Abstract
The development of central neuropathic pain varies among patients with spinal cord injury (SCI). The factors contributing to the development and perpetuation of segmental pain (at-level allodynia) has been the focus of ongoing experiments in our laboratory. One such factor is hormonal status. We have shown previously, using a male rat model of SCI, that a severe contusion injury is necessary for the development of allodynia in trunk regions at and just above the level of a T8 injury. In this study, we examined at-level sensitivity for SCI ovariectomized (ovx) and cycling female rats as well as for SCI males implanted with either a placebo pellet or one that slowly releases 17β-estradiol. The proportion of ovx SCI female rats and placebo-treated SCI males displaying pain-like behaviors to touch/pressure of at-level dermatomes up to six weeks post-injury (67% and 75%, respectively) was similar to our previous studies on SCI males (69%). In contrast, significantly fewer cycling SCI female rats and 17β-estradiol treated SCI male rats showed sensitivity to touch at-level (26% and 30%, respectively). These results implicate 17β-estradiol as a potential target that can readily be modulated to prevent segmental pain following SCI.
Keywords: Neuropathic Pain, Contusion, Estradiol, Neuroprotection
Chronic central pain develops in the majority of spinal cord injury (SCI) patients [9, 43] and is ranked highest by paraplegics as the function whose recovery would dramatically improve quality of life [2], yet relatively few studies are being done and current drug therapies and surgical interventions are inadequate [12, 47]. According to the National SCI Statistical Center at the University of Alabama (www.nscisc.uab.edu –April 2009), 259,000 people in the U.S. have SCI’s. The cost to the nation is estimated at $9.7 billion per year [3]. Classification of SCI pain by the International Association for the Study of Pain includes nociceptive pains such as musculoskeletal and visceral pain, and neuropathic pains above-level, at-level and below-level of lesion [44]. Neuropathic pains could be spontaneous or mechanically evoked by normally innocuous mechanical stimulation (allodynia) [10, 22]. An understanding of the variables involved in the development and perpetuation of SCI-related pain will lead to more effective strategies for pain management.
In one longitudinal study, 81% of patients reported the presence of pain, half of whom experienced at-level neuropathic pain [43]. A high percentage of male rats with mid-thoracic spinal contusion lesions develop allodynia at-level [22, 23, 27]. At-level pain-like behavioral responses post-SCI may be associated with hyperactivity of remaining intact spinothalamic dorsal horn neurons [7]. After chronic SCI, these dorsal horn neurons exhibit higher background activities, altered discharge patterns, increases in responsiveness to brush and pinch, and longer after-discharges [10, 18]. A significant (62%) increase in metabotropic glutamate receptor subtype 1 immunoreactivity has been demonstrated for spinothalamic tract neurons located immediately rostral to the epicenter of a T9 contusion [36], implicating the thalamus as an important supraspinal site contributing to chronic SCI pain. Thalamic changes occur in SCI patients experiencing segmental pain [32, 38] and we have shown in male rats a significant correlation between hyperexcitability of somatovisceral convergent thalamic neurons to at-level stimulation and behavioral signs of allodynia at-level following spinal contusion at T8 [23].
Although our studies on SCI pain use male rats, most animal models of SCI use females as subjects [20], despite a 80.9% prevalence of SCI among men (www.nscisc.uab.edu –April 2009). The effect of hormonal status on the development of allodynia after SCI is not known. In the first part of this study, sex differences in the incidence of SCI-related pain in at-level dermatomes were evaluated for SCI cycling and ovariectomized (ovx) female rats and the results compared with our previous studies on male rats. In the second part, to examine the potential contribution of ovarian steroids, the incidence of pain-like behaviors evoked by touch/pressure at-level was examined in SCI male rats treated with 17-β estradiol versus a placebo SCI control group.
A total of 56 female and 30 male Wistar rats were used and all procedures approved by the Institutional Animal Care and Use Committee at the University Of Louisville in accordance with USDA regulations. Four female and two male rats were excluded from the study as they did not survive for the entire 6-week post-SCI testing period (these rats are not included in values provided). For the first part of the study, ovx via small lumbar incisions [19, 21, 37] were done on 22 of the rats two weeks prior to SCI surgeries (SCI on 15 of these 22 rats). The remaining 34 rats made up the cycling groups (11 intact controls and 23 SCI rats). The estrus cycle was monitored [21] and the stage noted at the time of testing for all normal cycling animals. Vaginal smear samples were taken from all ovx rats to verify those rats were non-cycling.
