Abstract
Background
In infected lungs of the cystic fibrosis (CF) patients, opportunistic pathogens and mutated cystic fibrosis transmembrane conductance regulator protein (CFTR) contribute to chronic airway inflammation that is characterized by neutrophil/macrophage infiltration, cytokine release and ceramide accumulation. We sought to investigate CF lung inflammation in the alveoli.
Methods
Lung tissue from 14 CF patients and four healthy individuals was analyzed for numbers of effector cells, elastin and collagen concentrations, inflammatory markers and density of Pseudomonas aeruginosa. Additionally, desmosine and isodesmosine concentrations were determined in 52 urine specimens from CF patients to estimate the burden of elastase activities in respiratory secretions.
Results
Elastin concentration was significantly decreased and collagen significantly increased in CF alveolar tissues as compared to age-matched, healthy individuals. Elastin split products were significantly increased in urine samples from patients with CF and correlated inversely with age, indicating local tissue remodelling due to elastin degradation by unopposed proteolytic enzymes. Alveolar inflammation was also characterized by a significant cell infiltration of neutrophils, macrophages and T cells, extensive nuclear factor-κB and insulin-like growth factor-1 activation in various cell types and increased intercellular adhesion molecule-1 expression, and increased numbers of myofibroblasts. Additionally, ceramide accumulated in type II alveolar epithelial cells, lacking CFTR. P. aeruginosa organisms were rarely present in inflamed alveoli.
Conclusions
Chronic inflammation and remodeling is present in alveolar tissues of the CF lung and needs to be addressed by anti-inflammatory therapies.
Keywords: Ceramide, pathophysiology, elastin, collagen, neutrophils, macrophages
1. Introduction
Functional deficiency of the CF transmembrane conductance regulator (CFTR) leads to airway surface liquid depletion due to abnormal ion flux and impaired mucociliary clearance in the CF lung [1-3]. Poor mucociliary clearance is thought to favor lung infection with opportunistic bacterial pathogens such as Staphylococcus aureus and Pseudomonas aeruginosa [4, 5], which leads to a pronounced influx of neutrophils into the airways [6].
Neutrophil-dominated airway inflammation has been implicated as a key feature of airway remodeling and bronchiectasis. This process is mediated by the production of reactive oxygen species and serine and metallo–proteases, which are associated with lung fibrosis [7]. Bronchial wall thickening, bronchiectasis and lung tissue fibrosis have been demonstrated in CF patients [8-11]. Although bronchial inflammation has been extensively studied [4, 12, 13], little is known about inflammation in the alveolar space of CF patients. A study of clinical findings and lung pathology in lungs explanted from children concluded that inflammation is centered in the airways, rather than the alveoli, and that bronchial inflammation and damage was the real “Achilles heel” of CF lung disease and that airway obstruction, chronic endobronchial infection and inflammation interact continually and eventually culminate in lung destruction [14]. This picture of CF lung pathology might suggest that the periphery of the lung is less affected in the chronic disease process.
We questioned this notion using qualitative and quantitative immunofluorescence and immunohistology methods to assess the presence of innate immune cells, particularly neutrophils, alveolar macrophages and CD3-positive T cells, and myofibroblasts in the alveolar tissue of CF patients post lung transplantation. Additionally we sought to know whether the pro-inflammatory molecules nuclear factor-κB (NF-κB), insulin-like growth factor-1 (IGF-1) and intercellular adhesion molecule-1 (ICAM-1) are expressed in the periphery of the CF lungs. Furthermore, we addressed the issue of tissue remodelling in CF alveoli with regard to elastin cleavage and collagen substitution. Finally, we pursued the notion that alveolar inflammation might be linked to CFTR dysfunction [15-18], particularly to the sphingolipid metabolism [18]. We therefore investigated the expression of the pro-inflammatory molecule ceramide in alveolar type II cells which express CFTR in healthy individuals [20-23]. Here we present evidence of extensive inflammation and tissue remodeling in end-stage CF lungs which warrants anti-inflammatory treatment strategies for CF patients.
2. Methods
2.1. Patients
Explanted lung tissue was obtained from 14 CF patients who were chronically infected with P. aeruginosa prior to undergoing lung transplantation (mean age±SD: 28±8 yrs, range: 13-38 yrs) (CF Centre of the University of North Carolina, Chapel Hill, USA, University of Southern California, Los Angeles, USA, Department of International Health, Immunology and Microbiology, University of Copenhagen, Copenhagen, Denmark). Lung tissues from the contralateral donor lung of four otherwise healthy individuals (mean age: 26±8 yrs; range: 18-32 yrs) were investigated as controls (University of Southern California, Los Angeles, USA). The genotypes of 4/14 CF patients were available. Three patients were ΔF508 homozygous and one patient carried the ΔF508 an the 1303K/W128X mutation. Study protocols were approved by the respective local Human Subjects Committees.
2.2. Immunohistochemical staining procedures
Tissue blocks of ∼10×10×10 mm were cut and stored at 4°C in DMEM/HAM's F12 medium (mixed 1:1) (Gibco, Eggenstein, Germany), supplemented with nystatin (10,000 units/ml, Gibco) and penicillin/streptomycin (50 μg/ml, Gibco). Blocks were washed in phosphate-buffered saline (PBS), pH 7.4, fixed in freshly prepared 4% or 10% formaldehyde (Sigma, Steinheim, Germany), embedded in paraffin or were shock frozen in liquid nitrogen. Blocks were cut in 5 μm thin sections (Microtome Jung HN 40, Leica, Frankfurt, Germany) and transferred to glycerin-coated slides (Langenbrinck, Emmendingen, Germany). Prior to the stainings, sections were deparaffinized using xylol (Merck, Darmstadt, Germany) and decreasing (100% to 70%) alcohol concentrations. Overall three to five blocks of alveolar lung tissue from different parts of the lung periphery of 14 CF patients and 4 healthy individuals were used for this study. From each lung tissue block every 6th or 10th section was stained. Consequently 90 to 150 sections of each CF patient and healthy individual were analyzed.
For immunohistochemical staining the Alkaline Phosphatase (AP) or Horseradish Peroxidase (HRP) system (Dako, Hamburg, Germany) was used. Tissue sections were incubated for 30 min with swine or goat serum, diluted 1:10 in PBS/Tween 20 (Sigma) to block non-specific binding of rabbit IgG antibodies directed against human elastin, collagen type I (Biotrend, Köln, Germany), human insulin-like growth factor I (hIGF-1, Mediagnost, Reutlingen, Germany), P. aeruginosa alginate and to block non-specific binding of monoclonal antibodies directed against human neutrophil elastase, CD3 and against alveolar macrophage CD68 receptors (Dako, diluted 1:100). After incubation with the appropriate second antibody and washing, sections were embedded in Faramount aqueous mounting medium (Dako). Controls for non-specific staining were performed without adding the first antibody.
For NF-κB and ICAM-1 staining, paraffin sections were incubated with Proteinase K for 10 min at 37 °C. After repeated washings the sections were incubated with Methanol-H2O2 for 10 min at room temperature and washed three times. The sections were than incubated with swine serum (dilution 1:10) for 30 min and after with the monoclonal antibody against NF-κB (diluted 1:50; BD Bioscience Pharmingen, Heidelberg, Germany) or the monoclonal antibody against ICAM1 (diluted 1:10; BD Bioscience Pharmingen, Heidelberg, Germany) for 1h at room temperature. After washing the sections were incubated with the second antibody (goat anti mouse IgG, HRP conjugated; diluted 1:100) for 1 h. The sections were washed and the dark brown-red colour was developed using the AEC peroxidase substrate.
