Abstract
Background & Aims
We aimed to identify the incidence and predictors of de novo gastroesophageal variceal formation and progression in a large cohort of patients with chronic hepatitis C (CHC) and advanced fibrosis.
Methods
All participants in the HALT-C Trial were offered an endoscopy before treatment and again after 4 years. Patients with varices at baseline also had a endoscopy at 2 years. Baseline laboratory and clinical parameters were analyzed as predictors of de novo variceal formation and variceal progression.
Results
De novo varices developed in 157 of the 598 (26.2%) patients. Most of the new varices were small (76.4%) and only 1% of patients developed variceal hemorrhage. The likelihood of developing varices was associated with subject race (Hispanic > Caucasian > African American, p= 0.0005), lower baseline levels of albumin (P=0.051), and higher levels of hyaluronic acid (P< 0.001) with an area under the receiver operating characteristic (AUROC) curve=0.70. Among 210 patients with existing gastroesophageal varices, 74 (35.2%) had variceal progression or bleeding during follow-up. Patients with a higher baseline ratios of serum aspartate /alanine aminotransferase (P=0.028) and lower platelet counts (P=0.0002) were at greatest risk of variceal progression (AUROC = 0.72). Prolonged, low-dose peginterferon α2a therapy and β-blockers did not influence the risk of developing new or enlarging varices.
Conclusion
Development of varices in patients with CHC is associated with patient race/ethnicity and laboratory markers of disease severity. Prolonged low dose peginterferon α2a therapy and β-blockers do not reduce the risk of variceal development nor progression.
Keywords: cirrhosis, portal hypertension, esophagogastroduodenoscopy, hyaluronic acid
Introduction
The presence of varices in the esophagus or stomach (i.e. gastroesophageal varices) in patients with chronic liver disease has important prognostic and therapeutic implications. For example, patients with moderate to large varices should receive prophylactic beta-blockers or undergo band ligation to reduce the risk of variceal hemorrhage (1). In addition, patients with varices should avoid aspirin, non-steroidal anti-inflammatory drugs, and other anticoagulants to reduce the risk of gastrointestinal bleeding. Although upper endoscopy is the “gold standard” for diagnosing varices, the cost, risk, and inconvenience associated with this invasive procedure have led many to seek alternative means to predict the presence and severity of varices (2, 3). Development of reliable and simple non-invasive tests that accurately predict the presence of varices could greatly improve the clinical care of the increasing number of patients with chronic liver disease. In addition, accurate laboratory or clinical predictors of variceal progression could help guide surveillance and interventional strategies in patients with known varices.
The severity of cirrhosis as determined by the Child-Turcotte-Pugh (CTP) score has consistently been associated with the likelihood of having gastroesophageal varices. For example, subjects with decompensated cirrhosis are significantly more likely to have varices or develop variceal bleeding during follow-up compared to subjects with compensated cirrhosis (4,5). However, many of these studies involved a limited number of patients with variable follow-up and included a large proportion of patients with alcoholic liver disease (5). In the United States and most western countries, chronic hepatitis C (CHC) infection has emerged as a leading cause of liver failure, hepatocellular carcinoma (HCC) and liver transplantation (6). Improved understanding of the factors that influence disease progression including the development of gastroesophageal varices are urgently needed.
The Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial is a prospective, multi-center study designed to determine if maintenance peginterferon can reduce the rate of disease progression in patients with CHC and advanced but compensated fibrosis (7). The overall rate of liver decompensation as well as histological fibrosis progression was similar in the peginterferon treated and untreated control patients (7). However, the large number of well-characterized patients provided a unique opportunity to prospectively study various aspects of liver disease progression including the development of gastroesophageal varices. In a prior report, esophageal varices were detected in 16% of the HALT-C Trial patients with bridging fibrosis and 39% of those with compensated cirrhosis at study entry (8). Correlates of baseline varices included male gender, non-African American race, and laboratory markers of disease severity. The aims of the current study are to identify the incidence of de novo gastroesophageal varix formation in HALT-C Trial patients without baseline varices as well as the incidence of variceal progression in patients with established varices during a median follow-up of 4 years. In addition, baseline laboratory and clinical data were analyzed to develop models that accurately and reliably predict the presence and progression of gastroesophageal varices.
Methods
The design of the HALT-C Trial has been previously described (7). Briefly, patients with prior non-response to standard interferon and advanced hepatic fibrosis on biopsy (i.e. Ishak fibrosis score > 3) with no history of hepatic decompensation or HCC were treated with pegylated interferon and ribavirin during the “lead-in” phase. Patients with persistently detectable HCV RNA at week 20, were randomized to maintenance peginterferon-α2a 90 ug/ week (Pegasys; Roche Laboratories, Nutley NJ) or no treatment for the next 3.5 years. Week 20 virological responders in the lead-in continued combination therapy for 48 weeks. However, patients with detectable HCV RNA during treatment (breakthrough) or after stopping treatment (relapse) were also eligible for randomization. In addition, “express” patients who had received at least 24 weeks of peginterferon and ribavirin were eligible for enrollment in the randomized phase. This study was approved by the Institutional Review Board at each site and all subjects provided written informed consent for endoscopy.
Endoscopic evaluation and endpoints
A written endoscopy protocol was developed that included standardized criteria for identifying and grading esophageal varices, gastric varices and portal hypertensive gastropathy. All of the physician investigators completed a tutorial prior to the study initiation. Furthermore, endoscopic findings were immediately recorded on a templated form on the date of the endoscopy and the data were queried and verified against source documents at annual audits. All subjects underwent a pretreatment baseline endoscopy prior to randomization and subjects with baseline gastroesophageal varices had a repeat endoscopy at 18 months following randomization which is deemed study year 2. In addition, all randomized patients who had not died or undergone liver transplantation underwent a follow-up endoscopy at year 3.5 following randomization which is deemed study year 4. The presence and grade of esophageal varices were evaluated with the esophagus insufflated as follows (9):
F1= small varices with mild impingement on the lumen (< 25%)
F2 = medium varices, intermediate in size between F1 and F3
F3= large varices with considerable impingement on the lumen (> 50%).
Gastric varices were recorded according to the Sarin classification (10)
De Novo varices were defined as newly identified esophageal or gastric varices or the development of variceal hemorrhage during follow-up in the patients without varices at baseline. Variceal progression was defined as an increase in esophageal varix size of at least 1 grade at the year 2 or year 4 endoscopy, development of variceal bleeding any time in the randomized phase, or development of new gastric varices. Regression of varices was defined as a decrease in esophageal varix size by 1 grade or more at the year 4 endoscopy.
Laboratory and clinical assessment
Lifetime alcohol consumption was estimated using a modification of the Skinner survey at enrollment (7). Routine baseline laboratory values (i.e. serum aminotransferases, albumin, bilirubin, platelet count) were obtained at the local laboratories. HOMA-IR was calculated as HOMA= [(insulin* glucose)/22.5]*0.5551. Serum HCV RNA testing was done at baseline and during the randomized phase using the quantitative COBAS Amplicor HCV Monitor Test (Roche Molecular Diagnostics, Branchburg, NJ) with a lower limit of detection of 600 IU/ml at a central laboratory. Serum iron, total iron binding capacity, total bile acids and hyaluronic acid levels were determined at a reference laboratory (WAKO Diagnostics, Richmond VA). Liver biopsies were evaluated by a panel of expert liver pathologists and scored for the degree of hepatic fibrosis and inflammation defined by the Ishak scoring system and the degree of hepatic steatosis was estimated as grade 0 to 4 (11). Splenomegaly was defined by a spleen length > 13 cm on sonography.
