Abstract
Binge drinking, defined as achieving blood ethanol concentrations (BEC) of 80 mg%, has been increasing in adolescents and was reported to predispose later physical dependence. The present experiments utilized an animal model of binge drinking to compare the effect of ethanol “binge” experience during adolescence or adulthood on subsequent ethanol intake in male and female C57BL/6 mice. Adolescent and adult mice were initially exposed to the scheduled high alcohol consumption procedure, which produces BECs that exceed the levels for binge drinking following a 30 min ethanol session every third day. Ethanol intake and BECs were significantly higher in the adolescent (∼3 g/kg, 199 mg%) versus adult (∼2 g/kg, 135 mg%) mice during the first three ethanol sessions, but were more equivalent during the final two ethanol sessions (1.85-2.0 g/kg, 129-143 mg%). Then, separate groups of the ethanol experienced mice were tested with ethanol naïve adolescent and adult mice for 2-hr limited access (10 and 20% solutions) or 24-hr (5, 10 and 20% solutions) ethanol preference drinking. Limited access ethanol intake was significantly higher in female versus male mice, but was not altered by age or ethanol experience. In contrast, 24-hr ethanol intake was significantly higher in the adolescent versus adult mice and in female versus male mice. Furthermore, binge drinking experience in the adolescent mice significantly increased subsequent ethanol intake, primarily due to intake in female mice. Thus, adolescent binge drinking significantly increased unlimited ethanol intake during adulthood, with female mice more susceptible to this effect.
Keywords: alcohol, preference, self-administration, blood ethanol concentration
Introduction
It is estimated that there are 10.8 million underage drinkers in the United States (http://www.niaaa.nih.gov/AboutNIAAA/NIAAASponsoredPrograms/StasticalSnapshotUnderage Drinking.htm). Data from the 2005 National Survey on Drug Use and Health indicate that the first use of alcohol (defined as drinking a whole drink) increased proportionally from age 12 (∼15%) to age 17 (∼70%), with similar increases reported in all levels of drinking (use or binge drinking) across these ages during adolescence (Substance Abuse and Mental Health Service Administration, 2006). The National Advisory Council on Alcohol Abuse and Alcoholism in 2004 defined binge drinking as drinking that brought blood ethanol concentration (BEC) to 80 mg% or above, and which is typically obtained by drinking 4 (female) or 5 (male) drinks in a 2-hr period (http://pubs.niaaa.nih.gov/publications/StrategicPlan/NIAAASTRATEGICPLAN.htm#Drinking_P atterns). Interestingly, this prior experience is a strong predictor of alcoholic dependence later on in life, with half to two-thirds of the people meeting the diagnostic criteria for alcohol dependence by age 21 and 25, respectively (Hingson et al., 2006).
Much of the literature points towards the adolescent period as a time of neurodevelopment that also is associated with concomitant increases in vulnerability and propensity towards experimentation of substances (Chambers et al., 2003). In fact, this type of increase in risk-taking and novelty seeking behavior during adolescence can be seen across many species (Csikszentmihalyi et al., 1977; Laviola et al., 1999; Pellis and Pellis, 1990, 1997; Primus and Kellogg, 1989; Trimpop et al., 1998). Despite the large body of literature, it is not fully known what causes adolescent humans to display a greater propensity for alcohol experimentation. Subsequently, rodent models of ethanol intake can be a valuable way to examine the factors contributing to the differences between adolescent and adult animals in ethanol consumption.
It has been hypothesized that alterations in relative sensitivity to ethanol's behavioral effects (i.e., adolescent versus adult) may contribute to the increase in ethanol consumption in adolescent animals (Spear and Varlinskaya, 2005). Related to this hypothesis, it is possible that an increase in sensitivity to the positive reinforcing effects or decrease in sensitivity to the negative reinforcing effects of ethanol in adolescent versus adult animals might contribute to the differences in ethanol consumption. Data in rats indicate that adolescent rats exhibit decreased sensitivity to ethanol-induced ataxia, hypothermia, anxiolysis and hypnosis (reviewed in Spear and Varlinskaya, 2005), but increased ethanol-induced social facilitation (Varlinskaya and Spear, 2006) and heart rate responses (Ristuccia and Spear, 2008). In contrast, recent data suggest that adolescent C57BL/6 mice exhibit increased sensitivity to ethanol's locomotor stimulant and anxiolytic effects following a 1.5 g/kg dose, with evidence for increased sensitivity to ethanol's ataxic effects following 1.75 or 2.0 g/kg doses (Hefner and Holmes, 2007; Linsenbardt et al., 2009). There was a suggestion of reduced sensitivity to ethanol-induced hypnosis, based on loss of righting reflex duration, but not when BEC at regain of righting reflex was considered (Hefner and Holmes, 2007; Linsenbardt et al., 2009). These studies also provided some evidence for age-related differences in ethanol metabolism, suggesting that adult versus adolescent differences in behavioral sensitivity to ethanol may stem from an interaction of pharmacokinetic and pharmacodynamic factors in the mouse.
With this in mind, it is also important to consider the mechanisms contributing to the sex differences in ethanol intake normally seen in mice. Female rodents almost ubiquitously consume higher doses of ethanol when compared to males, and this has been attributed in part to differences in circulating hormone levels (see reviews by Becker & Hu, 2008; Carroll et al., 2004; Fattore et al., 2009; Lynch et al., 2002). The fact that gonadectomy can eliminate these differences in ethanol intake (Almeida et al., 1998; Caihol and Morméde, 2001) is consistent with the idea that the hormone milieu contributes to these sex differences. Related to this point, the emergence of sex differences in ethanol intake during the post-pubertal period coincides with changes in circulating steroid levels (Lancaster et al., 1996).
With regard to age differences in ethanol intake, there is modest evidence for increased ethanol consumption in adolescent versus adult rats (reviewed in Vetter et al., 2007) and mice (e.g., Chester et al., 2008; Hefner and Holmes, 2007; Tambour et al., 2008). In two of the studies in mice, ethanol intake in female adolescent mice was significantly higher than that in their age-matched male counterparts (Chester et al., 2008; Tambour et al., 2008), consistent with data in female versus male adult mice from our laboratory and others (e.g., Belknap et al., 1993; Yoneyama et al., 2008). With this in mind, it would be of interest to determine whether ethanol experience during the adolescent period would alter the pattern of sex differences normally seen in ethanol intake during the periods of adolescence and adulthood. That is, we were interested to see if adolescent binge-drinking experience would produce a significant increase in overall adult consumption in the general sense, but also to see if it would produce a significant increase in the consumption normally seen in male mice. Consistent with this idea, early forced ethanol consumption was positively correlated with increased ethanol preference in male adolescent rats (Schramm-Sapyta et al., 2008).
Studies in mice and rats examining the effect of ethanol exposure during adolescence on subsequent ethanol intake have found mixed results (reviewed in Tambour et al., 2008; Vetter et al., 2007). In general, ethanol exposure during adolescence increased ethanol intake during adulthood, but some of the studies did not have a separate adult comparison group. When ethanol intake was compared in groups of rats at the same chronological age (postnatal days 71 – 90) following differing experience with ethanol either during adolescence (postnatal days 28 – 90) or adulthood (postnatal days 71 – 90), there were no age-related differences (Vetter et al., 2007). When the time course of ethanol consumption was examined in adolescent (drinking onset on postnatal day 28) and adult (drinking onset on postnatal day 70), the increased ethanol intake in adolescent versus adult mice had dissipated by week 8 of drinking (Tambour et al., 2008). It should be noted that the majority of studies to date have exposed animals to ethanol with either limited or unlimited access procedures. Given that “binge drinking” is prevalent among adolescent humans (Substance Abuse and Mental Health Service Administration, 2006), it was suggested that future studies should model the impact of more “binge like” ethanol exposures during adolescence on later ethanol consumption during adulthood (Vetter et al., 2007).
