Abstract
Stimulant abuse continues to be a growing problem among women. Over the last 10-15 years, an increasing number of studies have focused on factors that may be implicated in stimulant abuse in women as compared to men, including the role of hormonal fluctuations across the menstrual cycle. Numerous preclinical studies have documented that female rodents are more sensitive than male rodents to the behavioral effects of stimulant administration and the hormone estradiol is involved in the enhanced response to stimulants observed in females. In contrast, fewer studies have been conducted in humans and non-human primates addressing the role of sex and gonadal hormones on the effects of cocaine. This review paper presents a recent update on data collected in our Human Cocaine Challenge Laboratory and our Non-human Primate Laboratory, including analysis of cocaine pharmacokinetics, sex differences, the menstrual cycle, and the role of progesterone in modulating the response to cocaine. Our studies indicate that there is minimal evidence that the response to intranasal cocaine varies across the menstrual cycle or between men and women. In contrast, the response to smoked cocaine is greater in the follicular phase than the luteal phase and differences between men and women generally only emerge when men are compared to women in the luteal phase. In terms of potential hormonal mechanisms for these differences, the hormone progesterone attenuates the subjective response to cocaine. With respect to cocaine self-administration, there are minimal changes across the menstrual cycle in both humans and non-human primates. Thus, there is converging evidence across a range of species that the behavioral effects of cocaine 1) differ between males and females, 2) differ in relation to hormonal fluctuations, 3) can be attenuated by progesterone (at least in females), and 4) do not appear to be related to differences in cocaine pharmacokinetics.
Keywords: cocaine, sex differences, humans, non-human primates, menstrual cycle, pharmacokinetics, self-administration, subjective response, estradiol, progesterone
Introduction
Cocaine abuse continues to be a growing problem among women, with the gender gap narrowing with respect to new cocaine use, even among those 12-17 years of age (SAMHSA, 2008). Although men are still more likely to be cocaine dependent than women, women exhibit a more rapid progression of the illness and they have a higher incidence of comorbid psychiatric disorders (McCance-Katz et al., 1999; Najavits and Lester, 2008; O’Brien and Anthony, 2005; Sinha and Rounsaville, 2002). These sex differences may contribute to women relapsing (Hyman et al., 2008) or dropping out of treatment sooner than men (Siqueland et al., 2002). These results parallel preclinical animal models using rodents showing that females are more vulnerable to the abuse-related effects of cocaine than males (see reviews by Becker et al., 2001; Festa and Quiñones-Jenab, 2004; Lynch et al., 2002; Mello and Mendelson, 2002).
This review paper will present a recent update using human and non-human primate models to assess differences in the response to cocaine including pharmacokinetics, sex differences, the menstrual cycle, and the role of progesterone in modulating the response to cocaine, with a specific emphasis on the research endeavors (both published and unpublished) conducted in our Human Cocaine Challenge Laboratory (Marian W. Fischman Behavioral Pharmacology Laboratory) and our Non-human Primate Laboratory. Accordingly, this will not be a comprehensive literature review, but relevant and related published research conducted by other laboratories will be mentioned. All human participants signed consent forms approved by the Institutional Review Boards of the College of Physicians and Surgeons of Columbia University or the New York State Psychiatric Institute and participants were financially compensated. All aspects of animal maintenance and experimental procedures of our non-human primate studies complied with the U.S. National Institutes of Health Guide for Care and Use of Laboratory Animals, and were approved by the New York State Psychiatric Institute Animal Care and Use Committee.