For quantitative determination of neutrophil and AM numbers, and elastin and collagen concentrations of alveolar tissue from CF patients and from control lungs, 10 to 20 digitalized images from every section were taken (magnification: 200; area: 400×600 μm). Images containing blood vessels or erythrocyte-filled alveoli were excluded. The number of neutrophils and CD3 positive cells were determined per mm of septum length and the content of elastin and collagen fibers were determined in percent per mm of septum length. Macrophages located in alveolar septa and in alveolar lumina were counted and expressed as macrophages/mm2 lung tissue. Between 73,000 μm and 173,000 μm of septa/patient and more than 1,000 alveoli of CF patients and controls were studied.
For the presence of P. aeruginosa, 1,000 digitalized images were taken from sections of peripheral lung tissue from CF patients (magnification: 1,000). Sections were analysed with an Axioplan microscope (Zeiss, Oberkochen, Germany) using Axiovision (Zeiss).
For immunofluorescence staining frozen sections of alveolar tissue from CF patients, and healthy individuals were blocked with goat serum (diluted 1:10, Dako) and double stained with a polyclonal antibody against surfactant protein C (diluted 1:500, Natu Tec GmbH, Frankfurt, Germany) for 1h at room temperature and a monoclonal antibody against CFTR (diluted 1:200, Mayo Clinic, Scottsdale, Arizona, USA) over night at 4°C or a monoclonal antibody against ceram ide clone MID 15B4, diluted 1:50, Alexis, Grünberg, Germany) for 1h at room temperature. Antibody binding was detected with Cy™2-conjugated goat anti mouse (diluted 1:100, Dianova, Hamburg, Germany) and Cy™3 – conjugated goat anti rabbit (diluted 1:800, Dianova). DNA was stained with DAPI (5 μg/ml, Sigma). Sections were visualized using a Axioplan microscope (Zeiss, Oberkochen, Germany) or a confocal microscope (Zeiss). Ceramide expression was quantitatively assessed in a blinded fashion and using the MetaMorph software (Visitron Systems GmbH, Puchheim, Germany).
2.3. Determination or elastin split products in CF urine specimens
To address the question whether matrix remodeling is present in lungs of CF patients with younger ages, we also quantified elastin split products in patient's urines. Urine specimens were obtained from 52 CF patients (mean age±SD: 22±11 yrs, range: 6-51 yrs), attending different CF centers (Department of Medicine, University of Wisconsin, Madison, USA; Children's Hospitals, University of Essen, and University of Düsseldorf, Germany). Written informed consent was obtained from all patients or their parents. Study protocols were approved by the respective local Human Subjects Committees. Desmosine (DES) and isodesmosine (IDES) concentrations were measured in mid-stream urine samples (200 μl total volume), and neutrophil elastase activity was determined in supernatant fluids of expectorated sputum specimens from CF patients using micellar electrokinetic chromatography [24]. DES and IDES values are expressed as μg/g creatinine, and MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide was used as substrate for neutrophil elastase. One unit of neutrophil elastase was defined as the conversion of 1 μmol p-nitroanilide/min.
2.4. Electron microscopy
For transmission electron microscopy, formalin fixed tissue blocks of peripheral lung tissue from a CF patient and a healthy individual were post-fixed with 2.5% glutaraldehyde (Agar Scientific Ltd., Plano GmbH, Wetzlar, Germany), 1% osmium tetroxide (Simec, Zofingen, Switzerland) and 0.5% uranyl acetate (Fluka Chemie GmbH, Sigma-Aldrich, Buchs, Switzerland), dehydrated in a graded series of ethanol and embedded in epon (Fluka), according to standard procedures. Ultrathin (60-80 nm) sections were cut from the tissue blocks, transferred to uncoated 200-mesh copper grids, stained with uranyl acetate and lead citrate (Ultrostain, Leica, Glattbrugg, Switzerland), and examined in a Philips 300 transmission electron microscope (Philips AG, Zürich, Switzerland), operating at 60 kV.
2.5. Statistics
For statistical analysis, raw data were checked for normality using the Shapiro-Wilks-test. Thereafter, significance was calculated using the Student's t-test or the Wilcoxon test. P-values of 0.05 or less were considered significant. The Pearson correlation coefficient r was calculated using the Winstat software (Microsoft, Redmont, Wash., U.S.A.). Results are given as means ± SD.
3. Results
3.1. Inflammatory cells and mediators are greatly increased in alveoli of CF patients
To assess whether innate and adaptive immune cells are present in increased numbers in the periphery of the CF lung, we stained neutrophils, alveolar macrophages and CD3 positive T cells with specific cell markers and determined the cell numbers quantitatively after immunostaining. Neutrophil numbers per mm septum were significantly higher in CF patients compared to controls (Fig. 1A). Neutrophils were exclusively located within alveolar septa in CF and healthy lung tissues. Also the percentages of alveolar macrophage in septae and intraluminally were greatly increased in CF patients compared to controls (Fig. 1B). In CF alveoli, the distribution of both cell types was inhomogeneous: areas with greatly increased cell counts were interspersed with areas with low cell numbers. In addition, CD3-positive T lymphocyte cell numbers were significantly higher in CF alveolar tissues compared to control tissues (Fig. 1C).
Figure 1. Increased numbers of neutrophils, alveolar macrophages and T lymphocytes are present in alveoli of CF patients.
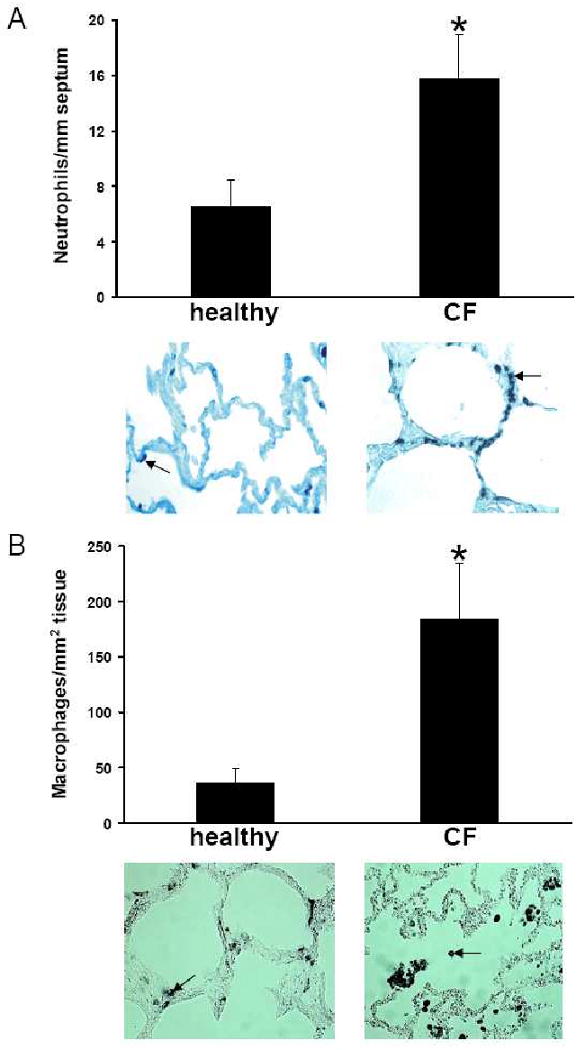
Neutrophils (A, arrow), stained with monoclonal antibodies directed against human neutrophil elastase, in CF alveolar lung tissue and in normal alveolar lung tissue differ significantly (p<0.0001) in numbers. Alveolar macrophages (B, arrow), stained with monoclonal antibodies directed against CD68 receptors, in CF alveolar lung tissue and in normal alveolar lung tissue differ significantly (p<0.0001) in numbers with regard to cells in septa (black bars) and intraluminally (white bars). T lymphocytes (C, arrow), stained with monoclonal antibodies directed against CD3, in CF alveolar lung tissue and in normal alveolar lung tissue differ significantly (p<0.0001) in numbers.
