Abstract
Increased cardiac ryanodine receptor (RyR)-dependent diastolic SR Ca leak is present in heart failure and in conditions when adrenergic tone is high. Increasing Ca leak from the SR could result in spontaneous Ca wave (SCaW) formation. SCaWs activate the inward Na/Ca exchanger (NCX) current causing a delayed afterdepolarization (DAD), potentially leading to arrhythmia. Here we examine SCaWs in ventricular myocytes isolated from failing and healthy rabbit hearts. Myocytes from healthy hearts did not exhibit SCaWs under baseline conditions versus 43% of those exposed to isoproterenol (ISO). This ISO-induced increase in activity was reversed by inhibition of Ca-calmodulin-dependent protein kinase II (CaMKII) by KN93. Inhibition of cAMP-dependent protein kinase (PKA) by H89 had no observed effect. Of myocytes treated with forskolin 50% showed SCaW activity, attributable to a large increase in SR Ca load ([Ca]SRT) versus control. At similar [Ca]SRT (121 µM) myocytes treated with ISO plus KN93 had significantly fewer SCaWs versus those treated with ISO or ISO plus H89 (0.2±0.28 vs. 1.1±0.28 & 1.29±0.39 SCaWs cell−1, respectively). In myocytes isolated from failing hearts ISO induced an increase in the percentage of cells generating SCaWs vs. baseline (74% vs. 11%) with no increase in [Ca]SRT. Inhibiting CaMKII reversed this effect (14%). At similar [Ca]SRT (71 µM) myocytes treated with ISO or ISO plus H89 had significantly more SCaWs per cell vs. untreated (2.5±0.5; 1.6±0.7 vs. 0.36±0.3, respectively). Treatment with ISO plus KN93 completely abolished this effect. The evidence suggests the ISO-dependent increase in SCaW activity in both healthy and failing myocytes is CaMKII-dependent, implicating CaMKII in arrhythmogenesis.
Keywords: Arrhythmia, CaMKII, SR Ca leak, heart failure, ryanodine receptor
Introduction
Patients diagnosed with heart failure (HF) face a five year mortality rate as high as 50%. The ultimate cause of death in these patients presents either as progressive decline in cardiac pump function (pump failure) or as sudden death attributable to arrhythmia [1]. Studies dating back over three decades have repeatedly shown dysfunctional Ca handling to be central to the disease. Decreased sarcoplasmic reticulum Ca content ([Ca]SRT) leading to blunted force-frequency dependency of Ca release, a decrease in twitch Δ[Ca]i and a general decrease in contractility are commonly associated with HF [2]. Among the causes for the decrease in [Ca]SRT may be the higher expression levels of Na/Ca exchange (NCX) protein observed in HF combined with the simultaneous increase in diastolic Ca leak from the SR, possibly due to elevated phosphorylation levels of the cardiac ryanodine receptor (RyR2) [3–5]. The coupling of these two alterations together shifts the balance of Ca-handling mechanisms more towards Ca efflux out of the myocyte (via the NCX) until the [Ca]SRT is lowered thereby attenuating the leak. Since the phosphorylated RyR has increased Ca sensitivity, the threshold for spontaneous release in response to either rising diastolic [Ca] ([Ca]d) or [Ca]SRT is decreased. Shifting the balance of Ca fluxes more in favor of SL flux lowers [Ca]T thereby avoiding complications associated with Ca overload.
The increased leak observed in HF could also have profound implications on arrhythmogenesis. Work in the 1990s demonstrated in humans and rabbits with nonischemic cardiomyopathy that >90% of fatal arrhythmias were initiated by a nonreentrant mechanism such as a delayed afterdepolarization (DAD) or early afterdepolarization (EAD) [6]. Both mechanisms are mediated through a transient inward depolarizing current (Iti). As early as 1976, Ca release from the SR has been shown to activate a Ca-dependent Iti [7]. Recent work has generally implicated the electrogenic NCX as the primary contributor to this Iti [8, 9].
Ca-induced Ca-release (CICR) in cardiac tissue is an inherently stable process. The many mechanisms coupling electrical activity and Ca handling to contraction of the heart are finely tuned and under tight regulation, and Ca induced arrhythmogenic activity is extremely rare [10]. However, recent evidence suggests that when an arrhythmogenic substrate is present—such as mutations in or phosphorylation of RyR2—Ca mishandling may promote arrhythmia [11, 12]. Mechanisms such as these result in a shift in Ca-sensitivity of the RyR array and increased SR Ca leak. However, it is important to realize that the arrhythmogenic substrate alone is insufficient to induce spontaneous activity. Cellular Ca must reach a level or threshold at which spontaneous activity is induced; i.e. Ca-overload [13].
Since contractility is blunted in HF, the sympathetic drive on the β-adrenergic receptor (β-AR) system becomes chronic. As a result Ca-calmodulin dependent protein kinase II (CaMKII) and cAMP-dependent protein kinase (PKA) are activated. Concurrently, protein phosphatases are down regulated. This combination leads to increased and chronic RyR2 phosphorylation in failing hearts [5, 14]. Phosphorylation of the RyR2 by either kinase results in a shift in Ca-sensitivity of the RyR, and could theoretically provide the necessary arrhythmogenic substrate. Controversy remains as to which of these kinases plays the more dominant role in mediating SR Ca leak.
We reported in intact ventricular myocytes the increased SR Ca leak observed in HF is CaMKII-dependent [5]. The same was reported in non-failing myocytes with acute stimulation of the β-ARs with isoproterenol (ISO) [15]. Very recently, using a transgenic mouse model of heart failure in which CaMKII is overexpressed, Sag et al concluded that CaMKII increases SR Ca leak which leads to pronounced ISO-dependent increases in both DADs and EADs [16]. Using a rabbit model of HF induced by aortic insufficiency and pressure overload here we focus on whether spontaneous Ca waves (SCaW) in ventricular myocytes isolated from failing and non-failing hearts are CaMKII-dependent. Ca waves act as a more proximal direct index of triggered DAD-like arrhythmias than leak or Ca sparks. We test the hypothesis that the CaMKII-dependent alterations to the EC coupling machinery are pro-arrhythmic. SCaWs were measured in intact ventricular myocytes from failing and non-failing hearts with and without β-AR stimulation by ISO. ISO stimulation greatly increases the propensity to generate SCaWs. Inhibition of CaMKII suppresses this ISO-dependent spontaneous activity, while inhibition of PKA had no effect. Direct activation of adenylate cyclase and PKA by forskolin had no effect on wave activity. All three observations were true in failing and non-failing myocytes. Interestingly, failing myocytes without ISO stimulation showed little SCaW activity; while those stimulated with ISO showed nearly a 7-fold increase in activity with no increase in [Ca]SRT.