Recently, we developed a model of “binge drinking” in adult mice whereby scheduling periods of fluid access produced consistent and high ethanol intake (∼2 g/kg in 30 min) and BECs ≥ 100 mg%, which exceed the criteria established by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) for “binge drinking” (Finn et al., 2005). Notably, this Scheduled High Alcohol Consumption (SHAC) procedure resulted in motor impairment (Cronise et al., 2005), indicating that mice were consuming intoxicating doses of ethanol during the period of ethanol access. This model has recently been used to examine the neural substrates in the nucleus accumbens that contribute to binge-like alcohol consumption (Cozzoli et al., 2009).
Based on the above, the purpose of the present set of experiments was to examine the effect of “binge drinking” experience during the adolescent period or adulthood with the SHAC procedure on subsequent ethanol intake following a 1 – 2 week period of abstinence, measured by limited or unlimited access ethanol preference drinking procedures. We chose these two procedures to assess ethanol consumption, since we were anticipating differences in patterns of intake between adolescent and adult mice. Both the 2-hour access and 24-hour access procedures have been well established as models of oral ethanol self-administration that can detect genetic (see Addiction Biology, vol 11 (3-4); Belknap et al., 1993; Blednov et al., 2005; Yoneyama et al., 2008), sex (e.g. Finn et al., 2004; Yoneyama et al., 2008), and age-related (e.g. Chester et al., 2008; McBride et al., 2005a; Tambour et al., 2008) differences in ethanol intake. When intake is limited to 2 hours, male and female C57BL/6J mice will orally consume pharmacologically relevant doses of ethanol (i.e., > 50 mg%). Thus, it has been suggested that the use of different procedures that can model a subset of the features seen in alcoholism can be a useful strategy (discussed in Rhodes et al., 2005).
The present studies compared ethanol intake and preference data in animals with “binge drinking” experience to data in separate groups of adolescent or adult animals that were “ethanol naïve” at the start of the preference drinking studies. Male and female mice were tested, so that we could examine the interaction of age, sex and “binge drinking” experience on subsequent ethanol intake. We hypothesized that “binge drinking” experience in adolescent mice would enhance ethanol intake during adulthood.
Materials and methods
Subjects
Male and female C57BL/6J mice were obtained from a breeding colony maintained by the Portland Alcohol Research Center in the Department of Veterans Affairs Veterinary Medical Unit (Portland, OR). Initially, mice were obtained from the Jackson Laboratory – West (Davis, CA), and every third generation, new breeders were obtained from Jackson Laboratory. Adolescent mice were obtained immediately upon weaning. Adult mice were obtained at approximately 6-7 weeks of age. Until the time of testing, mice were group housed (3-4 per cage, separated by sex) in clear polycarbonate cages (28 × 18 × 13 cm) on Bedicob or Ecofresh bedding. Unless noted, mice were maintained on a 12-hr light/dark cycle (lights on 0600) in a temperature (22 ± 2°C) and humidity controlled environment. Rodent chow (Labdiet 5001 rodent diet; PMI International, Richmond, IN) and water were available ad libitum, except for the SHAC procedure where water availability was scheduled. All rodent cages were changed once per week. One week prior to testing, mice were individually housed and allowed to acclimate to the procedure room. All procedures complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the local Institutional Animal Care and Use Committee.
General Experimental Design
The adolescent and adult mice with “binge” ethanol experience were tested initially in the SHAC procedure (details below). Following a one-week period of exposure to water, separate groups of the ethanol-experienced animals were tested for 2-hr or 24-hr ethanol preference drinking (details below). For comparative purposes, independent cohorts of ethanol naïve adolescent and adult animals were tested for 2-hr or 24-hr ethanol preference drinking. Depending on the study, ages of the adolescent animals ranged from 26-38 days at the start of the study. This age range was chosen, based on Spear (2000), where postnatal days 28 – 42 were defined as the prototypical adolescent period based on neurobehavioral changes. See Table 1 for details on animal ages upon the initiation of each phase of testing. For all studies, fluids were presented in inverted 25 ml glass graduated cylinders with metal sippers. Fluid intake (to the nearest 0.2 ml) was recorded by measuring the level of the meniscus on the graduated drinking tube at the beginning and end of each access period to ethanol or water (depending on the procedure). Ethanol and water tubes placed on empty cages allowed for the measurement of leakage and evaporation from the tubes. The average volume depleted from these “control” tubes was subtracted from the individual drinking volumes each day.
Table 1.
Animal Ages Upon Initiation of Each Phase of Testing
| Group | SHAC Procedure (5% ethanol) | 2 hr Ethanol Preference (10 & 20% ethanol) | 24 hr Ethanol Preference (5, 10 & 20% ethanol) |
|---|---|---|---|
| Adolescent – “binge” ethanol experience | 26 – 28 days (n=33) | 66 – 68 days (n=17) | 58 – 60 days (n=16) |
| Adolescent – naïve | 32 days (n=8) | 35 – 38 days (n=16) | |
| Adult – “binge” ethanol experience | 58 – 71 days (n=32) | 104 – 117 days (n=16) | 96 – 109 days (n=16) |
| Adult – naïve | 66 – 90 days (n=8) | 58 – 82 days (n=8) |
Scheduled High Alcohol Consumption (SHAC) Procedure
Our scheduled fluid access procedure has been described at length elsewhere (Finn et al., 2005). It uses mild fluid restriction to condition mice to drink their daily fluid requirement on a schedule, and animals are weighed daily. Food is available ad libitum throughout the experiment. Briefly, animals have varying amounts of total fluid access per day, ranging from 4 – 10 hrs, with 30 min access to a 5% v/v ethanol solution in tap water every 3rd day. Following the ethanol access period, water is provided for the remainder of the period of fluid availability. Water is the only fluid available on the intervening days. This 3-day cycle of fluid access was repeated (21 days total) so that animals received a total of 7 ethanol sessions, with the time of fluid access gradually increasing. There were a total of 12 days of 4 hr fluid access (3 ethanol sessions), 3 days of 6 hr fluid access (1 ethanol session), 3 days of 8 hr fluid access (1 ethanol session), and 6 days of 10 hr fluid access (2 ethanol sessions), with access to ethanol remaining at 30 min per session for each ethanol session. An orbital blood sample (20 μl) was taken following the 3rd and 7th (final) ethanol session to assess BEC. This procedure was chosen because the high levels of ethanol intake produced in mice (>100 mg%) would model the “binge drinking” experience in which adolescent humans are known to engage. Additionally, prior experience in our lab using this procedure in adult mice convinced us of its efficacy.
Limited Access Ethanol Preference Procedure
For this procedure, mice were habituated to a reverse light/dark cycle (lights off 0900) for a minimum of one week. Animals were never fluid restricted, and water and food were freely available. The procedure was a modification of that described by Sinnott et al. (2002), with mice having access to 2 inverted bottles with metal sippers placed on the stainless steel cage top. Food was distributed near both bottles to avoid food associated tube preferences. Initially, both tubes contained tap water. Then, one tube was replaced with a tube containing an increasing concentration of ethanol (10%, then 20%) in tap water for a period of 2-hrs, beginning 3 hrs after lights off. Following the measurement of 2-hr ethanol intake, the ethanol tube was replaced with a water tube for the remainder of the day. Mice had access to each ethanol concentration for 4 days, with bottle positions alternated every 2nd day. Fresh fluids were provided each time the concentration was changed, and body weight was recorded simultaneously with each concentration change. A total of 49 mice were tested (Table 1): adolescent-binge (n=17), adolescent-naïve (n=8), adult-binge (n=16), and adult-naïve (n=8).