For the second part of the study on 30 male rats, hormones were delivered continuously throughout the recovery period with 60 day time release capsules obtained from Innovative Research of America (IRA; Sarasota, Florida - www.innovrsrch.com). Pellet insertion, one week prior to SCI, was done with a small incision in the skin of the neck just behind the right ear (closure with one wound clip) under brief anesthesia with isofluorane. Each pellet (for 10 rats) contained 0.25 mg of 17-β estradiol to maintain serum levels similar to the proestrus stage of the female animals’ cycle [14, 28]. Ovarian hormones, such as progesterone and estradiol, reach their highest level during proestrus [4, 14]. The pellets are known to yield continuous serum hormone levels that are reached during proestrus [8, 40]. The placebos (20 rats) are made of only the vehicle, which contains cholesterol, cellulose, lactose, phosphates and stearates.
Serum estradiol concentrations were determined with radioimmunoassays on blood samples taken during euthanasia at the end of the 6 week testing period. Blood was collected directly from the heart, allowed to clot at room temperature, centrifuged, then frozen and stored at −20°C. Samples were subsequently shipped overnight on dry ice to the Ligand Assay and Analysis Core Laboratory at the Center for Research in Reproduction, University of Virginia Health Sciences Center (ligandcore@virginia.edu). Sample labels were coded, so the assays were done blinded.
For SCI (female and male) rats, each animal was anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10mg/kg), injected intraperitonealy. All surgeries were performed as previously described [22, 23]. A T7 laminectomy exposed the underlying T8 cord. Contusions were done using an Infinite Horizon (IH) Impactor (Precision Systems & Instrumentation, LLC, Fairfax, VA). All animals received 5 mg/kg of gentamicin (Abbott Laboratories, North Chicago, IL) once/day for five days post-surgery to control for possible bladder infections, and 2.5 mg/kg of ketoprofen (Ketofen®, Fort Dodge Laboratories, Fort Dodge, IA) once/day for two days to alleviate post-surgical pain. The urinary bladder was expressed every 8 hours until reflexive voiding occurred (up to about 12 days [24]).
The dorsolateral trunk sensitivity in the T5–T7 dermatomes was tested once a week for six weeks as previously described [17]. Briefly, testing for pain-like behaviors (freezing and/or escape and/or grab/push probe with forepaw) began with a #5 paint brush (17 g force) stimulus. If a pain-like behavior occurred for 60% of the trials (6 of 10, 5 each being alternating between left and right sides of the trunk), the response threshold was determined using a series of 15 Semmes-Weinstein Monofilaments with forces ranging between 0.008 g and 15 g (Stoelting Co., Wood Dale, IL). If the 60% brush response criteria was not met, the rat was tested further using gentle pressure (60g) with a pair of modified Adson tissue forceps (2.0 mm wide tips) having a load cell mounted for force measurement [17]. Note that the mean pain-like response threshold for intact rats is 101.3 ± 3.2 g/cm2, so all sensory testing in the SCI rats used normally innocuous levels of stimulation (≤ 60 g/cm2). A five week mean allodynia score was calculated using our 10 point scoring system [17], which accounts for the type of pain-like behavior exhibited as well as the degree of sensitivity.
At the end of the experiment, each animal was perfused and 2 cm of cord containing the lesion at the center (based upon surface bruising and visible cord atrophy) extracted. The entire piece of cord was then sectioned transversely at 30 μm thickness using a cryostat, and stained with both luxol fast blue and cresyl violet (Kluver-Barrera Method [35]). All slides were viewed under a light microscope using the 4X objective to locate the section with the largest area of damage (lesion epicenter). A SPOT RT3 camera mounted on a Nikon E400 microscope was used to capture images and SPOT version 4.6 software (Diagnostic Instruments, Inc.) for area measurements. The epicenter section image and one from a more intact portion of spinal cord (located 1 mm rostral to the epicenter) was saved for comparison and quantification for extent of white matter area sparing [17, 26]. As previously described, landmarks used for white matter area measurements included medial and lateral edges of the dorsal horn, tips of the ventral horn, and surface notches where the dorsal and ventral roots enter and exit, respectively.