Materials and Methods
Experiments were conducted in accordance with the guidelines for the care and use of experimental animals at Rush University Medical Center and conformed to the Guide for the Care and Use of Laboratory Animals published by NIH (publication No. 85-23, revised 1985). Data were collected with PClamp (Axon Instruments, Foster City, CA). Mathematical data manipulation was performed using Microsoft Excel (Microsoft Corporation, USA) and GraphPad Prism (GraphPad Software, San Diego, CA).
All chemicals were purchased from Sigma Aldrich unless indicated. Normal tyrode (NT) solution was made up as follows (all concentrations in mM): 2 Ca, 140 NaCl, 4 KCl, 1 MgCl, 10 glucose, 5 HEPES, pH 7.4 with NaOH. 0 Na/0 Ca NT with caffeine was made up as NT with LiCl substituted for NaCl, with 10 EGTA, no Ca added, 10 caffeine, pH 7.4 with LiOH.
Arrhythmogenic Rabbit Nonischemic HF Model
All experiments were conducted using ventricular myocytes from New Zealand White rabbits. HF was induced in rabbits by aortic insufficiency followed by aortic constriction as previously described [5, 17, 18]. Hearts were rapidly excised and LV myocytes were isolated as previously described [17]. The protocol was approved by the University of Illinois at Chicago Animal Studies Committee.
Experimental Protocol
Spontaneous Ca Wave Measurement
Myocytes were loaded with calcium-dependent fluorescent indicator, fluo-4 AM (10 µM, Invitrogen) for 30 minutes at room temperature and electrically field stimulated for at least 5 minutes before data acquisition. Grading [Ca]SRT was achieved by stimulating at frequencies from 0.25 Hz to 1.0 Hz in 2 Ca NT solution. After 20 beats a rapid switch to 0 Na/0 Ca NT solution + 10 mM caffeine was applied for 2 seconds to empty the SR of Ca. The difference between basal and peak total cytosolic [Ca2+] in the presence of caffeine is therefore total SR [Ca2+].
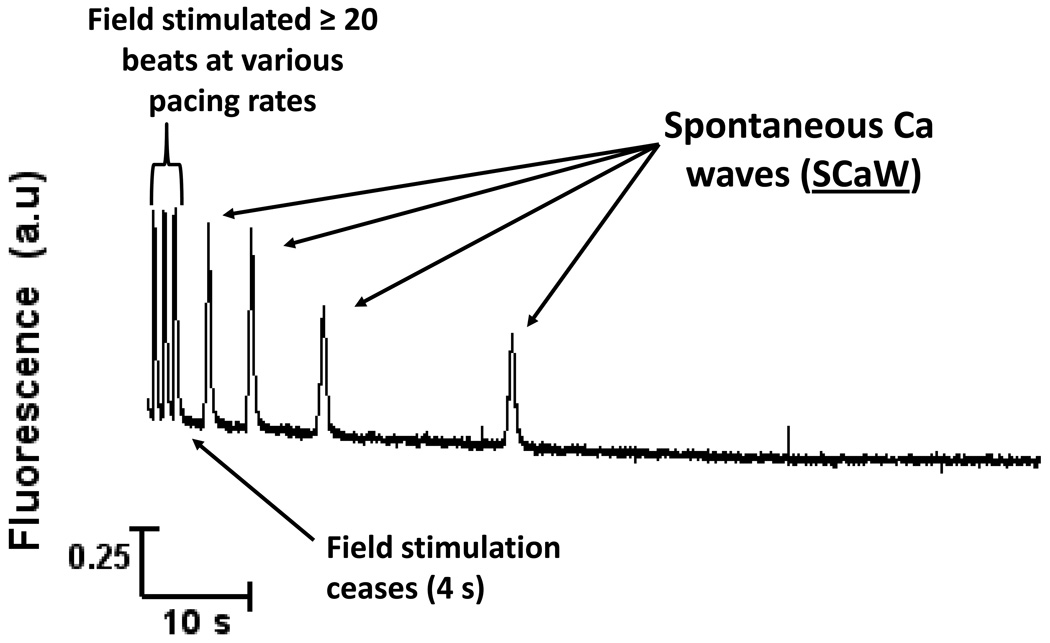
After assessing [Ca]SRT the myocyte was loaded under the same conditions. After loading, field stimulation was terminated and [Ca]i was continuously monitored for 90 seconds. Spontaneous calcium release was determined by visual inspection of the fluo-4 signal, and confirmed if the peak signal was greater than two standard deviations above the average signal for the preceding 50 ms. Experiments were conducted at room temperature. A typical raw data trace shows in Figure 1.
FIGURE 1.
General protocol to assess SCaW activity. Intact ventricular myocytes were loaded with fluo-4 and field stimulated at varying pacing rates to vary SR Ca load. After bringing the myocytes to steady state by pacing for at least 20 beats, 4 seconds of stimulated activity was recorded at which point field stimulation was ceased and the cell was allowed to come to rest. Continuous monitoring for spontaneous activity took place for a further 90 seconds. Data showing here was acquired in a healthy myocyte stimulated with ISO.
To activate β-ARs 250 nM isoproterenol (ISO) was added to the NT and superfused normally. PKA inhibitor H-89 (1µM) and CaMKII inhibitor KN-93 (1µM, CalBiochem) were independently added to the NT + ISO solution and were constantly present. As a negative control for KN-93 the inactive analog, KN-92 (1µM, Calbiochem), was substituted. To stimulate cAMP production, forskolin (1 µM) was added to the NT solution and was constantly present.
SR Ca Leak Measurement
The protocol used to measure SR Ca leak was as previously described [15, 19]. See supplementary material for full discussion.
Statistical Analysis
Data are reported as mean ± SEM. Student t test was applied when appropriate. P<0.05 was considered statistically significant.
