Abstract
The architecture and functioning of the mammalian nervous system is partly based on the complexity of combinatorial gene expression in the developing brain that results in a tremendous diversity of neural cells. MicroRNAs are small non-coding RNAs that are particularly abundant in the brain and are emerging as influential regulators of neural gene expression. This mini-review summarizes the recently discovered role of microRNAs in the development and maintenance of the nervous system. MicroRNAs are temporally expressed during neural differentiation, spatially regulated and embedded in molecular feedback loops that may contribute to the robustness of the neural networks.
Keywords: microRNAs, post-transcriptional regulation, development, nervous system, neurogenesis, differentiation
1. Introduction
MicroRNAs (miRNAs) are small (~ 21 nucleotides), non-coding RNAs that recognize binding sites located in the 3’ Untranslated Region (3’UTR) of mRNA targets (Chekulaeva and Filipowicz, 2009). The miRNA genes are transcribed as primary miRNAs (pri-miRNAs) in the nucleus by RNA polymerase II and are further processed by the Rnasen/Dgcr8 microprocessor complex into ~ 70 nucleotides (nts) long precursor miRNAs (pre-miRNAs), which fold into a classical stem-loop structure (Figure 1). These pre-miRNAs are then exported to the cytoplasm via the Xpo5/Ran complex, where they are further cleaved into ~21 nts duplexes by the RNase III-type enzyme Dicer1. Only one strand of the miRNA duplex, referred to as the miRNA, is incorporated into the RNA-induced silencing complex (RISC) while the opposite strand, also known as miRNA*, is generally not active – rather, it is poised for degradation. In animals, miRNAs predominantly bind to their targets by following imperfect complementary base pairing rules; of particular importance is the base pairing between positions 2 and 7 of the miRNAs (also known as the seed sequence) and their targets.
Figure 1.
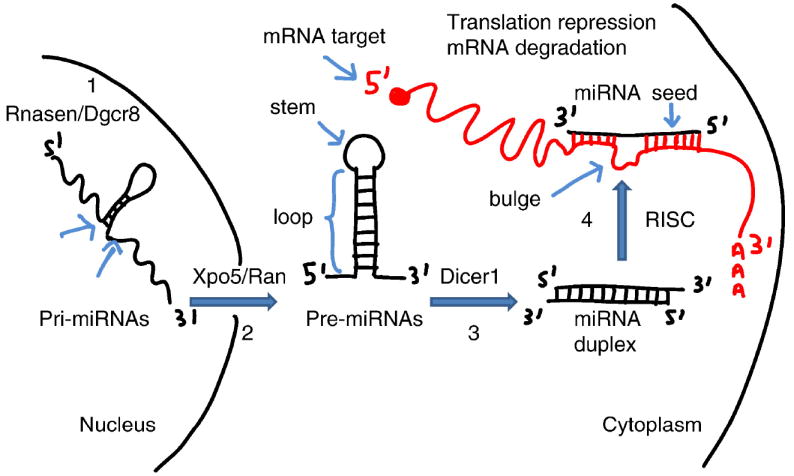
The biogenesis of miRNAs. The pri-miRNAs are composed of a stem, a terminal loop and two flanking sequences which are subject to cleavage by the Rnasen/Dgcr8 microprocessor complex to produce pre-miRNAs (step 1). Through the Xpo5/Ran pathway, pre-miRNAs are translocated from the nucleus into the cytoplasm (step 2) where they are processed by Dicer1 into miRNAs (step 3). The miRNA duplexes are unwound and one strand of the duplex (namely the miRNA) is incorporated into the RISC complex (step 4). MiRNAs trigger post-transcriptional gene silencing through base pairing with the 3’UTR of mRNA targets, thereby leading to mRNA target degradation or translation repression.
The complexity of the nervous system originally arises during embryogenesis from a small number of neural stem cells (NSCs) (Merkle and Alvarez-Buylla, 2006). The large diversity of neural cell types present in the embryonic and adult brains is partly supported by the combinatorial complexity of the genetic programs orchestrated at the transcriptional level by transcription factors. Furthermore, the high abundance of miRNAs in the mammalian brain (Sempere et al., 2004), (Miska et al., 2004) strongly suggests that miRNAs may represent important post-transcriptional regulators of gene expression by adding subtle but influential changes to the genetic programs that normally occur in neural cells. In this mini-review, we will discuss the growing importance of miRNAs during neural proliferation and differentiation.