Behavioral testing for dorsal trunk sensitivity to low threshold mechanical stimuli revealed that only 26% (6 out of 23) of contused females with intact ovaries developed allodynia at-level, which is significantly (χ2, p < 0.05) lower than ovx SCI female rats and SCI male rats (Table 1). Those ovx female rats meeting the criteria for at-level allodynia had a mean allodynia score of 5.1 ± 0.4 (out of a possible 10). The few cycling female rats that had pain-like behavioral responses to touch had a mean score of 6.5 ± 1.5, which was not significantly different. Those rats responding to brush had response thresholds to monofilament sizes ranging between 0.008g and 1.4g. Note that the readout impactor forces for the cycling and ovx groups were not significantly different (232.1±11.2 Kdyn versus 252.4±9.6 Kdyn, respectively; t test, p>0.05). There were also no significant differences in the percent white matter sparing between SCI ovx and cycling groups of rats (11.5 ± 2.6% and 10.4 ± 2.8%, respectively), a finding consistent with the lack of impactor force differences from the computer generated read-outs obtained at the time of injury.
Table 1.
Summary of animals with at level allodynia
| GROUP | CONDITION | # with at level allodynia |
|---|---|---|
| Male* | Intact Control | 0/7 (0%) |
| Female – ovx | Intact Control | 0/7 (0%) |
| Female – cycling | Intact Control | 0/11 (0%) |
| Male** | Complete T8 Transection | 0/15 (0%) |
| Male* | Contusion at T8 | 18/26 (69%) |
| Female – ovx | Contusion at T8 | 10/15 (67%) |
| Female – cycling | Contusion at T8 | 6/23 (26%)^ |
| Male - placebo | Contusion at T8 | 15/20 (75%) |
| Male – 17β estradiol | Contusion at T8 | 3/10 (30%)^^ |
Hubscher & Johnson (1999, 2006)
Hubscher et al. 2008
significantly different from contused males (χ2 = 3.99; p<0.05) and ovx females (χ2 = 2.49; p<0.05)
significantly different from contused placebo males (χ2 = 3.91; p<0.05)
For the SCI male rats, the percentage having at-level sensitivity to touch post-contusion was significantly reduced (30%; χ2, p < 0.05) for the group receiving the 17-β estradiol treatment. For those responding to touch at-level, the mean allodynia score was 3.9 ± 0.5 for the placebo group and 6.3 ± 1.1 for the three estradiol treated males (approaching significance; p=0.052). Note that serum levels of estradiol were significantly lower (p<0.001) for placebos (8.5 ± 1.1 pg/ml) than for the estradiol-treated (60.5 ± 9.6 pg/ml) males. Although the serum levels were lower for the three allodynic estradiol-treated rats (48.5 ± 10.1 pg/ml), the difference relative to the non-allodynic estradiol-treated rats was not significant (p>0.05).
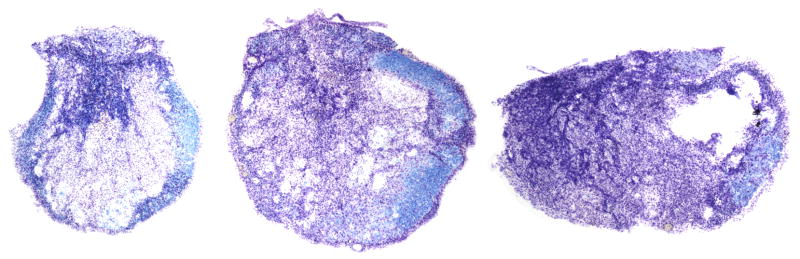
The placebo male allodynia scores is not significantly different from ovx females (t-test; p = 0.083). Male rats responding to brush also had similar thresholds at the low end (0.008g and 2.0g). Note that the forces for the male estradiol and placebo groups were also not different (221.8±2.7 Kdyn versus 224.9±2.7 Kdyn, respectively; t test, p>0.05). Interestingly, displacement values were significantly higher for the EB-treated group, despite randomization and group blindness at time of injury (1.22 ± 0.03 mm placebo; 1.44 ± 0.06 mm estradiol). However, no significant differences were found for percent white matter sparing at the lesion epicenter between SCI placebo and estradiol treated groups of male rats (5.3 ± 1.5% and 7.3 ± 2.3%, respectively). One would predict greater damage for the estradiol rats based on the displacement difference between groups. This was not the case, suggesting that neuroprotection may have occurred (a finding consistent with several published studies – discussed below). Alternatively, the % damage is so high (5–7% sparing) that the displacements for the placebos (1.2 mm) may be near maximum so that any further amount may not have produced a significant difference in the extent of tissue damage. An example showing typical lesion epicenters of rats having at level allodynia is provided in Figure 1.