Results
Spontaneous Diastolic Ca Waves in Non-Failing Myocytes Are CaMKII-dependent
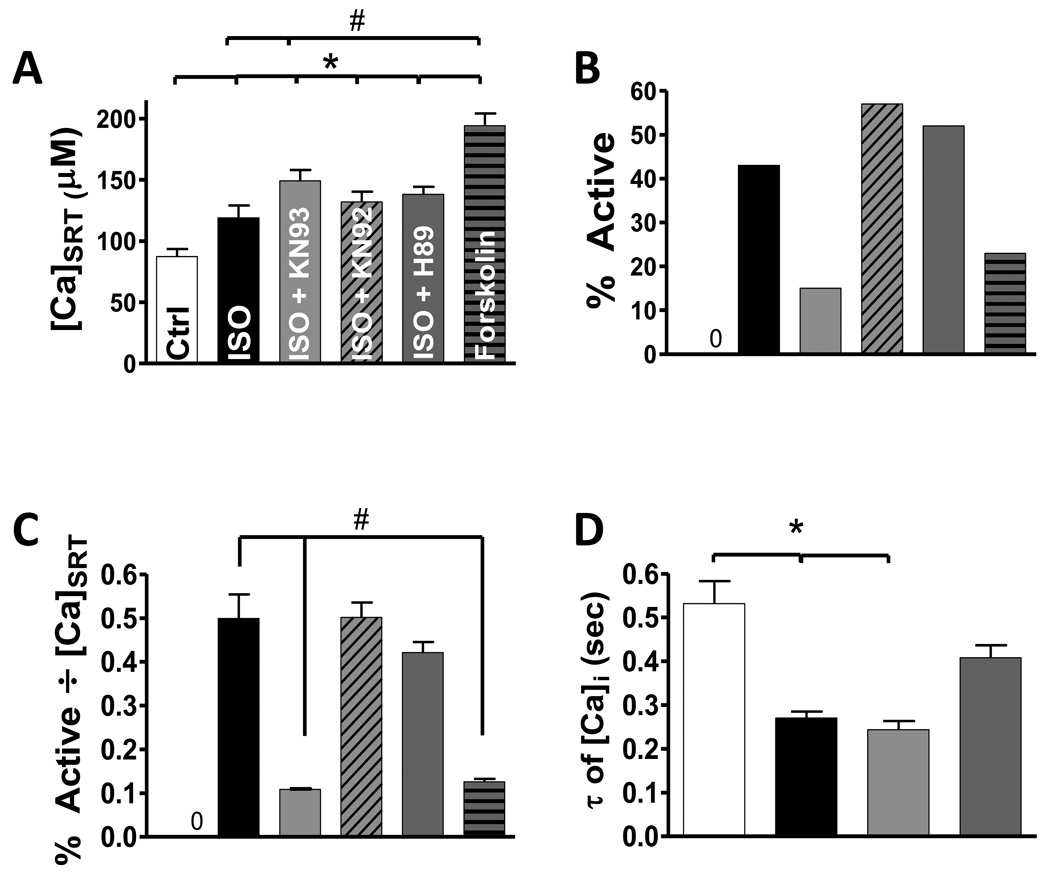
The propensity for SCaW generation in intact ventricular myocytes isolated from non-failing hearts (NF) was assessed using the general protocol showing in Figure 1. Figure 2A–B shows that under baseline conditions NF myocytes showed no SCaWs regardless of [Ca]SRT, whereas 250 nM ISO caused an increase in [Ca]SRT (from 87 ± 6 to 119 ± 10 µmol/l cytosol; µM) and the appearance of SCaWs in 43% of cells. Note that 1 mM tetracaine prevented SCaWs, but Na-free, Ca-free solution did not (not shown), assuring that SCaWs were RyR-dependent. CaMKII inhibition with 1 µM KN-93 prevented the ISO-induced SCaWs (15%), and further enhanced [Ca]SRT (149 ± 9 µM, n=46, Figure 2A). The ISO-induced effects were not reversed by either KN-92 (an inactive KN-93 analogue, 132 ± 8 µM, 57%, n=35) or 1 µM H-89 (a potent PKA inhibitor, 138 ± 6 µM, 52%, n=46). This suggests that the ISO-induced increase in SCaWs was mediated by CaMKII and not PKA. Notably, H-89 was sufficient here to inhibit the classic lusitropic effect of ISO that is mediated by phospholamban phosphorylation (twitch [Ca]i decline τ retuned to control; Fig 2D), indicating that PKA was effectively inhibited by the H-89 treatment. The higher [Ca]SRT with ISO+KN-93 is a logical consequence of the inhibition of SR Ca leak and waves by CaMKII inhibition. When 1 µM forskolin was used (to activate PKA independent of β-AR activation), [Ca]SRT was dramatically increased (194 ± 10 µM, n=27), and SCaWs were seen in only 23% of myocytes. It is well recognized that increasing [Ca]SRT enhances SR Ca leak and SCaWs. Figure 2C normalizes the appearance of SCaWs for altered [Ca]SRT in the cases shown in Fig 2A–B. This shows that ISO increased SCaWs (0.5 ± 0.06) even in the presence of H-89 or KN-92 (0.42 ± 0.02, 0.5 ± 0.03, respectively) whereas CaMKII inhibition reversed the ISO effect on SCaWs (0.1 ± 0.001). Similar results were obtained using CaMKII- and PKA-specific peptide inihibtors AIP and PKI, respectively (see Supplementary Figure 4S). Forskolin did not mimic the ISO effect (0.13 ± 0.007). To assure the results we observed were not due to random animal to animal variation, we analyzed how the myocytes from each animal used responded to the relative treatments. The results showed no unexpected animal to animal variation (i.e. ISO treated myocytes from all animals were always more active; ISO plus KN93 always resulted in minimized activity, see Supplementary Data discussion, Figure 9S).
FIGURE 2.