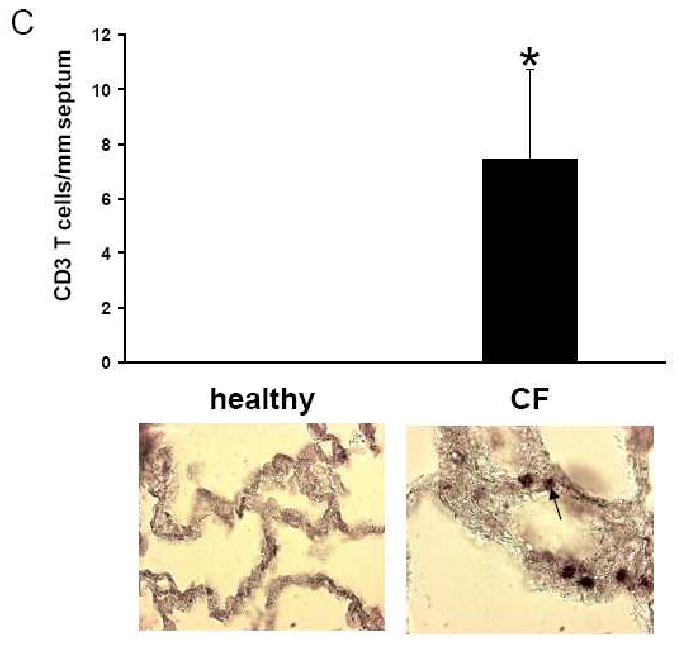
Concentrations of pro-inflammatory cytokines were increased in parallel fashion with innate and adaptive immune cell numbers in alveolar tissues and in the lumen of alveoli of CF patients. Increased expression of the cell surface protein ICAM-1 (Fig. 2 A-D) was demonstrated in CF tissues compared to non-CF control lung tissue. In contrast to lung tissues from healthy individuals, many cells in alveolar septa, presumably macrophages and the lumen of alveoli of CF patients showed nuclear translocation of the transcription factor NF-κB (Fig. 2 E-H).
Figure 2. Inflammatory mediators and transcription factor expression in CF versus normal alveolar lung tissue.
Immunohistochemical detection of ICAM-1 expression in alveolar tissue of a CF patient (A, C) and a healthy individual (B, D). In the CF patient, positively stained (dark brown) ICAM-1 expression was present in blood vessels (A, arrow) and alveolar septae (C, arrow), whereas blood vessels (B) and alveolar septae (D) of a healthy individual were negative. E, G: Immunohistochemical detection of NF-κB in the alveolar tissue of a CF patient. All alveolar macrophages in alveolar lumina (E) and many cells in the alveolar epithelium (G) of CF lungs were positive for NF-κB staining in the nuclei (dark brown spots, arrow). These cells were negative for nuclear NFκB in a healthy control (F, H). Original magnifications: D-J: × 200
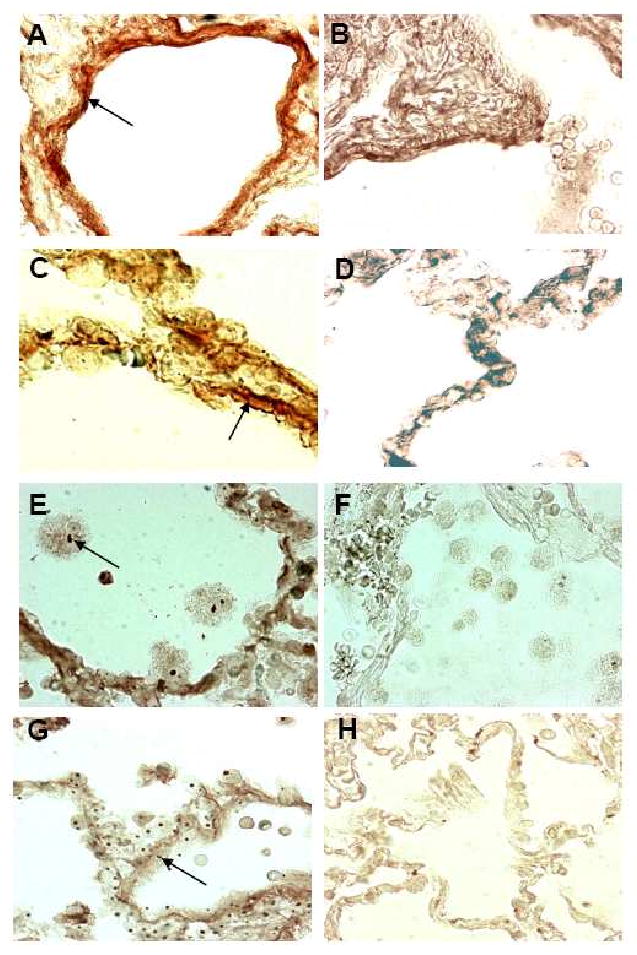
Because activated epithelial cells and macrophages may produce various mediators of inflammation, we stained alveolar tissues of CF patients and controls for the pro-fibrotic growth factor IGF-1. In contrast to lung tissues from healthy individuals, many epithelial cells and macrophages of CF patients stained positive for IGF-1 (Fig. 3 A-D). Furthermore, increased numbers of myofibroblasts were detectable in CF, but not in control tissues (Fig. 3E-H).
Figure 3. IGF-1 and myofibroblast expression in CF versus normal alveolar lung tissue.
IGF-1, stained with a polyclonal antibody (red), differed significantly (p<0.0001) between CF alveolar (A, C) and normal alveolar lung tissue (B, D). Myofibroblasts, stained with an antibody (dark brown) in CF alveolar lung tissue (E, G) and in normal alveolar lung tissue (F, H) differed largely in expression. Original magnifications: A, B, F, H × 400; C, D, E, G × 200.
