Abstract
Candida albicans is an important cause of morbidity in hospitalized and immunosuppressed patients. Virulence factors of C. albicans include: filamentation, proteinases, adherence proteins and biofilm formation. The objective of this work was to use Galleria mellonella as a model to study the roles of C. albicans filamentation in virulence. We focused our study to five genes BCR1, FLO8, KEM1, SUV3 and TEC1 that have been shown to play a role in filamentation. Filaments are necessary for biofilm formation and evading interaction with macrophages in mammalian infections. Among the five mutant strain tested, we found that only the flo8/flo8 mutant strain did not form filaments within G. mellonella. This strain also exhibited reduced virulence in the larvae. Another strain that exhibited reduced pathogenicity in the G. mellonella model was tec1/tec1 but by contrast, the tec1/tec1 strain retained the ability to form filaments. Overexpression of TEC1 in the flo8/flo8 mutant restored filamentation but did not restore virulence in the larvae as well as in a mouse model of C. albicans infection. The filamentation phenotype did not affect the ability of hemocytes, the immune cells of G. mellonella, to associate with the various mutant strains of C. albicans. The capacities of the tec1/tec1 mutant and the flo8/flo8 TDH3-TEC1 strains to form filaments with impaired virulence suggest that filamentation alone is not sufficient to kill G. mellonella and suggest other virulence factors may be associated with genes that regulate filamentation.
Keywords: Candida albicans, Galleria mellonella, Filamentation, Hyphae
1. Introduction
Candida albicans is the fourth most common cause of bloodstream infections in patients. Candida infections carry a mortality rate that can reach over 35% [1]. Infections can be provoked by the presence of medical implants that provide a niche for the development of a biofilm which is resistant to antifungal agents [1, 2]. The development of a biofilm relies on filamentation whereby hyphae form and extend from a basal layer of cells followed by the production of an extracellular matrix. Several virulence traits are associated with C. albicans hyphal-development and biofilm development, including adherence proteins, proteinases, phospholipases, and filamentation [3, 4].
A study by Brennan et al., (2002) demonstrated that genes important for the transition from yeast to hyphal forms could be tested for their role in virulence using a Galleria mellonella (Lepidoptera: the greater wax moth) infection system [5]. C. albicans causes a lethal infection in G. mellonella [6] and elicits an immune response [7, 8] that is useful in evaluating differences in pathogenicity between Candida species [6]. We continued the pursuit of this model as a means of studying the role of filamentation in virulence.
Our study focused on a collection of genes previously shown to be necessary for filamentation during biofilm formation. These genes included BCR1, FLO8, KEM1, SUV3 and TEC1 [9-11]. Examination of the kem1/kem1 mutant revealed it was capable of forming pseudohyphae rather than hyphae in an in vitro study by Richard and colleagues [10] and the abnormal filaments integrate into a biofilm [10]. The suv3/suv3 mutant also exhibited hyphal-defects in the same study for which hyphae formation was blocked and produced only a thin layer of cells and occasionally pseudohyphae [10]. The transcription factor mutant strains tec1/tec1 and bcr1/bcr1 which were identified for their roles in filamentation as it pertains to biofilm development in a targeted screen by Nobile and Mitchell [9, 10]. The tec1/tec1 mutant was defective in hyphal-development, which resulted in a thin biofilm in vitro [9]. The bcr1/bcr1 mutant produced hyphae, but these hyphae lacked the ability to adhere, which led to the formation of filaments that could not support biofilm development in vitro [9]. Flo8 was characterized in a separate study by Cao et al. and was described as being involved in hyphal-specific gene expression [11]. The flo8/flo8 mutant was unable to form hyphae in vitro in hyphal-induction growth conditions [11]. Hence our virulence assay included mutant strains that represent a spectrum of filamentation development defects.
In the G. mellonella infection model, bcr1/bcr1 and suv3/suv3 strains were virulent and able to form filaments associated with G. mellonella internal structures. In contrast, the flo8/flo8 mutant strain, which did not produce filaments within G. mellonella, exhibited reduced virulence. The mutant strains tec1/tec1 and kem1/kem1 exhibited reduced pathogenicity but unlike the flo8/flo8 mutant the tec1/tec1 and kem1/kem1 strains retained the ability to form filaments within G. mellonella. To determine if these mutations affect the interactions between C. albicans and immune system of G. mellonella, we tested the association between hemocytes and the mutant strains of C. albicans in vitro. We found that all mutant strains included in our study associated with hemocytes in a similar capacity. Thus defects in C. albicans filamentation do not alter the capacity for hemocytes recognition of the pathogen and therefore, aspects of filamentation that lead to reduced pathogenicity in the G. mellonella model are associated with fungal cell virulence.
2. Materials and Methods
2.1. Strains and media
All fungal strains used in this work are listed in Table 1. C. albicans strains were grown in YPD (1% yeast extract, 2% peptone and 2% dextrose) at 30°C.