Summary data for SCaW activity in NF myocytes. A) Average [Ca]SRT over all treatments (n = 27–46). B) Percentage of all myocytes tested that showed at least one SCaW. C) Average of the percent activity, data in B, normalized to each individual myocyte’s [Ca]SRT. D) Tau of decline of twitch [Ca]i. (*different from control, # different from ISO)
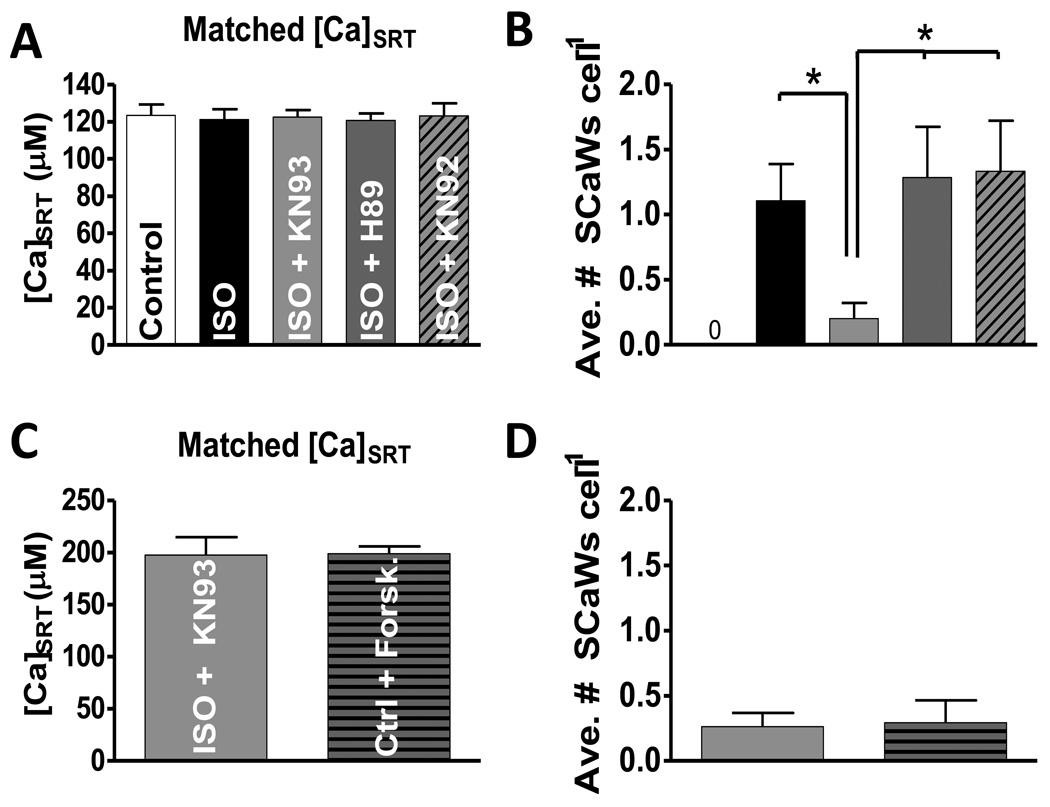
Because the [Ca]SRT vs. SR Ca leak relationship is non-linear[15] this normalization method may be imperfect for these comparisons. Reporting just normalized data may falsely lead to the conclusion that PKA inhibition had no effect on the overall activity of the myocytes, which could manifest itself as the number of waves per myocyte. Therefore, we grouped myocytes so [Ca]SRT was matched between each condition (Fig 3A), and measured the frequency of SCaWs (Fig 3B). The results are essentially the same. The ISO-induced increase in Ca wave frequency (1.1 ± 0.3 waves cell−1) was prevented by CaMKII inhibition with KN-93 (0.2 ± 0.1), but not with H-89 (1.3 ± 0.4) or KN-92 (1.3 ± 0.4). Similar results were obtained using AIP and PKI (see Supplementary Figure 5S). It was difficult to reduce [Ca]SRT in the presence of forskolin, so the only practical comparison was with ISO+KN-93 (Fig 3C). Forskolin did not increase SCaWs compared to ISO+KN-93 at the same [Ca]SRT (0.23 ± 0.19 & 0.25 ± 0.18, Fig 3D). This data is in line with our previous study which showed a CaMKII-dependent increased leak at similar [Ca]SRT [5, 15]. The data suggest that increased ISO-dependent CaMKII activity is proarrhythmic in NF myocytes.
FIGURE 3.
Controlling for SR Ca load and statistical analysis of SCaW activity. A) Data is matched such that all treatments were examined over the same [Ca]SRT (n = 19–21). B) Average number of SCaWs exhibited by those myocytes grouped in panel A. C) The average [Ca]SRT for myocytes from forskolin was too high to enable grouping with all other treatments. For this reason myocytes treated with forskolin were compared to myocytes treated with ISO + KN93 and matched such that the average load did not vary (n = 16 and 22, respectively). D) Average number of SCaWs in myocytes grouped in panel C.
Coupling Ca Overload to the CaMKII-dependent Arrhythmogenic Substrate Produces Waves
Diastolic SR Ca leak is a potentially regenerative process. As Ca leaks out of the SR it is free to bind to and activate a neighboring RyR2 in the junctional cleft and induce a spontaneous opening of that channel (leak) by CICR. The likelihood of this occurring may be proportional to the Ca-sensitivity of the RyR. For this reason we postulated that A) those cells exhibiting higher levels of [Ca]d would show higher levels of leak, B) these cells would show higher SCaW activity and C) this effect would be exaggerated in the presence of ISO and reversed by CaMKII inhibition.
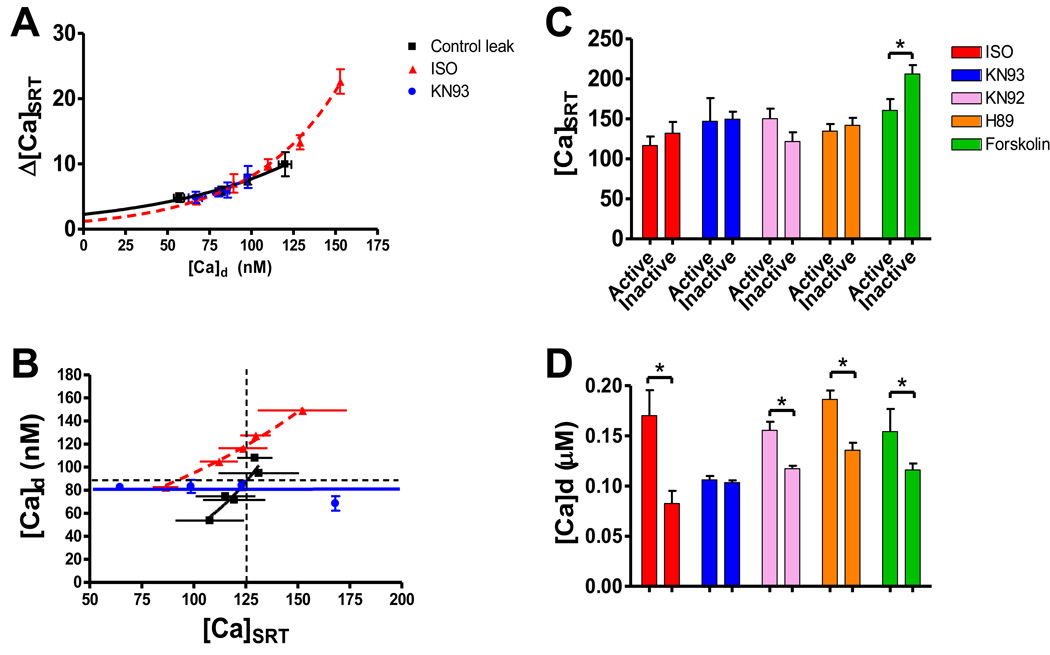
To address whether leak increases along with [Ca]d we used our leak assessment protocol [15]. Figure 4A shows that as [Ca]d increases, leak tends to increase in a nonlinear fashion. This was true over all treatments. The relationship of leak vs. [Ca]d was superimposable over all treatments, but the relationship of [Ca]d vs. [Ca]SRT was not (Figure 4B). ISO left-shifted this such that for any given [Ca]SRT myocytes had a significantly higher [Ca]d (horizontal dashed line). Likewise, at any given [Ca]d ISO had a lower [Ca]SRT (vertical dashed line). This suggests that increased SR Ca leak in ISO is leading to increased [Ca]d at lower SR Ca loads. Treatment with ISO and KN93 flattens this relationship. We attribute this difference to in the decrease in SR Ca leak associated with CaMKII inhibition. ISO also increases the rate of the SR Ca pump (SERCA) via PKA stimulation. Together with the reduction of SR Ca leak greater SR Ca loading at any [Ca]d can occur [20]. This suggests an ISO-dependent increase in RyR Ca-sensitivity mediated by CaMKII. PKA inhibition in the presence of ISO resulted in a relationship similar to ISO alone (data not shown).