Menstrual Cycle of Human and Non-human Primates
While the vast majority of research on sex differences and the role of reproductive hormones on the response to cocaine has been conducted in rats, the rat estrous cycle is quite different from the menstrual cycle of humans and laboratory primates. The rat estrous cycle is typically 4 days, separated into 4 phases: diestrus, proestrus, estrus and metestrus (see Lynch et al., 2002 for an excellent review comparing the rat estrous cycle and human menstrual cycle). During proestrus the levels of both estradiol and progesterone are elevated, whereas during estrus, the levels of both of these hormones are minimal (Butcher, Collins, and Fugo, 1974; Smith, Freeman, and Neill, 1975). In contrast, the human female has a 28-day menstrual cycle separated into 2 broad phases: the follicular phase and the luteal phase. During the follicular phase, progesterone levels are minimal and estradiol levels rise gradually, peaking just before ovulation (around day 14). The luteal phase begins after ovulation, and during this time progesterone levels increase, peaking 3-8 days after ovulation and then declining several days prior to menses. Given these species differences in cycle length and gonadal hormone fluctuations, it is difficult to directly compare data collected in rodents and humans. Fortunately, female rhesus monkeys provide an ideal laboratory animal model for assessing changes across the menstrual cycle because they have a menstrual cycle almost identical in terms of ovarian hormone fluctuations (e.g., progesterone and estradiol) and length to that of humans (Yoshida, 1999; Shimizu, 2008).
Cocaine Pharmacokinetics
There is growing evidence, primarily from rodent studies, that there are sex differences in the behavioral responses to cocaine. Further, fluctuations in estradiol and progesterone underlie these behavioral differences that may be related to differences in cocaine pharmacokinetics. Clearly, when investigating and interpreting behavioral differences in response to a drug, whether it is differences between males and females, or differences as a function of hormonal status, it is important to ascertain whether the behavioral differences are due to changes in the pharmacokinetics of the drug. Unfortunately, due to the invasiveness of collecting multiple blood samples and the financial expense of analyzing these samples, few studies have carefully measured cocaine and metabolite plasma levels. In rodents, the results from cocaine pharmacokinetic studies have been inconsistent (e.g., Bowman et al., 1999; Festa et al., 2004; Niyomchai et al., 2006; Van Haaren et al., 1997). The following section will summarize what our studies have contributed to the understanding of cocaine pharmacokinetics in relation to sex differences and hormonal fluctuations in human and non-human primates.
Human Studies
Our first study exploring whether there were sex differences in response to cocaine was a retrospective analysis of men and women who had participated in cocaine challenge studies (Evans et al., 1999). In that initial study, we found that women had significantly higher cocaine plasma levels than men after repeated doses of smoked cocaine, but similar benzoylecgonine plasma levels to men. This sex difference in cocaine plasma levels was most likely due to women receiving larger doses of cocaine, women had lower body weights than men (61 vs. 78 kg, respectively) and cocaine dose was not adjusted for body weight. In contrast, as shown in Table 1, subsequent studies conducted in our laboratory have failed to show differences in peak cocaine plasma levels between men and women following either repeated doses of smoked cocaine (Evans and Foltin, 2006a) or intranasal cocaine (Collins et al., 2007). Dosing was not adjusted for body weight with smoked cocaine (Evans and Foltin, 2006a), but it was for intranasal cocaine (Collins et al., 2007). These two studies also addressed changes in cocaine plasma levels across the menstrual cycle. As shown in Table 1, peak cocaine plasma levels do not vary between the follicular and luteal phases of the menstrual cycle in women for either smoked or intranasal cocaine (Collins et al., 2007; Evans and Foltin, 2006a). Similarly, there were no sex differences or menstrual cycle phase differences for cocaine metabolite plasma levels in either of these studies. These cocaine pharmacokinetic findings are consistent with most other studies in humans (Evans et al., 2002; Kosten et al., 1996; Mendelson et al., 1999a; Sofuoglu et al., 1999; but see Lukas et al., 1996).
TABLE 1.