Table 1. C. albicans strains.
| Strain | Description | Genotype | Reference or source | |||
|---|---|---|---|---|---|---|
| CAN14 | SC5314 | Wild type strain SC5314 | [21] | |||
| BWP17 | Parent strain | ura3∷λimm434 | arg4∷hisG | his1∷hisG | [22] | |
| ura3∷λimm434 | arg4∷hisG | his1∷hisG | ||||
| DAY185 | Arg+ Ura+ His+ Reference strain | ura3∷λimm434 | ARG4:URA3∷arg4∷hisG | his1∷hisG∷pHIS1 | [23] | |
| ura3∷λimm434 | arg4∷hisG | his1∷hisG | ||||
| DAY286 | Arg+ Ura+ His- Reference strain | ura3∷λimm434 | ARG4:URA3∷arg4∷hisG | his1∷hisG | [24] | |
| ura3∷λimm434 | arg4∷hisG | his1∷hisG | ||||
| CJN702 | bcr1/bcr1 | ura3∷λimm434 | arg4∷hisG | his1∷hisG∷pHIS1 | bcr1∷ARG4 | [9, 17] |
| ura3∷λimm434 | arg4∷hisG | his1∷hisG | bcr1∷URA3 | |||
| GKO443 | suv3/suv3 | ura3∷λimm434 | arg4∷hisG | his1∷hisG | suv3∷Tn7-UAU1 | [10] |
| ura3∷λimm434 | arg4∷hisG | his1∷hisG | suv3∷Tn7-URA3 | |||
| MLR74 | kem1/kem1 | ura3∷λimm434 | arg4∷hisG | his1∷hisG | kem1∷ARG4 | [10] |
| ura3∷λimm434 | arg4∷hisG | his1∷hisG | kem1∷URA3 | |||
| CJN896 | tec1/tec1 | ura3∷λimm434 | arg4∷hisG | his1∷hisG∷pHIS1 | tec1∷Tn7-UAU1 | [17] |
| ura3∷λimm434 | arg4∷hisG | his1∷hisG | tec1∷Tn7-URA3 | |||
| CJN1023 | tec1/tec1 + pTEC1 | ura3∷λimm434 | arg4∷hisG | his1∷hisG∷pHIS1-TEC1 | tec1∷Tn7-UAU1 | [9, 17] |
| ura3∷λimm434 | arg4∷hisG | his1∷hisG | tec1∷Tn7-URA3 | |||
| CA2 | flo8/flo8 | ura3∷1 imm434 | flo8∷hisG | [11] | ||
| ura3∷1 imm434 | flo8∷hisG-URA3-hisG | |||||
| BF131 | flo8/flo8 TDH3-TEC1 | ura3∷1 imm434 | flo8∷hisG-URA3-hisG | TDH3-TEC1∷NAT1 | This study | |
| ura3∷1 imm434 | flo8∷hisG | TEC1 | ||||
2.2. C. albicans strains construction
To construct BF131, a genomic fusion was created with the TDH3 promoter to the TEC1 coding region. For construction of the flo8/flo8 TDH3-TEC1 strain, CA2 (previously reported as strain CCF3 ura3∷1 imm434/ura3∷1 imm434 flo8∷hisG/flo8∷hisG-URA3-hisG) [11] was transformed, with a PCR product generated from template NAT1-TDH3 promoter plasmid pCJN542 [12] and primers ElMy 257 (5′-TCAGGTAGAAATTTACAAAGGCGACAAAAACCAAGAAGCAGAATAAGTTAA ATGAAGTAAAGAAAAAAAAATAAAGAGAGAGTAAAAAAAAAAATGCACCAT CAAGCTTGCCTCGTCCCC-3′) and ElMy 258 (5′-TACCAATTGCAACATCAACAATTAATGGAAGCTTCTTGCCTTTCTTTTGTCCA TCGGCATTTCTCACGGGAGTAGCACTAGGAGTAGCTTGCGACATCATATTTGA ATTCAATTGTGATG-3′). These primers amplified the Ashbya gossypii TEF1 promoter, the C. albicans NAT1 ORF, the A. gossypii TEF1 terminator and the C. albicans TDH3 promoter with 100 bp of hanging homology to 500 bp upstream into the promoter of TEC1 and 100 bp of hanging homology from the start codon of the TEC1 ORF. The generated PCR product provides the necessary sequence for homologous recombination of the entire cassette directly upstream of the TEC1 locus so that TEC1 was overexpressed with the TDH3 promoter instead of its endogenous promoter. The cassette was transformed into CA2 using a standard C. albicans transformation protocol described previously [13]. Transformants were selected on YPD+clonNAT (1% yeast extract, 2% peptone, 2% dextrose and 400 μg/ml nourseothricin [NAT] [Werner Bioagents]). Integration of the construct was verified by PCR using primers ElMy 299 (5′-GGGAATTAATGGGCAATAGAAGTG-3′) annealing to the promoter and ElMy 216 (5′-GCAGTATCATCCAAAGTAGTA-3′) annealing to a sequence within the NAT1 gene.
2.3. G. mellonella survival assay
Inocula were prepared by growing C. albicans strains at 30°C overnight with agitation. Cells were collected with centrifugation and washed twice with PBS. Yeast cells were counted using a hemocytometer. G. mellonella larvae (Vanderhorst Wholesale) at their final instar stage were inoculated with 106 cfu of C. albicans suspended in phosphate buffered saline (PBS). Each infection group contained 16 randomly chosen larvae of the appropriate weight (330 ± 25 mg). The inoculum was injected in a 10 μl volume directly to the last left pro-leg using a Hamilton syringe [14]. Larvae were incubated at 37°C and the number of dead larvae was scored daily. Killing curves were plotted and statistical analysis was performed by the Kaplan-Meier method using STATA 6 statistical software (Stata). Killing curves were performed in duplicate and a representative graphs were reported.
2.4. Fungal cell staining with Periodic Acid Schiff (PAS)
Larvae were infected as described above. Two larvae per infection group were cut open to release the internal solid structures into a collection tube. The structures were formalin fixed and embedded in paraffin. Sections were cut and then stained with PAS. The stained tissue was observed with microscopy at 60× magnification using an Olympus microscope.