FIGURE 4.
Analysis of Ca dynamics. A) Increasing SR Ca leak (Δ[Ca]SRT) depends on increasing diastolic Ca ([Ca]d) in myocytes grouped together by [Ca]d. Curves are best fit non-linear regression curves. All treatments were superimposable. Only data for control, ISO and ISO + KN93 are showing. B) Interdependency of [Ca]d and [Ca]SRT. Horizontal arrow shows for any given [Ca]d ISO has a significantly lower [Ca]SRT. Vertical arrow shows for any given [Ca]SRT ISO exhibits significantly higher [Ca]d. Both are indicative that Ca sensitivity is increased in ISO thereby increasing diastolic SR Ca leak. C) Average [Ca]SRT of myocytes. First grouped by treatment, then subdivided into two separate subgroups: Active and Inactive. D) Average [Ca]d over the 50 ms preceding the 1st SCaW event. For inactive myocytes, [Ca]d was determined by taking the average [Ca]d over the time period corresponding with the average time to first SCaW of the active pool (Table 1).
All myocytes from the entire data pool for each treatment were then divided into two sub-groups per treatment, those showing SCaW events (active) and those showing no SCaW events (inactive). Figure 4C shows the average [Ca]SRT was not different between the two subgroups with the exception of forskolin (160.7 ± 14.2 & 206.2 ± 11.3 µM). However, when the same groups were analyzed by [Ca]d an interesting pattern emerged (Figure 4D). Mean [Ca]d for active myocytes over the 50 ms preceding the 1st SCaW event was compared to mean [Ca]d in inactive myocytes at the average time to first SCaW event from the active pool (see Table 1). All active groups had significantly higher [Ca]d compared to their inactive counterparts. The only exception to this was myocytes treated with ISO and KN93.
Table 1.
Average time to first SCaW
| Treatment | Ave. Time to 1st SCaW (sec) |
n Active (total) |
% Active which were paced at ≥ 0.5 Hz |
|---|---|---|---|
|
Control, non- failing |
n/a | 0 (44) | n/a |
| ISO | 3.29 ± 0.51 | 18 (46) | 78% |
| ISO + KN93 | 17.6 ± 8.2 | 7 (46) | 100% |
| ISO + KN92 | 4.31 ± 0.74 | 20 (35) | 75% |
| ISO + H89 | 2.15 ± 0.33 | 24 (46) | 92% |
| Forskolin | 8.59 ± 4.14 | 7 (27) | 86% |
| HF | 4.1 ± 0.25 | 3 (26) | 67% |
| HF + ISO | 3.83 ± 0.74 | 19 (26) | 84% |
| HF + ISO + KN93 | 18.65 ± 8.18 | 3 (28) | 100% |
| HF + ISO + H89 | 7.43 ± 3.92 | 9 (17) | 89% |
| HF + Forskolin | 1.97 ± 0.28 | 9 (18) | 89% |
As leak increases in magnitude more Ca will pool in the cytosol. This effect is exaggerated at faster pacing rates as total cellular Ca ([Ca]T) is increased leading to larger [Ca]SRT and a subsequently larger leak. We hypothesized that this effect of increased pacing rate on leak and cytosolic Ca pooling would result in an increase in SCaW generation. We are able to show evidence in support of this hypothesis. The majority of the active cells generated SCaWs at field stimulation rates of 0.5 Hz or above (See Table 1), the range we have previously observed diastolic leak to be the largest and SR Ca loads to be limited in ISO and in HF [15]. We propose a mechanism in which the Ca sensitivity of the RyR array is increased in a CaMKII-dependent manner, making it a potentially arrhythmogenic substrate; coupling this to an increase in [Ca]d leads directly to SCaW activity.
Spontaneous Diastolic Ca Waves in Myocytes Isolated from Failing Hearts
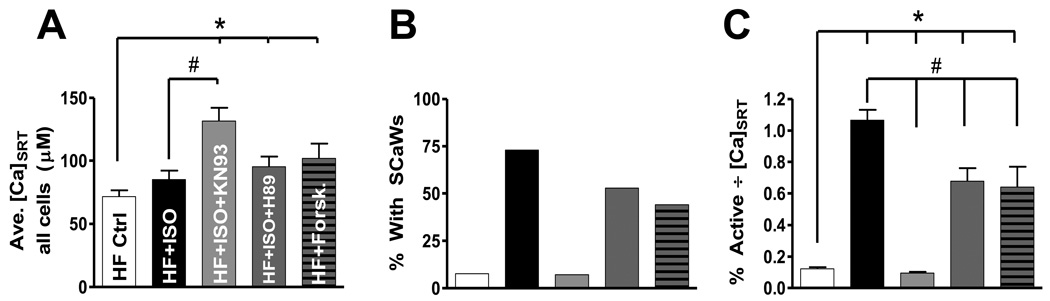
We isolated ventricular myocytes from failing rabbit hearts and applied the same wave assessment protocol. Similar to NF myocytes, those isolated from failing hearts did not have a tendency to wave under control conditions. Only 11% of non-treated HF myocytes showed SCaWs. Applying ISO did not increase the average [Ca]SRT (85.1 ± 6.99 µM vs. 71.5 ± 5.0 µM, n.s., Figure 5A) but caused a near 7-fold increase in SCaW activity in ISO to 73% (Figure 5B). This dramatic rise in SCaW activity in the presence of ISO was completely reversed by the addition of KN93 (7%), while significantly increasing [Ca]SRT (131.7 ± 10.4 µM, Figure 5A & B). Normalizing this activity to [Ca]SRT (Figure 5C) reveals activity in ISO plus KN93 was significantly lower than even non-treated HF myocytes.
FIGURE 5.