Peak Cocaine Plasma Levels as a Function of Route, Cocaine Dose, Sex and Menstrual Cycle Phase*
| Males | Females Combined |
Females Follicular |
Females Luteal |
|
|---|---|---|---|---|
| Smoked Cocaine Levels (ng/ml) | N = 10 | N = 11 | N = 11 | N = 11 |
| 0 mg | 5.8 (± 3.2) | 11.1 (± 2.5) | 12.4 (± 4.5) | 10.1 (± 4.8) |
| 6 mg | 106.9 (± 9.3) | 107.1 (± 8.2) | 104.6 (± 13.9) | 102.2 (± 14.7) |
| 12 mg | 214.5 (± 14.5) | 222.2 (± 11.8) | 211.4 (± 12.5) | 238.7 (± 25.7) |
| 25 | 455.3 (± 39.0) | 455.2 (± 24.9) | 442.4 (± 49.8) | 486.8 (± 37.3) |
| Intranasal Cocaine Levels (ng/ml) | N = 10 | N = 8 | N = 8 | N = 8 |
| 0.06 mg/kg | 36.0 (± 6.4) | 31.1 (± 18.8) | 28.2 (± 29.8) | 44.5 (± 33.5) |
| 0.34 mg/kg | 84.7 (± 8.6) | 71.2 (± 9.1) | 80.7 (± 15.6) | 72.7 (± 19.3) |
| 0.69 mg/kg | 155.4 (± 19.6) | 115.7 (± 8.3) | 114.7 (± 16.0) | 129.7 (± 17.4) |
| 1.37 /kg | 342.4 (± 32.0) | 319.5 (± 55.5) | 284.7 (± 73.7) | 354.3 (± 94.4) |
These data were adapted from two previously published studies with repeated doses of smoked cocaine (Evans & Foltin, 2006a) and repeated doses of intranasal cocaine (Collins et al., 2007).
Rhesus Monkey Studies
With humans it is difficult and time consuming to assess cocaine pharmacokinetics comprehensively across the entire menstrual cycle. However, female rhesus monkeys have menstrual cycles that are remarkably similar to human menstrual cycles and they can be tested repeatedly over extensive lengths of time, making them an ideal laboratory animal model for this type of research. Consequently, we used female rhesus monkeys to assess the pharmacokinetics of a range of cocaine doses across the menstrual cycle. In contrast to previous cocaine pharmacokinetic studies in monkeys (e.g., Mello et al., 1993, 2000, 2002; Saady et al., 1995), our monkeys were trained to enter and remain in primate chairs during the sessions so that repeated blood samples could be obtained with alert monkeys to avoid sedation, which could potentially alter pharmacokinetics.
Our first study (Evans and Foltin, 2004) assessed the acute effects of intravenous cocaine (0, 0.25, 0.50 and 1.00 mg/kg) in 5 female rhesus monkeys. Monkeys were tested at four hormonally distinct phases: menses, midfollicular, periovulatory and midluteal. Each session before cocaine administration, a blood sample was obtained to measure the ovarian hormones estradiol, progesterone and luteinizing hormone (LH) to confirm menstrual cycle phase. Cocaine and cocaine metabolite plasma levels were measured repeatedly after cocaine administration (i.e., 5, 15, 30, 45, 60 and 90 min). Given that previous studies in both humans and laboratory animals have shown that cocaine increases LH (see Mello and Mendelson, 2002 for a comprehensive review), we also measured LH repeatedly after cocaine administration. While cocaine and cocaine metabolite levels increased as a function of cocaine dose, cocaine plasma levels were relatively consistent across the menstrual cycle. Further, the half-life of cocaine ranged between 36 to 49 min, regardless of cocaine dose or menstrual cycle phase. These results are similar to a previous study by Mello et al. (2000) that compared an acute dose of cocaine in midfollicular phase and midluteal phase female monkeys. In addition, our study showed that the cocaine metabolites, benzoylecgonine and ecgonine methyl ester, were increased during the luteal phase, but only following the highest dose of cocaine. Menstrual cycle phase and cocaine dose did modulate changes in LH plasma levels, with the greatest changes observed following 1.0 mg/kg cocaine; LH plasma levels increased during menses and the follicular phase, but decreased during the periovulatory and luteal phases. For reasons that are unclear, these results are not in line with previous studies showing that cocaine consistently increases LH levels (see review by Mello and Mendelson, 2002). While these data suggest that there are minimal differences in the pharmacokinetics of cocaine across the menstrual cycle, only acute doses were tested. Thus, these data may not reflect the natural ecology where multiple doses of cocaine are commonly used.