All patients were seen every 3 months during the randomized phase for laboratory and clinical assessment. In addition, annual liver ultrasounds were obtained to screen for HCC and serum alpha fetoprotein levels were obtained every 6 months as well. Clinical endpoints for the study included an increase in CTP score to > 7 on two separate occasions 3 months apart, variceal bleeding, ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, HCC, or death. For the subgroup of patients with bridging fibrosis at baseline, a two point increase in the Ishak Fibrosis score was considered histological progression.
Statistical methods
Statistical analyses were performed at the data coordinating center with the use of SAS software, 9.2 (SAS Institute, Cary, NC). Log transformation of non-normally distributed variables was undertaken when indicated. Multivariate logistic regression methods were used to explore factors associated with new varices development, variceal progression, and variceal regression. For multivariate models, individual factors that were associated with the outcome on bivariate analysis with a p < 0.20 were initially included. Then only variables that were significant with p < 0.05 were retained in the final multivariate model. The area under the receiver operating curve (AUROC) was calculated from a plot of sensitivity versus 1-specificity and can range from 0.5 (no predictive ability) to 1 (perfect discrimination).
Results
Patient population
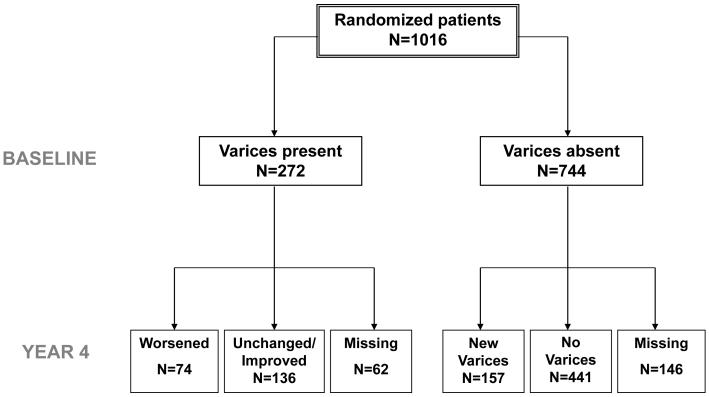
A pretreatment screening endoscopy was available in 1,016 of the 1,050 (96.8%) patients randomized in the HALT-C Trial. Gastroesophageal varices were absent in 744 (73.2%) patients while 272 (26.8%) had either baseline esophageal varices (261) or gastric varices alone (11) (Figure 1). The 34 patients who did not undergo a pretreatment screening endoscopy did not differ from the patients included in this analysis except for having significantly lower BMI, baseline serum AST/ ALT and being younger (p< 0.05 for each).
Figure 1.
Description of the HALT-C Trial cohort. Amongst the 744 patients without baseline varices, 441 continued to have no detectable varices during follow-up while 157 developed new gastroesophageal varices. Amongst the 272 patients with baseline gastroesophageal varices, 136 patients had stable or improved varices while 74 subjects had worsening varices.
In the 744 subjects without baseline gastroesophageal varices, 146 patients did not undergo a follow-up endoscopy by year 4 due to liver disease progression (n=40), study withdrawal (n=66), or patient refusal (n= 40). The 146 excluded patients had similar baseline demographic features to the 598 patients included in this analysis. However, the excluded patients also had a greater lifetime history of alcohol consumption (p=0.0002), were more likely to be smokers (p=0.0024), and had laboratory markers of more severe liver disease at entry (i.e. higher AST/ ALT, p= 0.038, lower albumin p= 0.0093) (data not shown). In addition, the excluded patients were more likely to develop a clinical outcome during follow-up in the HALT-C Trial (27% vs 8%, p < 0.0001). Clinical characteristics of the 598 patients without pretreatment gastroesophageal varices include a mean age of 50.1 years, 72% were Caucasian, and 32% had histological cirrhosis (Table 1).
Table 1.
Characteristics of the 598 HALT-C Trial patients without gastroesophageal varices at pretreatment screening endoscopy
| Characteristic | All patients N=598 |
De Novo varices n=157 |
Non- progressors n=441 |
p-value * | OR (95% CI) |
|---|---|---|---|---|---|
| Demographics | |||||
|
| |||||
| Age (yrs) | 50.1 (7.1) | 50.9 (7.2) | 49.8 (7.1) | 0.10 | 1.02 (1.00,1.05) |
|
| |||||
| % Male | 69% | 68% | 69% | 0.77 | 0.94 (0.64,1.40) |
|
| |||||
| Race | 0.0003 | ||||
| % Hispanic | 6% | 13% | 4% | 3.72 (1.89,7.31) |
|
| % Black | 22% | 19% | 23% | 0.86 (0.54,1.37) |
|
| % Caucasian | 72% | 68% | 73% | 1 Ref | |
|
| |||||
| Co-morbidities | |||||
|
| |||||
| % Diabetes mellitus | 23% | 28% | 22% | 0.11 | 1.40 (0.92,2.12) |
|
| |||||
| BMI (kg/m2) | 29.8 (5.3) | 30.6 (5.5) | 29.5 (5.2) | 0.0330 | 1.04 (1.00,1.07) |
|
| |||||
| % Truncal obesity | 51% | 56% | 49% | 0.1524 | 1.31 (0.91,1.90) |
|
| |||||
| % Current smoker | 28% | 24% | 29% | 0.25 | 0.78 (0.51,1.19) |
|
| |||||
| Total lifetime drinks | 15358 (23940) | 12793 (18289) |
16273 (25614) | 0.12 | 1.00 (1.00,1.00) |
|
| |||||
| % Baseline NSAID/Cox- 2 Inhibitor |
5% | 4% | 5% | 0.43 | 0.69 (0.28,1.72) |
|
| |||||
| % B-Blocker at baseline | 7% | 7% | 8% | 0.84 | 0.93 (0.46,1.89) |
|
| |||||
| % Statins at baseline | 1.3% | 2% | 1% | 0.44 | 1.70 (0.40,7.19) |
|
| |||||
| HCV parameters | |||||
|
| |||||
| Prior HALT-C status | 0.37 | ||||
| % Non-responder | 63% | 67% | 62% | 1.09 (0.68,1.74) |
|
| % Breakthru/ relapser | 17% | 13% | 18% | 0.75 (0.40,1.40) |
|
| % Express | 20% | 20% | 20% | 1 Ref | |
|
| |||||