Unlimited Access Ethanol Preference Procedure
Animals were never fluid restricted, and water and food were freely available. The procedure was a modification of that described by Yoneyama et al. (2008), with mice having 24-hr access to 2 inverted bottles with metal sippers placed on the stainless steel cage top. Food was distributed near both bottles to avoid food associated tube preferences. Initially, both tubes contained tap water. Then, one tube was replaced with a tube containing an increasing concentration of ethanol (5%, 10%, then 20%) in tap water. Mice had 24-hr access to each ethanol concentration for 4 days, with bottle positions alternated every 2nd day. At each concentration change, fresh fluids were provided and body weights were measured. We chose to test a range of ethanol concentrations (5-20%) in both access paradigms because we felt that since C57BL/6J mice are considered to be an alcohol preferring strain (Belknap et al., 1993; Yoneyama et al., 2008) we had the flexibility to incrementally increased the solution concentration and not have the animals experience a taste-aversion effect. This also allowed us to examine a concentration-dependent group differences. A total of 56 mice were tested (Table 1): adolescent-binge (n=16), adolescent-naïve (n=16), adult-binge (n=16), and adult naïve (n=8).
Blood Ethanol Concentration
The orbital blood samples (20 μl) were analyzed immediately after collection, as described in Finn et al. (2007). Briefly, the blood samples were diluted into 500 μl of a solution containing 4 mM n-propanol in deionized water. The 2 ml vials were capped and vortexed thoroughly. Gas chromatography (Agilent 6890N GC, using a DB-ALC1 column) was performed on a 30 μl ambient headspace aliquot. Six pairs of ethanol standards (0.1 – 3.0 mg/ml), which included n-propanol as an internal standard, were run before the samples.
Data Analysis
For the SHAC procedure, the dependent variables were BEC, volume of ethanol or water consumed, ethanol dose consumed, and body weight. The effect of the period of fluid access as well as the sex and age of the animal on these dependent measures was analyzed by analysis of variance (ANOVA) with day as a repeated measures factor. When appropriate, simple main effects analysis, followed by post-hoc comparisons, was conducted.
For the preference drinking procedures, the dependent variables were ethanol dose consumed, volume of ethanol or water consumed, body weight and ethanol preference ratio (calculated as the volume of ethanol consumed divided by the total volume consumed). These drinking data were analyzed with our standard laboratory procedure, where the average of the 2nd and 4th days of each set of solution concentrations was used. This allows the mouse to stabilize its consumption after a concentration and tube position change. ANOVA assessed age (adolescent vs. adult), sex (male vs. female), ethanol experience (“binge” vs. naïve), and concentration (repeated measure) effects on the dependent variables. Significant interactions were pursued with one-way ANOVAs at each concentration and post-hoc tests. Due to our a priori hypothesis that ethanol intake in the ethanol experienced adolescent mice would exceed that of naïve animals or of ethanol experienced adults, separate analyses were conducted on the adolescent versus adult animals in the absence of a significant interaction.
In all cases, data are presented as the mean ± SEM. The level of significance was set at P ≤ 0.05. Statistical analyses were conducted with the computer program SYSTAT (version 11, SYSTAT Software, Inc., Richmond, CA).
Results
SHAC Procedure
Data for the ethanol dose consumed during each 30 min session is depicted in Figure 1A. Analysis across all 7 ethanol sessions indicated that the ethanol dose consumed was significantly higher in adolescent versus adult mice [F(1,60) = 52.53, P < 0.001], with a significant interaction between age and sex [F(1,60) = 4.73, P < 0.05]. The interaction between age and sex appeared to be due to the slight increase in ethanol intake in male versus female adolescent mice, whereas ethanol intake in the adult mice was comparable or slightly higher in female versus male mice (not shown). There also was a main effect of ethanol session [F(6,360) = 8.36, P < 0.001], with an interaction between session and age [F(6,360) = 9.01, P < 0.001], on the ethanol dose consumed. As can be seen from Figure 1A, the interaction between session and age was due in large part to the higher ethanol dose consumed by adolescent mice in the earlier ethanol sessions, which decreased down to levels consumed by the adult mice in the later sessions. Subsequent analyses confirmed that the ethanol dose consumed was significantly higher in the adolescent versus adult mice on sessions 1 – 3 (P < 0.001; days 3, 6 and 9), session 5 (P < 0.01; day 15) and session 6 (P < 0.05; day 18). Ethanol intake did not differ on session 7 (day 21) and was significantly higher in the adult versus adolescent mice on session 4 (P = 0.05; day 12).
Figure 1. “Binge drinking” with the SHAC procedure is significantly higher in adolescent versus adult mice.
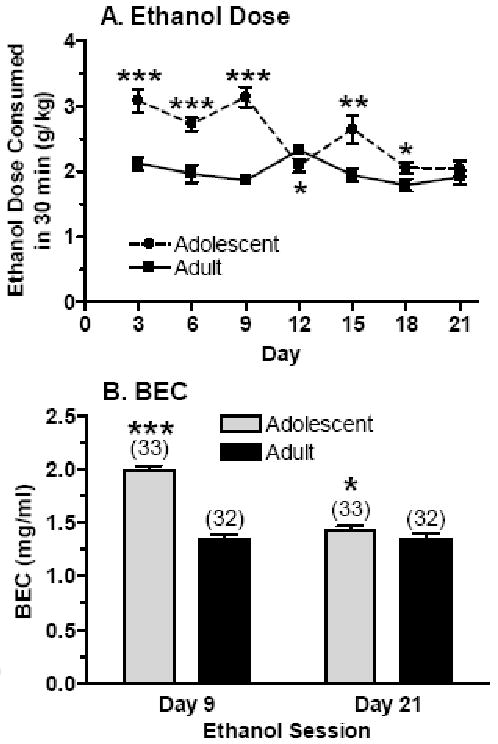
Mice had 30 min access to a 5% ethanol solution every 3rd day. Panel A depicts the ethanol dose consumed during the access period, whereas Panel B depicts the corresponding BEC after the period of ethanol access on day 9 and day 21. Values are the mean ± SEM for the number of animals in parentheses (panel B), collapsed across sex. *P ≤ 0.05, **P < 0.01, ***P < 0.001 vs. respective value in adult mice
BEC was assessed immediately following the ethanol session on day 9 and on day 21 (Figure 1B). Analysis of these data revealed that BEC was higher in the adolescent versus adult mice [F(1,61) = 63.79, P < 0.001] and was significantly different on the two days that BEC was assessed [F(1,61) = 53.30, P < 0.001]. The significant interaction between days and age [F(1,61) = 34.52, P < 0.001] was due to the differential change in BEC across days in the adolescent versus adult mice (↓ in adolescent mice, no change in adult mice). Notably, the age-related differences in BEC were consistent with the corresponding ethanol intake data. On day 9, mean ethanol intake and BEC were significantly higher in the adolescent (3.14 g/kg, 1.99 mg/ml) versus adult (1.86 g/kg, 1.35 mg/ml) mice. However, ethanol dose and BEC were more comparable on day 21 in the adolescent (2.04 g/kg, 1.43 mg/ml) and adult (1.92 g/kg, 1.29 mg/ml) mice.
