Abstract
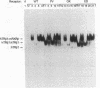
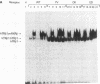
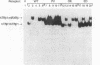
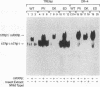
Mutations in the human beta thyroid hormone receptor (h-TR beta) gene are associated with the syndrome of generalized resistance to thyroid hormone. We investigated the interaction of three h-TR beta 1 mutants representing different types of functional impairment (kindreds ED, OK, and PV) with different response elements for 3,3',5-triiodothyronine (T3) and with retinoid X receptor beta (RXR beta). The mutant receptors showed an increased tendency to form homodimers on a palindromic T3-response element (TREpal), a direct repeat (DR + 4), and an inverted palindrome (TRElap). On TRElap, wild type TR binding was decreased by T3, while the mutant receptors showed a variably decreased degree of dissociation from TRElap in response to T3. The extent of dissociation was proportional to their T3 binding affinities. RXR beta induced the formation of h-TR beta 1:RXR beta heterodimers equally well for mutants and the wild type h-TR beta 1 on these T3 response elements. However, the T3-dependent increase in heterodimerization with RXR beta was absent or reduced for the mutant TRs. Transient transfection studies indicated that the dominant negative potency was several-fold more pronounced on the TRElap as compared to TREpal or DR + 4. In CV-1 and HeLa cells, transfection of RXR beta could not reverse the dominant negative action. These results demonstrate that the binding of mutant h-TRs to DNA, as well as their dominant negative potency, are TRE dependent. In addition, competition for DNA binding, rather than for limiting amounts of RXR beta, is likely to mediate the dominant negative action.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baniahmad A., Steiner C., Köhne A. C., Renkawitz R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990 May 4;61(3):505–514. doi: 10.1016/0092-8674(90)90532-j. [DOI] [PubMed] [Google Scholar]
- Chatterjee V. K., Nagaya T., Madison L. D., Datta S., Rentoumis A., Jameson J. L. Thyroid hormone resistance syndrome. Inhibition of normal receptor function by mutant thyroid hormone receptors. J Clin Invest. 1991 Jun;87(6):1977–1984. doi: 10.1172/JCI115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsetti A., Desvergne B., Hallenbeck P., Robbins J., Nikodem V. M. Characterization of myelin basic protein thyroid hormone response element and its function in the context of native and heterologous promoter. J Biol Chem. 1992 Aug 5;267(22):15784–15788. [PubMed] [Google Scholar]
- Flug F., Copp R. P., Casanova J., Horowitz Z. D., Janocko L., Plotnick M., Samuels H. H. cis-acting elements of the rat growth hormone gene which mediate basal and regulated expression by thyroid hormone. J Biol Chem. 1987 May 5;262(13):6373–6382. [PubMed] [Google Scholar]
- Forman B. M., Casanova J., Raaka B. M., Ghysdael J., Samuels H. H. Half-site spacing and orientation determines whether thyroid hormone and retinoic acid receptors and related factors bind to DNA response elements as monomers, homodimers, or heterodimers. Mol Endocrinol. 1992 Mar;6(3):429–442. doi: 10.1210/mend.6.3.1316541. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Samuels H. H. Interactions among a subfamily of nuclear hormone receptors: the regulatory zipper model. Mol Endocrinol. 1990 Sep;4(9):1293–1301. doi: 10.1210/mend-4-9-1293. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M., Devary O. V., Rosenfeld M. G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988 Jul 29;54(3):313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. L., Marks M. S., Lippoldt R. E., Ozato K., Nikodem V. M. Heterodimerization of thyroid hormone (TH) receptor with H-2RIIBP (RXR beta) enhances DNA binding and TH-dependent transcriptional activation. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5572–5576. doi: 10.1073/pnas.89.12.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck P. L., Phyillaier M., Nikodem V. M. Divergent effects of 9-cis-retinoic acid receptor on positive and negative thyroid hormone receptor-dependent gene expression. J Biol Chem. 1993 Feb 25;268(6):3825–3828. [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Holloway J. M., Glass C. K., Adler S., Nelson C. A., Rosenfeld M. G. The C'-terminal interaction domain of the thyroid hormone receptor confers the ability of the DNA site to dictate positive or negative transcriptional activity. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8160–8164. doi: 10.1073/pnas.87.20.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- Magner J. A., Petrick P., Menezes-Ferreira M. M., Stelling M., Weintraub B. D. Familial generalized resistance to thyroid hormones: report of three kindreds and correlation of patterns of affected tissues with the binding of [125I] triiodothyronine to fibroblast nuclei. J Endocrinol Invest. 1986 Dec;9(6):459–470. doi: 10.1007/BF03346968. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Borgmeyer U., Heyman R. A., Zhou J. Y., Ong E. S., Oro A. E., Kakizuka A., Evans R. M. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992 Mar;6(3):329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- Marks M. S., Hallenbeck P. L., Nagata T., Segars J. H., Appella E., Nikodem V. M., Ozato K. H-2RIIBP (RXR beta) heterodimerization provides a mechanism for combinatorial diversity in the regulation of retinoic acid and thyroid hormone responsive genes. EMBO J. 1992 Apr;11(4):1419–1435. doi: 10.1002/j.1460-2075.1992.tb05187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier C. A., Dickstein B. M., Ashizawa K., McClaskey J. H., Muchmore P., Ransom S. C., Menke J. B., Hao E. H., Usala S. J., Bercu B. B. Variable transcriptional activity and ligand binding of mutant beta 1 3,5,3'-triiodothyronine receptors from four families with generalized resistance to thyroid hormone. Mol Endocrinol. 1992 Feb;6(2):248–258. doi: 10.1210/mend.6.2.1569968. [DOI] [PubMed] [Google Scholar]