| Log HCV RNA (IU/ml) | 6.5 (0.5) | 6.37 (0.5) | 6.54 (0.5) | 0.0004 | 0.52 (0.37,0.75) |
|
| |||||
| % HCV genotype 1 | 94% | 90% | 95% | 0.0253 | 0.45 (0.23,0.91) |
|
| |||||
| Laboratory parameters | |||||
|
| |||||
| Hemoglobin (g/dl) | 15.0 (1.4) | 14.9 (1.6) | 15.1 (1.4) | 0.25 | 0.93 (0.82, 1.05) |
|
| |||||
| Serum AST/ALT | 0.85 (0.3) | 0.91 (0.3) | 0.83 (0.3) | 0.0027 | 2.55 (1.38, 4.70) |
|
| |||||
| Alk phos ratio (ULN) | 0.82 (0.4) | 0.91 (0.4) | 0.79 (0.4) | 0.0011 | 2.12 (1.35, 3.33) |
|
| |||||
| Total bilirubin (mg/dl) | 0.73 (0.4) | 0.81 (0.4) | 0.7 (0.4) | 0.0014 | 2.29 (1.38, 3.82) |
|
| |||||
| INR | 0.0005 | ||||
| % >1.0 | 31% | 42% | 27% | 1.96 (1.34-2.87) |
|
| % ≤ 1.0 | 69% | 58% | 73% | 1 Ref | |
|
| |||||
| Albumin (g/dl) | 3.93 (0.4) | 3.83 (0.4) | 3.96 (0.3) | <.0001 | 0.33 (0.20, 0.57) |
|
| |||||
| Platelets (103/ml) | 177 (61.5) | 159 (67.9) | 183 (57.8) | <.0001 | 0.50 (0.36, 0.70) |
|
| |||||
| Total Bile acids (umol/l) | 14.1 (16.5) | 20.5 (24) | 11.7 (12) | <.0001 | 1.03 (1.02, 1.04) |
|
| |||||
| HOMA-IR | 14.1 (17.7) | 15.2 (17.3) | 13.7 (17.9) | 0.42 | 1.00 (0.99, 1.02) |
|
| |||||
| Log HA (ng/ml) | 1.89 (0.5) | 2.12 (0.5) | 1.81 (0.4) | <.0001 | 4.69 (2.98, 7.37) |
|
| |||||
| % Splenomegaly on ultrasound |
28% | 38% | 25% | 0.0018 | 1.87 (1.26, 2.76) |
|
| |||||
| Baseline Histology | |||||
|
| |||||
| % Cirrhosis (Ishak 5/6) | 32% | 42% | 29% | 0.0025 | 1.79 (1.23, 2.62) |
|
| |||||
| Mean HAI Baseline | 7.4 (2.1) | 7.5 (2) | 7.4 (2.1) | 0.49 | 1.03 (0.94, 1.13) |
|
| |||||
| % Hepatic steatosis ≥ 2 | 42% | 54% | 37% | 0.0005 | 1.93 (1.33, 2.78) |
|
| |||||
| % Mallory Bodies | 14% | 21% | 12% | 0.0064 | 1.95 (1.21, 3.15) |
|
| |||||
| % Zone 3 fibrosis | 0.15 | ||||
|
| |||||
| 0 | 64% | 67% | 63% | 0.64 (0.30,1.36) |
|
|
| |||||
| 1 | 30% | 25% | 32% | 0.47 (0.21,1.06) |
|
|
| |||||
| 2 | 6% | 38% | 5% | 1 Ref | |
|
| |||||
| Randomized phase | |||||
|
| |||||
| % Peginterferon arm | 50% | 50% | 49% | 0.81 | 1.05 (0.73,1.51) |
|
| |||||
| % Clinical outcome | 8% | 20% | 4% | <0.0001 | 5.46 (2.99,10.01) |
|
| |||||
| % Two-point increase in Ishak fibrosis score |
26% | 53% | 18% | <0.0001 | 5.20 (3.11,8.68) |
Data reported as mean + std dev or %
comparing de novo vs non-progressors
Sixty-two of the 272 subjects with pretreatment gastroesophageal varices failed to undergo a follow-up endoscopy due to liver disease progression (n= 32), study withdrawal (n=26), or patient refusal (n= 4). The 62 excluded patients had similar demographic features to the 210 patients included in this analysis but also had greater likelihood of smoking (p=0.015), higher serum alkaline phosphatase levels (p=0.025) and lower serum albumin levels (p=0.0013) (data not shown). In addition, the excluded patients were more likely to develop a primary clinical outcome during follow-up (52% vs 27%, p=0.0003). The mean age of the 210 patients included in this analysis was 50.8 years, 78% were male, 81% were Caucasian, 64% had cirrhosis, and only 9.5 % (20) had gastroesophageal varices while the remainder (190) had esophageal varices alone (Table 2). Adherence to the endoscopy schedule was excellent with 203 (97%) of the patients undergoing a surveillance endoscopy at year 2 and 192 (91%) undergoing a surveillance endoscopy at study year 4.
Table 2.
Characteristics of the 210 HALT-C Trial patients with gastroesophageal varices at pretreatment screening endoscopy
| Characteristic | All patients N=210 |
Variceal progressors n=74 |
Non- progressors n=136 |
p-value * |
OR (95% CI) |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (yrs) | 50.8 (7.0) | 51.5 (7.6) | 50.5 (6.7) | 0.35 | 1.02 (0.98, 1.06) |
|
| |||||
| % Male | 78% | 77% | 79% | 0.78 | 0.91 (0.46, 1.79) |
|
| |||||
| Race | 0.188 | ||||
| % Hispanic | 10% | 15% | 8% | 1.88 (0.77, 4.60) |
|
| % Black | 9% | 5% | 10% | 0.54 (0.17, 1.71) |
|
| % Caucasian | 81% | 80% | 82% | 1 Ref | |
|
| |||||
| Co-morbidities | |||||
|
| |||||
| % Diabetes mellitus | 26% | 28% | 25% | 0.60 | 1.19 (0.63, 2.25) |
|
| |||||
| BMI (kg/m2) | 29.8 (4.5) | 29.5 (4.4) | 30 (4.6) | 0.44 | 0.94 (0.80, 1.10) |
|
| |||||
| % Truncal obesity | 50% | 47% | 52% | 0.52 | 0.83 (0.47, 1.47) |
|
| |||||
| % Current smoker | 26% | 30% | 24% | 0.33 | 1.38 (0.73, 2.60) |
|
| |||||
| Total lifetime drinks | 20113 (36096) | 25283 (52592) | 17300 (22336) | 0.155 | 1.00 (1.00, 1.00) |
|
| |||||
| % Baseline NSAID/ Cox-2 Inhibitor |
4% | 3% | 4% | 0.54 | 0.60 (0.12, 3.06) |
|
| |||||
| % B-Blocker at baseline |
15% | 19% | 13% | 0.28 | 1.53 (0.71, 3.29) |
|
| |||||
| % Statins at baseline | 1% | 0% | 1.5% | 0.54 | NA |
|
| |||||
| HCV parameters | |||||
|
| |||||
| Prior HALT-C status | 0.28 | ||||
| % Non-responder | 63% | 69% | 60% | 1.29 (0.67, 2.51) |
|
| % Breakthru/relapse | 11% | 7% | 13% | 0.57 (0.18, 1.79) |
|
| % Express | 26% | 24% | 27% | 1 Ref | |
|
| |||||
| Log HCV RNA (IU/ml) | |||||
|
| |||||
| % HCV genotype 1 | 93% | 97% | 90% | 0.086 | 3.78 (0.83, 17.24) |
|
| |||||
|