Figure 1.
Lesion Epicenters - Typical lesion epicenters from three rats with pain-like responses to touch/pressure at-level. The cross-section to the left is from an ovariectomized female (mean five-week allodynia score of 5.0; 224 Kdyn impactor force), the middle section is from a placebo-treated male (mean allodynia score of 7.0; 221 Kdyn), and the section to the right is from an estradiol-treated male (mean allodynia score of 6.8; 216 Kdyn). The sections were stained with both luxol fast blue (indicates the presence of myelin) and cresyl violet (to stain Nissl substance in nervous tissue).
The results demonstrate that ovarian steroids likely account for at least some of the variability related to the presence/absence of SCI related pain. The similarity between data from ovx female and placebo-treated male rats in combination with a significant reduction in the incidence of allodynia in male rats treated with estradiol implicates hormones as potential therapeutic targets for at-level central neuropathic pain.
Mechanisms underlying a reduction in the incidence of allodynia by estradiol are unknown. There are two main possibilities. First, the presence of estradiol in male rats, given prior to the SCI (for proof of principle), may be neuroprotective. There is a growing literature from rat models of traumatic brain injury and stroke on the neuroprotective effects of ovarian steroids, including estradiol [1, 33, 39, 45]. One study on SCI female rats demonstrated improved recovery of hind limb locomotion, increased white matter sparing, and decreased apoptosis when given estradiol [5]. Another study on SCI ovx and male rats supplemented with estradiol [46] failed to show an effect on hind limb locomotion or percent of spared tissue. However, for both studies [5, 46], the time frame for treatment and testing was short (tested only up to 21 days post-SCI). They also did not test for allodynia or any other function aside from over-ground locomotion using a behavioral outcome measure (BBB locomotor scale) that has limited value when used on its own. We did not find any significant differences in hind limb locomotion between estradiol (BBB 9.6 ± 0.5) and placebo (BBB 10.0 ± 0.7) treated males at 6 weeks post-SCI. Though we may have found some differences in tissue sparing at the lesion epicenter, more subtle differences in the degree of axonal damage might have escaped the morphometric analysis. Very labor intensive examination and quantification in 1 micron plastic sections for a higher degree of anatomical resolution of individual myelinated axons (as done for a previous study - [23]) will need to be done in future studies.
A second possible mechanism underlying the reduction in the incidence of allodynia by estradiol may involve its known effects on both antinociceptive and nociceptive processes. In cycling female rats, for example, thresholds to somatic and visceral nociceptive stimuli vary at different stages of estrus [16, 19, 29, 41]. Simulation of pregnancy serum levels of ovarian sex steroids induces a substantial elevation in nociceptive thresholds in female and male rats, which is not surprising given well documented elevations in maternal pain thresholds in rats and humans during pregnancy [34]. Such an elevation may be occurring in the present study with the peak physiological dosages being used and the continuous length of treatment time with the slow release pellets. Studies are in progress to not only examine the mechanisms underlying the neuroprotective and/or anti-nociceptive effect(s) of estradiol in the acute phase post-SCI on the outcome of chronic pain at level but also the potential benefit on other functional deficits that occur following chronic SCI.
The contusion injury for all groups was severe with less than 15% white matter sparing. In a previous study, none of the animals receiving complete spinal cord transections showed mechanical hypersensitivity (allodynia) at-level during the 30 day recovery period post-injury [25]. Whereas there is some sparing even with a severe anatomically discomplete contusion injury, the anatomically complete spinal transection eliminated all axonal connection between the cord below and above the lesion. The transection result supports the necessity of having intact central axons at the site of injury in order for allodynia to occur. We have recently shown a very strong correlation between the development of allodynia and connections that are asymmetrically spared within the ventrolateral funiculus at the lesion epicenter [17]. This asymmetry can be seen in all the typical examples shown in the Figure 1 cross sections. Note that some sensory communication across the lesion site was demonstrated even in persons that developed neuropathic pain who had presumably clinically complete SCI’s [13].
We have anecdotal evidence from our behavioral testing that cycling SCI female rats are either more sensitive to touch on the day of proestrus than during diestrus (n=4) or are only sensitive during proestrus (n=2), observations that are consistent with other studies showing increased sensitivity to pain in proestrus [11, 15, 16, 29]. Thus, although acute treatment with estradiol post-SCI may prevent allodynia (i.e., possibility that it is neuroprotective), its presence if allodynia develops may exacerbate it.