Therefore, our second study in female monkeys (Evans and Foltin, 2006b) used a similar design, but monkeys were administered 4 injections of intravenous cocaine (0.00, 0.25 or 0.50 mg/kg), spaced 15 min apart. Again, monkeys were tested at the four menstrual cycle phases mentioned above and cocaine, cocaine metabolite, LH and prolactin plasma levels were measured repeatedly, with the last sample obtained 120 min after the last cocaine injection. Similar to our previous study, repeated injections of cocaine increased cocaine and metabolite levels in a dose-related manner, and there were minimal differences across the menstrual cycle. Overall, LH plasma levels either remained stable or decreased during the session although cocaine did produce transient increases in LH in the luteal phase regardless of cocaine dose. Lastly, as expected, repeated doses of cocaine substantially attenuated prolactin levels at all menstrual cycle phases.
Summary
Our pharmacokinetic studies in humans, in combination with previous studies from other laboratories, indicate that there are minimal differences in the pharmacokinetics of both smoked and intranasal cocaine across the menstrual cycle or between men and women (Collins et al., 2007; Evans et al., 2002; Evans and Foltin, 2006a; Kosten et al., 1996; Mendelson et al., 1999a; Sofuoglu et al., 1999). Similarly, in non-human primates, our studies (Evans and Foltin, 2004, 2006b) and others (Mello et al., 2000) have shown no changes in cocaine pharmacokinetics as a function of menstrual cycle phase. While a limitation of our pharmacokinetic studies in monkeys is that we did not include a group of males for comparison, previous studies in monkeys have shown minimal sex differences in the pharmacokinetics of cocaine following intravenous cocaine (Mello et al., 1993, 2002: Mendelson et al., 1999b). Consistent with these findings, Niyomchai et al. (2006) reported that although progesterone attenuated the cocaine-induced locomotor response in female rats, this effect was not related to changes in cocaine pharmacokinetics. Therefore, the existing data indicate that the pharmacokinetics of cocaine play a limited role in mediating the behavioral responses to cocaine.
Subjective Response to Cocaine: Sex and Menstrual Cycle Effects
We, and several other research groups have investigated whether there are differences in the subjective response to cocaine between men and women and whether these differences are related to hormonal fluctuations across the menstrual cycle (Collins et al., 2007; Evans et al., 1999, 2002; Evans and Foltin, 2006a; Haney et al., 1998; Kosten et al., 1996; Lukas et al., 1996; Mendelson et al., 1999a; Sofuoglu et al., 1999). As mentioned above, our initial retrospective study that combined data across three studies (Evans et al., 1999) assessed sex differences in response to repeated doses of 50 mg smoked cocaine in 11 men and 9 women. Despite higher cocaine plasma levels in women, the subjective response to cocaine was virtually identical for men and women, with the exception that after the last dose of cocaine each session, ratings of “I want cocaine” were lower in women compared to men. In another study from our laboratory that assessed a range of intravenous cocaine doses in a relatively equal number of men and women (Haney et al., 1998), there were minimal differences in subjective response to cocaine between men and women, although women reported being less “stimulated” and reported that the cocaine was of lower quality. One major limitation with both of these studies was that menstrual cycle phase was not controlled for. In fact, at that time, our laboratory was just beginning to obtain information related to the menstrual cycle in women. Based on prospective daily ratings of mood and menstrual cycle (that were not confirmed with hormone levels), of the 9 women included in the Evans et al. 1999 paper, 2 women were in the ovulatory phase, 5 were in the luteal phase and 2 were in the menstrual phase.