2. Neural stem cells: At the root of the neural lineage tree
During brain development, NSCs give rise to neurons, astrocytes and oligodendrocytes of the central nervous system (CNS). Typically, NSCs are multipotent and self-renewing (Gotz and Huttner, 2005). Before neurogenesis, the neural plate is composed of a single layer of neuroepithelial cells, which are highly polarized cells that first expand by symmetrical divisions. At the onset of neurogenesis, these neuroepithelial cells switch to an asymmetrical mode and produce radial glia cells and intermediate progenitor cells. Radial glia cells in the ventricular zone (VZ) undergo symmetric and asymmetric divisions to generate neurons (Anthony et al., 2004). In contrast, most intermediate progenitor cells are neuronal committed progenitors and divide symmetrically in the subventricular zone (SVZ) (Noctor et al., 2008). In the adult brain, neurogenesis becomes more limited even though some populations of NSCs are persisting through adult life either as a subset of astrocytes in restricted brain regions (Doetsch et al., 1999) or as Sox2+ cells residing in the subgranular zone (SGZ) of the hippocampus (Suh et al., 2007).
3. MiRNAs and early neurogenesis
The role of miRNAs in early neurogenesis has been assessed using mouse models in which the Dicer1 gene has been inactivated, resulting in a global loss of miRNAs in specific neuronal cell types such as Purkinje cells (Schaefer et al., 2007), hippocampal and cortical neurons (Davis et al., 2008). For instance, deletion of Dicer1 in Purkinje cells resulted in neuronal cell death suggesting an essential role of miRNAs in the survival of differentiated neurons. The deletion of Dicer1 in excitatory forebrain neurons led to reduced dendritic branch elaboration, increase in dendritic spines, microcephaly and ataxia by P15, confirming that miRNAs are important for the maintenance of differentiated neuronal cells and brain homeostasis. To determine whether miRNAs play a role at the earliest stages of neural differentiation, a mouse line with a Dicer1 floxed allele was crossed with an Emx1-Cre strain, resulting in the specific deletion of Dicer1 in neuroepithelial cells (De Pietri Tonelli et al., 2008). This deletion of Dicer1 in the E9.5 cortex led to a reduction in the size of the cortex by E12.5 and an increase in neuronal apoptosis in the cortical wall. By E14.5, there was depletion of apical and basal intermediate progenitor cells, thus showing that miRNAs play an indispensable role in the production of neurons during embryonic corticogenesis. Given that the miRNA repertoires of these neuronal cells have not been characterized, it would be of high interest to identify the specific miRNAs that are crucial during the transition from neuroepithelial cells to neuronal progenitor cells. Very recently, the miRNA repertoires of E11 and E13 rat neuronal progenitor cells revealed that several miRNAs were either up- or down-regulated at the onset of neurogenesis (Nielsen et al., 2009). Interestingly, miR-9 and miR-124, which have been initially reported as up-regulated during brain development (Krichevsky et al., 2003), were also found up-regulated during the lineage progression of neuronal progenitor cells, thus supporting a critical role for these two miRNAs during neuronal proliferation and differentiation (Table 1).
Table 1.