The incidence of tactile allodynia following peripheral nerve ligation [6, 30] has been shown to decrease post-ovx. However, the mechanisms underlying the presence/absence of tactile allodynia in this peripheral neuropathic pain model are likely very different than those for our injury model of central neuropathic pain at-level. In the peripheral ligation model, progesterone supplementation maintained the hypersensitivity to thermal and tactile stimuli following L5 nerve root ligation [31], which may be associated with known interactions between ovarian hormones and inhibitory neurotransmitters in the spinal cord [6]. Estradiol and progesterone have been shown to modulate the activity of the γ-aminobutyric acid and glycine [42, 48], which may also explain our anecdotal observations of cyclical variations in tactile sensitivity in the small number of rats that developed allodynia.
Acknowledgments
We thank James Armstrong and Christine Nunn for excellent technical assistance and Dr. Richard Johnson for his input. This study was supported by grant RR015576 from NCRR, a component of NIH. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) Grant U54-HD28934.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz M, O’Leary P, Kruse D, Harvey C. Spinal cord injury: An analysis of medical and social costs. Demos Medical Publishing Inc; New York: 1998. [Google Scholar]
- 4.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 5.Chaovipoch P, Jelks KA, Gerhold LM, West EJ, Chongthammakun S, Floyd CL. 17beta-estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J Neurotrauma. 2006;23:830–852. doi: 10.1089/neu.2006.23.830. [DOI] [PubMed] [Google Scholar]
- 6.Coyle DE, Sehlhorst CS, Behbehani MM. Intact female rats are more susceptible to the development of tactile allodynia than ovariectomized female rats following partial sciatic nerve ligation (PSNL) Neurosci Lett. 1996;203:37–40. doi: 10.1016/0304-3940(95)12259-1. [DOI] [PubMed] [Google Scholar]
- 7.Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Westlund KN, Hulsebosch CE. Upregulation of the phosphorylated form of CREB in spinothalamic tract cells following spinal cord injury: relation to central neuropathic pain. Neurosci Lett. 2005;384:139–144. doi: 10.1016/j.neulet.2005.04.066. [DOI] [PubMed] [Google Scholar]
- 8.Dantas AP, Tostes RC, Fortes ZB, Costa SG, Nigro D, Carvalho MH. In vivo evidence for antioxidant potential of estrogen in microvessels of female spontaneously hypertensive rats. Hypertension. 2002;39:405–411. doi: 10.1161/hy0202.102993. [DOI] [PubMed] [Google Scholar]
- 9.Davidoff G, Roth EJ. Clinical characteristics of central (dysesthetic) pain in spinal cord injury patients. In: Casey KL, editor. Pain and Central Nervous System Disease: The Central Pain Syndromes. Raven Press; New York: 1991. pp. 77–83. [Google Scholar]
- 10.Drew GM, Siddall PJ, Duggan AW. Responses of spinal neurones to cutaneous and dorsal root stimuli in rats with mechanical allodynia after contusive spinal cord injury. Brain Res. 2001;893:59–69. doi: 10.1016/s0006-8993(00)03288-1. [DOI] [PubMed] [Google Scholar]
- 11.Drury RA, Gold RM. Differential effects of ovarian hormones on reactivity to electric footshock in the rat. Physiol Behav. 1978;20:187–191. doi: 10.1016/0031-9384(78)90071-9. [DOI] [PubMed] [Google Scholar]
- 12.Eaton MJ. Cell and molecular approaches to the attenuation of pain after spinal cord injury. J Neurotrauma. 2006;23:549–559. doi: 10.1089/neu.2006.23.549. [DOI] [PubMed] [Google Scholar]
- 13.Finnerup NB, Gyldensted C, Fuglsang-Frederiksen A, Bach FW, Jensen TS. Sensory perception in complete spinal cord injury. Acta Neurol Scand. 2004;109:194–199. doi: 10.1034/j.1600-0404.2003.00219.x. [DOI] [PubMed] [Google Scholar]
- 14.Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Vol. 2. Raven; New York: 1994. pp. 613–658. [Google Scholar]