3.2. Expression of elastin and collagen in CF alveolar septae and elastin split products in CF urine
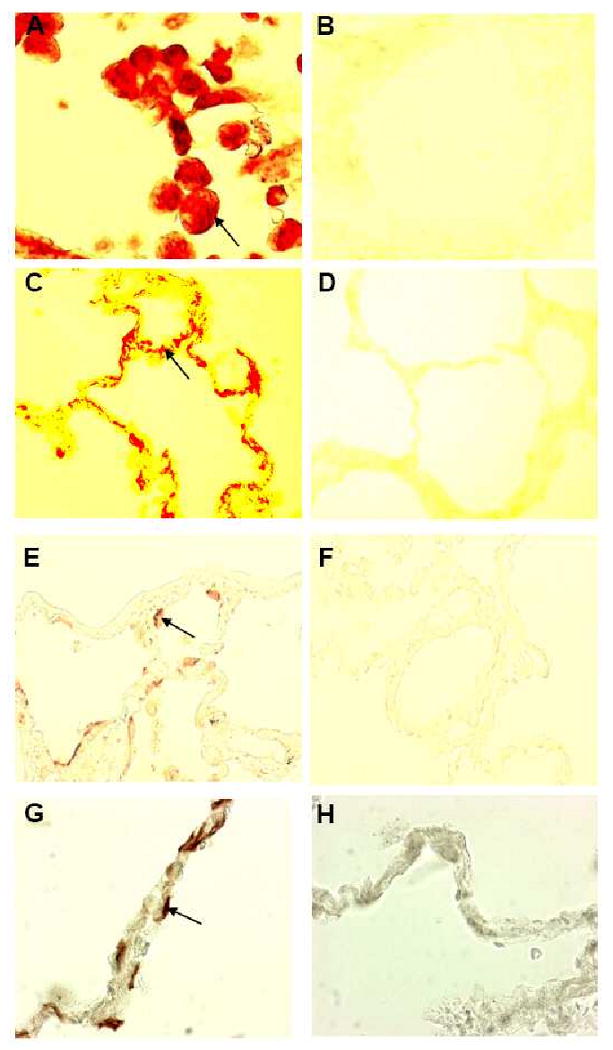
Based on the findings of increased neutrophil/alveolar macrophage numbers of CF alveoli, as well as the increased concentrations of the profibrotic mediator IGF-1 and the collagen producing myofibroblasts, we addressed the question whether matrix remodeling is present in CF alveoli by quantifying α-elastin in alveolar septa and elastin split products in patient's urine specimens. Elastin was significantly reduced in CF alveoli as compared to healthy control lungs (Fig. 4 A-C). Corroborating the extensive loss of elastin, significantly increased desmosine (DES) (23.5 ± 6.1 μg/g creatinine) and isodesmosine (IDES) (20.6 ± 5.5 μg/g creatinine) levels were present in urines of CF patients of all ages as compared to levels of 12 healthy controls (DES: 9.31±2.75 μg/g creatinine, p< 0.0001; IDES: 7.34±1.95 μg/g creatinine, p< 0.0001), published previously [24]. The data reflect ongoing degradation of elastin. When the DES and IDES values in single patients were compared to each other, both markers of elastin degradation were highly correlated (r = 0.977; p<0.0001). Additionally, DES and IDES values were inversely correlated to age of the CF patients (DES r= -0.500, p<0.0001; IDES r= -0.550, p<0.0001) (Fig. 4 D). Because DES and IDES values in CF urines correlated with human neutrophil elastase levels in sputum samples of CF patients (DES: r=0.491, p=0.004; IDES: r=0.555, p=0.001) (Fig. 4 E), this serine proteinase likely contributes to elastin cleavage in CF airways.
Figure 4. Increased elastin degradation and collagen substitution in alveolar septa and increased elastin degradation in urines of CF patients.
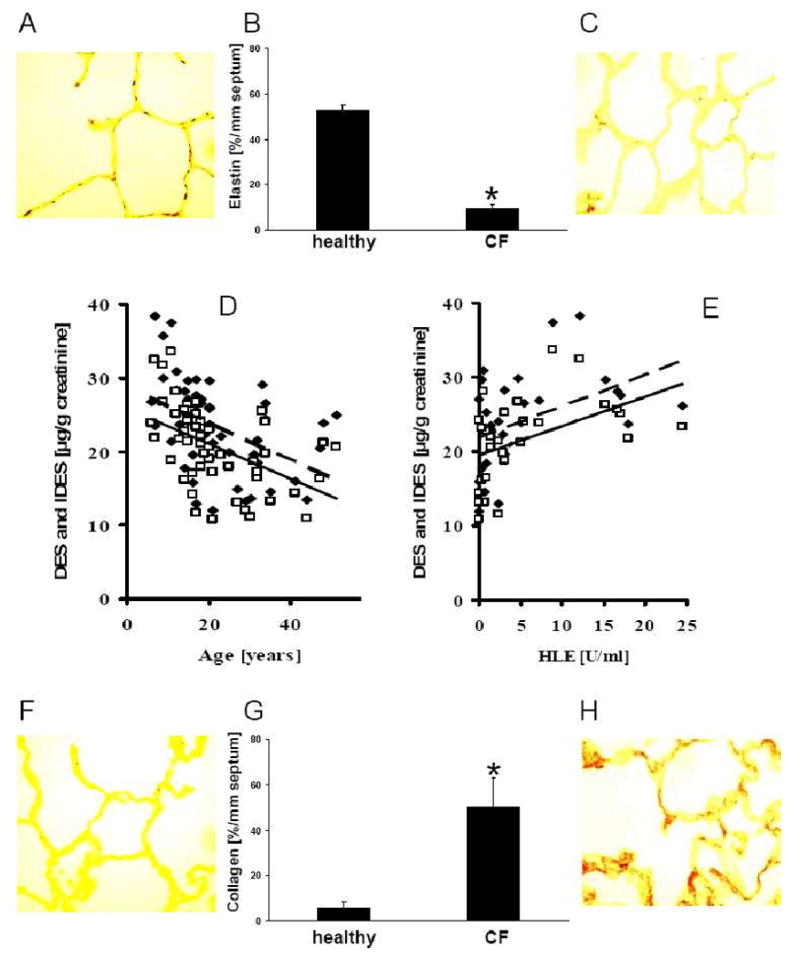
Elastin fibers, stained with a rabbit IgG antibody directed against human α-elastin, in CF alveolar lung tissue (C) and in normal alveolar lung tissue (A) differ significantly (p<0.0001) in concentration (B). In CF patients mean elastin values ± SD were 9.6±1.5% elastin/mm septum (range: 7.6-12.6% elastin/mm septum), and 52.5±2.3% elastin/mm septum (range: 50.3-55.1% elastin/mm septum) in healthy control lungs. Urinary concentrations of the elastin split product desmosine (DES □) and isodesmosine (IDES ◆), determined by micellar electrokinetic chromatography, decrease with age in 52 CF patients (D). Both markers of elastin degradation were highly correlated. DES and IDES concentrations correlate with human neutrophil elastase (HLE) levels in sputum samples of CF patients (E). Collagen fibers, stained with a rabbit IgG antibody directed against human collagen type 1, in CF alveolar lung tissue (H) and in normal alveolar lung tissue (F) differ significantly (p<0.0001) in concentration (G). Mean collagen type 1 expression ± SD in alveolar septa of CF patients was 49.9±13.2% collagen/mm septum (range: 30.2-74.6% collagen/mm septum) and 7.3±2.6% collagen/mm septum, range: 4.0-9.6% collagen/mm septum) in tissues of healthy controls. Normal collagen deposition in alveolar septae of a healthy individual (I) and increased deposition of collagen in alveolar septae of a CF patient (J). Arrows: Insert. Original magnifications: A, C, G, I × 100; Original magnifications: A, C, F, H × 100; I, J × 3.190.