miRNAs and neural development
| miRNA | Species | Targets | Function | Reference |
|---|---|---|---|---|
| miR-9 | Human | REST | Promotes neurogenesis | Packer, 2008 |
| miR-9 | Mouse | Tlx | Promotes NSC differentiation | Zhao, 2009 |
| miR-9 | Mouse | Foxg1 | Differentiation of CR+ neurons | Shibata, 2008 |
| miR-9 | Zebrafish | Fgf8, Fgfr1 | Maintains organizer activity at the midbrain-hindbrain boundary | Leucht, 2008 |
| miR-124 | Mouse | Sox9 | Promotes neurogenesis in the adult SVZ | Cheng, 2009 |
| miR-124 | Mouse | Actl6a | Neural specific chromatin-remodeling Swi/Snf-like complexes | Yoo, 2009 |
| miR-124 | Mouse | Ptbp1 | Inclusion of neuron-specific exons in neuronal transcripts | Makeyev, 2007 |
| miR-124 | Chicken | Ctdsp1 | Neural induction in the spinal cord of embryos | Visvanathan, 2007 |
| miR-133b | Mouse | Pitx3 | Differentiation of dopaminergic neurons | Kim, 2007 |
| miR-219 | Mouse | Sox6, Hes5 | Promotes oligodendrocyte differentiation | Zhao, 2010 |
| miR-219 | Mouse | Sox6, Foxj3, Zfp238, Pdgfra | Promotes oligodendrocyte differentiation | Dugas, 2010 |
4. Temporal and spatial expression of miRNAs and control of neural differentiation
MicroRNAs are dynamically regulated during neural differentiation. For example, miR-138, miR-219 and miR-338 were found to be up-regulated during oligodendrocyte differentiation (Lau et al., 2008). These miRNAs are required for myelination, as demonstrated by two recent studies using conditional inactivation of Dicer1 in oligodendrocytes. Loss of myelin occurred after crossing the Dicer1 floxed mice with the PlpERT line, resulting in the dysregulation of miR-219 which normally down-regulates Sox6 and Hes5, two inhibitors of oligodendrocyte differentiation (Zhao et al., 2010). In another study, Dicer1 inactivation obtained after crossing the Dicer1 floxed line with Cnp-cre or Olig2-cre lines resulted in abnormal CNS myelination (Dugas et al., 2010). In this transgenic model, lack of Dicer1 led to delay or disruption of oligodendrocyte differentiation; moreover, miR-219 was shown to regulate the expression of Sox6, Foxj3 and Zfp238, three transcription factors with potential role during oligodendrocyte proliferation. Pdgfra was also found to be a target of miR-219 and this observation is of importance since it has been known for decades that Pdgf is an important mitogenic signal for oligodendrocyte progenitor cells (Noble et al., 1988).
The levels at which miRNAs are expressed are critical for normal neural development, as documented by modulating the amounts of miR-9 in NSCs. One target of miR-9 is Tlx, a nuclear receptor involved in NSC renewal (Shi et al., 2004). In utero electroporation of miR-9 in the VZ of E13.5 mouse led to decreased Ki67+ cells and increased Dcx+ positive cells, suggesting that miR-9 accelerates neuronal differentiation by fine-tuning the expression of genes involved in the differentiation of neuronal progenitor cells (Zhao et al., 2009). Similar results were observed with another target of miR-9, Foxg1, a transcription factor involved in the generation of Cajal–Retzius (CR+) neurons (Hanashima et al., 2004). In the E12 mouse brain, miR-9 is located in the medial pallium and the VZ of the ganglionic eminences. Over-expression of miR-9 caused premature neuronal differentiation in the VZ, as identified by the early emergence of CR+ cells (Shibata et al., 2008). Of note, Foxg1 is expressed in the entire mouse telencephalon at E12 but is absent in the most medial pallium where miR-9 is highly expressed, introducing the notion that miRNAs are not only regulated during cellular differentiation, but also show defined expression patterns at precise time points during development. This spatial distribution may explain how miRNAs effectively dual-task: by being present in some tissues to actively repress gene expression and by being absent in the surrounding areas to allow a genetic program to occur by default in these adjacent tissues. In the zebrafish, miR-9 is absent from the midbrain-hindbrain boundary (MHB), an organization center of the neural tube, while present in adjacent areas of the midbrain and hindbrain (Leucht et al., 2008). Remarkably, the over-expression of miR-9 in the MHB triggered loss of the territory integrity, suggesting that the expression of miR-9 surrounding the MHB defines the boundaries of this territory. Furthermore, the inhibition of miR-9 in the surrounding areas led to reduction of HuC+ neuronal cells, consistent with an active role of miR-9 during the differentiation of neurons.