- 15.Frye CA, Bock BC, Kanarek RB. Hormonal milieu affects tailflick latency in female rats and may be attenuated by access to sucrose. Physiol Behav. 1992;52:699–706. doi: 10.1016/0031-9384(92)90400-v. [DOI] [PubMed] [Google Scholar]
- 16.Giamberardino MA, Berkley KJ, Iezzi S, de Bigontina P, Vecchiet L. Pain threshold variations in somatic wall tissues as a function of menstrual cycle, segmental site and tissue depth in non-dysmenorrheic women, dysmenorrheic women and men. Pain. 1997;71:187–197. doi: 10.1016/s0304-3959(97)03362-9. [DOI] [PubMed] [Google Scholar]
- 17.Hall BJ, Lally JE, Vukmanic EV, Armstrong JE, Fell JD, Gupta DS, Hubscher CH. Spinal cord injuries containing asymmetrical damage in the ventrolateral funiculus is associated with a higher incidence of at level allodynia. J Pain. 2010 doi: 10.1016/j.jpain.2009.12.008. EPub Mar. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoheisel U, Scheifer C, Trudrung P, Unger T, Mense S. Pathophysiological activity in rat dorsal horn neurones in segments rostral to a chronic spinal cord injury. Brain Res. 2003;974:134–145. doi: 10.1016/s0006-8993(03)02571-x. [DOI] [PubMed] [Google Scholar]
- 19.Hubscher CH. Estradiol-associated variation in responses of rostral medullary neurons to somatovisceral stimulation. Exp Neurol. 2006;200:227–239. doi: 10.1016/j.expneurol.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Hubscher CH, Armstrong JE, Johnson JR. Effects of spinal cord injury on the rat estrous cycle. Brain Res. 2006;1100:118–124. doi: 10.1016/j.brainres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Hubscher CH, Brooks DL, Johnson JR. A quantitative method for assessing stages of the rat estrous cycle. Biotech Histochem. 2005;80:79–87. doi: 10.1080/10520290500138422. [DOI] [PubMed] [Google Scholar]
- 22.Hubscher CH, Johnson RD. Changes in neuronal receptive field characteristics in caudal brain stem following chronic spinal cord injury. J Neurotrauma. 1999;16:533–541. doi: 10.1089/neu.1999.16.533. [DOI] [PubMed] [Google Scholar]
- 23.Hubscher CH, Johnson RD. Chronic spinal cord injury induced changes in the responses of thalamic neurons. Exp Neurol. 2006;197:177–188. doi: 10.1016/j.expneurol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Hubscher CH, Johnson RD. Effects of acute and chronic midthoracic spinal cord injury on neural circuits for male sexual function. II. Descending pathways. J Neurophysiol. 2000;83:2508–2518. doi: 10.1152/jn.2000.83.5.2508. [DOI] [PubMed] [Google Scholar]
- 25.Hubscher CH, Kaddumi EG, Johnson RD. Segmental neuropathic pain does not develop in male rats with complete spinal transections. J Neurotrauma. 2008;25:1241–1245. doi: 10.1089/neu.2008.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hubscher CH, Reed WR, Kaddumi EG, Armstrong JE, Johnson RD. Select spinal lesions reveal multiple ascending pathways in the rat conveying input from the male genitalia. J Physiol. 2010;588.7:1073–1083. doi: 10.1113/jphysiol.2009.186544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hulsebosch CE, Xu GY, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J Neurotrauma. 2000;17:1205–1217. doi: 10.1089/neu.2000.17.1205. [DOI] [PubMed] [Google Scholar]
- 28.Kalra SP, Kalra PS. Temporal interrelationships among circulating levels of estradiol, progesterone and LH during the rat estrous cycle: effects of exogenous progesterone. Endocrinology. 1974;95:1711–1718. doi: 10.1210/endo-95-6-1711. [DOI] [PubMed] [Google Scholar]
- 29.Kayser V, Berkley KJ, Keita H, Gautron M, Guilbaud G. Estrous and sex variations in vocalization thresholds to hindpaw and tail pressure stimulation in the rat. Brain Res. 1996;742:352–354. doi: 10.1016/s0006-8993(96)01108-0. [DOI] [PubMed] [Google Scholar]