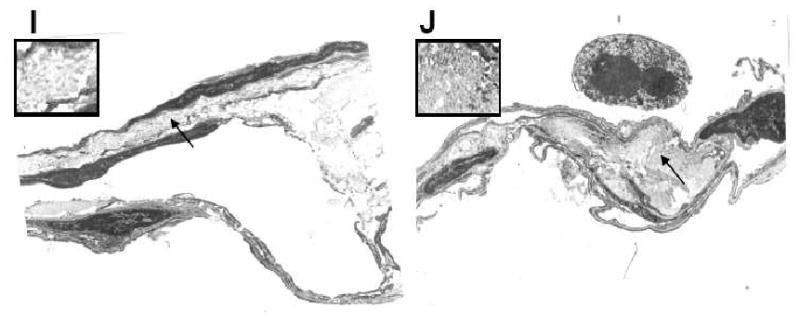
The extensive loss of elastin led us to hypothesize that elastin may be substituted by collagen in alveolar septae of CF patients. Indeed, staining with a specific antibody for collagen type 1 revealed that its concentration was significantly higher in alveolar septae of CF patients versus tissues from healthy controls (Fig. 4 F-H). These finding support the hypothesis that alveolar matrix remodeling and fibrosis is present in the CF lung and leads to stiffening of alveolar tissues. Elastin was ∼5-fold decreased and collagen ∼9-fold increased in CF patients versus healthy controls. When lung tissue from a CF patient and from a healthy individual was investigated by electron microscopy (Fig. 4. I,J), thickened septae with extensive collagen deposition were visible in CF but not in age-matched normal lung tissue.
3.3. Human alveolar type II epithelial cells from CF patients accumulate ceramide
We tested the hypothesis that inflammation in the alveolar space of CF patients may occur as a consequence of the accumulation of the pro-inflammatory sphingolipid ceramide [18]. Since CFTR has been detected in alveolar type II cells [19-23] of normal human airways, we focused on this cell type. Immunostaining of alveolar type II cells with antibodies specific for CFTR (Fig. 5 A,D) and surfactant C (Fig. 5 B), demonstrated the presence of CFTR in alveolar type II cells from healthy individuals (Fig. 5 C). In contrast, CFTR staining of alveolar type II cells was absent in lung tissues from ΔF508 homozygous CF patients (Fig. 5 E). Staining of peripheral lung tissue from CF patients and control tissues from healthy individuals with an antibody specific for ceramide revealed ceramide accumulation in alveolar cells of CF patients (Fig. 5 F), but not in the corresponding cells from healthy individuals (Fig. 5 I). Using the MetaMorph software ceramide accumulation was significantly higher in cells from CF patients (mean fluorescence intensity ± SD: 97.1±19.9) compared to that in cells of healthy individuals (mean fluorescence intensity ± SD: 49.5±5.9) (p=0.001). Double staining of the tissues with an antibody specific for ceramide and surfactant C identified these cells as type II alveolar epithelial cells (Fig. 5 G,H). These findings show that CF alveolar type II cells accumulate ceramide.
Figure 5. Ceramide accumulates in type II alveolar epithelial cells of CF patients.
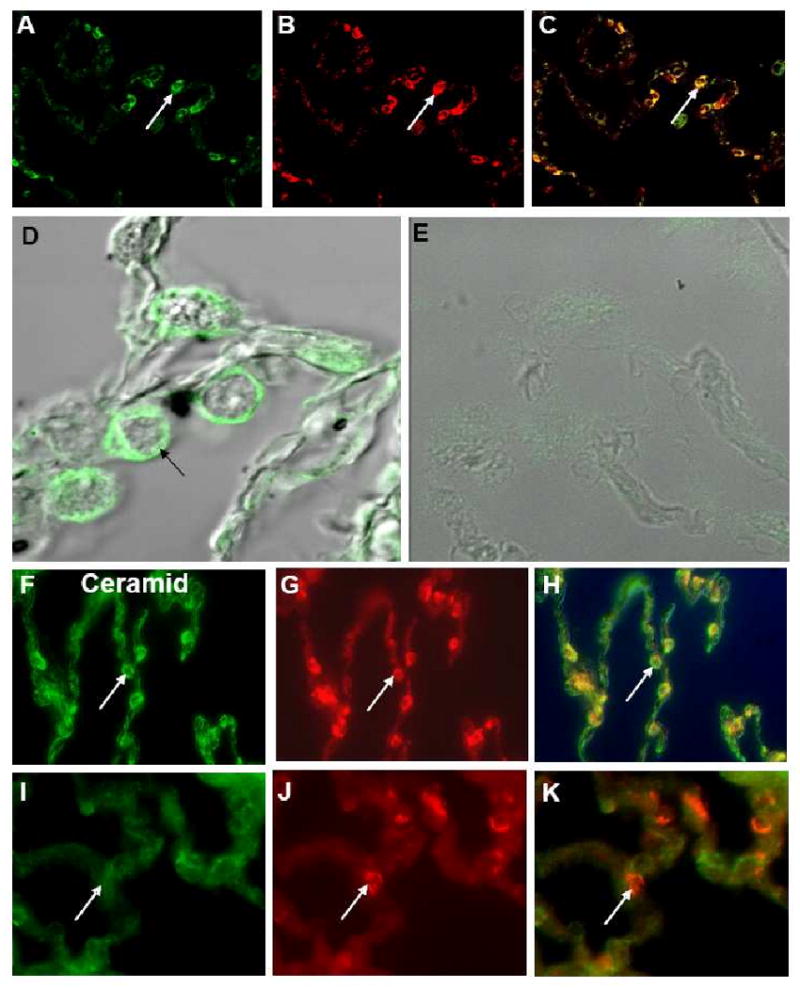
Immunostaining of alveolar type II cells from healthy individuals with a monoclonal antibody specific for CFTR (A) and a rabbit antibody against surfactant C (B); C: overlay of A and B. D, E: Confocal microscopy of alveolar type II cells from a healthy individual (D) and a ΔF508 homozygous CF patient. (E) with a monoclonal antibody specific for CFTR. Immunostaining of alveolar type II cells from CF patients (F, G) and healthy individuals (I, J) with a monoclonal antibody specific for ceramide (F, I) and a rabbit antibody against surfactant C (G, J). H: overlay of F, G, K: overlay of I,J. Original magnifications: A-C, F-K: × 200; D, E × 400.
3.4. P. aeruginosa in alveoli of CF patients
P. aeruginosa is a potent stimulant of various pathways of inflammation including stimulation of acid sphingomyelinase resulting in ceramide accumulation [25]. To address the question whether P. aeruginosa cells would reach the alveoli from sites of endobronchial infection, we stained alveolar tissues from nine CF patients with chronic P. aeruginosa infection with an antibody specific for alginate. Only 74 P. aeruginosa cells were detected in eight alveolar tissue sections of three CF patients, indicating a relative paucity of bacteria in alveolar spaces.
4. Discussion
While inflammation and tissue damage within conductive airways has been well-demonstrated and repeatedly reported, surprisingly little is known about alterations at the cell and tissue level in the peripheral lung in CF patients [8-12]. Our study provides evidence for extensive tissue inflammation and remodeling in the alveolar space of CF patients with advanced lung disease. Our findings are supported by (i) the detection of increased numbers of neutrophils, macrophages and T cells located in alveolar septa, (ii) an up-regulation of ICAM-1 in these tissues, (iii) the activation of NF-κB and IGF-1 in macrophages and alveolar epithelial cells, (iv) an increased presence of collagen-producing myofibroblasts, and (vi) extensive elastin degradation and collagen substitution in alveolar tissues. Additionally, and in line with the notion that airway inflammation is detectable very early in the lives of CF patients [26-28], our data indicate that lung elastin degradation is not limited to end stage disease. This notion is evidenced by the determination of the crosslink amino acids DES and IDES using the validated MEKC technique [24] in urine specimens from CF patients with different ages including younger patients with milder lung disease. Our data confirm those of previous investigators [29], who using an isotope dilution method found similar levels of increased urinary DES and IDES in CF patients. In contrast to their study, we were able to correlate urinary DES and IDES levels with levels of active neutrophil elastase in CF sputa. While urinary DES and IDES are not tissue specific, the correlation with neutrophil elastase which is abundantly present in inflamed and infected CF airways [29, 30], strongly suggests that the site of excess elastin degradation in patients with CF is the lung. Also in COPD patients, urinary DES levels have been inversely correlated with the severity of lung disease, suggesting an irreversible loss of lung elastin [31].