2.5. Labeling of C. albicans with Fluorescein-5-iosthiocyanate (FITC)
Overnight cultures of C. albicans grown at 30°C with agitation were collected with centrifugation and washed twice with PBS. C. albicans were counted with a hemocytometer and 106 cells in PBS were incubated with 0.1 mg/ml FITC (Invitrogen, Molecular Probes) by adding 10 μl of 10 mg/ml FITC in DMSO to 990 μl of PBS. Cells were incubated for 30 min in the dark at room temperature. Cells were washed three times with PBS containing 1.5% fetal bovine serum (FBS).
2.6. Isolation of G. mellonella hemocytes
Using a procedure modified from Bergin and colleagues (2005) [15], healthy final instar larvae were bled by incision at the base of the right last true leg. Hemolymph was collected in tubes containing cold, sterile insect physiologic saline (IPS) (150 mM sodium chloride; 5 mM potassium chloride; 100 mM tris hydrochloride, pH 6.9 with 10 mM EDTA and 30 mM sodium citrate). The suspension was centrifuged at 700 × g for five min at 4°C and washed twice with cold IPS then suspended in cold PBS containing 100 mg/ml calcium chloride, 100 mg/ml magnesium chloride and 5 mM glucose and then counted with a hemocytometer and used immediately.
2.7. Opsonization of fungal cells
Hemolymph was collected from larvae into cold IPS as described above. Cells were pelleted with centrifugation at 700 × g for five min at 4°C. The supernatant was passed through a 0.45 μm filter and retained as cell-free hemolymph. FITC labeled C. albicans were then suspended in 10% cell-free hemolymph in IPS for 60 min at 37°C in the dark with agitation for opsonization.
2.8. Visualization of C. albicans co-incubated with hemocytes
Hemocytes were permitted to adhere to glass chamber slides by suspending 5 × 104 cells in 500 μl of Grace's media. After the hemocytes incubated for two h at 37°C on the glass chamber slides, the media was aspirated. Adherent hemocytes received 400 μl of Grace's media to the glass chamber slide. To the hemocytes, 100 μl of opsonized, FITC labeled C. albicans in 10% hemolymph diluted in IPS were added for an MOI of 2:1 (fungal cells: hemocytes) and incubated at 37°C for an additional two h. The chamber slides were then washed twice with PBS containing 100 mg/ml calcium chloride, 100 mg/ml magnesium chloride and 5 mM glucose and fixed overnight with 4% paraformaldehyde. The slides were viewed with a confocal laser microscope (TCS; NT Leica).
2.9. FACS
Hemocytes were isolated as described above suspended in PBS with 100 mg/ml calcium chloride, 100 mg/ml magnesium chloride and 5mM glucose; incubated with FITC labeled, opsonized C. albicans cells at a MOI of 2:1 (fungal cells: hemocytes) for 30 min at 37°C in the dark and then submitted to FACS. FACS analysis was performed with a Becton Dickenson FACSCalibur flow cytometer.
2.10. Mouse model of candidiasis
Mice were infected with C. albicans according to an established protocol [16]. Strains CAN14, flo8/flo8 or flo8/flo8 TDH3-TEC1 were used to infect mice. Cultures were grown overnight then washed twice with PBS. CD-1, six week old female mice were infected with 1.5 × 106 cfus suspended in PBS via a tail vein injection in a 100 μl volume. Twelve mice were inoculated per strain tested. Mice were observed daily. The murine protocol was approved by Massachusetts General Hospital, Subcommittee on Research Animal Care.
3. Results
3.1. Infection with hyphal-defective mutants
Our goal for this study was to investigate whether G. mellonella is a useful model to study the role of C. albicans filamentation in virulence. We focused our study to five genes that play various roles in filamentation. Four of the genes included in our study, KEM1, SUV3, FLO8 and TEC1 are required for hyphal morphology. The final gene included in our study, BCR1, is not required for hyphal morphology but does play a role in filament-associated adherence during biofilm development. All of the genes included in this study play varying roles in filamentation in the context of biofilm development. We determined if these genes were also required for virulence by observing the survival of G. mellonella infected with C. albicans mutant strains and compared survival rates to infections by the appropriate reference strains or wild-type CAN14. The bcr1/bcr1 and tec1/tec1 strains were each derived from the prototrophic reference strain DAY185 and therefore were compared to DAY185 to determine changes to pathogenicity (Table 1). Strains kem1/kem1 and suv3/suv3 were constructed from the auxotrophic reference strain DAY286 and were thus compared to DAY286 to determine changes in pathogenicity caused by KEM1 or SUV3 deletions (Table 1). G. mellonella infected with the flo8/flo8 strain was compared to the survival of G. mellonella infected with the wild-type strain CAN14.