In our experience, a mouse that is not fluid restricted will consume ∼ 5 ml water in a 24-hr period. In the present study, 24-hr water consumption was 4.9 mls in the adolescent mice and 5.5 mls in the adult mice. Scheduling fluid availability with the SHAC procedure enabled mice to consume 57 – 79% of their total fluid on the 4 hr schedule, 73 – 90% of their total fluid on the 6 hr schedule, and 91 – 100% of their total fluid on the 8 or 10 hr schedules. Thus, mice were conditioned to drink on a schedule rather than being fluid restricted. Additionally, the adult mice maintained their body weight during the SHAC procedure, while the adolescent mice gained weight. The mean ± SEM body weights at baseline were 22.8 ± 0.7 g (adult) and 13.0 ± 0.3 g (adolescent). In the adult mice, body weight dropped 14% during the first 3 days of the experiment, but recovered to within baseline values within the next 7 days of the experiment (not shown). In contrast, body weight in the adolescent mice increased over baseline across the days of the experiment. Upon completion of the study, body weights were 22.2 ± 0.6 g (adult) and 17.4 ± 0.4 g (adolescent).
Limited Access Ethanol Preference Procedure
Upon completion of the SHAC procedure, half of the adolescent and adult mice with “binge drinking” experience were tested for 2-hr ethanol intake of a 10% and 20% solution with independent cohorts of ethanol naïve adolescent and adult mice. See Table 1 for the ages of the animals upon testing. The initial analyses, conducted across both ethanol solutions, revealed that ethanol intake was significantly higher in female than in male mice [F(1,41) = 4.84, P < 0.05] and in adolescent versus adult mice [F(1,41) = 4.16, P < 0.05]. There was a significant interaction between age and sex [F(1,41) = 4.01, P = 0.05]. The ethanol dose consumed was significantly higher with intake of the 20% solution [F(1,41) = 76.82, P < 0.001], with a strong trend for an interaction between solution and sex [F(1,41) = 3.62, P = 0.06]. Data are depicted in Figure 2. Based on these interactions, separate analyses were conducted for each ethanol solution.
Figure 2. Limited access ethanol intake of a 10% (A) or 20% (B) ethanol solution in male and female adolescent and adult mice that were ethanol naïve or had prior “binge drinking” ethanol experience.
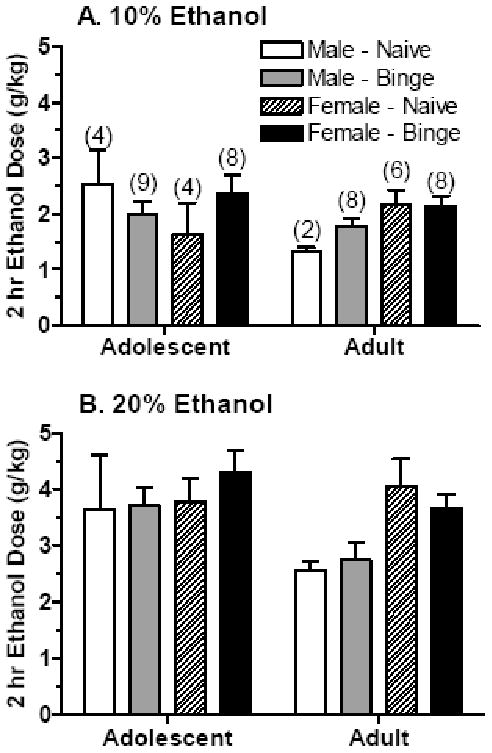
Values are the mean ± SEM for the number of animals in parentheses.
Analyses of the 10% ethanol solution (Figure 2A) revealed a strong trend for an interaction between age and sex [F(1,41) = 3.50, P < 0.07] as well as between age, sex, and experience [F(1,41) = 3.53, P < 0.07]. Subsequent analyses were conducted across each age. In the adolescent mice, there was no significant effect of sex on ethanol intake. However, the analysis of adult mice revealed a main effect of sex on ethanol dose consumed [F(1,20) = 7.10, P < 0.05], with ethanol intake significantly higher in female than in male mice. As depicted in Figure 2B, consumption of the 20% ethanol solution was significantly higher in female than in male mice [F(1,41) = 6.32, P < 0.05] and tended to be higher in adolescent versus adult animals [F(1,41) = 3.83, P < 0.06].
The analysis of ethanol preference yielded a slightly different pattern of results (Figure 3). Experience with “binge drinking” significantly increased preference for both ethanol solutions [F(1,41) = 5.37, P < 0.05], but there was no overall effect of age, sex, or solution on ethanol preference. Planned comparisons indicated that preference for the 20% ethanol solution was significantly increased in adolescent mice with “binge drinking” experience (P < 0.05).
Figure 3. Ethanol preference during 2-hr limited access to a 10% (A) or 20% (B) ethanol solution in male and female adolescent and adult mice that were ethanol naïve or had prior “binge drinking” ethanol experience.
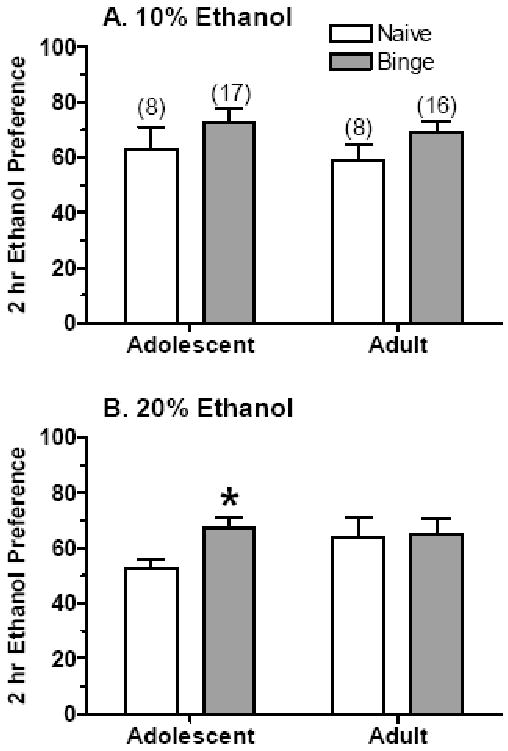
Shown are mean ± SEM corresponding ethanol preference ratios for the ethanol intake data depicted in Figure 2. The number of animals per group are the same as in Figure 2 (in parentheses), but are collapsed across sex. *P < 0.05 versus respective naïve group
Unlimited Access Ethanol Preference Procedure
Upon completion of the SHAC procedure, half of the adolescent and adult mice with “binge drinking” experience were tested for 24-hr ethanol intake of a 5%, 10% and 20% solution with independent cohorts of ethanol naïve adolescent and adult mice. See Table 1 for the ages of the animals upon testing. An initial repeated measures ANOVA was conducted across the three ethanol solutions. The ethanol dose consumed was significantly higher in adolescent versus adult mice [F(1,48) = 6.60, P < 0.02], in female than in male mice [F(1,48) = 24.29, P < 0.001], and in mice with “binge drinking” experience versus naïve animals [F(1,48) = 4.60, P < 0.05]. As expected, the ethanol dose consumed was significantly increased by the concentration of the ethanol solution [F(2,96) = 162.33, P < 0.001],. Data are depicted in Figure 4, and separate analyses were conducted on each ethanol solution.
Figure 4. Unlimited access ethanol intake of a 5%, 10%, or 20% ethanol solution in male and female adolescent (A) and adult (B) mice that were ethanol naïve or had prior “binge drinking” ethanol experience.
