerically calculated as an increase above baseline, normalized to the baseline.
Urethane (ethyl carbamate) is a widely used anesthetic for animal studies. The advantages of using urethane as an anesthetic include that: 1) it exerts minimal effects on respiratory, cardiovascular and autonomic nervous systems, and numerous spinal reflexes as well, so that anesthetized animals show similar physiologic and pharmacologic behaviours to those observed in un-anesthetized animals; 2) it can be easily administrated via several routes, producing a long lasting, steady level of surgical anesthesia without additional doses; and 3) it is relatively inexpensive [7, 11]. The anesthetic mechanism of urethane has not been well understood. Urethane at an anesthetic concentration enhanced the function of GABAA and glycine receptors, and inhibited the function of NMDA and AMPA receptors expressed in Xenopus oocytes [7], suggesting that urethane achieves anesthesia by modestly changing both inhibitory and excitatory systems (most injectable and volatile anesthetics achieve anesthesia by markedly changing either the inhibitory or excitatory system). It should be emphasized that although our results show that urethane inhibits gLTF in un-paralyzed rats, it is still unclear how exactly urethane inhibits gLTF and what role paralysis plays, i.e., why urethane inhibits gLTF in un-paralyzed rats but not hypoglossal LTF in paralyzed rats.
LTF was pursued early on mainly as a model for long-term respiratory plasticity without a clear physiological significance [9, 20]. However, in the last decade, investigators have begun to appreciate its potential clinical implications in diseases, such as OSA. This is because the AIH-induced LTF in pharyngeal dilator muscles is present in OSA patients during sleep [1], an important time when all OSA events occur. AIH also preferentially induces LTF in pharyngeal dilating muscles vs. pump muscles in humans during sleep, as AIH induced LTF in EMGgg activity [6], upper airway conductance [1] and ventilation [2] without concomitant pump muscle LTF. It should be noted however, that whether LTF plays an important role in OSA is still uncertain. Whether LTF is beneficial or detrimental to OSA is also controversial. There is some evidence suggesting that repeated hypocapnia accompanied with OSA events may inhibit LTF expression in OSA patients; other evidence also suggests that LTF may even exacerbate OSA [cf. 14, 23]. Thus, more evidence from clinical and basic research is needed to clarify this issue.
Recently, we found that AIH induced a progressive and persistent decrease in pharyngeal airway collapsibility, which correlated inversely with gLTF [15], suggesting that repeated apneas/hypopneas may decrease pharyngeal collapsibility via LTF mechanism in OSA patients. These experiments have to be carried out in un-paralyzed anesthetized animals with sound upper airway muscle activities, since the AIH-induced collapsibility decrease was eliminated by paralysis [5]. The “urethane + alphaxalone” approach can provide reasonable anesthesia, which not only minimizes the inhibitory effect of urethane on LTF, but also reduces the inhibitory effect of alphaxalone on pharyngeal muscle activities.
Acknowledgements
This research was supported by the National Institute of Health (NIH) grant HL64912. 102-67=35
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol. 2001;91:2751–2757. doi: 10.1152/jappl.2001.91.6.2751. [DOI] [PubMed] [Google Scholar]
- 2.Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep. 1998;21:709–716. [PubMed] [Google Scholar]
- 3.Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- 4.Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci U S A. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y, Liu C, Ling L. Acute intermittent hypoxia-induced decrease in pharyngeal collapsibility requires 5-HT2 and NMDA receptors in rats. (Abstract) Program Number: 799.11. The Experimental Biology Annual Meeting. 2010 [Google Scholar]
- 6.Chowdhuri S, Pierchala L, Aboubakr SE, Shkoukani M, Badr MS. Long-term facilitation of genioglossus activity is present in normal humans during NREM sleep. Respir Physiol Neurobiol. 2008;160:65–75. doi: 10.1016/j.resp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94:313–318. doi: 10.1097/00000539-200202000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Janssen PL, Fregosi RF. No evidence for long-term facilitation after episodic hypoxia in spontaneously breathing, anesthetized rats. J Appl Physiol. 2000;89:1345–1351. doi: 10.1152/jappl.2000.89.4.1345. [DOI] [PubMed] [Google Scholar]
- 9.Ling L. Serotonin and NMDA receptors in respiratory long-term facilitation. Respir Physiol Neurobiol. 2008;164:233–241. doi: 10.1016/j.resp.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1. General considerations. Experientia. 1986;42:109–114. doi: 10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- 12.Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- 13.Mateika JH, Fregosi RF. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J Appl Physiol. 1997;82:419–425. doi: 10.1152/jappl.1997.82.2.419. [DOI] [PubMed] [Google Scholar]
- 14.Mateika JH, Narwani G. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Exp Physiol. 2009;94:279–296. doi: 10.1113/expphysiol.2008.045153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuire M, Ling L. Pharyngeal airway collapsibility progressively decreases for about 1 hour following acute intermittent hypoxia in rats. Sleep Med. 2006;7:S5–S6. [Google Scholar]
- 16.McGuire M, Zhang Y, White DP, Ling L. Effect of hypoxic episode number and severity on ventilatory long-term facilitation in awake rats. J Appl Physiol. 2002;93:2155–2161. doi: 10.1152/japplphysiol.00405.2002. [DOI] [PubMed] [Google Scholar]
- 17.McGuire M, Zhang Y, White DP, Ling L. Phrenic long-term facilitation requires NMDA receptors in the phrenic motonucleus in rats. J Physiol. 2005;567:599–611. doi: 10.1113/jphysiol.2005.087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKay LC, Janczewski WA, Feldman JL. Episodic hypoxia evokes long-term facilitation of genioglossus muscle activity in neonatal rats. J Physiol. 2004;557:13–18. doi: 10.1113/jphysiol.2004.064006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- 21.Ryan S, Nolan P. Episodic hypoxia induces long-term facilitation of upper airway muscle activity in spontaneously breathing anaesthetized rats. J Physiol. 2009;587:3329–3342. doi: 10.1113/jphysiol.2009.169680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokolowska B, Pokorski M. Ventilatory augmentation by acute intermittent hypoxia in the rabbit. J Physiol Pharmacol. 2006;57 Suppl 4:341–347. [PubMed] [Google Scholar]
- 23.White DP. Long-term facilitation (LTF) and obstructive sleep apnea. Respir Physiol Neurobiol. 2007;158:112–113. doi: 10.1016/j.resp.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, McGuire M, White DP, Ling L. Episodic phrenic-inhibitory vagus nerve stimulation paradoxically induces phrenic long-term facilitation in rats. J Physiol. 2003;551:981–991. doi: 10.1113/jphysiol.2003.048157. [DOI] [PMC free article] [PubMed] [Google Scholar]