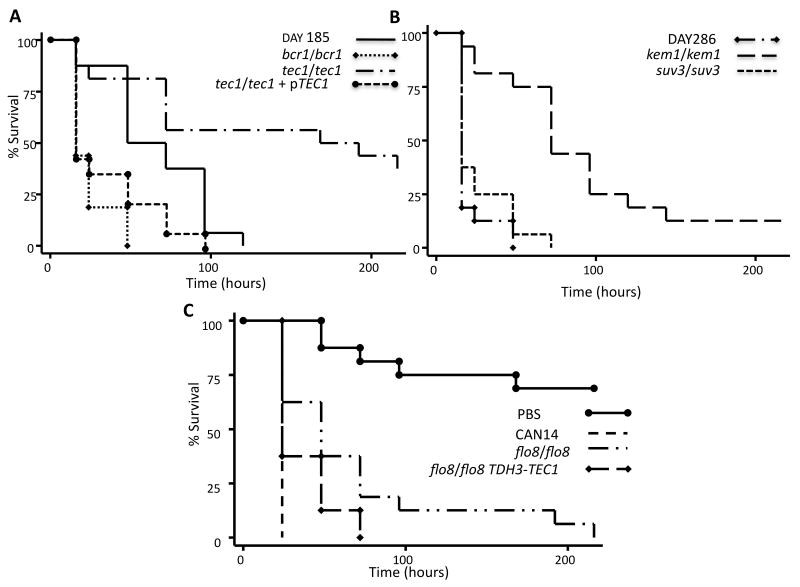
By observing G. mellonella survival after C. albicans infection, we found that TEC1 was associated with pathogenicity whereby the tec1/tec1 mutant exhibited a significant reduction in virulence compared to the reference strain DAY185 (P<0.01) (Fig. 1A). G. mellonella infected with the tec1/tec1 strain reached 50% mortality by 168 h post inoculation. Infection of G. mellonella with a complemented strain carrying an ectopic copy of TEC1 (tec1/tec1 + pTEC1) restored virulence to levels equivalent to the DAY185 reference strain with 50% mortality reached by 24 h (Fig. 1A). The tec1/tec1 mutant strain remained less virulent when compared to the complement strain tec1/tec1 + pTEC1 (P<0.01). At 216 h post infection, when the experiment was concluded, 6 out of 16 G. mellonella in the tec1/tec1 infected group (37.5%) remained alive.
Fig. 1. The effects of hyphal defects on C. albicans pathogenicity in a G. mellonella infection model.
A Kaplan-Meier plot of G. mellonella survival after injection with 106 cfu/larva of C. albicans demonstrated which mutant strains were defective in pathogenicity. A. Strains bcr1/bcr1 and tec1/tec1 were compared to the DAY185 reference strain. The hyphal defective mutant tec1/tec1 demonstrated slower larvae killing compared to the DAY185 reference strain (P<0.01). The TEC1 compliment strain was also evaluated for pathogenicity and found to be virulent. B. Larvae infected with kem1/kem1 or suv3/suv3 were compared to infections with the reference strain DAY286. The kem1/kem1 mutant resulted in slower killing rate compared to the DAY286. Infections with the suv3/suv3 mutant did not adversely affect pathogenicity. C. The hyphal defective mutant flo8/flo8 was compared to the wild-type strain CAN14 and exhibited a slower rate of killing. Injections with PBS were included as a control.
The kem1/kem1 strain demonstrated reduced pathogenicity when compared to the reference strain DAY286 (P<0.0001). The kem1/kem1 infected G. mellonella exhibited 50% mortality by 72 h after infection (Fig. 1B).
FLO8 also contributes to pathogenicity. The flo8/flo8 mutant exhibited a slower rate of killing G. mellonella compared to CAN14 (P<0.001) (Fig. 1C). To explore the roles of TEC1 and FLO8 in virulence in our model, we attempted to determine if they control a similar set of genes as transcription factors by overexpression of TEC1 in the flo8/flo8 mutant by generating the strain flo8/flo8 TDH3-TEC1. Expression of TEC1 restored filamentation ability (see below) but did not restore virulence to the level of a wild type infection to the flo8/flo8 strain in the G. mellonella model (Fig. 1C). The flo8/flo8 TDH3-TEC1 strain was slightly more virulent than the flo8/flo8 strain (P>0.05), but was still less virulent than CAN14 (P=0.0001). We confirmed that flo8/flo8 TDH3-TEC1 did not restore virulence using a mouse model of candidiasis. All twelve flo8/flo8 TDH3-TEC1 inoculated mice remained alive at 62 days post inoculation (P<0.01) compared to CAN14, which exhibited 100% mortality by day 9 post infection (Supplemental Fig. 1).
The deletion of either SUV3 or BCR1, do not reduce C. albicans virulence in the G. mellonella model. The rate of killing by suv3/suv3 and bcr1/bcr1 was not significantly different from the references strains DAY286 and DAY185 respectively. Strains suv3/suv3 and bcr1/bcr1 each reached 50% mortality by 24 h post infection (Fig. 1A and B).
3.2. Filamentation
We endeavored to determine if the less virulent mutant strains filament in G. mellonella. We observed that virulent strains CAN14 and DAY185 produced a mixture of yeast cells and filaments within G. mellonella (Fig. 2). Observations of G. mellonella tissue collected from larvae infected with tec1/tec1, kem1/kem1, suv3/suv3 or bcr1/bcr1 revealed that each of these strains was capable of forming filaments within G. mellonella. We observed a mixture of both yeast and filamenting cells within the tissue (Fig. 2).
Fig. 2. PAS staining of fungal cells in G. mellonella tissue.