5. Fine-tuning of developmental transitions through temporal negative feedback loops
How is miR-9 up-regulated in progenitor cells committed to the neuronal lineage? In Huntington’s Disease (HD), a polyglutamine expansion in the Htt protein abrogates its binding to REST, leading to its abnormal nuclear translocation in neuronal cells and decreased neuronal gene expression (Zuccato et al., 2003). Several miRNAs including miR-9, -9*, -29b, -124a and -132 were found as down-regulated in HD cases (Packer et al., 2008), suggesting that Rest normally regulates their expression in non-neuronal cells and NSCs by binding to upstream RE1 silencing transcription factor (REST) elements of these miRNA genes. The existence of a feedback loop was suggested by the observation that REST inhibits miR-9 expression in NSCs and the subsequent down-regulation of REST during neuronal differentiation led to increased level of miR-9. Furthermore, miR-9 was also shown to down-regulate REST, thereby confirming a feedback loop between miR-9 and REST and a role for miR-9 in the removal of residual REST in neuronal committed progenitor cells. Other examples of feedback loops have been reported for miR-133b and Pitx3, which is a transcription factor required during the maturation of midbrain dopaminergic neurons (Kim et al., 2007) and for miR-9 and Tlx (Zhao et al., 2009). Of note, several of the miRNAs which are controlled by REST are also embedded in feedback loops and target REST or other components of the REST complex such as Rcor1/2 (Packer et al., 2008), Mecp2 (Klein et al., 2007) and Ctdsp1 (Visvanathan et al., 2007). For instance, Ctdsp1 normally acts as an anti-neural factor together with REST (Yeo et al., 2005) in non-neuronal cells and NSCs. Electroporations of 2’-O-methyl oligoribonucleotides targeting miR-124 in the chick neural tube increased the number of BrdU+ proliferating cells in the lateral post-mitotic zone and reduced NeuN+ neuronal cells, thus showing that miR-124 modulates neuronal cell differentiation (Visvanathan et al., 2007).
6. MiRNAs and functional homeostasis in neurons
When miR-124 was over-expressed in HeLa cells, the gene expression profile of these non-neuronal cells was directed toward a neuronal phenotype (Lim et al., 2005). There were ~170 down-regulated genes, each of which is generally absent or expressed at low levels in the brain, thus suggesting that a function for miR-124 in the nervous system is to repress unwanted or leaky transcripts. Such a fail-safe function (or “expression buffering”) is further supported by the observation that inhibition of miR-124 in primary cultures of terminally differentiated cortical neurons resulted in the increase of 10 non-neuronal transcripts (Conaco et al., 2006). However, the targets identified by these two studies are only the tip of the iceberg and the interplay between miR-124 and neurons extends beyond the primary down-regulation of targets. For example, miR-124 globally impacts splicing of neuronal genes by regulating the splicing factor Ptbp1, which allows inclusion of neuron-specific exons in transcripts (Makeyev et al., 2007) and is normally down-regulated in mature neurons (Boutz et al., 2007). Furthermore, miR-124 and Ptbp1 display reciprocal patterns of expression in the embryo, consistent with a function of miR-124 in maintaining neuronal integrity. Accordingly, the inactivation of Dicer1 in the telencephalon led to a high level of Ptbp1 protein throughout the entire span of the lateral telencephalic wall by E13.5, whereas Ptbp1 expression is normally restricted to the VZ of the telencephalon. This ectopic expression was accompanied by a loss of Map2+ post-mitotic neurons; however, this study did not address whether this resulted from abnormal proliferation of progenitor cells or increased neuronal apoptosis. Other important targets of miR-124 have been also identified during embryonic and adult neurogenesis. For example, over-expression of miR-124 during embryogenesis causes reduced proliferation of neural progenitor cells, repression of Actl6a, a component of the chromatin-remodeling Swi/Snf-like complex (Kuroda et al., 2002), and its replacement by Actl6b in post-mitotic neurons of the neural tube (Yoo et al., 2009). During adult neurogenesis, miR-124 also regulates neuronal progression at the transition of type C transit-amplifying cells to type A migrating neuroblasts (Cheng et al., 2009). Blocking miR-124 led to hyperplasia of type C cells, suggesting that these cells failed to differentiate into type A neuroblasts. Conversely, over-expression of miR-124 led to cell-cycle exit, increased neuronal differentiation and down-regulation of the transcription factor Sox9. The dynamics of the temporal and spatial regulation of miRNAs in the brain are schematized in Figure 2.
Figure 2.