Both neutrophils and macrophages, located in high numbers within the alveolar septae contain proteases that can be released and cleave structural proteins such as lung elastin [7, 32, 33], and increased numbers of these cells have been detected in CF bronchoalveolar lavage (BAL) fluids, including BAL fluids from very young children with CF [12, 34-38]. In the light of the findings of Hilliard and colleagues who reported that elastase activity and matrix metalloproteinase-9 (MMP-9) concentrations correlated with increased concentrations of collagen, glycosaminoglycans and elastin in BAL fluids of CF patients [38], the mechanisms of airway remodelling in children with CF may differ regionally between central and peripheral areas. A reasonable explanation for the observed alveolar tissue inflammation and remodeling in CF lungs is that single bacterial cells from the large burden of bacterial pathogens causing chronic endobronchial infection in the conductive airways enter the 5 μm opening of the alveoli, thereby triggering and sustaining inflammation in alveolar spaces. However, we could only detect a surprisingly small number of P. aeruginosa cells in CF alveoli. P. aeruginosa was present in only three of 14 CF patients chronically infected with this pathogen within the alveolar lung tissue. Additionally, only 74 P. aeruginosa cells were detected in tissue from the 3 patients with bacteria in their alveoli. This finding may be a consequence of the high number of professional phagocytes in CF alveoli that may rapidly phagocytose and kill the invading bacteria. However, neutrophils, present only in alveolar septa, did not reveal the presence of internalized P. aeruginosa when alveolar tissues were investigated with a P. aeruginosa-specific antibody. Additionally, none of the luminal or intraseptal alveolar macrophages which have been reported to have an impaired killing activity towards P. aeruginosa due to defective acidification in phagolysosomes [39], revealed the presence of internalized P. aeruginosa. Alternatively, alveoli may be kept sterile by the intense antibiotic regimen which all of the patients received, and planktonic P. aeruginosa may be readily cleared in distal alveolar tissues as reported previously [40], in contrast to more proximal airways where they tend to form alginate-protected colonies that are resistant to immune cell attack and penetration by antibiotics.
The large number of P. aeruginosa-free alveoli that, nonetheless, reveal extensive fibrosis may support the hypothesis that pulmonary inflammation in CF patients is triggered by endogenous factors. This hypothesis is supported by reports demonstrating that lung inflammation is detectable in some CF patients who lack evidence of ongoing bacterial infection [26-28] and that uninfected CF airway grafts, but not grafts from matched non-CF controls, undergo a time-dependent, neutrophil-mediated inflammation that leads to progressive lung tissue destruction when these grafts are placed in severe combined immunodeficiency mice [41]. Furthermore, our previous data reveal that CFTR-mediated ceramide accumulation triggered an increased synthesis and release of cytokines and a profound recruitment of neutrophils and macrophages in bronchial tissues of uninfected CF mice in contrast to control mice [18]. However, the “intrinsic hyper-inflammation” hypothesis is still controversely discussed, since conflicting results, ranging from increased NF-κB activation and production of IL-8 to no changes or even decreased levels of inflammatory cytokines have been reported in investigations using nasal or bronchial epithelial cells from CF or non-CF individuals or immortalized cell lines in which the CFTR status has been manipulated [42, 43]. Our study does not add significant information about the aetiology of alveolar-based inflammation in CF. This is basically a consequence of the major difficulty to obtain sequential lung tissues or lung tissues at all from CF patients without lung infection. Because CF mice are strikingly different compared to CF patients concerning pulmonary pathophysiology (44), they may not represent a particularly good model to study alveolar-based inflammation in CF. Samples from non-CF bronchiectasis patients undergoing heart-lung transplant may be valuable to shed more light on the infection-inflammation question. Our data reveal that ceramide accumulation is also present in alveolar epithelial cells of CF patients. Whether ceramide accumulation is a consequence of defective CFTR, as demonstrated previously in uninfected mice [18], or triggered by bacteria [25] or other inflammatory stimuli, as observed in patients with COPD [45], is difficult to decide when examining lung tissue retrieved from chronically infected CF patients. However, our finding that ceramide accumulation is restricted to alveolar type II cells of CF patients discriminates CF from COPD, where ceramide accumulation has also been observed in type I alveolar cells [45].
Our observation that large numbers of macrophages and epithelial cells reveal nuclear translocation of NF-κB further supports our notion of alveolar inflammation in CF lungs. Several factors including ceramide [46, 47], tumor necrosis factor(TNF)-α [48] and bacterial factors [49] activate this transcription factor. In CF knockout (KO) mice, challenged intratracheally with P. aeruginosa, a prolonged and excessive activation of NF-κB, high concentrations of pro-inflammatory cytokines and higher neutrophil numbers were detected in BAL fluids compared to wild type mice, suggesting that dysregulation of the IκB/NF-κB pathway in the CF lung leads to prolonged cytokine secretion and persistent inflammation in response to acute challenges [49]. Up-regulated NF-kB has also been detected in cultures of CF airway epithelial cells [15-17]. Nevertheless, conflicting results have been published concerning this topic [42, 43]. Thus, again both exogenous and endogenous factors may be responsible for the increased NF-κB activation in CF alveolar tissues.
Another important mediator of fibrosis, which we detected in high concentrations in macrophages and alveolar epithelial cells of CF patients, is IGF-1. IGF-1 stimulates proliferation of fibroblasts, presumably by inhibition of apoptosis, and is also a potent inducer of collagen synthesis [50, 51]. Increased IGF-1 mRNA has been detected in lung tissues following the induction of bleomycin-induced pulmonary fibrosis in mice [52]. Additionally, other growth factors including transforming growth factor-ß (TGF-ß) have been detected in inflamed CF airways [12].
In summary, we have demonstrated extensive inflammation and tissue remodeling in alveolar tissues from CF patients with advanced lung disease, and these changes are likely to occur and progress despite antibacterial therapies and need to be countered via other therapeutic strategies that target inflammation and fibrosis. Bronchial inflammation has been the main target of anti-inflammatory therapeutic regimens. Such strategies, which consist of primarily of delivering anti-inflammatory drugs including corticosteroids [53, 54] and protease inhibitors [55, 56] via inhalation, may have a therapeutic impact on bronchial inflammation, but they may not necessarily down-regulate alveolar inflammation, especially in patients with more advanced disease where deposition of inhaled agents in the lung periphery is limited and shows marked regional heterogeneity because of bronchial obstruction. Future studies should focus on whether suppurative non-CF lung disease exhibits similar or different abnormalities.