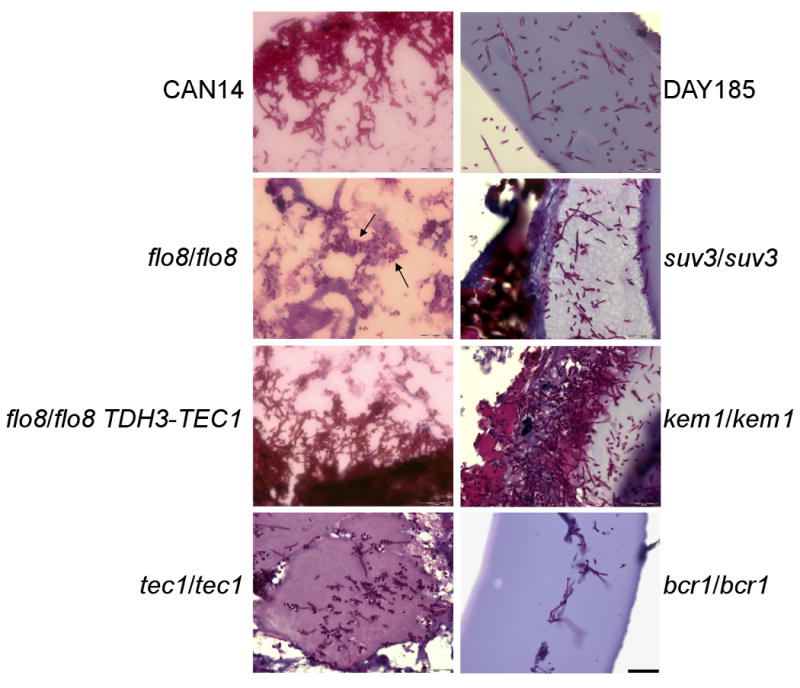
G. mellonella were infected with 106 cfus/larvae and maintained at 37°C for 48 h. Tissue was collected from larvae, formalin fixed and embedded in paraffin. Sections were stained with PAS. Hyphae were observed in G. mellonella tissue infected with each of the mutant strains except for the flo8/flo8 strain. Infection with the flo8/flo8 strain remained in the yeast form. Arrows indicate the location of yeast cells. Scale bar, 20 μm (bottom right corner).
By contrast, flo8/flo8 did not form filaments within the G. mellonella body (Fig. 2). Thus, although all the genes examined play some role in hyphal-development in the context of biofilm formation only FLO8 is necessary for filament formation in G. mellonella tissue.
The filamentation phenotype of the flo8/flo8 mutant was recovered by overexpressing TEC1 in the flo8/flo8 mutant background, where we observed filamentation within G. mellonella (Fig. 2). We next endeavored to determine if TEC1 overexpression could restore the ability of flo8/flo8 to form a biofilm using silicone pads as a platform for in vitro biofilm growth according to a previously described method [10, 17]. However, TEC1 overexpression did not restore the biofilm phenotype (data not shown).
We extended our examination to in vitro conditions. To determine if our C. albicans strains of interest interact with hemocytes, we first determined if C. albicans were able to filament under the in vitro test conditions. FITC labeled C. albicans suspended in 10% hemolymph were added to glass slides containing adherent hemocytes for two h. The wild-type strain was indeed able to filament. We found that many of the strains included in our study were able to filament in vitro with a few notable exceptions. The flo8/flo8, flo8/flo8 TDH3-TEC1 and suv3/suv3 strains did not form hyphae (Fig. 3). The flo8/flo8 strains appeared to remain in a yeast-like form as it did within the G. mellonella tissue. Unlike the in vivo conditions in which overexpression of TEC1 recovered the filament phenotype of the flo8/flo8 mutant, overexpression of the TEC1 gene in the flo8/flo8 mutant in the in vitro conditions did not restore the filamenting phenotype.
Fig. 3. In vitro filamentation.
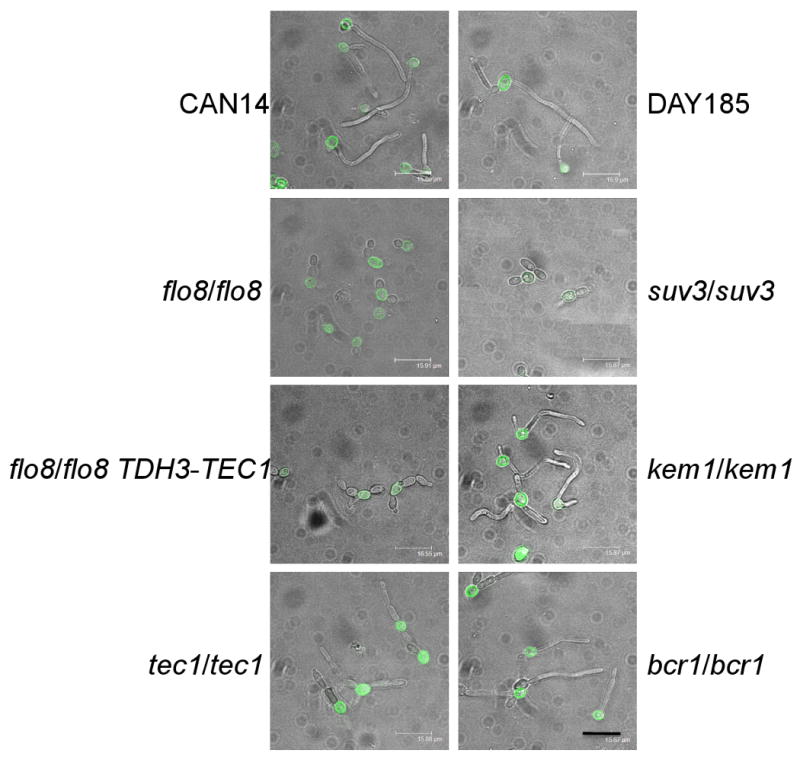
C. albicans strains were incubated for two h with hemolymph and Grace's media at 37°C to induce filamentation. C. albicans were labeled with FITC. Scale bar, 16 μm (bottom right corner).