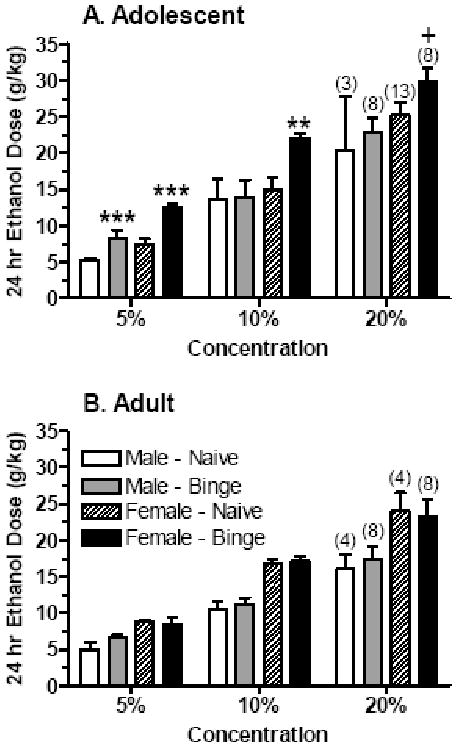
Values are the mean ± SEM for the number of animals in parentheses.
+P ≤ 0.10, **P < 0.01, ***P < 0.001 versus respective naïve group
Consumption of the 5% ethanol solution was significantly higher in female versus male mice [F(1,48) = 20.68, P < 0.001] and in mice with “binge drinking” experience versus naïve animals [F(1,48) = 12.69, P = 0.001]. There was a significant interaction between age and experience [F(1,48) = 5.93, P < 0.02]. Subsequent analyses in adolescent mice confirmed that the ethanol dose consumed from the 5% solution was significantly higher in female than in male mice [F(1,28) = 10.20, P < 0.01]. As depicted in Figure 4A, experience with “binge drinking” significantly increased ethanol intake over values in naïve animals in both male and female adolescent mice [F(1,28) = 15.37, P = 0.001]. In contrast, ethanol intake of the 5% solution in adult mice only was higher in female versus male mice [F(1,20) = 12.81, P < 0.01] (Figure 4B).
Analysis of the 10% solution revealed that the ethanol dose consumed was significantly higher in female than in male mice [F(1,48) = 16.33, P < 0.001]. Subsequent analyses in the adolescent mice confirmed that ethanol consumption was higher in female versus male mice [F(1,28) = 4.35, P < 0.05],. Planned comparisons confirmed that ethanol intake in adolescent female mice with “binge drinking” experience was significantly higher than in respective naïve mice (P < 0.01; Figure 4A). In contrast, ethanol intake in adolescent male mice was not significantly altered by prior ethanol drinking experience. In adult mice, consumption of the 10% ethanol solution only was significantly higher in female than in male mice [F(1,20) = 40.35, P < 0.001].
Ethanol intake from the 20% solution was significantly higher in adolescent versus adult mice [F(1,48) = 5.72, P < 0.05] and in female versus male mice [F(1,48) = 11.90, P = 0.001]. Subsequent analyses confirmed this sex difference in the adolescent [F(1,28) = 4.76, P < 0.05] and adult [F(1,20) = 8.07, P = 0.01] mice (Figure 4).
The analysis of preference ratio across the three ethanol solutions (Figure 5) revealed that ethanol preference was significantly higher in female than in male mice [F(1,48) = 7.06, P = 0.01], and significantly lower in mice consuming the 20% solution [F(2,96) = 51.20, P < 0.001].
Figure 5. Ethanol preference during 24-hr access to a 5%, 10%, or 20% ethanol solution in male and female adolescent (A) and adult (B) mice that were ethanol naïve or had prior “binge drinking” ethanol experience.
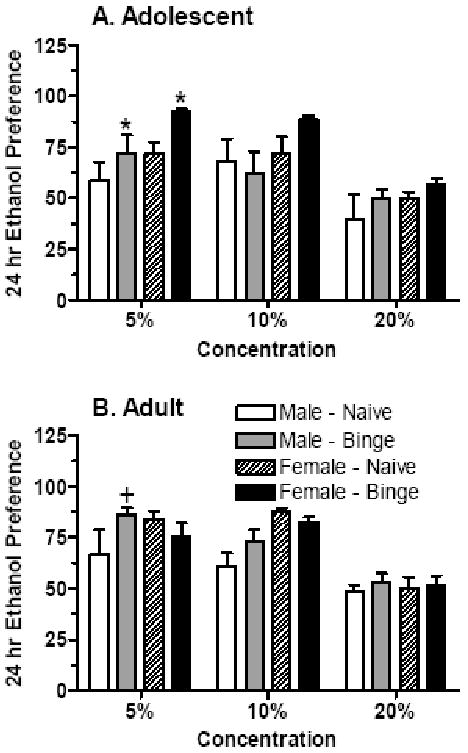
Shown are the mean ± SEM corresponding ethanol preference ratios for the ethanol intake data depicted in Figure 4. The numbers of animals per group are the same as in Figure 4.
+P < 0.10, *P < 0.05 versus respective naïve group
Preference for the 5% ethanol solution was significantly higher in mice with “binge drinking” experience versus naïve animals [F(1,48) = 4.40, P < 0.05] and tended to be higher in female versus male mice [F(1,48) = 3.50, P < 0.07]. Subsequent analyses in adolescent mice confirmed that ethanol preference was significantly higher in female versus male mice [F(1,28) = 4.79, P < 0.05] and in mice with “binge drinking” experience versus naïve animals [F(1,28) = 4.69, P < 0.05] (Figure 5A). In adult mice, there was a strong trend for an interaction between sex and ethanol experience [F(1,20) = 4.09, P < 0.06]. Planned comparisons indicated that preference in adult male mice with ethanol experience tended to be higher than values in respective naïve animals (P = 0.06; Figure 5B).
Preference for the 10% ethanol solution was primarily influenced by sex [F(1,48) = 7.44, P < 0.01],. And subsequent analyses indicated that preference for the 10% ethanol solution was significantly higher in adult female versus male mice [F(1,20) = 11.75, P < 0.01]. While preference for the 20% ethanol solution tended to be higher in adolescent female versus male mice [F(1,28) = 3.04, P = 0.09], there was no effects of sex or ethanol experience on ethanol preference in adult mice (Figure 5).
Discussion
It has been known for many years that sensitivity and tolerance to ethanol's pharmacological effects differs between adolescents and adults, across species (reviewed in Spear, 2000). However, the neurobiological mechanisms behind these differences are largely unknown, and most of the research examining differences in ethanol consumption in adolescents and adults use rodent models. Our studies sought to further corroborate the work done in rodents, by examining the effect of “binge drinking” experience during the adolescent period or adulthood on subsequent ethanol intake. Consistent with recent data in adolescent mice (Chester et al., 2008; Tambour et al., 2008), we found that ethanol naïve adolescent mice consumed significantly higher doses of ethanol, when compared to adult naïve mice, and that intake was higher in female versus male mice of both age groups. Furthermore, initial “binge drinking” exposure to ethanol during the adolescent phase produced high ethanol consumption as an adult under a two bottle choice, 24 hour access procedure, with adolescent female mice more susceptible to this effect than male mice.