Spontaneous Ca wave activity in myocytes isolated from failing hearts. A) Average [Ca]SRT over all treatments (n = 17–28). B) Percentage of all myocytes tested that showed at least one SCaW. C) Average of the percent activity, data in B, normalized to each individual myocyte’s [Ca]SRT. (*different from control, # different from ISO)
Treating HF myocytes with ISO plus H89, or with forskolin, resulted in a modest but significant rise in average [Ca]SRT (95.2 ± 8.1 and 101.8 ± 12.3 µM respectively)without the dramatic reduction in overall SCaW activity (53% and 44%, respectively; Figures 5A&B) seen during CaMKII inhibition. Unlike NF myocytes, normalized activity in the presence of ISO+H89 or forskolin was significantly lower than ISO alone, but still higher than control. Taken together these results indicate that PKA may play a small role in the generation of SCaW in HF; though the dominant role is clearly CaMKII-mediated.
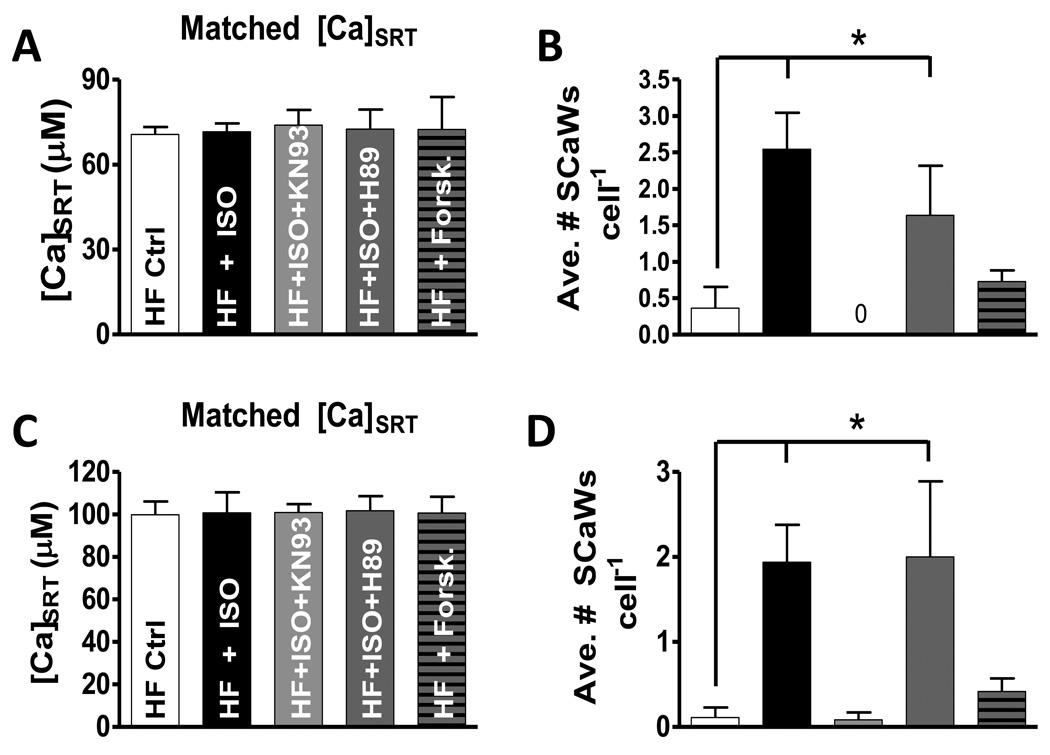
Figure 6 shows SCaW activity at matched [Ca]SRT amongst all treatments (Figure 6A). Figure 6B shows those myocytes stimulated with ISO generated significantly more SCaWs cell−1 vs. control (2.55 ± 0.50 vs. 0.36 ± 0.29, respectively). When stimulated with ISO in the presence of KN93, zero SCaWs were observed. Inhibition of PKA in the presence of ISO was similar to ISO alone (1.64 ± 0.68). Forskolin did not induce SCaW activity different from control (0.73 ± 0.16 SCaWs cell−1). To demonstrate that this pattern was observed over a range of SR Ca loads we selected myocytes for a higher average [Ca]SRT (Figure 6C). Very similar results were found (Figure 6D).
FIGURE 6.
Controlling for SR Ca load and statistical analysis of SCaW activity. A) Data is matched so all treatments were examined over the same [Ca]SRT (n = 7–11). B) Average number of SCaWs exhibited by those cells grouped in panel A. C) Data is matched at a larger, but similar [Ca]SRT (n = 9–15). D) Average number of SCaWs exhibited by those cells grouped in panel C.
These results indicate that ISO is both exacerbating the arrhythmogenic substrate and promoting the Ca overload necessary to induce SCaW activity. In HF the RyR array exhibits increased diastolic Ca leak at lower [Ca]SRT, indicative a shift in Ca sensitivity. Stimulating myocytes isolated from failing hearts with ISO would only exaggerate this sensitivity all the while promoting cellular Ca overload. Like NF myocytes, the active pools in HF were dominated by myocytes field stimulated at or above 0.5 Hz (See Table 1).
Discussion
The major findings of this report are 1) the observed ISO-dependent spontaneous Ca waves in intact, ventricular myocytes isolated from both failing and non-failing rabbit hearts are strongly dependent on CaMKII activity; 2) there was no observed role for PKA activity in ISO-dependent wave generation in non-failing myocytes and only a limited role observed in failing myocytes; 3) failing myocytes stimulated through the β-AR by ISO are nearly 7 times more likely to generate spontaneous wave activity at a similar SR Ca load and these waves are dependent upon the activity of both kinases with CaMKII playing the dominant role.
We attribute this increase in SCaW activity to the increased CaMKII-dependent diastolic SR Ca leak observed in both HF and non-failing myocytes acutely stimulated through the β-AR system [5, 15]. Higher diastolic SR Ca leak will increase the likelihood of generating a SCaW. As Ca leaks out of the SR a positive feedback mechanism is set into motion. Leak increases [Ca]d which, in turn, promotes spontaneous openings of other RyR channels through CICR. This feedback process continues leading to greater instability of the RyR2 array (exhibited as increased spontaneous activity) until full spontaneous release is elicited in the form of a Ca wave. Figure 4 shows evidence for this as myocytes which generated SCaW events tended to have higher [Ca]d. This SR unloading via waves would reduce [Ca]T to subthreshold levels and stabilize CICR (e.g. by preventing alternans).