Since there were differences in filamentation between the various mutant strains we tested if the filamentation phenotype would affect the association between C. albicans strains and G. mellonella hemocytes, which could potentially be a factor in the reduced virulence of the tec1/tec1 and flo8/flo8 strains. Our observations for associations between C. albicans and hemocytes did not distinguishing between phagocytosis and adherence; both types of interaction were considered an association. FITC labeled C. albicans were co-incubated with hemocytes in vitro and observed with microscopy. All of the tested C. albicans strains were capable of associating with the hemocytes regardless of whether they were in a yeast or filamenting form (Fig. 4A). The C. albicans strains that were previously observed to be able to form hyphae in hemolymph were capable of forming hyphae when associated with hemocytes. Although the flo8/flo8 and tec1/tec1 were less virulent than the other strains, there was no correlation between virulence and hemocytes association (Fig. 4B). The scatter plots were divided into three regions and we determined the percentage of cells within each region. Region 3 (R3) depicts hemocytes, region 1 (R1) depicts C. albicans cells and region 5 (R5) indicates associations between hemocytes and C. albicans (Fig. 4B). The average percentage in R5 for three replicates for each mutant strain was compared to the appropriate reference strain and expressed as a percentage of cells in region 5 relative to the reference strain (Fig. 4C).
Fig. 4. C. albicans association with G. mellonella hemocytes.
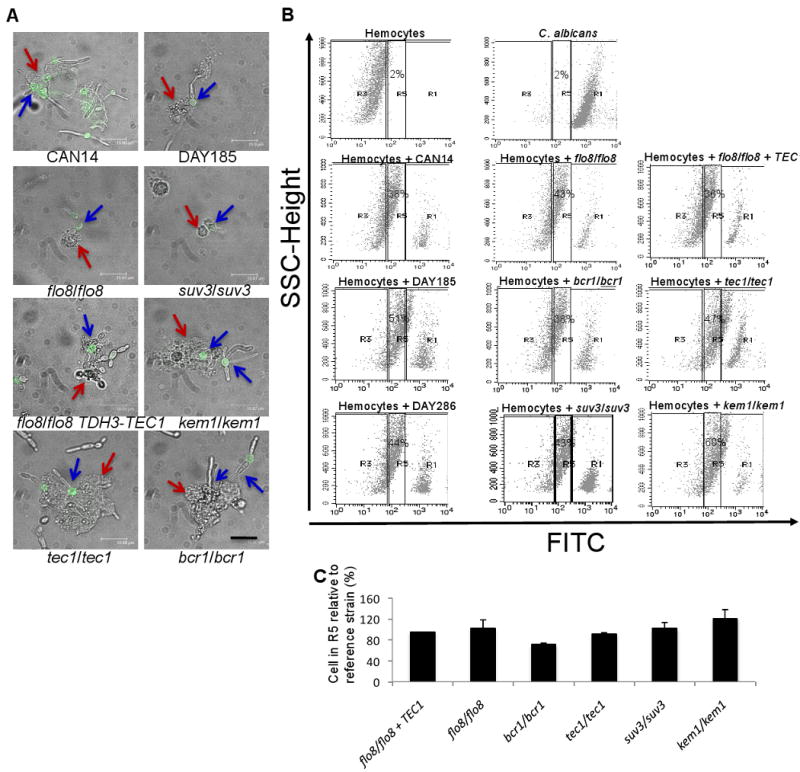
A. C. albicans were observed to bind to hemocytes when incubated in hemolymph and Grace's media for 2 h at 37°C. C. albicans were labeled with FITC. Red arrows indicate G. mellonella hemocytes and blue arrows indicate C. albicans cells. B. G. mellonella hemocytes were combined in suspension with FITC-labeled fungal cells and analyzed by FACS for association between hemocytes and fungal cells. Y-axis is side scatter (SSC). X-axis is FITC fluorescence. Hemocytes were submitted to FACS to obtain a characteristic plot for hemocytes in suspension without the addition of fungal cells. A characteristic scatter plot was also generated for fungal cells without hemocytes. Hemocytes were then combined with fungal cells to examine the shift in the plot due to associations between hemocytes and fungal cells. The gated regions of scatter plots represent hemocytes (R3), C. albicans (R1) and C. albicans associating with hemocytes (R5). C. An average of three independent FACS analyses were used to compare the association between C. albicans and hemocytes (R5) for each of the C. albicans mutant strains to the association between C. albicans and hemocytes (R5) for the appropriate reference strain. Therefore the flo8/flo8 and flo8/flo8 + TEC1 strains were both compared to the CAN14 strain; the bcr/bcr1 and tec1/tec1 strains were both compared to strain DAY185; and the suv3/suv3 and kem1/kem1 strains were both compared to strain DAY286.
4. Discussion
G. mellonella provides a facile model to further the study of C. albicans. Brennan and colleagues (2002) established that the G. mellonella model was applicable for examining the genetic components of pathogenicity for C. albicans. We expanded on their study by examining the role of BCR1, FLO8, KEM1, SUV3 and TEC1 in virulence using the G. mellonella infection model. These genes are required for filamentation as it relates to biofilm development. We find that FLO8 and TEC1 are associated with pathogenicity. Under in vitro conditions the strains included in this study show similar association with G. mellonella hemocytes. There were defects in filamentation associated with the flo8/flo8, flo8/flo8 TDH3-TEC1 and suv3/suv3 strains to the degree that these strains were unable to form hyphae. By contrast the flo8/flo8 TDH3-TEC1 and suv3/suv3 strains did produce filaments in G. mellonella tissue that was isolated post infection. Although flo8/flo8 TDH3-TEC1 forms filaments, this formation is not sufficient for virulence at the level of a wild type infection. Similarly, the tec1/tec1 strain also produced filaments but was hindered in killing larvae.