In the initial phase of the experiment, male and female adolescent and adult mice received “binge drinking” ethanol experience with the SHAC procedure. Notably, the adolescent mice consumed significantly more ethanol than their adult counterparts, and this difference was further corroborated by a significant elevation in BECs over the adult mice. In both age groups, the dose of ethanol consumed not only met, but greatly exceeded the NIAAA's criteria for “binge drinking,” with BECs above 80 mg% in all cases. Thus, both age groups received initial ethanol experience consistent with “binge drinking”. Even in light of the modest evidence for age-related differences in ethanol-induced ataxia (Hefner and Holmes, 2007; Linsenbardt et al., 2009), the BECs achieved with the SHAC procedure would be expected to produce visible signs of intoxication (Cronise et al., 2005). It is possible that since the SHAC procedure was performed in C57BL/6J mice, an alcohol preferring strain, we saw a more robust effect than would be observed in other strains of mice. However, we believe that this is unlikely, given that the SHAC procedure has been shown to produce high levels of intake (BECs > 100mg%) in genetically heterogeneous mice (Finn et al., 2005).
In fact, our studies revealed that this early exposure to an intoxicating dose of ethanol during adolescence had a profound effect on later consumption in the same animals. This pre-exposure to ethanol in adolescent mice elevated the subsequent consumption of ethanol in an unlimited access procedure when the animals were tested as adults, when compared to ethanol intake in naïve adolescent mice as well as ethanol intake in naïve and ethanol pre-exposed adult mice. Notably, female adolescent mice were more vulnerable to this effect of prior “binge drinking” experience on subsequent ethanol intake, with the ethanol dose consumed from the 20% solution approaching 30 g/kg (mean = 29.8 g/kg; range in take 23.3 – 38.5 g/kg) in a 24-hr period. In general, female adolescent mice with “binge drinking” experience exhibited elevated drinking over all other experimental groups and following consumption of all three ethanol solutions. In male adolescent mice, “binge drinking” experience only increased consumption of the 5% ethanol solution, but intake remained lower than in similarly treated female mice. Conversely, there was no effect of prior ethanol experience on subsequent ethanol intake in the adult mice, with analyses revealing an overall main effect for intake to be higher in female than in male adult mice, regardless of ethanol experience. To our knowledge, this is the first demonstration that initial “binge drinking” experience selectively increases subsequent ethanol intake during adulthood when the initial exposure occurred during the adolescent period of development.
The mechanism(s) underlying the effect of “binge drinking” experience during adolescence to increase subsequent ethanol intake during adulthood are not known. It is unlikely that the age-related differences in ethanol sensitivity are contributing to the differences in consumption during the 24-hr access period, since at that point in testing all mice were adults and age differences were negligible. Even with the proviso that there was a difference in the range of ages upon initiation of testing for preference drinking during adulthood (adolescent experienced mice = postnatal days 58 – 60; adult experienced mice = postnatal 96 – 109 days; adult naïve mice = postnatal 58 – 82 days), the age range of the adult mice with adolescent ethanol “binge drinking” experience is compatible with reports measuring sensitivity to ethanol's behavioral effects (e.g., Linsenbardt et al., 2009). This would suggest that the behavioral sensitivity of the adolescent experienced mice (tested during adulthood) would be comparable to that of adult rather than adolescent animals.
We did not measure BEC in animals during the unlimited access procedure, since it is difficult to pinpoint a specific time for assessment. Given that recent work provide some modest evidence for age-related differences in ethanol metabolism in C57BL/6 mice (Hefner and Holmes, 2007; Linsenbardt et al., 2009), it is possible that pharmacokinetic factors contributed to the enhanced 24-hr ethanol intake in the mice with prior “binge drinking” experience as adolescents. Based on the time points examined (Hefner and Holmes, 2007; Linsenbardt et al., 2009), there appeared to be a slight enhancement in ethanol metabolism in adolescent mice. However, the BEC measurements during the SHAC procedure would suggest that ethanol metabolism was not altered by age in the initial portion of the study, given that the age-related differences in BEC corresponded to the differences in ethanol intake. Additionally, ethanol metabolism studies conducted in our laboratory in male and female adult C57BL/6 mice following a high dose of ethanol indicated that there was no sex difference in ethanol clearance rate (Gorin-Meyer et al., 2007), suggesting that the enhanced ethanol intake in female mice was not due to a sex difference in ethanol metabolism. Nonetheless, future studies will need to determine whether the enhanced ethanol intake during adulthood in the adolescent mice with “binge drinking” experience corresponded to enhanced metabolism.
Another possible explanation for the elevated consumption in mice subsequent to adolescent “binge drinking” experience is that age-related differences in the neurobiological effects of ethanol differ in adolescent versus adult animals. For example, rats who exhibit an early adolescent preference to alcohol displayed higher densities of serotonin receptors in cerebral-cortical and hippocampal regions, and lower densities of dopamine receptors in the ventral tegmental area (McBride et. al, 2005b), suggesting that the neurochemistry of the brain changed as the rodents matured. Stamford (1989) suggested that adolescent rodents have greater storage capacity for dopamine, possibly resulting in larger amounts of dopamine release during novel or risk-taking behavior in adolescent versus adult mice. In fact, it may not be the novel taste of ethanol that is rewarding to adolescents, but the pharmacodynamic properties of ethanol in motivational circuits. There is some evidence to suggest that there is an ontogenetic difference in the expression of type 1 endocannabinoid receptors, which are directly implicated in a hierarchial system involving opioid, cannabinoid, and some of the GABA systems that are thought to mediate the hedonic properties of rewarding stimuli (Peciña et al., 2006). Endocannabinoid receptors peak during adolescence in striatum, ventral mesencephalon, and limbic systems, but are lower in the nucleus accumbens and hippocampus compared to adults (Rodríguez de Fonseca et al., 1993; Romero et al., 1997). Adolescents may be more sensitive to endocannabid receptor agonists due to lower levels in certain areas, which results in compensatory upregulation of these receptors in “hedonic hot spots”. Subsequently this could contribute to the higher hedonic sensitivity seen in adolescents (see Wilmouth & Spear 2009). Related to this point, a recent review of the rodent literature suggests that the balance of rewarding and aversive effects of drugs of abuse is tipped toward reward in adolescence (Schramm-Sapyta et al., 2009).
Evidence shows that adolescents across a wide range of species are known to participate more frequently in “risky” behaviors such as substance abuse, sexual promiscuity and other novelty seeking behavior (Arnett 1992; Wilson and Daly, 1985), which could predispose some individuals to later addiction (see Introduction). This has recently been characterized in mice (for a review, see Laviola et al., 2003) with regard to substance abuse. Specifically, Adriani et. al (2002) reported greater nicotine intake by adolescents, without a concomitant increase in anxiety (measured by circulating levels of corticosterone) that could lead to stress related increases in nicotine intake. Adolescent rodents have also shown an increase in sensitivity to the effects of cocaine (Zakharova et al., 2009). This propensity to engage in risk-taking and novelty-seeking behavior without an apparent concomitant increase in anxiety is common in adolescents, and may be related to the increase in ethanol intake reported in this study.