Acknowledgments
The authors would like to thank John R. Riordan, Chapel Hill, NC, USA, for a gift of a monoclonal antibody against CFTR and Scott Randell and James R. Yankaskas, Chapel Hill, NC, USA, for lung tissues from CF patients. The excellent work of Simone Greiner, Jan Ruprecht, Martin Glöckler, Silja Schönleber and Christiane Wolz, Institute of Medical Microbiology and Hygiene, Universitätsklinikum Tübingen, Germany, is greatly acknowledged.
This investigation was supported, in part, by NIH grant AI48917-04 for GBP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N, Zsiga M, Buchwald M, Riordan JR, Tsui LC, Collins F. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–65. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 2.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzu MC, Collins FS, Tsui LC. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 3.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–80. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 4.Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–58. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 5.Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, Birrer P, Bellon G, Berger J, Weiß T, Botzenhart K, Yankaskas JR, Randell S, Boucher RC, Döring G. Reduced oxygen concentrations in airway mucus contribute to the early and late pathogenesis of Pseudomonas aeruginosa cystic fibrosis airways infection. J Clin Invest. 2002;109:295–09. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Döring G, Knight R, Bellon G. Immunology of cystic fibrosis. In: Hodson ME, Geddes D, editors. Cystic Fibrosis. London, England: Arnold; 2000. pp. 109–41. [Google Scholar]
- 7.Hogg JC, Senior RM. Chronic obstructive pulmonary disease - part 2: pathology and biochemistry of emphysema. Thorax. 2002;57:830–4. doi: 10.1136/thorax.57.9.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobonya RE, Taussig LM. Quantitative aspects of lung pathology in cystic fibrosis. Am Rev Respir Dis. 1986;134:290–5. doi: 10.1164/arrd.1986.134.2.290. [DOI] [PubMed] [Google Scholar]
- 9.Bedrossian CWM, Greenberg SD, Singer DB, Hansen JJ, Rosenberg HS. The lung in cystic fibrosis. A quantitative study including prevalence of pathologic findings among different age groups. Human Pathol. 1976;7:195–04. doi: 10.1016/s0046-8177(76)80023-8. [DOI] [PubMed] [Google Scholar]
- 10.Tiddens HA, Koopman LP, Lambert RK, Elliott WM, Hop WC, van der Mark TW, de Boer WJ, de Jongste JC. Cartilaginous airway wall dimensions and airway resistance in cystic fibrosis lungs. Eur Respir J. 2000;15:735–42. doi: 10.1034/j.1399-3003.2000.15d18.x. [DOI] [PubMed] [Google Scholar]
- 11.Durieu I, Peyrol S, Gindre D, Bellon G, Durand DV, Pacheco Y. Subepithelial fibrosis and degradation of the bronchial extracellular matrix in cystic fibrosis. Am J Respir Crit Care Med. 1998;158:580–8. doi: 10.1164/ajrccm.158.2.9707126. [DOI] [PubMed] [Google Scholar]
- 12.Shute J, Marshall L, Bodey K, Bush A. Growth factors in cystic fibrosis – when more is not enough. Paediatr Respir Rev. 2003;4:120–7. doi: 10.1016/s1526-0542(03)00028-9. [DOI] [PubMed] [Google Scholar]
- 13.Ratjen F, Döring G. Cystic Fibrosis. Lancet. 2003;361:681–9. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 14.Hamutcu R, Rowland JM, Horn MV, Kaminsky C, MacLaughlin EF, Starnes VA, Woo MS. Clinical findings and lung pathology in children with cystic fibrosis. Am J Respir Crit Care Med. 2002;165:1172–5. doi: 10.1164/ajrccm.165.8.2104090. [DOI] [PubMed] [Google Scholar]
- 15.Weber AJ, Soong G, Bryan R, Saba S, Prince A. Activation of NF–kappaB in airway epithelial cells is dependent on CFTR trafficking and Cl− channel function. Am J Physiol Lung Cell Mol Physiol. 2001;281:L71–L8. doi: 10.1152/ajplung.2001.281.1.L71. [DOI] [PubMed] [Google Scholar]
- 16.Joseph T, Look D, Ferkol T. NF–kappaB activation and sustained IL–8 gene expression in primary cultures of cystic fibrosis airway epithelial cells stimulated with Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2005;288:L471–L9. doi: 10.1152/ajplung.00066.2004. [DOI] [PubMed] [Google Scholar]
- 17.Tabary O, Escotte S, Couetil JP, Hubert D, Dusser D, Puchelle E, Jacquot J. Relationship between IkappaBalpha deficiency, NFkappaB activity and interleukin-8 production in CF human airway epithelial cells. Pflugers Archiv – Eur. J Physiol. 2001;443(Suppl 1):S40–S4. doi: 10.1007/s004240100642. [DOI] [PubMed] [Google Scholar]
- 18.Teichgräber V, Ulrich M, Riethmüller J, Grassme H, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr M, von Kürthy G, Schmid KW, Weller M, Tümmler B, Lang F, Döring G, Gulbins E. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nature Med. 2008;14:381–92. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- 19.Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR, Boucher RC. Characterization of wild–type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell. 2005;16:2154–67. doi: 10.1091/mbc.E04-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regnier A, Dannhoffer L, Blouquit-Laye S, Bakari M, Naline E, Chinet T. Expression of cystic fibrosis transmembrane conductance regulator in the human distal lung. Human Pathol. 2008;39:368–76. doi: 10.1016/j.humpath.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. PNAS. 2006;103:4964–9. doi: 10.1073/pnas.0600855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCray PB, Jr, Wohlford-Lenane CL, Snyder JM. Localization of cystic fibrosis transmembrane conductance regulator mRNA in human fetal lung tissue by in situ hybridization. J Clin Invest. 1992;90:619–25. doi: 10.1172/JCI115901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang X, Song Y, Hirsch J, Galietta LJ, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L242–L9. doi: 10.1152/ajplung.00178.2005. [DOI] [PubMed] [Google Scholar]
- 24.Viglio S, Iadarola P, Lupi A, Trisolini R, Tinelli C, Balbi, Grassi V, Worlitzsch D, Döring G, Meloni F, Meyer KC, Dowson L, Hill SL, Stockley RA, Luisetti M. MEKC of desmosine and isodesmosine in urine of chronic destructive lung disease patients. Eur Respir J. 2000;15:1039–45. doi: 10.1034/j.1399-3003.2000.01511.x. [DOI] [PubMed] [Google Scholar]
- 25.Grassme H, Jendrossek V, Riehle A, von Kurthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E. Host defense against Pseudomonas aeruginosa requires ceramide–rich membrane rafts. Nature Med. 2003;9:322–30. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- 26.Balough K, McCubbin M, Weinberger M, Smits W, Ahrens R, Fick R. The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatr Pulmonol. 1995;20:63–70. doi: 10.1002/ppul.1950200203. [DOI] [PubMed] [Google Scholar]