However, spontaneous waves are shown to induce significant membrane depolarizations in single ventricular myocytes and in whole heart preparations [21, 22]. This may be exaggerated in HF cells for a number of reasons. Because NCX expression is substantially increased in HF the system will have increased sensitivity to changes in [Ca]i. Further, the inward rectifier potassium current (IK1) has been shown to be reduced by nearly 50% of normal in HF, destabilizing the resting membrane potential [17]. Lastly, the Ca needed to activate this current could be supplied by the observed increased diastolic SR Ca leak in HF. Localized spontaneous release events such as Ca sparks could act as a proximal source of this activating Ca. However, the magnitude and duration of such events would not likely produce a significant membrane depolarization. Ca waves, on the other hand, illicit a near full Ca release from the SR. Such an abrupt, large rise in [Ca]i can activate INCX with enough amplitude to depolarize the membrane and locally reach threshold ultimately causing a spontaneous AP [23]. While such events were rare in this study, SCaWs were observed in both NF and HF which showed similar characteristics (Δ[Ca]T, full duration half maximum, d[Ca]T/dt) to those observed in electrically stimulated transients (see supplementary data figure 1S and 2S). This suggests that a full, electrically unified release had occurred. Myocytes isolated from failing hearts exhibit increased leak even without β-AR stimulation [5, 24]. However, because [Ca]SRT is low in these HF myocytes, the smaller local events may be insufficient to propagate as Ca waves. As we see here, for the higher SR Ca leak in HF to translate to waves, it may require SERCA stimulation and additional RyR sensitivity. Working concurrently in HF, CaMKII-dependent phosphorylation of the RyR could provide the arrhythmogenic substrate while PKA stimulation of SERCA could provide the requisite Ca overload.
During HF, the sympathetic drive on the heart becomes chronic. The heart’s attempt to maintain contractility results in the sustained stimulation of the various components of the EC coupling machinery. One pathological side-effect of this is the increased propensity for spontaneous waves, and arrhythmogenesis. In the clinical setting, 50% of patients who exhibited sustained ventricular tachycardia did so as a result of a focal nonreentrant mechanism [6]. Follow up work showed this focal mechanism to mediated through the NCX [17]. The time during which lethal arrhythmias appear is often linked with times of increased cardiac demand such as exercise or stress. Our results reinforce this observation. HF myocytes did not have a high tendency to exhibit SCaWs under control, or non-stressed conditions. However, when β-AR drive was stimulated by ISO a nearly 7-fold increase in SCaW activity was observed. This increase in activity occurred with no rise in [Ca]SRT (figure 5). This emphasizes that SR Ca leak limits [Ca]SRT, and that CaMKII block by KN-93 increases [Ca]SRT.
Isoproterenol Acts to Lower the Threshold of Spontaneous Ca Release
We believe this observation reveals something fundamental about the nature of arrhythmogenesis. When phosphorylated, the RyR2 may become increasingly sensitive to Ca leading to an increase of channel activity at rest, thereby supplying a potential arrhythmogenic substrate [11]. This would mean that at similar SR Ca loads a myocyte with an increased phosphorylation of its RyR2 array would exhibit appreciably more spontaneous Ca release. Release would continue until a sufficient amount of Ca was extruded out of the myocyte, establishing a lower steady-state level of Ca in which spontaneous activity was minimized. This type of autoregulation at the level of the individual myocyte was unmasked in a series of elegant studies by Eisner et al [25, 26]. This mechanism shares similar characteristics with the store-overload-induced Ca2+ release (SOICR) hypothesis proposed by Chen and colleagues explaining their observations in mice with RyR2 mutations leading to catecholaminergic polymorphic ventricular tachycardia [27]. In both frameworks SCaWs occur when [Ca]SRT rises above a certain threshold for activation of the RyR. Anything that would increase the RyR sensitivity to Ca would effectively lower this threshold.
We believe this is the root cause of the observed increase in SCaW activity in failing myocytes stimulated with ISO. Using a back-phosphorylation assay, we have previously shown enhanced but incomplete phosphorylation of the RyR in this HF model [5]. Our data support a mechanism in which ISO stimulation may result in further phosphorylation of the RyR array, further increasing the channels’ sensitivity to Ca, thereby lowering threshold for SCaW generation. When matching for load (Figure 6) the application of ISO increased SCaW activity in failing myocytes. More activity at the same Ca load is indicative of an increase in Ca sensitivity. This could explain why ISO induced a large increase in SCaW activity without an increase in [Ca]SRT (Figure 5). This β-AR dependent mechanism of arrhythmogenic Ca2+ waves may partially explain the efficacy of β-blockers in the clinical setting.
We propose that by inhibiting CaMKII the RyR becomes less sensitive to Ca and thereby restores the threshold for spontaneous release to a level comparable with control (or even higher). The result—as was observed—would be augmented SR Ca loading with a minimized propensity for SCaW activity (Figures 2, 5 and 6).
A Limited Role for PKA in Arrhythmogenesis in Failing Hearts
The observed increase in SR Ca load-independent SCaW activity in NF myocytes could be attributed to CaMKII activity; such was not the case in HF myocytes. While CaMKII clearly plays the dominant role in HF, a lesser effect was attributable to PKA (Figures 5 & 6). This observation is consistent with data from both our lab and others. Using the same rabbit HF model as here, Ai et al[5] showed a modest increase in RyR2 phosphorylation at Ser2809. This serine residue on RyR2 has both PKA- and CaMKII-specificity [14, 28]. It has been demonstrated that PKA-dependent phosphorylation of RyR2 at this residue increases the Ca-sensitivity and spontaneous activity of the channel [14]. Using spontaneously hypertensive rats, Chen-Izu et al[29] recently demonstrated that Ser2808 (Ser2809 analogue in rat) becomes hyperphosphorylated only in the late stages of HF, suggesting PKA regulation of the RyR might have a temporal component associated with the severity of failure [29]. The PKA-dependent effect observed in this report is consistent with this data as the rabbits used here are in the later stages of HF [18]. While no PKA-dependent increase in SR Ca leak was observed using our leak assessment protocol in HF,[5] we cannot rule out a subtle shift in Ca sensitivity leading to the effect observed here.
The possibility that temperature may be affecting these results must be addressed. First, it is likely that the observed delay to the first SCaW in all treatments is temperature dependent. Increasing the temperature from 25° to 35° results in a Q10 of 2.5–2.6 for the SERCA [30]. This would increase the time needed to load the SR to a superthreshold [Ca]SRT necessary to induce spontaneous release.
It is known that less than physiological temperature causes [Na]i to rise [31]. This [Na]i leads to increased [Ca]SRT [32]. Since CaMKII is activated by increases in Ca, concern arises that temperature may be preferentially activating CaMKII and biasing the results. We do not believe this to be the case. First, in assessing [Ca]d we found no evidence for cytosolic Ca overload amongst any treatments (see Supplement for data and discussion, Figure 6S).
We further investigated the effect temperature may play in a series of leak experiments. We hypothesized that CaMKII was being preferentially activated due to temperature and this activation would lead to a measurable increase in leak without ISO stimulation. This was not the case. All three experimental groups—control, control + KN93, and control + H89—did not vary. (See Figures 7S and 8S in Supplement). This suggests that neither kinase is preferentially activated.