Infection of G. mellonella with the various mutants used in this study revealed that the tec1/tec1 and flo8/flo8 mutants were defective in virulence, thus implicating an aspect of hyphal morphogenesis in pathogenicity. Although both TEC1 and FLO8 were required for virulence they exhibited differing morphologies. The flo8/flo8 strain was deficient in filament production in vitro or when associated with G. mellonella tissue structures however the tec1/tec1 strain was observed to form filaments associated with G. mellonella internal structures and under in vitro conditions. These contrasting filament morphologies indicate that filamentation alone is not enough to kill G. mellonella since tec1/tec1 produced filaments that do not result in virulence.
Like Tec1, Bcr1 is also a transcription factors but unlike TEC1, BCR1 is not required for virulence. The bcr1/bcr1 mutant, defective in expression of many hyphal-specific genes but not in hyphal morphogenesis [9, 10], is fully virulent. Thus our findings argue that Bcr1-independent aspects of filamentation, including Bcr1-independent genes as well as morphogenesis per se, are critical for virulence. The difference in pathogenicity between the two mutant strains argues for a Bcr1-independent and Tec1-dependent mechanism of virulence.
Pathogenicity in the G. mellonella infection model was attributed to factors that are Kem1, Flo8 and Tec1-dependent but Bcr1 and Suv3-independent. Our findings with the G. mellonella model agree with previous assessments of FLO8 and TEC1 that found both were involved in C. albicans pathogenicity in mammalian infection models [11, 18]. Observations previously reported with a mouse model of infection showed that a tec1/tec1 strain was able to filament in a localized infection but exhibited a reduction in virulence in a systemic infection of candidiasis [18]. Although both FLO8 and TEC1 are important to virulence, overexpression of TEC1 in the flo8/flo8 mutant strain did not restore the virulence phenotype, the capacity to form a biofilm in vitro or filament in vitro. The strains were kept in in vitro conditions for two h and perhaps with a longer incubation filamentation could have been achieved by flo8/flo8 TDH3-TEC1 but we did not extend the time because the time allotted was sufficient for the wild-type and reference strains to form filaments. TEC1 overexpression did however restore the capacity to form filaments during infection of G. mellonella since filaments were observed in the tissue structures isolated from infected G. mellonella. Thus Flo8-dependent genes that respond to Tec1 expression for the restoration of filaments could potentially be dependent upon the conditions in which TEC1 is expressed.
The recovery of filament formation in the G. mellonella tissue by overexpressing TEC1 in the flo8/flo8 mutant suggests that Tec1 or Tec1-dependent genes control a similar set of genes as Flo8 in filament formation. Since the recovery of filamentation within G. mellonella does not lead to biofilm development or filamentation in vitro, Tec1 does not support the full recovery of genes activated by Flo8 but perhaps a subset of genes controlled by Flo8. Also, the inability for TEC1 overexpression to fully recover the virulence aspect of the flo8/flo8 phenotype suggests that Tec1 does not influence the virulence factors that are Flo8-dependent. Thus, it is reasonable to assume that there are Tec1-dependent and Flo8-dependent virulence factors, and that the Flo8-dependent virulence factors are Tec1-independent.
The other strain that showed differential filamentation between in vivo and in vitro conditions was suv3/suv3. Although the suv3/suv3 strain retained the pathogenicity phenotype and formed filaments in vivo, it too did not form filaments in our in vitro conditions. Our findings indicate that pathogenicity and in vivo filamentation are Suv3-independent but filamentation in vitro is Suv3-dependent. Thus there is potentially a genetic difference in filamentation between in vitro and in vivo conditions. It cannot be discounted that host factors could contribute to filamentation and that these factors were present in the in vivo conditions but absent in our in vitro experiments or that filamentation was delayed for the suv3/suv3 strain within the in vitro conditions. This same reasoning can be applied to the flo8/flo8 TDH3-TEC1 strain that failed to produce filaments in in vitro conditions.
The in vivo formation of filaments by the virulence deficient strains tec1/tec1 and flo8/flo8 TDH3-TEC1 does not necessarily mean that filaments are completely without a role in pathogenicity. Others have shown that filaments provide a means of escaping host defenses in order to establish an infection. In a study of mammalian cell phagocytosis of C. albicans, the formation of filaments by yeast cells contributed to phagocytic cell death [19]. We were not able to establish a correlation between association with hemocytes and pathogenicity for the strains examined in this study.
In conclusion, G. mellonella was a useful model in evaluating the role of filamentation in C. albicans pathogenicity. We determined that the hyphae formation gene KEM1 [20], hyphal formation regulator gene TEC1, which is important for biofilm formation and hyphal formation, as well as virulence in a mouse model [18], and the hyphal-development gene FLO8 [11] are important for C. albicans virulence in G. mellonella. Our findings argue that a subset of filamentation properties are required for virulence, and we anticipate that a more refined comparison of the targets of Tec1, Flo8 and Bcr1 will provide mechanistic insight into C. albicans virulence traits.
Supplementary Material
CD-1, six week old, female mice were infected with C. albicans through the tail vein. Inoculum was given at 1.5 × 106 cfu in a 100 μl volume. CAN14 caused a lethal infection but flo8/flo8 and flo8/flo8 TDH3-TEC1 were avirulent after monitoring survival for 62 days.