Laboratory
parameters |
|||||
|
| |||||
| Hemoglobin (g/dl) | 15.1 (1.4) | 14.8 (1.4) | 15.2 (1.4) | 0.068 | 0.83 (0.67, 1.01) |
|
| |||||
| Serum AST/ALT | 0.94 (0.3) | 1.03 (0.3) | 0.89 (0.3) | 0.0014 | 5.19 (1.87,14.27) |
|
| |||||
| Alk phos ratio (ULN) | 0.87 (0.4) | 0.92 (0.4) | 0.84 (0.4) | 0.189 | 1.65 (0.78, 3.50) |
|
| |||||
| Total bilirubin (mg/dl) | 0.97 (0.5) | 1.10 (0.5) | 0.90 (0.5) | 0.0065 | 2.25 (1.26, 4.04) |
|
| |||||
| INR | 0.0811 | ||||
| % > 1.0 | 60% | 68% | 55% | 1.69 (0.94, 3.06) |
|
| % ≤ 1.0 | 40% | 32% | 45% | ||
|
| |||||
| Albumin (g/dl) | 3.8 (0.5) | 3.7 (0.4) | 3.8 (0.5) | 0.0487 | 0.52 (0.27, 1.00) |
|
| |||||
| Platelets (103/ml) | 132.5 (60.7) | 107.8 (44.1) | 146.0 (64.3) | <0.0001 | 0.24 (0.12, 0.47) |
|
| |||||
| Total Bile acids (umol/L) |
22.4 (22) | 26.7 (23.7) | 20.2 (20.8) | 0.0524 | 1.01 (1.00, 1.03) |
|
| |||||
| HOMA-IR | 20.6 (29.8) | 25.8 (33.6) | 17.6 (27.2) | 0.116 | 1.01 (1.00, 1.02) |
|
| |||||
| Log HA (ng/ml) | 2.18 (0.5) | 2.37 (0.4) | 2.09 (0.5) | <0.0001 | 2.12 (1.47, 3.07) |
|
| |||||
| % Splenomegaly on ultrasound |
49% | 62% | 42% | 0.0062 | 2.28 (1.26, 4.12) |
|
| |||||
| Baseline Endoscopy | |||||
|
| |||||
| % Gastric varices | 10% | 29% | 3% | <0.0001 | 13.2 (4.17, 41.74) |
|
| |||||
| Esophageal varix size | 0.30 | ||||
| % Grade 1 | 76% | 70% | 79% | 1.03 (0.25, 4.14) |
|
| % Grade 2 | 19% | 25% | 16% | 1.80 (0.41, 8.03) |
|
| % Grade 3 | 5% | 5% | 5% | 1 Ref | |
|
| |||||
| Baseline Histology | |||||
|
| |||||
| % Cirrhosis (Ishak 5/6) |
64% | 78% | 56% | 0.0015 | 2.86 (1.50, 5.48) |
|
| |||||
| Mean HAI Baseline | 7.90 (2) | 8.07 (2.1) | 7.82 (2) | 0.39 | 1.06 (0.92, 1.23) |
|
| |||||
| % Hepatic steatosis ≥ 2 |
40% | 31% | 46% | 0.0420 | 0.54 (0.30, 0.98) |
|
| |||||
| % Mallory Bodies | 16% | 14% | 18% | 0.44 | 0.73 (0.33, 1.62) |
|
| |||||
| % Zone 3 fibrosis | 0.40 | ||||
|
| |||||
| 0 | 65% | 68% | 64% | 2.43 (0.66, 8.98) |
|
|
| |||||
| 1 | 27% | 28% | 26% | 2.50 (0.63, 9.88) |
|
|
| |||||
| 2 | 8% | 4% | 10% | 1 Ref | |
|
| |||||
| Randomized phase | |||||
|
| |||||
| % Peginterferon arm | 47% | 41% | 51% | 0.1584 | 0.66 (0.37, 1.17) |
|
| |||||
| % Clinical outcome | 27% | 49% | 15% | < 0.0001 |
5.50 (2.85, 10.61) |
|
| |||||
| % Two-point increase in Ishak fibrosis score |
40% | 69% | 32% | 0.0119 | 4.64 (1.40, 15.39) |
Data reported as mean + std dev or %
comparing de novo vs non-progressors
Development of de novo gastroesophageal varices
During a median follow-up of 48 months (range: 1.7 to 74 months), 157 of the 598 HALT-C patients (26.2%) without baseline varices developed gastroesophageal varices and only 8 (5.1%) developed new gastric varices alone. New varices were detected at year 2 in 3 patients and at year 4 in 150 patients while 4 additional patients presented with variceal hemorrhage. The size of the de novo esophageal varices at diagnosis was grade 1 in 120 patients (76.4%), grade 2 in 21 patients (13.4%), and grade 3 in 4 patients (2.5%). Subjects who developed new varices were significantly more likely to be Hispanic, less likely to be African-American, have a higher BMI, and have a lower serum albumin level (Table 1). In addition, subjects with new varices had significantly higher baseline serum AST/ ALT, total bilirubin, INR, alkaline phosphatase, hyaluronic acid (HA), and total bile acid levels compared to patients without new varices (Table 1). The pretreatment liver biopsies of patients with new onset varices were also more likely to demonstrate cirrhosis, Mallory-Denk bodies, and more severe hepatic steatosis. During follow-up, patients with de novo varices were also significantly more likely to develop a clinical outcome (Odds ratio 5.46: p< 0.0001). Conversely, the rate of de novo varices was significantly higher in the 50 (8%) patients with clinical progression compared to the 127 (92%) patients without a clinical outcome (62% vs 23%, p< 0.0001)
Multivariate modeling of baseline clinical and laboratory predictors identified subject race and pretreatment serum albumin and log HA levels as significantly associated with developing varices. Specifically, the odds ratio for Hispanics versus Caucasians was 4.20 (95% CI: 1.92, 9.20) and for African Americans versus Caucasians was 0.77 (95% CI: 0.47, 1.28). Similarly, for every one gm/dl unit decrease in albumin, the odds ratio was 0.55 (95% CI: 0.30,1.00) and for every one unit increase in serum HA levels, the odds ratio was 3.84 (95% CI: 2.36, 6.26). The AUROC of the multivariate model for identifying patients at risk of developing varices was 0.721 (95% CI: 0.65, 0.79). The addition of the pretreatment Ishak fibrosis, Mallory-Denk bodies, and steatosis scores to the model did not substantially change the model performance nor the inclusion of serial HA levels (Data not shown). Finally, the frequency of beta-blocker use (44% vs 34%, p= 0.07) during the randomized phase was not significantly different in those with and without denovo varices.