- 27.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–82. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, Hiatt P, McCoy K, Wilson CB, Inglis A, Smith A, Martin TR, Ramsey BW. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol. 2001;32:356–66. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- 29.Stone PJ, Konstan MW, Berger M, Dorkin HL, Franzblau C, Snider GL. Elastin and collagen degradation products in urine of patients with cystic fibrosis. Am J Respir Crit Care Med. 1995;152:157–62. doi: 10.1164/ajrccm.152.1.7599816. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein W, Döring G. Lysosomal enzymes from polymorphonuclear leukocytes and proteinase inhibitors in patients with cystic fibrosis. Am Rev Respir Dis. 1986;134:49–56. doi: 10.1164/arrd.1986.134.1.49. [DOI] [PubMed] [Google Scholar]
- 31.Cocci F, Miniati M, Monti S, Cavarra E, Gambelli F, Battolla L, Lucattelli M, Lungarella G. Urinary desmosine excretion is inversely correlated with the extent of emphysema in patients with chronic obstructive pulmonary disease. Intern J Biochem Cell Biol. 2002;34:594–04. doi: 10.1016/s1357-2725(02)00015-8. [DOI] [PubMed] [Google Scholar]
- 32.Chapman HA. Disorders of lung matrix remodeling. J Clin Invest. 2004;113:148–57. doi: 10.1172/JCI20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tetley TD. Macrophages and the pathogenesis of COPD. Chest. 2002;121:156S–9S. doi: 10.1378/chest.121.5_suppl.156s. [DOI] [PubMed] [Google Scholar]
- 34.Taggart CC, Greene CM, Smith SG, Levine RL, McCray PB, Jr, O'Neill S, McElvaney NG. Inactivation of human beta-defensins 2 and 3 by elastolytic cathepsins. J Immunol. 2003;171:931–7. doi: 10.4049/jimmunol.171.2.931. [DOI] [PubMed] [Google Scholar]
- 35.Ratjen F, Hartog CM, Paul K, Wermelt J, Braun J. Matrix metalloproteases in BAL fluid of patients with cystic fibrosis and their modulation by treatment with dornase alpha. Thorax. 2002;57:930–4. doi: 10.1136/thorax.57.11.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Power C, O'Connor CM, McFarlane D, O'Mahoney S, Gaffney K, Hayes J, FitzGerald MX. Neutrophil collagenase in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med. 1994;50:818–22. doi: 10.1164/ajrccm.150.3.8087357. [DOI] [PubMed] [Google Scholar]
- 37.Delacourt C, Le Bourgeois M, D'Ortho MP, Doit C, Scheinmann P, Navarro J, Harf A, Hartmann DJ, Lafuma C. Imbalance between 95 kDa type IV collagenase and tissue inhibitor of metalloproteinases in sputum of patients with cystic fibrosis. Am J Respir Crit Care Med. 1995;52:765–74. doi: 10.1164/ajrccm.152.2.7633740. [DOI] [PubMed] [Google Scholar]
- 38.Hilliard TN, Regamey N, Shute JK, Nicholson AG, Alton EWFW, Bush A, Davies JC. Airway remodelling in children with cystic fibrosis. Thorax. 2007;62:1074–80. doi: 10.1136/thx.2006.074641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, Huang P, Tong J, Naren AP, Bindokas V, Palfrey HC, Nelson DJ. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nature Cell Biol. 2006;8:933–44. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 40.Bjarnsholt T, Jensen PO, Fiandaca MJ, Pedersen J, Hansen CR, Andersen CB, Pressler T, Givskov M, Hoiby N. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Ped Pulmonol. 2009;44:547–58. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 41.Tirouvanziam R, Khazaal I, Peault B. Primary inflammation in human cystic fibrosis small airways. Am J Physiol. 2002;283:L445–51. doi: 10.1152/ajplung.00419.2001. [DOI] [PubMed] [Google Scholar]
- 42.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol. 2006;291:C218–30. doi: 10.1152/ajpcell.00605.2005. [DOI] [PubMed] [Google Scholar]
- 43.Becker MN, Sauer MS, Muhlebach MS, Hirsh AJ, Wu Q, Verghese MW, Randell SH. Cytokine secretion by cystic fibrosis airway epithelial cells. Am J Respir Crit Care Med. 2004;169:645–53. doi: 10.1164/rccm.200207-765OC. [DOI] [PubMed] [Google Scholar]
- 44.Grubb BR, Boucher RC. Pathophysiology of Gene-targeted mouse models for cystic fibrosis. Physiological Rev. 1999;79(suppl 1):S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- 45.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nature Med. 2005;11:491–8. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sreenivasan Y, Sarkar A, Manna SK. Oleandrin suppresses activation of nuclear transcription factor-kappa B and activator protein-1 and potentiates apoptosis induced by ceramide. Biochem Pharmacol. 2003;66:2223–39. doi: 10.1016/j.bcp.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Yang Z, Costanzo M, Golde DW, Kolesnick RN. Tumor necrosis factor activation of the sphingomyelin pathway signals nuclear factor kappa B translocation in intact HL-60 cells. J Biol Chem. 1993;268:20520–3. [PubMed] [Google Scholar]
- 48.Dbaibo GS, Hannun YA. Signal transduction and the regulation of apoptosis: roles of ceramide. Apoptosis. 1998;3:317–34. doi: 10.1023/a:1009668802718. [DOI] [PubMed] [Google Scholar]
- 49.Saadane A, Soltys J, Berger M. Acute Pseudomonas challenge in cystic fibrosis mice causes prolonged nuclear factor-kappa B activation, cytokine secretion, and persistent lung inflammation. J Allergy Clin Immunol. 2006;117:1163–9. doi: 10.1016/j.jaci.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 50.Goldstein RH, Poliks CF, Pilch PF, Smith BD, Fine A. Stimulation of collagen formation by insulin and insulin-like growth factor I in cultures of human lung fibroblasts. Endocrinology. 1989;124:964–70. doi: 10.1210/endo-124-2-964. [DOI] [PubMed] [Google Scholar]
- 51.Harrison NK, Cambrey AD, Myers AR, Southcott AM, Black CM, du Bois RM, Laurent GJ, McAnulty RJ. Insulin-like growth factor-I is partially responsible for fibroblast proliferation induced by bronchoalveolar lavage fluid from patients with systemic sclerosis. Clin Sci (London) 1994;86:141–8. doi: 10.1042/cs0860141. [DOI] [PubMed] [Google Scholar]
- 52.Cao B, Guo Z, Zhu Y, Xu W. The potential role of PDGF, IGF-1, TGF-beta expression in idiopathic pulmonary fibrosis. Chin Med J. 2000;113:776–82. [PubMed] [Google Scholar]
- 53.Balfour-Lynn IM, Klein NJ, Dinwiddie R. Randomised controlled trial of inhaled corticosteroids (fluticasone propionate) in cystic fibrosis. Arch Dis Child. 1997;77:124–30. doi: 10.1136/adc.77.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bisgaard H, Pedersen SS, Nielsen KG, Skov M, Laursen EM, Kronborg G, Reimert CM, Høiby N, Koch C. Controlled trial of inhaled budesonide in patients with cystic fibrosis and chronic bronchopulmonary Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 1997;156:1190–6. doi: 10.1164/ajrccm.156.4.9612044. [DOI] [PubMed] [Google Scholar]
- 55.McElvaney NG, Hubbard RC, Birrer P, Chernick MS, Caplan DB, Frank MM, Crystal RG. Aerosol α1-antitrypsin treatment for cystic fibrosis. Lancet. 1991;337:392–4. doi: 10.1016/0140-6736(91)91167-s. [DOI] [PubMed] [Google Scholar]
- 56.McElvaney NG, Nakamura H, Birrer P, Hebert CA, Wong WL, Alphonso M, Baker JB, Catalano MA, Crystal RG. Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin-8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J Clin Invest. 1992;90:1296–01. doi: 10.1172/JCI115994. [DOI] [PMC free article] [PubMed] [Google Scholar]