- Nagaya T., Madison L. D., Jameson J. L. Thyroid hormone receptor mutants that cause resistance to thyroid hormone. Evidence for receptor competition for DNA sequences in target genes. J Biol Chem. 1992 Jun 25;267(18):13014–13019. [PubMed] [Google Scholar]
- O'Donnell A. L., Rosen E. D., Darling D. S., Koenig R. J. Thyroid hormone receptor mutations that interfere with transcriptional activation also interfere with receptor interaction with a nuclear protein. Mol Endocrinol. 1991 Jan;5(1):94–99. doi: 10.1210/mend-5-1-94. [DOI] [PubMed] [Google Scholar]
- Parrilla R., Mixson A. J., McPherson J. A., McClaskey J. H., Weintraub B. D. Characterization of seven novel mutations of the c-erbA beta gene in unrelated kindreds with generalized thyroid hormone resistance. Evidence for two "hot spot" regions of the ligand binding domain. J Clin Invest. 1991 Dec;88(6):2123–2130. doi: 10.1172/JCI115542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentoumis A., Chatterjee V. K., Madison L. D., Datta S., Gallagher G. D., Degroot L. J., Jameson J. L. Negative and positive transcriptional regulation by thyroid hormone receptor isoforms. Mol Endocrinol. 1990 Oct;4(10):1522–1531. doi: 10.1210/mend-4-10-1522. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Miyamoto T., Refetoff S., DeGroot L. J. Dominant negative transcriptional regulation by a mutant thyroid hormone receptor-beta in a family with generalized resistance to thyroid hormone. Mol Endocrinol. 1990 Dec;4(12):1988–1994. doi: 10.1210/mend-4-12-1988. [DOI] [PubMed] [Google Scholar]
- Sakurai A., Takeda K., Ain K., Ceccarelli P., Nakai A., Seino S., Bell G. I., Refetoff S., DeGroot L. J. Generalized resistance to thyroid hormone associated with a mutation in the ligand-binding domain of the human thyroid hormone receptor beta. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8977–8981. doi: 10.1073/pnas.86.22.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfelder H. J., Hauser P., Nakayama Y., Radovick S., McClaskey J. H., Taylor T., Weintraub B. D., Wondisford F. E. Thyrotropin-releasing hormone regulation of human TSHB expression: role of a pituitary-specific transcription factor (Pit-1/GHF-1) and potential interaction with a thyroid hormone-inhibitory element. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3130–3134. doi: 10.1073/pnas.88.8.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Sakurai A., DeGroot L. J., Refetoff S. Recessive inheritance of thyroid hormone resistance caused by complete deletion of the protein-coding region of the thyroid hormone receptor-beta gene. J Clin Endocrinol Metab. 1992 Jan;74(1):49–55. doi: 10.1210/jcem.74.1.1727829. [DOI] [PubMed] [Google Scholar]
- Usala S. J., Bale A. E., Gesundheit N., Weinberger C., Lash R. W., Wondisford F. E., McBride O. W., Weintraub B. D. Tight linkage between the syndrome of generalized thyroid hormone resistance and the human c-erbA beta gene. Mol Endocrinol. 1988 Dec;2(12):1217–1220. doi: 10.1210/mend-2-12-1217. [DOI] [PubMed] [Google Scholar]
- Usala S. J., Tennyson G. E., Bale A. E., Lash R. W., Gesundheit N., Wondisford F. E., Accili D., Hauser P., Weintraub B. D. A base mutation of the C-erbA beta thyroid hormone receptor in a kindred with generalized thyroid hormone resistance. Molecular heterogeneity in two other kindreds. J Clin Invest. 1990 Jan;85(1):93–100. doi: 10.1172/JCI114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Yen P. M., Darling D. S., Carter R. L., Forgione M., Umeda P. K., Chin W. W. Triiodothyronine (T3) decreases binding to DNA by T3-receptor homodimers but not receptor-auxiliary protein heterodimers. J Biol Chem. 1992 Feb 25;267(6):3565–3568. [PubMed] [Google Scholar]
- Yen P. M., Sugawara A., Chin W. W. Triiodothyronine (T3) differentially affects T3-receptor/retinoic acid receptor and T3-receptor/retinoid X receptor heterodimer binding to DNA. J Biol Chem. 1992 Nov 15;267(32):23248–23252. [PubMed] [Google Scholar]
- Yen P. M., Sugawara A., Refetoff S., Chin W. W. New insights on the mechanism(s) of the dominant negative effect of mutant thyroid hormone receptor in generalized resistance to thyroid hormone. J Clin Invest. 1992 Nov;90(5):1825–1831. doi: 10.1172/JCI116058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zenke M., Muñoz A., Sap J., Vennström B., Beug H. v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell. 1990 Jun 15;61(6):1035–1049. doi: 10.1016/0092-8674(90)90068-p. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Lehmann J., Hoffmann B., Dawson M. I., Cameron J., Graupner G., Hermann T., Tran P., Pfahl M. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature. 1992 Aug 13;358(6387):587–591. doi: 10.1038/358587a0. [DOI] [PubMed] [Google Scholar]