- 30.LaCroix-Fralish ML, Tawfik VL, DeLeo JA. The organizational and activational effects of sex hormones on tactile and thermal hypersensitivity following lumbar nerve root injury in male and female rats. Pain. 2005;114:71–80. doi: 10.1016/j.pain.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacroix-Fralish ML, Tawfik VL, Nutile-McMenemy N, Deleo JA. Progesterone mediates gonadal hormone differences in tactile and thermal hypersensitivity following L5 nerve root ligation in female rats. Neuroscience. 2006;138:601–608. doi: 10.1016/j.neuroscience.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 32.Lenz FA, Kwan HC, Martin R, Tasker R, Richardson RT, Dostrovsky JO. Characteristics of somatotopic organization and spontaneous neuronal activity in the region of the thalamic principal sensory nucleus in patients with spinal cord transection. J Neurophysiol. 1994;72:1570–1587. doi: 10.1152/jn.1994.72.4.1570. [DOI] [PubMed] [Google Scholar]
- 33.Liao S, Chen W, Kuo J, Chen C. Association of serum estrogen level and ischemic neuroprotection in female rats. Neurosci Lett. 2001;297:159–162. doi: 10.1016/s0304-3940(00)01704-3. [DOI] [PubMed] [Google Scholar]
- 34.Liu NJ, Gintzler AR. Prolonged ovarian sex steroid treatment of male rats produces antinociception: identification of sex-based divergent analgesic mechanisms. Pain. 2000;85:273–281. doi: 10.1016/s0304-3959(99)00278-x. [DOI] [PubMed] [Google Scholar]
- 35.Luna L. Manual of histologic staining methods of the Armed Forces Institute of Pathology. McGraw Hill; New York: 1968. [Google Scholar]
- 36.Mills CD, Hulsebosch CE. Increased expression of metabotropic glutamate receptor subtype 1 on spinothalamic tract neurons following spinal cord injury in the rat. Neurosci Lett. 2002;319:59–62. doi: 10.1016/s0304-3940(01)02551-4. [DOI] [PubMed] [Google Scholar]
- 37.Olson ME, Bruce J. Ovariectomy, ovariohysterectomy and orchidectomy in rodents and rabbits. Can Vet J. 1986;27:523–527. [PMC free article] [PubMed] [Google Scholar]
- 38.Pattany PM, Yezierski RP, Widerstrom-Noga EG, Bowen BC, Martinez-Arizala A, Garcia BR, Quencer RM. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am J Neuroradiol. 2002;23:901–905. [PMC free article] [PubMed] [Google Scholar]
- 39.Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- 40.Samuel CS, Coghlan JP, Bateman JF. Effects of relaxin, pregnancy and parturition on collagen metabolism in the rat pubic symphysis. J Endocrinol. 1998;159:117–125. doi: 10.1677/joe.0.1590117. [DOI] [PubMed] [Google Scholar]
- 41.Sapsed-Byrne S, Ma D, Ridout D, Holdcroft A. Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Res. 1996;742:10–16. doi: 10.1016/s0006-8993(96)00989-4. [DOI] [PubMed] [Google Scholar]
- 42.Schumacher M, Coirini H, McEwen BS. Regulation of high-affinity GABAa receptors in specific brain regions by ovarian hormones. Neuroendocrinology. 1989;50:315–320. doi: 10.1159/000125239. [DOI] [PubMed] [Google Scholar]
- 43.Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 44.Siddall PJ, Taylor DA, Cousins MJ. Classification of pain following spinal cord injury. Spinal Cord. 1997;35:69–75. doi: 10.1038/sj.sc.3100365. [DOI] [PubMed] [Google Scholar]
- 45.Stein DG. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 2001;24:386–391. doi: 10.1016/s0166-2236(00)01821-x. [DOI] [PubMed] [Google Scholar]
- 46.Swartz KR, Fee DB, Joy KM, Roberts KN, Sun S, Scheff NN, Wilson ME, Scheff SW. Gender differences in spinal cord injury are not estrogen-dependent. J Neurotrauma. 2007;24:473–480. doi: 10.1089/neu.2006.0167. [DOI] [PubMed] [Google Scholar]
- 47.Widerstrom-Noga EG, Turk DC. Types and effectiveness of treatments used by people with chronic pain associated with spinal cord injuries: influence of pain and psychosocial characteristics. Spinal Cord. 2003;41:600–609. doi: 10.1038/sj.sc.3101511. [DOI] [PubMed] [Google Scholar]
- 48.Wu FS, Gibbs TT, Farb DH. Inverse modulation of gamma-aminobutyric acid- and glycine-induced currents by progesterone. Mol Pharmacol. 1990;37:597–602. [PubMed] [Google Scholar]