Further support of this conclusion is found in the experiments using forskolin to directly stimulate PKA production (Figures 2 & 3). Here, at room temperature, we have robustly activated PKA, yet no effect on SCaW activity was observed. We conclude from this data that even if CaMKII was preferentially activated under the experimental conditions—which we believe it is not—PKA-dependent effects on RyR still play little to no role in SCaW generation.
We conclude that ISO-dependent SCaWs in both failing and non-failing ventricular myocytes are CaMKII-dependent. Stimulation by ISO acts to increase the Ca sensitivity of the RyR array, lowering the threshold for spontaneous release and provides an arrhythmogenic substrate. Simultaneously ISO stimulates SERCA activity and facilitates SR Ca loading potentially leading to Ca overload. Coupling together the arrhythmogenic substrate with Ca overload induces conceivably lethal SCaWs.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of Cited References
- 1.Packer M. Sudden unexpected death in patients with congestive heart failure: a second frontier. Circulation. 1985;72:681–685. doi: 10.1161/01.cir.72.4.681. [DOI] [PubMed] [Google Scholar]
- 2.Sipido KR, Stankovicova T, Flameng W, Vanhaecke J, Verdonck F. Frequency dependence of Ca2+ release from the sarcoplasmic reticulum in human ventricular myocytes from end-stage heart failure. Cardiovasc Res. 1998;37:478–488. doi: 10.1016/s0008-6363(97)00280-0. [DOI] [PubMed] [Google Scholar]
- 3.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ.Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 4.Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- 5.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ.Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 6.Pogwizd SM, Hoyt RH, Saffitz JE, Corr PB, Cox JL, Cain ME. Reentrant and focal mechanisms underlying ventricular tachycardia in the human heart. Circulation. 1992;86:1872–1887. doi: 10.1161/01.cir.86.6.1872. [DOI] [PubMed] [Google Scholar]
- 7.Lederer WJ, Tsien RW. Transient inward current underlying arrhythmogenic effects of cardiotonic steroids in Purkinje fibres. J Physiol. 1976;263:73–100. doi: 10.1113/jphysiol.1976.sp011622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bers DM, Pogwizd SM, Schlotthauer K. Upregulated Na/Ca exchange is involved in both contractile dysfunction and arrhythmogenesis in heart failure. Basic Res.Cardiol. 2002;97 Suppl 1:I36–I42. doi: 10.1007/s003950200027. [DOI] [PubMed] [Google Scholar]
- 9.Koster OF, Szigeti GP, Beuckelmann DJ. Characterization of a [Ca2+]i-dependent current in human atrial and ventricular cardiomyocytes in the absence of Na+ and K+ Cardiovasc.Res. 1999;41:175–187. doi: 10.1016/s0008-6363(98)00202-8. [DOI] [PubMed] [Google Scholar]
- 10.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Kluwer Academic Publishers; 2001. [Google Scholar]
- 11.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang D, Chen W, Wang R, Zhang L, Chen SR. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc.Natl.Acad.Sci.U.S.A. 2007;104:18309–18314. doi: 10.1073/pnas.0706573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venetucci LA, Trafford AW, Eisner DA. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ Res. 2007;100:105–111. doi: 10.1161/01.RES.0000252828.17939.00. [DOI] [PubMed] [Google Scholar]
- 14.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 15.Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ.Res. 2007;100:391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 16.Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann KO, J. Backs MK, Olson EN, Brown JH, Neef S, Maier SKG, Maier LS. Calcium/Calmodulin-Dependent Protein Kinase II Contributes to Cardiac Arrhythmogenesis in Heart Failure. Circulation: Heart Failure. 2009;2:664–675. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ.Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 18.Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation. 1995;92:1034–1048. doi: 10.1161/01.cir.92.4.1034. [DOI] [PubMed] [Google Scholar]
- 19.Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ.Res. 2002;91:594–600. doi: 10.1161/01.res.0000036914.12686.28. [DOI] [PubMed] [Google Scholar]
- 20.Shannon TR, Bers DM. Assessment of intra-SR free [Ca] and buffering in rat heart. Biophys.J. 1997;73:1524–1531. doi: 10.1016/S0006-3495(97)78184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, Reiken S, Wronska A, Drew LJ, Ward CW, Lederer WJ, Kass RS, Morley G, Marks AR. Leaky Ca release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008 doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujiwara K, Tanaka H, Mani H, Nakagami T, Takamatsu T. Burst Emergence of Intracellular Ca2+ Waves Evokes Arrhythmogenic Oscillatory Depolarization via the Na+-Ca2+ Exchanger Simultaneous Confocal Recording of Membrane Potential and Intracellular Ca2+ in the Heart. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.108.176677. [DOI] [PubMed] [Google Scholar]
- 23.Schlotthauer K, Bers DM. Sarcoplasmic reticulum Ca(2+) release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ Res. 2000;87:774–780. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- 24.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ.Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 25.Dibb KM, Graham HK, Venetucci LA, Eisner DA, Trafford AW. Analysis of cellular calcium fluxes in cardiac muscle to understand calcium homeostasis in the heart. Cell Calcium. 2007;42:503–512. doi: 10.1016/j.ceca.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Trafford AW, Sibbring GC, Diaz ME, Eisner DA. The effects of low concentrations of caffeine on spontaneous Ca release in isolated rat ventricular myocytes. Cell Calcium. 2000;28:269–276. doi: 10.1054/ceca.2000.0156. [DOI] [PubMed] [Google Scholar]
- 27.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SR. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc.Natl.Acad.Sci.U.S.A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J.Biol.Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- 29.Chen-Izu Y, Ward CW, Stark JW, Banyasz T, Sumandea MP, Balke CW, Izu LT, Wehrens XH. Phosphorylation of RyR2 and shortening of RyR2 cluster spacing in spontaneously hypertensive rat with heart failure. Am.J.Physiol Heart Circ.Physiol. 2007 doi: 10.1152/ajpheart.00562.2007. [DOI] [PubMed] [Google Scholar]
- 30.Puglisi JL, Bassani RA, Bassani JW, Amin JN, Bers DM. Temperature and relative contributions of Ca transport systems in cardiac myocyte relaxation. Am J Physiol. 1996;270:H1772–H1778. doi: 10.1152/ajpheart.1996.270.5.H1772. [DOI] [PubMed] [Google Scholar]
- 31.Eisner DA, Lederer WJ. Characterization of the electrogenic sodium pump in cardiac Purkinje fibres. J Physiol. 1980;303:441–474. doi: 10.1113/jphysiol.1980.sp013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shattock MJ, Bers DM. Inotropic response to hypothermia and the temperature-dependence of ryanodine action in isolated rabbit and rat ventricular muscle: implications for excitation-contraction coupling. Circ Res. 1987;61:761–771. doi: 10.1161/01.res.61.6.761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.