The risk of developing varices in patients of varying ethnicity and baseline hyaluronic acid levels was estimated from the logistic regression model (Figure 2). It was assumed that all patients would have a baseline serum albumin level that was similar to the median value of the HALT-C Trial patients of 3.9 g/dl. In subjects with a baseline hyaluronic acid level of < 50 ng/ml (i.e. log < 1.7), the risk of developing varices ranged from 13% in African Americans to 22% in Caucasians and 44% in Hispanics. Similarly, the risk of developing varices in patients with a baseline hyaluronic acid level of > 126 ng/ml (i.e. log > 2.1) varied from 36% to 51% and 75% amongst the African American, Caucasian, and Hispanic subgroups, respectively.
Figure 2.
Logistic regression model to predict the likelihood of developing new varices in HALT-C Trial patients. The predicted probability of developing new varices over 4 years according to baseline hyaluronic acid level and subject ethnicity using an imputed serum albumin level of 3.9 g/dl in the model. (log HA < 1.7 is < 50 ng/ml, log HA 1.7 to < 2.1 is 50 to < 126 ng/ml, and log HA ≥ 2.1 is > 126 ng/ml).
Progression of baseline varices
During a median follow-up of 48 months, 74 (35.2%) subjects experienced variceal progression which included 46 detected at year 2, 23 detected at year 4, and 5 presenting with variceal bleeding. On univariate analysis, subjects with variceal progression had significantly higher baseline serum AST/ ALT, total bilirubin, and hyaluronic acid levels and significantly lower serum albumin and platelet levels compared to non-progressors (Table 2). In addition, the patients with variceal progression were more likely to have splenomegaly on their pretreatment liver ultrasound. Administration of beta-blockers during the randomized phase was more common in those with compared to those without variceal progression (78% vs 50%, p= 0.0003). Patients with variceal progression were also more likely to experience a clinical outcome compared to those without variceal progression (Table 2). Conversely, the rate of variceal progression was also significantly higher in the 56 (27%) patients with clinical progression compared to the 154 (73%) patients without a clinical outcome (64% vs 25%, p< 0.0001). The overall risk of bleeding among those with baseline varices was ~ 7% over 4 years as compared to 0.7% in those without baseline varices (p < 0.001).
On multivariate analysis, baseline serum AST/ALT and platelet counts were the only parameters independently associated with variceal progression. Specifically, for every one unit increase in the serum AST/ ALT ratio, the odds ratio was 3.39 (95% CI: 1.17, 9.84) and for every decrease in baseline platelet counts of 50,000/ml the odds ratio for variceal progression was 0.53 (95% CI: 0.38, 0.75). The addition of baseline histological parameters including hepatic steatosis did not improve model performance (data not shown). For every decrease in the platelet count by 50,000/ ml, the likelihood of variceal progression during follow-up increased by 88%. The impact of baseline platelet counts and varying serum AST/ ALT levels on the risk of variceal progression can be seen in supplemental figure 1. In subjects with a baseline serum AST/ ALT level < 0.8, the impact of baseline platelet count varies from 8% risk in subjects with platelets > 150,000/ml to 37% in subjects with platelet counts < 100,000/ml. In contrast, in subjects with a baseline serum AST/ ALT > 1.5, indicative of more severe liver disease, the baseline platelet count can vary the risk of variceal progression from 23% to 66%.
Impact of pegylated Interferon on development and progression of varices
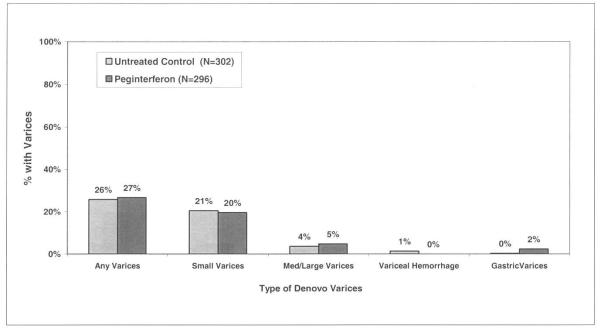
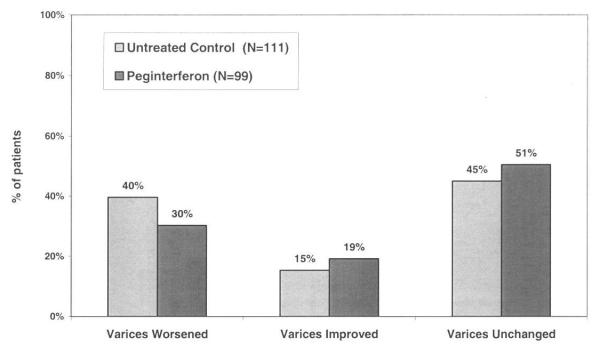
The HALT-C Trial was designed to determine if maintenance peginterferon would reduce the rate of clinical and histological disease progression. If effective, it was anticipated that variceal development and progression would also be less frequent in the peginterferon treated patients. However, peginterferon therapy was not a predictor of new varices formation compared to no therapy (27% vs 26%, p=0.81) (Figure 3A). Similarly, the frequency of variceal progression was similar in the treated and untreated patients during the randomized phase (30% vs 40%, p=0.157) and the overall likelihood of changes in variceal status were not different (p=0.353) (Figure 3B).
Figure 3.
Gastroesophageal varices in peginterferon treated and untreated patients. 3A). Amongst the 598 patients without baseline varices, 302 were randomized to receive peginterferon and 296 to receive no further treatment. By year 4, the proportion of patients with new varices in the treated and untreated groups was similar (27% vs 26%, p= 0.81). In addition, the distribution of the types of varices that were detected was similar (p=0.06) 3B). Amongst the 210 patients with baseline gastroesophageal varices, 99 received peginterferon and 111 received no further antiviral treatment. By year 4, the proportion of patients with worsening, stable, and improved varices was similar (p=0.35).
Finally, the role of medication adherence amongst the peginterferon treated patients was explored in relationship to variceal outcomes by defining adherent patients as those who were able to take > 80% of the prescribed dose of peginterferon for at least 80% of the time. In this analysis, 62% of the peginterferon treated patients without varices were adherent but medication adherence was not associated with the risk of developing de novo varices (25% vs 30%, P=0.30). Similarly, 47% of the peginterferon treated patients with baseline varices were adherent but again medication adherence was not associated with the risk of variceal progression (26% vs 35%, p= 0.33).
Discussion
Prior studies had suggested that interferon therapy in patients with CHC that did not clear virus may lead to histological improvement (12, 13). As a result, several large randomized controlled trials of peginterferon therapy were initiated with the hope that the anti-inflammatory and anti-fibrotic effect of treatment in persistently viremic patients would slow the rate of disease progression. However, the HALT-C Trial and other studies have failed to demonstrate any significant benefit regarding clinical and histological disease progression with maintenance interferon therapy (7, 14-16). Previous studies have also demonstrated that administration of interferon to cirrhotic patients with CHC can lead to a reduction in portal pressure (17, 18). Although reductions in portal pressure were consistently greatest in patients with an initial or sustained virological response, some non-responders with improved liver histology also experienced improved portal pressures. Therefore, we hypothesized that antiviral therapy would be associated with a reduction in the rate of development of de novo varices as well as a reduced rate of variceal progression compared to no treatment.