In addition to the effect of age on ethanol consumption, a significant sex difference emerged. In both adolescent and adult mice, females showed a marked increase in 24-hr ethanol intake versus respective male mice. As already noted, pre-exposure to “binge drinking” experience only increased ethanol intake in the adolescent females. Sex differences in ethanol intake has been well-documented in rodents, with intake in females higher than that in males (e.g., Belknap et al., 1993; Chester et al., 2008; Finn et al., 2004; Lancaster et al., 1996; Yoneyama et al., 2008). Sex-dependent sensitivity to other drugs has been shown, with females generally being more sensitive to the rewarding effects of drug than males (see reviews by Becker & Hu, 2008; Carroll et al., 2004; Fattore et al., 2009). For example, female rats acquired intravenous self-administration of cocaine, methamphetamine and nicotine faster than males. Female rats also self-administered more cocaine with longer duration of “binges” and greater loss of circadian control over drug intake in an escalation model, and showed greater extinction responding on the drug associated lever after drug removal and greater reinstatement after drug priming than males (see Becker & Hu, 2008; Carroll et al., 2004; Fattore et al., 2009 and references therein). Along these same lines, women tend to increase rates of consumption of alcohol, marijuana, opioids and cocaine more rapidly than men do, and also have a harder time quitting after addiction has been established than do men (Brady et al., 1999). In contrast, women abuse drugs at lower rates when compared with men, but this could reflect differences in opportunity, rather than vulnerability to drug use (Becker & Hu, 2008). So it is possible that the mechanisms of sex differences in alcohol abuse in human subjects has not been fully characterized and that rodent studies conducted under more controlled circumstances can point clinical researchers in a direction not yet fully explored.
Certainly, the endogenous milieu as well as the endogenous steroid responses to alcohol could be contributing to the differences in intake that we have observed. Laviola et al. (2002) reported that corticosterone (CORT) levels in adolescent mice were higher than in adults, an effect that was particularly robust in the males, and that the CORT response to acute stress was reduced in females when compared with males. One possibility is that this sex and age-dependent stress contributes to the present findings. Catherine Rivier has shown that the hypothalamic-pituitary-adrenal axis is activated by ethanol consumption, but differentially between the sexes. Females generally have higher circulating levels of CORT, adrenocorticotrophic hormone (ACTH; an upstream regulator of CORT) and estrogen and secrete more CORT and ACTH than males in response to stress and alcohol administration (Ogilvie & Rivier, 1997; Handa et al., 1994; Galluci et al., 1993). Levels of testosterone are also altered by ethanol administration. In addition to sex differences, there seems to be a variable effect. Both acute and chronic ethanol administration was reported to lower plasma testosterone levels in males, mainly due to the inhibition of luteinizing hormone in the testes (Ylikahri et al., 1980; Rivier, 1999). Apter & Eriksson (2003) reported a decrease in plasma testosterone levels after acute ethanol treatment in alcohol-preferring and non-alcohol-preferring rats, but mean testosterone levels were higher in the alcohol-preferring rats. Conversely, Alomary et al., (2003), one of the only studies to also investigate brain levels of testosterone, reported increases in both plasma and brain testosterone after acute ethanol administration. Along these same lines, Sarkola et al. (2000) reported an increase in plasma testosterone in premenopausal women after an acute ethanol administration. Consistent with the idea that there are sex differences in the effect of ethanol on testosterone levels, recent preliminary findings indicate that chronic ethanol exposure and withdrawal significantly increase testosterone levels in female mice, while decreasing levels in male mice (Hashimoto et al., 2009).
With regard to female sex hormones, alcohol has been shown to precipitate a rise in estrogen levels in premenopausal women (reviewed in Gill, 2000). Acute administration of ethanol also increased levels of pregnenolone (a precursor to progesterone) and progesterone (O'Dell et al., 2004; Korneyev et al., 1993). In light of the present studies, sex hormones could be partially responsible for the differences in alcohol consumption we report, but since the literature is inconclusive at the present time, it is difficult to make any direct interpretations.
We did not monitor estrus cycles in the present study, but there was no change in drinking patterns that could be related to hormonal changes associated with stage of the estrous cycle. Results by Tambour et al. (2008) indicated that ethanol intake was significantly higher in female adult and adolescent mice versus respective male counterparts after approximately 3 weeks of alcohol experience. This finding suggests that alcohol history (experience) was more important than the onset of puberty to the sex differences in ethanol intake. Additionally, the higher ethanol intake in female mice during adolescence, which likely exhibited inconsistent estrous or non-existent estrous cycles, would suggest that the overall higher ethanol intake in female mice was not related to a specific stage of the estrous cycle.
Interestingly, no significant effect of age or sex on ethanol intake was observed in the limited access two-bottle choice paradigm. This result was surprising, considering the highly significant divergence seen in the continuous access 24-hr ethanol preference procedure. One possible explanation could be that the pattern of ethanol intake differed in the adolescent versus adult animals so that an examination of 2-hr ethanol intake might not capture the true differences in consumption that would be evident over a 24-hr period. For instance, we have used lickometers to examine the microarchitecture of ethanol drinking behavior in male and female C57BL/6 mice and observed sex differences in bout frequency versus bout size when animals were on a 2-hr limited access schedule (Ford et al., 2005, 2008). Perhaps more relevant to the present findings, we recently examined ethanol intake patterns in male and female wildtype and knockout mice with a null mutation in the 5α-reductase-1 gene and found that the effect of sex and genotype on ethanol intake differed, when assessed with 24-hr versus 2-hr periods of ethanol preference drinking (Nickel et al., 2006). Thus, an alteration in the pattern of ethanol intake across time might not reveal a sex or age-related difference in ethanol intake, when the analysis was limited to a 2-hr limited access session. This possibility could be examined in future studies utilizing lickometers to assess the pattern of ethanol intake across 24-hr ethanol sessions.
Taken together, “binge drinking” ethanol experience during the adolescent period significantly increased subsequent ethanol intake during adulthood, with female mice more susceptible to this effect. The enhanced vulnerability of female mice is consistent with evidence indicating that female rodents are more sensitive to the rewarding effects of drugs than males during all phases/models of drug abuse (see Becker & Hu, 2008; Carroll et al., 2004; Fattore et al., 2009). While the potential contribution of pharmacokinetic mechanisms to these differences in ethanol intake are unclear, it is possible that fundamental differences in neurobiology, as well as an increased propensity for novelty-seeking and “risky” adolescent behavior could be contributing to the differences seen in adolescent versus adult mice. Based in the important interaction between sex, age, and “binge drinking” ethanol experience on subsequent ethanol intake, research aimed at understanding the mechanisms contributing to the effects of these factors (alone or in combination) on ethanol drinking behavior will be important in the development of pharmacotherapies for the treatment of high alcohol intake.