Acknowledgments
The flo8/flo8 mutants strain CA2 was provided as a gift from Dr. Haoping Liu. CAN14 was provided by Dr. Gerald Fink. This work was supported by a R01 award AI075286 from the NIH and a R21 award R21A1070569 to EM. BBF was supported by a Massachusetts General Hospital Executive Committee on Research Fund for Medical Discovery Fellowship.
Abbreviations
- FBS
fetal bovine serum
- FITC
fluorescein-5-isothiocyanate
- IPS
insect physiological saline
- PAS
Periodic Acid Schiff
- PBS
phosphate buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–6. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 2.Hawser SP, Douglas LJ. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother. 1995;39:2128–31. doi: 10.1128/aac.39.9.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berman J, Sudbery PE. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat Rev Genet. 2002;3:918–30. doi: 10.1038/nrg948. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi SD, Cutler JE. Candida albicans hyphal formation and virulence: is there a clearly defined role? Trends Microbiol. 1998;6:92–4. doi: 10.1016/s0966-842x(98)01218-9. [DOI] [PubMed] [Google Scholar]
- 5.Brennan M, Thomas DY, Whiteway M, Kavanagh K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol. 2002;34:153–7. doi: 10.1111/j.1574-695X.2002.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 6.Cotter G, Doyle S, Kavanagh K. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol Med Microbiol. 2000;27:163–9. doi: 10.1111/j.1574-695X.2000.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 7.Bergin D, Brennan M, Kavanagh K. Fluctuations in haemocyte density and microbial load may be used as indicators of fungal pathogenicity in larvae of Galleria mellonella. Microbes Infect. 2003;5:1389–95. doi: 10.1016/j.micinf.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Bergin D, Murphy L, Keenan J, Clynes M, Kavanagh K. Pre-exposure to yeast protects larvae of Galleria mellonella from a subsequent lethal infection by Candida albicans and is mediated by the increased expression of antimicrobial peptides. Microbes Infect. 2006;8:2105–12. doi: 10.1016/j.micinf.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Nobile CJ, Mitchell AP. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol. 2005;15:1150–5. doi: 10.1016/j.cub.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 10.Richard ML, Nobile CJ, Bruno VM, Mitchell AP. Candida albicans biofilm-defective mutants. Eukaryot Cell. 2005;4:1493–502. doi: 10.1128/EC.4.8.1493-1502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao F, Lane S, Raniga PP, Lu Y, Zhou Z, Ramon K, Chen J, Liu H. The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol Biol Cell. 2006;17:295–307. doi: 10.1091/mbc.E05-06-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nobile CJ, Solis N, Myers CL, Fay AJ, Deneault JS, Nantel A, Mitchell AP, Filler SG. Candida albicans transcription factor Rim101 mediates pathogenic interactions through cell wall functions. Cell Microbiol. 2008;10:2180–96. doi: 10.1111/j.1462-5822.2008.01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nobile CJ, Mitchell AP. Large-scale gene disruption using the UAU1 cassette. Methods Mol Biol. 2009;499:175–94. doi: 10.1007/978-1-60327-151-6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, Ausubel FM, Diener A. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun. 2005;73:3842–50. doi: 10.1128/IAI.73.7.3842-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergin D, Reeves EP, Renwick J, Wientjes FB, Kavanagh K. Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect Immun. 2005;73:4161–70. doi: 10.1128/IAI.73.7.4161-4170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breger J, Fuchs BB, Aperis G, Moy TI, Ausubel FM, Mylonakis E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007;3:e18. doi: 10.1371/journal.ppat.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nobile CJ, Andes DR, Nett JE, Smith FJ, Yue F, Phan QT, Edwards JE, Filler SG, Mitchell AP. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2006;2:e63. doi: 10.1371/journal.ppat.0020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schweizer A, Rupp S, Taylor BN, Rollinghoff M, Schroppel K. The TEA/ATTS transcription factor CaTec1p regulates hyphal development and virulence in Candida albicans. Mol Microbiol. 2000;38:435–45. doi: 10.1046/j.1365-2958.2000.02132.x. [DOI] [PubMed] [Google Scholar]
- 19.Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–49. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 20.An HS, Lee KH, Kim J. Identification of an exoribonuclease homolog, CaKEM1/CaXRN1, in Candida albicans and its characterization in filamentous growth. FEMS Microbiol Lett. 2004;235:297–303. doi: 10.1016/j.femsle.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 21.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae Ura3 and E. coli PyrF mutations. Mol Gen Genet. 1984;198:179–82. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RB, Davis D, Mitchell AP. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol. 1999;181:1868–74. doi: 10.1128/jb.181.6.1868-1874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis D, Edwards JE, Jr, Mitchell AP, Ibrahim AS. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect Immun. 2000;68:5953–9. doi: 10.1128/iai.68.10.5953-5959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis DA, Bruno VM, Loza L, Filler SG, Mitchell AP. Candida albicans Mds3p, a conserved regulator of pH responses and virulence identified through insertional mutagenesis. Genetics. 2002;162:1573–81. doi: 10.1093/genetics/162.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CD-1, six week old, female mice were infected with C. albicans through the tail vein. Inoculum was given at 1.5 × 106 cfu in a 100 μl volume. CAN14 caused a lethal infection but flo8/flo8 and flo8/flo8 TDH3-TEC1 were avirulent after monitoring survival for 62 days.