Overall, 26.2% of the HALT-C Trial patients without baseline varices developed de novo varices during a median follow-up of 4 years. As expected, patients that developed varices were also significantly more likely to experience a clinical outcome or histological progression compared to patients without de novo varices (Table 1). The majority of these new esophageal varices were small (76%) and only 1% of patients developed variceal hemorrhage. The annualized rate of new varices formation of 6.5% per year is slightly lower compared to that reported in a randomized controlled trial of beta-blockers (8.9% per year) as well as in a natural history study of variceal development (10% per year) (5, 19). Of note, all of the patients in the latter studies had cirrhosis and underwent annual endoscopy whereas only ~ 33% of the HALT-C patients at risk for varices had cirrhosis and an endoscopy was repeated at 4 years (Table 1). Also, the HALT-C Trial patients who did not undergo a follow-up endoscopy had clinical profiles that suggested they were at greater risk of developing varices. Thus, our observed rates of variceal formation may be underestimates. Interestingly, African American patients in the HALT-C Trial were at a reduced risk of developing de novo varices compared to Caucasians while Hispanics were at the highest risk (Figure 2). These data are consistent with our prior analysis demonstrating that African Americans are also less likely to have varices on screening endoscopy after controlling for disease severity and liver histology (8). The reduced risk of developing varices amongst African Americans may be related to their tendency to have less severe necroinflammation or hepatic steatosis compared to Caucasians with HCV at initial presentation and during follow-up (20, 21). The increased risk of Hispanic patients to develop varices should be interpreted with caution due to the small number of Hispanics enrolled (i.e. only 6% of HALT-C patients but 13.3% of general US population). Nonetheless, the increased prevalence of metabolic syndrome, hepatic steatosis, and advanced fibrosis reported in cross-sectional studies of Hispanic compared to Caucasian patients with CHC may, in part, explain our findings (22). In support of this, the frequency of significant hepatic steatosis (i.e ≥ 2) was 32.5% in the African Americans, 42.5% in Caucasians, and 54.8% in Hispanic HALT-C patients (p = 0.002). When we controlled for subject BMI and liver histology, Hispanic ethnicity remained an independent risk factor for developing varices. Additional longitudinal studies of larger numbers of Hispanic and African-American HCV patients are eagerly awaited to follow-up on these findings.
Baseline serum albumin and hyaluronic acid levels were also significantly associated with an increased risk of developing varices which is consistent with prior studies demonstrating that these analytes correlate with the extent of hepatic fibrosis and disease severity in patients with CHC (23). Increases in serum hyaluronic acid levels are believed to be due to reduced endothelial clearance of HA from hepatic sinusoids as well as from increased hepatic stellate cell production with advancing fibrosis. A number of laboratory parameters associated with the likelihood of developing varices in prior studies (e.g. platelet counts, total bilirubin levels, serum AST/ ALT) were also associated with the likelihood of developing varices in our patient population (Table 1) (24). However, their lack of independent contribution to our model likely reflects their collinearity with serum albumin and hyaluronic acid levels. Similarly, histological markers of disease severity were also associated with the risk of developing varices on univariate analysis but were not on multivariate modeling presumably for the same reasons.
Administration of low dose peginterferonα2a did not reduce the risk of developing new varices (Figure 4A). Although only low doses of peginterferonα2a were used, the likelihood of developing varices was also not influenced by medication adherence amongst the treated patients. Therefore, our results demonstrate that maintenance peginterferon does not reduce the risk of developing varices. These results are consistent with the overall HALT-C Trial results wherein the rate of liver disease progression by clinical and histological assessment was not reduced in treated compared to untreated patients despite improvements in serum aminotransferase and HCV RNA levels (7). Our study results differ from preliminary reports of other studies of low-dose peginterferonα2b compared to no treatment or colchicine wherein a reduced risk of variceal development and bleeding have been noted (16, 25). The differing results may, in part, relate to the differing entry criteria of the studies. For example, 100% of the patients in the EPIC3 trial and 83% of the Colchicine versus PegIntron Long-Term (CoPilot) study patients had histological cirrhosis while only 40% of the HALT-C patients had cirrhosis. In addition, Co-Pilot allowed patients with a CTP score of < 8 while HALT-C limited enrollment to patients with a CTP < 7. In addition, the lower reported rate of de novo varices/ variceal progression with peginterferonα2b in CoPilot may relate to the absence of retreatment with full dose peginterferon and ribavirin prior to randomization. This could result in the CoPilot patients receiving peginterferonα2b having a greater chance of virological suppression and reduced necrointlammation compared to those treated with peginterferonα2a in HALT-C. However, both of the trials involving peginterferonα2b also failed to demonstrate a reduction in the overall rate of disease progression and clinical outcomes compared to the control arm (15, 16).
A second aim of our study was to determine the rate of variceal progression amongst patients with CHC. Overall, 35.2% of the 210 patients with baseline gastroesophageal varices experienced variceal progression and these patients were also significantly more likely to develop a clinical outcome or histological progression compared to the patients without variceal progression (Table 2). The annualized rate of 8.8% per year is similar to that reported in other studies of patients with small varices followed over time (19). On multivariate analysis, baseline serum AST/ ALT and platelet counts were independently associated with variceal progression. Since the serum AST/ ALT ratio is associated with more advanced hepatic fibrosis in patients with CHC, it is not surprising that this parameter was also associated with variceal progression. In fact, baseline serum AST/ ALT levels are a component of a model developed in the HALT-C cohort to distinguish patients with bridging fibrosis from those with cirrhosis as well as to identify patients at increased risk of clinical outcomes during follow-up (26, 27) In addition, subjects with variceal progression were more likely to have cirrhosis (Table 2) although the addition of liver histology parameters did not substantially improve the model’s performance.
Use of these simple and widely available laboratory parameters may be of value to practitioners. For example, the likelihood of developing variceal progression can be categorized as low, medium and high using various platelet levels for a patient with a given serum AST/ ALT level (Supplemental Figure 1). However, contrary to expectations, maintenance peginterferon therapy was not associated with a reduced likelihood of variceal progression (Figure 3B). In addition, adherence to peginterferon was not associated with a reduced risk of variceal progression amongst the treated patients although the trend was in the expected direction (26% vs 35%, p= 0.33). The lack of an association between subject ethnicity and variceal progression may relate to the limited number of patients with baseline varices in this analysis.
The serial endoscopic data also demonstrated that 17% of patients with baseline varices had a reduction in the size of their varices by at least 1 grade and remarkably 3% had a reduction in variceal size by more than 2 stages (See supplemental material). It is possible that the regression in varices may relate to inter-observer variability in the grading of varix size which is known to vary by 10 to 15% (28). Alternatively, the reproducibility of assessing esophageal varices in a given patient due to technical factors of insufflating the esophagus may have also played a role. However, on univariate analysis patients with lower INR levels and higher platelet counts, indicative of less severe liver disease, were more likely to experience regression. Nonetheless, the use of maintenance peginterferon was not associated with variceal regression (Figure 3B). In addition, baseline use of beta-blockers and statins were also not associated with variceal regression.