Acknowledgments
The research was supported by INIA Consortium grant AA13478 and AA016981 from NIAAA and the Department of Veterans Affairs. We thank the Portland Alcohol Research Center for supplying the C57BL/6 mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Macrì S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–224. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, Patcher VK. Gender differences in ethanol preferences and ingestion in rats: the role of the gonadal steroid environment. J Clin Invest. 1998;101:2677–2685. doi: 10.1172/JCI1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alomary AA, Vallée M, O'Dell LE, Koob GF, Purdy RH, Fitzgerald RL. Acutely administered ethanol participates in testosterone synthesis and increases testosterone in rat brain. Alcohol Clin Exp Res. 2003;27:38–43. doi: 10.1097/01.ALC.0000047304.28550.4F. [DOI] [PubMed] [Google Scholar]
- Apter SJ, Peter Eriksson CJ. The effect of alcohol on testosterone concentrations in alcohol-preferring and non-preferring rat lines. Alcohol Clin Exp Res. 2003;27:1190–1193. doi: 10.1097/01.ALC.0000075832.83254.81. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: a developmental perspective. Dev Rev. 1992;12:339–373. [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Metten P, Finn DA, Rhodes JS, Bergeson SE, Harris RA, Crabbe JC. Hybrid C57BL/6J × FVB/NJ mice drink more alcohol than do C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1949–1958. doi: 10.1097/01.alc.0000187605.91468.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Caihol SH, Mormède P. Sex and strain differences in ethanol drinking: effects of gonadectomy. Alcohol Clin Exp Res. 2001;25:594–599. [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Barrenha GD, Hughes ML, Keuneke KJ. Age- and sex-dependent effects of footshock stress on subsequent alcohol drinking and acoustic startle behavior in mice selectively bred for high-alcohol preference. Alcohol Clin Exp Res. 2008;32:1782–1794. doi: 10.1111/j.1530-0277.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK. Binge drinking up-regulates accumbens mGluR5-Homer2-PI3K signaling: Functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronise K, Finn DA, Metten P, Crabbe JC. Scheduled access to ethanol results in motor impairment and tolerance in female C57BL/6 mice. Pharmacol Biochem Behav. 2005;81:943–953. doi: 10.1016/j.pbb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R, Prescott S. The ecology of adolescent activity and experience. J Youth Adolescence. 1977;6:281–294. doi: 10.1007/BF02138940. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Fratta W. Sex differences in the self-administration of cannabinoids and other drugs of abuse. Psychoneuroendocrinology. 2009 Sept 8; doi: 10.1016/j.psyneuen.2009.08.008. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Finn DA, Belknap JK, Cronise K, Yoneyama N, Murillo A, Crabbe JC. A procedure to produce high alcohol intake in mice. Psychopharmacology. 2005;178:471–480. doi: 10.1007/s00213-004-2039-8. [DOI] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck M, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Ford MM, Beckley EH, Nickel JD, Eddy S, Finn DA. Ethanol intake patterns in female mice: Influence of allopregnanolone and the inhibition of its synthesis. Drug Alcohol Depend. 2008;97:73–85. doi: 10.1016/j.drugalcdep.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J. The effects of moderate alcohol consumption on female hormone levels and reproductive function. Alcohol Alcohol. 2000;35:417–423. doi: 10.1093/alcalc/35.5.417. [DOI] [PubMed] [Google Scholar]
- Gorin-Meyer RE, Wiren KM, Tanchuck MA, Long SL, Yoneyama N, Finn DA. Sex differences in the effects of finasteride on acute ethanol withdrawal severity in C57BL/6J and DBA/2J mice. Neuroscience. 2007;146:1302–1315. doi: 10.1016/j.neuroscience.2007.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto JG, Forquer MR, Wiren KM. Sexually dimorphic hormone profiles after chronic alcohol exposure in WSP and WSR mice: Testosterone levels drop in males but increase in females. Alcohol Clin Exp Res. 2009;33:32A. [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology. 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration and severity. Arch Pediatr Adolesc Med. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Korneyev AY, Costa E, Guidotti A. During anesthetic-induced activation of the hypothalamic pituitary adrenal axis, blood-borne steroids contribute to the anesthetic effect. Neuroendocrinology. 1993;57:559–565. doi: 10.1159/000126405. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Morley-Fletcher S, Terranova M. Peculiar response of adolescent mice to acute and chronic stress and to amphetamine: evidence of sex differences. Behav Brain Res. 2002;130:117–125. doi: 10.1016/s0166-4328(01)00420-x. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova M, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: Psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL., II Sensitivity and tolerance to the hypnotic and ataxic effects of ethanol in adolescent and adult C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 2009;33:464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Bull RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences: studies with animal models. Recent Dev Alcohol. 2005a;17:123–142. doi: 10.1007/0-306-48626-1_6. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Kerns RT, Rodd ZA, Strother WN, Edenberg HJ, Hashimoto JG, Wiren KM, Miles MF. Alcohol effects on central nervous system gene expression in genetic animal models. Alcohol Clin Exp Res. 2005b;29:167–175. doi: 10.1097/01.alc.0000153539.40955.42. [DOI] [PubMed] [Google Scholar]
- Nickel JD, Ford MM, Yoneyama N, Murillo AR, Finn DA. Modulation of ethanol intake patterns in male and female Srd5a1 knockout mice. Alcohol Clin Exp Res. 2006;30:65A. [Google Scholar]
- O'Dell LE, Alomary AA, Vallée M, Koob GF, Fitzgerald RL, Purdy RH. Ethanol-induced increases in neuroactive steroids in the rat brain are absent in adrenalectomized and gonadectomized rats. Eur J Pharmacol. 2004;484:241–247. doi: 10.1016/j.ejphar.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Pecina S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev Psychobiol. 1990;23:215–231. doi: 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. The prejuvenile onset of play fighting in laboratory rats (Rattus norvegicus) Dev Psychobiol. 1997;31:193–205. [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22:633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Adolescent and adult heart rate responses to self-administered ethanol. Alcohol Clin Exp Res. 2008;32:180–1815. doi: 10.1111/j.1530-0277.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. Alcohol rapidly lowers plasma testosterone levels in the rat: evidence that a neural brain-gonadal pathway may be important for decreases testicular responsiveness to gonadotropin. Alcohol Clin Exp Res. 1999;23:38–45. doi: 10.1111/j.1530-0277.1999.tb04021.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Ramos JA, Bonnin A, Fernández-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4:135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Romero J, Garcia-Palomero E, Berrendero F, Garcia-Gil L, Hernandez ML, Ramos JA. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse. 1997;26:317–323. doi: 10.1002/(SICI)1098-2396(199707)26:3<317::AID-SYN12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Sarkola T, Tatsushige F, Heikke M, Peter Eriksson CJ. Acute effect of alcohol on androgens in premenopausal women. Alcohol Alcohol. 2000;35:84–90. doi: 10.1093/alcalc/35.1.84. [DOI] [PubMed] [Google Scholar]
- Sarkola T, Peter Eriksson CJ. Testosterone increases in men after a low dose of alcohol. Alcohol Clin Exp Res. 2003;27:682–685. doi: 10.1097/01.ALC.0000060526.43976.68. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology. 2002;162:438–447. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Stamford JA. Development and ageing of the rat nigrostriatal dopamine system studied with fast cyclic voltammetry. J Neurochem. 1989;52:1582–1589. doi: 10.1111/j.1471-4159.1989.tb09212.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2005 National Survey on Drug Use and Health: National Findings. Rockville, MD: Substance Abuse and Mental Health Services Admiistration; 2006. NSDUH Series H-30, DHHS publication SMA 06-4194. [Google Scholar]
- Tambour S, Brown LL, Crabbe JC. Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol Clin Exp Res. 2008;32:2100–2106. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- Trimpop R, Kerr J, Kirkcaldy BD. Comparing personality constructs of risk-taking behavior. Pers Individ Differ. 1998;26:237–254. [Google Scholar]
- Schramm-Sapyta NL, Kingsley MA, Rezvani AH, Propst K, Swartzwelder HS, Kuhn CM. Early ethanol consumption predicts relapse-like behavior in adolescent male rats. Alcohol Clin Exp Res. 2008;32:754–762. doi: 10.1111/j.1530-0277.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology. 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Hedonic sensitivity in adolescent and adult rats: Taste reactivity and voluntary sucrose consumption. Pharmacol Biochem Behav. 2009;92:566–573. doi: 10.1016/j.pbb.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Daly M. Competitiveness, risk taking, and violence: the young male syndrome. Ethology Sociobiol. 1985;6:59–73. [Google Scholar]
- Ylikahri RH, Huttunen MO, Härkönen M. Hormonal changes during alcohol intoxication and withdrawal. Pharmacol Biochem Behav. 1980;13:131–137. doi: 10.1016/s0091-3057(80)80021-9. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biocehm Behav. 2009;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