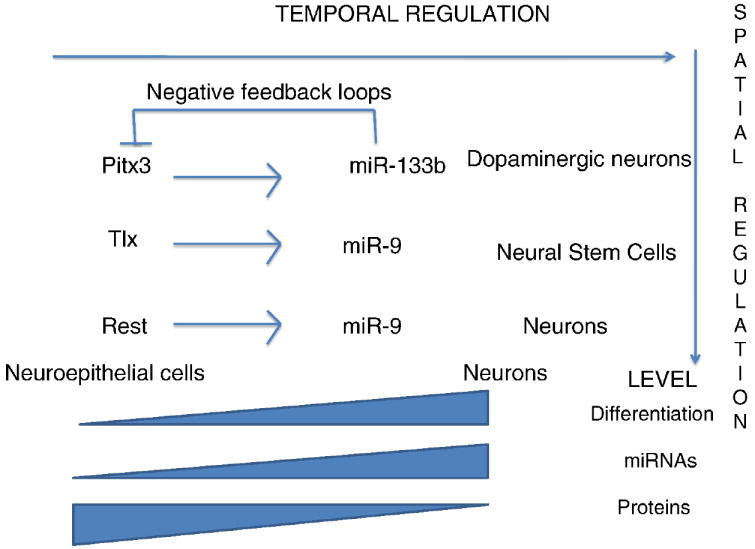
The miRNAs and the existence of molecular feedback loops. Three examples of negative feedback loops are shown for Pitx3-miR-133b, Tlx-miR-9 and Rest-miR-9. In the hypothetical model, the transcription factors (Pitx3, Tlx and REST) induce transcription of the miRNAs during neural differentiation. Those miRNAs are embedded in negative feedback circuits that normally suppress the expression of transcription factors. The proposed model also implies that miRNAs are dynamically regulated during neural differentiation (e.g. increase inexpression) and expressed regionally or specifically in some cell types (dopaminergic neurons, neural stem cells or neurons). Because transcription factors and miRNAs are co-expressed during a specific time window, the proposed function of these miRNAs is to insure the proper timing of differentiation, as opposed to a more prevalent fail-safe function in maintaining cellular homeostasis in neural cells by preventing the expression of leaky transcripts (i.e. expression buffering characterized by mutual exclusion of miRNAs and mRNA targets, not described here).
7. Concluding remarks
It is now clear that miRNAs play a critical role during neural cell proliferation and differentiation and that their dysregulation may lead to neurodegeneration (Lau and de Strooper, 2010). Moreover, the developmental regulation of gene expression by miRNAs in NSCs may be abnormally recapitulated in cancer stem cells. For example, miR-9 is not only highly expressed in fetal brain but also in oligodendroglioma and glioblastoma when compared to adult brain, normal oligodendrocytes and astrocytes (Nelson et al., 2006), (Nass et al., 2009). This snapshot of miRNA action thus captures not only the sophisticated regulatory network that operates during the nervous system development but also the miRNA dysregulation that could occur in pathophysiological conditions such as neurodegeneration or cancer. Further understanding of the complexities of these microregulators will uncover new developmental pathways and spawn innovative strategies aimed at promoting adult neurogenesis in neurodegenerative and demyelinating diseases as well as controlling brain cancers.
Acknowledgments
We sincerely apologize to all those colleagues whose important work was not cited in this mini-review because of space considerations. This work was supported by intramural funds from the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–90. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Boutz PL, Stoilov P, Li Q, Lin CH, Chawla G, Ostrow K, Shiue L, Ares M, Jr, Black DL. A post-transcriptional regulatory switch in polypyrimidine tract-binding proteins reprograms alternative splicing in developing neurons. Genes Dev. 2007;21:1636–52. doi: 10.1101/gad.1558107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekulaeva M, Filipowicz W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol. 2009;21:452–60. doi: 10.1016/j.ceb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A. 2006;103:2422–7. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, Ullian EM. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28:4322–30. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pietri Tonelli D, Pulvers JN, Haffner C, Murchison EP, Hannon GJ, Huttner WB. miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development. 2008;135:3911–21. doi: 10.1242/dev.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Dugas JC, Cuellar TL, Scholze A, Ason B, Ibrahim A, Emery B, Zamanian JL, Foo LC, McManus MT, Barres BA. Dicer1 and miR-219 Are Required for Normal Oligodendrocyte Differentiation and Myelination. Neuron. 2010;65:597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–88. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Hanashima C, Li SC, Shen L, Lai E, Fishell G. Foxg1 suppresses early cortical cell fate. Science. 2004;303:56–9. doi: 10.1126/science.1090674. [DOI] [PubMed] [Google Scholar]
- Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–4. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein ME, Lioy DT, Ma L, Impey S, Mandel G, Goodman RH. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci. 2007;10:1513–4. doi: 10.1038/nn2010. [DOI] [PubMed] [Google Scholar]
- Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9:1274–81. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y, Oma Y, Nishimori K, Ohta T, Harata M. Brain-specific expression of the nuclear actin-related protein ArpNalpha and its involvement in mammalian SWI/SNF chromatin remodeling complex. Biochem Biophys Res Commun. 2002;299:328–34. doi: 10.1016/s0006-291x(02)02637-2. [DOI] [PubMed] [Google Scholar]
- Lau P, Verrier JD, Nielsen JA, Johnson KR, Notterpek L, Hudson LD. Identification of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J Neurosci. 2008;28:11720–30. doi: 10.1523/JNEUROSCI.1932-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau P, de Strooper B. Dysregulated microRNAs in neurodegenerative disorders. Semin Cell Dev Biol. 2010 doi: 10.1016/j.semcdb.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Leucht C, Stigloher C, Wizenmann A, Klafke R, Folchert A, Bally-Cuif L. MicroRNA-9 directs late organizer activity of the midbrain-hindbrain boundary. Nat Neurosci. 2008;11:641–8. doi: 10.1038/nn.2115. [DOI] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27:435–48. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Alvarez-Buylla A. Neural stem cells in mammalian development. Curr Opin Cell Biol. 2006;18:704–9. doi: 10.1016/j.ceb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, Constantine-Paton M, Horvitz HR. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5:R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass D, Rosenwald S, Meiri E, Gilad S, Tabibian-Keissar H, Schlosberg A, Kuker H, Sion-Vardy N, Tobar A, Kharenko O, Sitbon E, Lithwick Yanai G, Elyakim E, Cholakh H, Gibori H, Spector Y, Bentwich Z, Barshack I, Rosenfeld N. MiR-92b and miR-9/9* are specifically expressed in brain primary tumors and can be used to differentiate primary from metastatic brain tumors. Brain Pathol. 2009;19:375–83. doi: 10.1111/j.1750-3639.2008.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Baldwin DA, Kloosterman WP, Kauppinen S, Plasterk RH, Mourelatos Z. RAKE and LNA-ISH reveal microRNA expression and localization in archival human brain. RNA. 2006;12:187–91. doi: 10.1261/rna.2258506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JA, Lau P, Maric D, Barker JL, Hudson LD. Integrating microRNA and mRNA expression profiles of neuronal progenitors to identify regulatory networks underlying the onset of cortical neurogenesis. BMC Neurosci. 2009;10:98. doi: 10.1186/1471-2202-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333:560–2. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol. 2008;508:28–44. doi: 10.1002/cne.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AN, Xing Y, Harper SQ, Jones L, Davidson BL. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington’s disease. J Neurosci. 2008;28:14341–6. doi: 10.1523/JNEUROSCI.2390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, O’Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, Greengard P. Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007;204:1553–8. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- Shibata M, Kurokawa D, Nakao H, Ohmura T, Aizawa S. MicroRNA-9 modulates Cajal-Retzius cell differentiation by suppressing Foxg1 expression in mouse medial pallium. J Neurosci. 2008;28:10415–21. doi: 10.1523/JNEUROSCI.3219-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–28. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvanathan J, Lee S, Lee B, Lee JW, Lee SK. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21:744–9. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo M, Lee SK, Lee B, Ruiz EC, Pfaff SL, Gill GN. Small CTD phosphatases function in silencing neuronal gene expression. Science. 2005;307:596–600. doi: 10.1126/science.1100801. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Staahl BT, Chen L, Crabtree GR. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature. 2009;460:642–6. doi: 10.1038/nature08139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16:365–71. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, He X, Han X, Yu Y, Ye F, Chen Y, Hoang T, Xu X, Mi QS, Xin M, Wang F, Appel B, Lu QR. MicroRNA-Mediated Control of Oligodendrocyte Differentiation. Neuron. 2010;65:612–626. doi: 10.1016/j.neuron.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L, Cataudella T, Leavitt BR, Hayden MR, Timmusk T, Rigamonti D, Cattaneo E. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35:76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]