Strengths of our study include the large number of well-characterized patients with CHC that were prospectively assessed using a standardized endoscopy protocol while being carefully monitored in a multi-center clinical trial. Furthermore, overall patient retention in the HALT-C Trial was excellent at 85% (7). In addition, the majority of patients with a pretreatment screening endoscopy did undergo a follow-up study as planned (80% of those without varices and 90% of those with varices). However, the subjects without baseline varices who did not get a follow-up endoscopy had more severe liver disease at entry and were significantly more likely to develop a clinical outcome during follow-up leading to a potential underestimation of the rate of varices formation. Similarly, amongst the 272 patients with baseline varices, the excluded subjects that did not undergo a follow-up endoscopy tended to have more severe liver disease at entry and were also more likely to develop a clinical outcome. Additional limitations regarding our study design was the lack of central review of endoscopic findings which may have led to substantial inter-individual variability in grading varices as has been shown in prior studies (28). To address these concerns, formal training on the grading of varices was undertaken at the initiation of the study and a standardized scoring form was used in all patients at all sites by a limited number of experienced investigators (8). Finally, direct portal pressure measurements were not obtained at baseline nor during follow-up. Although these measurements are closely associated with the risk of developing new or worsening varices, the incremental risk, cost, and inconvenience of this invasive method precluded us from incorporating it into this trial (1, 5).
In summary, 26.2% of HALT-C Trial participants developed new gastroesophageal varices and 35.2% of those with baseline varices experienced variceal progression during a median follow-up of 4 years. The risk of developing varices was significantly influenced by patient ethnicity and laboratory markers of disease severity at entry but not influenced by the use of maintenance peginterferon. Assessment of baseline serum albumin and hyaluronic acid levels can help stratify patients with CHC into low, medium, and high risk of developing varices (Figure 2). Use of these models may improve patient and physician adherence with recommendations for endoscopic screening and assist in targeting the highest risk patients for the most frequent follow-up. Similarly, determination of serum AST/ ALT and platelet counts in patients with CHC and known varices may help identify those that may benefit from beta-blocker prophylaxis or pre-emptive band ligation as well as more frequent endoscopic surveillance.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center and Clinical and Translational Science Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed below). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc., through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Barbara F. Banner, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Herbert L. Bonkovsky, MD, Gloria Borders, RN, Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Adrian M. Di Bisceglie, MD, Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Elizabeth M. Brunt, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066; Grant 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center) Jules L. Dienstag, MD, Raymond T. Chung, MD, Atul K. Bhan, MD, Wallis A. Molchen, Cara C. Gooch
University of Colorado School of Medicine, Denver, CO: (Contract N01-DK-9-2327, Grant M01RR-00051, Grant 1 UL1 RR 025780-01) Gregory T. Everson, MD, S. Russell Nash, MD, Jennifer DeSanto, RN, Carol McKinley, RN
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) Timothy R. Morgan, MD, John C. Hoefs, MD, John R. Craig, MD, M. Mazen Jamal, MD, MPH, Muhammad Sheikh, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633, Grant 1 UL1 RR024982-01, North and Central Texas Clinical and Translational Science Initiative) Thomas E. Rogers, MD, Peter F. Malet, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN, Nancy Liston, MPH
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Karen L. Lindsay, MD, MMM, Sugantha Govindarajan, MD, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042, Grant 1 UL1 RR024986, Michigan Center for Clinical and Health Research) Anna S. Lok, MD, Joel K. Greenson, MD, Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Mitchell L. Shiffman, MD, Richard K. Sterling, MD, Melissa J. Contos, MD, A. Scott Mills, MD, Charlotte Hofmann, RN, Paula Smith, RN
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: T. Jake Liang, MD, David Kleiner, MD, PhD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: James E. Everhart, MD, Leonard B. Seeff, MD, Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD, Elizabeth C. Wright, PhD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318) Chihiro Morishima, MD, David R. Gretch, MD, PhD, Minjun Chung Apodaca, BS, ASCP
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Kristin K. Snow, MSc, ScD, Anne M. Stoddard, ScD, Teresa M. Curto, MSW, MPH
Armed Forces Institute of Pathology, Washington, DC: Zachary D. Goodman, MD, PhD
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
Abbreviations
- BMI
Body mass index
- CHC
Chronic hepatitis C
- CTP
Child-Turcotte-Pugh
- HA
Hyaluronic acid
- HALT-C
Hepatitis C Antiviral Long-term Treatment against Cirrhosis Trial
- HCC
Hepatocellular cancer
- HCV
Hepatitis C virus
Footnotes
Conflict of Interest
Section A. The following are disclosures that pertain to the industrial sponsors who have partnered with the NIDDK to support this study, which are also listed in the text of the manuscript.
Financial relationships of the authors with Hoffmann-La Roche, Inc., are as follows:
R.J. Fontana is on the speaker’s bureau; W.M. Lee receives research support; G. T. Everson is a consultant, on the speaker’s bureau, and receives research support; A.M. Di Bisceglie is a consultant and receives research support; G. Szabo receives research support; and T.R. Morgan is consultant, on the speaker’s bureau, and receives research support.
Section B. In addition, many of the HALT-C Trial investigators have other associations with industry relating to the area of hepatitis C, and, to achieve the highest level of disclosure, we list these for you as well.
R.J. Fontana: Bristol-Meyers Squibb – Speaker’s bureau and consultant. Abbott Pharmaceuticals – Consultant. Bayer/Siemens – Consultant.
A.J. Sanyal has served on ad hoc Advisory Boards of Bayer, Salix, Gilead, Astellas, Sanofi, Exhalenz in the last year. He has participated in clinical trials by Bayer, Sanofi, Gilead, Exhalenz, Salix and Roche.
W.M. Lee receives research support from Aegerion, Globeimmune, Orasure, Schering-Plough, Siemens Diagnostics and Vertex Pharmaceuticals.
G. T. Everson receives research support from Schering-Plough, Pharmasset, GlobeImmune, Source, Novartis/Human Genome Sciences, and GlaxoSmithKline; and is a consultant and receives research support from Vertex Pharmaceuticals.
A.M. Di Bisceglie is a consultant and receives research support from Vertex Pharmaceuticals, Idenix Pharmaceuticals, Gilead Sciences, Anadys, GlobeImmune and Pharmasset; has served as a consultant to Schering-Plough, Novartis, Bristol-Myers-Squibb and Abbott.
G. Szabo receives research support from Idenix Pharmaceuticals, Vertex Pharmaceuticals, GlaxoSmithKline, Bristol-Myers-Squibb, IDERA, Novartis, and Schering Plough Corporation.
T.R. Morgan is a consultant, serves on an advisory board, and receives research support from Vertex Pharmaceuticals; and receives research support from Merck, Schering Plough Corporation, and WAKO Diagnostics.
This is publication #48 from the HALT-C Trial Group.
The HALT-C Trial was registered with clinicaltrials.gov (#NCT00006164).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of Gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 2.de Franchis R. Non-invasive (and minimally invasive) diagnosis of esophageal varices. J Hepatology. 2008:520–527. doi: 10.1016/j.jhep.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Qamar A, Grace ND, Groszmann RJ, et al. Platelet count is not a predictor of the presence or development of gastroesophageal varices. Hepatology. 2008;47:153–159. doi: 10.1002/hep.21941. [DOI] [PubMed] [Google Scholar]
- 4.Burton JR, Liangpunsakul S, Lapidus J, et al. Validation of a multivariate model predicting presence and size of varices. J Clin Gastroenterol. 2007;41:69–615. doi: 10.1097/01.mcg.0000225669.84099.04. [DOI] [PubMed] [Google Scholar]
- 5.Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–2261. doi: 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- 6.Wise M, Bialek S, Finelli L, et al. Changing trends in hepatitis C-related mortality in the United States, 1995-2004. Hepatology. 2008;31:777–782. doi: 10.1002/hep.22165. [DOI] [PubMed] [Google Scholar]
- 7.DiBisceglie AM, Shiffman ML, Everson GT, et al. Prolonged therapy of advanced chronic hepatitis C with low dose peginterferon. N Engl J Med. 2008;359:2429–2441. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanyal AJ, Fontana RJ, DiBisceglie AM, et al. The prevalence and risk factors associated with esophageal varices in subjects with hepatitis C and advanced fibrosis. Gastrointest Endosc. 2006;64:855–864. doi: 10.1016/j.gie.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Zoli M, Merkel C, Magalotti D, et al. Evaluation of a new endoscopic index to predict first bleeding from the upper gastrointestinal tract in patients with cirrhosis. Hepatology. 1996:1047–1052. doi: 10.1053/jhep.1996.v24.pm0008903373. [DOI] [PubMed] [Google Scholar]
- 10.Sarin SK, Lahoti D, Saxena SP, et al. Prevalence, classification, and natural history of Gastric varices: A long-term follow-up study in 568 portal hypertension Patients. Hepatology. 1992;16:1343–1349. doi: 10.1002/hep.1840160607. [DOI] [PubMed] [Google Scholar]
- 11.Lok ASF, Everhart JE, Chung RT, et al. Hepatic steatosis in hepatitis C: Comparison of diabetic and non-diabetic patients in the Hepatitis C Antiviral Long-term Treatment against Cirrhosis Trial. Clin Gastroenterol Hepatol. 2007;5:245–254. doi: 10.1016/j.cgh.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Shiffman ML, Hofmann CM, Contos MJ, et al. A randomized controlled trial of maintenance interferon therapy for patients with chronic hepatitis C virus and persistent viremia. Gastroenterology. 1999;117:1164–1172. doi: 10.1016/s0016-5085(99)70402-6. [DOI] [PubMed] [Google Scholar]
- 13.Alric L, Duffaut M, Selves J, et al. Maintenance therapy with a gradual reduction of the interferon dose over one year improves histological response in patients with chronic hepatitis C with biochemical response: results of a randomized trial. J Hepatology. 2001;35:272–278. doi: 10.1016/s0168-8278(01)00110-6. [DOI] [PubMed] [Google Scholar]
- 14.Fartoux L, Degos F, Trepo C, et al. Effect of prolonged interferon therapy on the outcome of hepatitis C virus-related cirrhosis: A randomized trial. Clinic Gastro & Hepatology. 2007;5:502–507. doi: 10.1016/j.cgh.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Afdhal NH, Levine R, Brown R, et al. Colchicine versus peginterferon alfa-2b long term therapy: results of the 4 year CoPilot Trial (Abstract) J Hepatology. 2008;48:S4. [Google Scholar]
- 16.Bruix J, Poynard T, Colombo M, Schiff ER, et al. Final results of the EPIC3 cirrhosis maintenance Trial: Pegintron maintenance therapy in cirrhotic (METAVIR F4) HCV patients, who failed to respond to Interferon/ ribavirin therapy. Gastroenterology. 2009;136(Suppl 1):#295. (Abstract) [Google Scholar]
- 17.Roberts S, Gordon A, McLean C, et al. Effect of sustained viral response on hepatic venous pressure gradient in Hepatitis-C related cirrhosis. Clin Gastro & Hep. 2007;5:932–937. doi: 10.1016/j.cgh.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Rincon D, Ripoll C, LoIacono O, et al. Antiviral therapy decreases hepatic venous pressure gradient in patients with chronic hepatitis C and advanced fibrosis. Am J Gastroenterol. 2006;101:2269–2274. doi: 10.1111/j.1572-0241.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 19.Merli M, NIcolini G, Angeloni S, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003;38:266–272. doi: 10.1016/s0168-8278(02)00420-8. [DOI] [PubMed] [Google Scholar]
- 20.Lepe R, Layden-Almer JE, Layden TJ, Cotler S. Ethnic differences in the presentation of chronic hepatitis C. J V Hepatitis. 2006;13:116–120. doi: 10.1111/j.1365-2893.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- 21.Tencate V, Layden-Almer JE, Wolfert M, et al. Paired biopsies illustrate racial differences in fibrosis progression in HCV patients (Abstract) Hepatology. 2009;50(Suppl 1):#1629. [Google Scholar]
- 22.Rodriguez-Torres M, Jeffers LJ, Sheikh MY, et al. Peginterferon alfa-2a and ribavirin in Latino and Non-latino whites with hepatitis C. N Engl J Med. 2009;360:257–267. doi: 10.1056/NEJMoa0805062. [DOI] [PubMed] [Google Scholar]
- 23.Fontana RJ, Goodman ZD, Dienstag JL, et al. Relationship of serum fibrosis markers with liver fibrosis stage and collagen content in patients with advanced chronic hepatitis C. Hepatology. 2008;47:789–798. doi: 10.1002/hep.22099. [DOI] [PubMed] [Google Scholar]
- 24.Giannini E, Zaman A, Kreil A, et al. Platelet count/spleen diameter ratio for the non-invasive diagnosis of esophageal varices: Results of a multicenter, prospective validation study. Am J Gastroenterol. 2006;101:2511–2519. doi: 10.1111/j.1572-0241.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 25.Cardenas A, Pritchett S, Brown RS, Levin RA, Curry MP, Afdhal NH. The effects of long-term PEG-Interferon therapy on the Development of esophageal varices and variceal bleeding in patients with chronic hepatitis C and Advanced fibrosis: Final results from the CoPilot Trial. Gastroenterology (Abstract) 2009;136(Suppl 1):#259. [Google Scholar]
- 26.Lok ASF, Ghany MG, Goodman ZD, et al. Predicting cirrhosis in patients with chronic hepatitis C based on standard laboratory tests: Results of the HALT-C cohort. Hepatology. 2005;38:518–526. doi: 10.1002/hep.20772. [DOI] [PubMed] [Google Scholar]
- 27.Ghany MG, Lok ASF, Everhart JE, et al. Predicting Clinical outcome and Progression to cirrhosis using standard laboratory Tests: Analysis of The HALT-C Cohort. Gastroenterology. 2009 (accepted) [Google Scholar]
- 28.Bendsten F, Skovgard LT, Sorensen TIA, et al. Agreement among multiple observers on endoscopic diagnosis of esophageal varices before bleeding. Hepatology. 1990;11:341–347. doi: 10.1002/hep.1840110302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.