Menstrual Cycle Phase and Subjective Response to Cocaine
Based on the ever increasing preclinical literature with laboratory animals (see other reviews in this issue), there is undeniable evidence that there are sex differences between males and females and that these differences are to some extent related to fluctuations in gonadal hormones. Therefore, based on several of these earlier studies (see early reviews by Becker et al., 2001; Lynch et al., 2002; Mello and Mendelson, 2002), there was sufficient evidence to warrant an investigation into the contribution of hormonal fluctuations to the effects of cocaine in humans. This information would be useful for understanding whether the failure to elucidate sex differences in previous cocaine challenge studies in humans was in part due to the fact that menstrual cycle phase had been ignored. We felt that the best approach to answer this question was to recruit female cocaine abusers and have them reside in the hospital at various phases of their menstrual cycle so that we could evaluate the effects of experimentally administered cocaine under controlled conditions. When we initially embarked on this research, we anticipated two major obstacles: 1) that it would be very difficult to recruit and retain women and 2) that cocaine-abusing women would have irregular menstrual cycles (Sofuoglu et al., 1999). While the former was true, i.e., the most difficult aspect of this research is the recruitment of medically and psychiatrically healthy female cocaine abusers, the latter was not a problem. While previous studies have shown that chronic cocaine use disrupts the reproductive cycle in rodents (e.g., Chen and Vandenbergh, 1994; King et al., 1993), non-human primates (Mello et al., 1997; Potter et al., 1999) and humans (Mello, 1998), across the various studies we have conducted with this population, most women have had remarkably regular menstrual cycles despite their chronic cocaine use.
In our first study (Evans et al., 2002) designed to assess the response to cocaine at different phases of the menstrual cycle, a range of repeated doses of smoked cocaine (0, 6, 12, 25 mg base) were tested in 11 normally cycling female cocaine abusers during the midfollicular phase (days 6-10 after onset of menses) and the midluteal phase (days 7-12 after the LH surge). In this study (Evans et al., 2002), and subsequent studies from our laboratory (Evans and Foltin, 2006a; Collins et al., 2007), menstrual cycle status was prospectively monitored, and menstrual cycle phase was verified using urinary ovulation kits and plasma levels of estradiol and progesterone. In our 2002 study (Evans et al., 2002), there was some evidence that the subjective effects of cocaine were increased more in the follicular phase than the luteal phase, including “Good Drug Effect,” “High,” “Stimulated,” and “Drug Quality Ratings.” However, it should be noted that these effects varied as a function of cocaine dose and were modest. Nevertheless, these data appeared to partially support the results from laboratory animal studies indicating that the effects of cocaine were greater in the presence of estradiol. In a subsequent study using a similar design, we were able to replicate these findings (Evans and Foltin, 2006a). As illustrated in the top panel of Figure 1, smoked cocaine produced dose-related increases on “Good Drug Effect” cluster scores (mean visual analog ratings of “I feel…,” “High,” “Stimulated,” and “a Good Drug Effect”) and these scores were significantly higher in the follicular phase compared to the midluteal phase. [The data for Figure 1 were based on combined data from 22 women (11 from Evans et al., 2002 and 11 from Evans and Foltin, 2006a).] A similar pattern was observed with other positive subjective effects of cocaine. These data confirm and extend those previously reported by Sofuoglu et al. (1999) with smoked cocaine. These studies suggest that the effects of smoked cocaine in women are attenuated in the luteal phase of the menstrual cycle when progesterone levels are elevated. Consistent with these results, one of the few studies conducted in normally cycling female rats showed that the lowest levels of cocaine seeking behavior occurred when progesterone levels were elevated (Feltenstein and See, 2007).
Figure 1.
Mean “Good Drug Effect” cluster scores as a function of menstrual cycle phase following repeated doses of cocaine. The top panel shows results for smoked cocaine (6, 12 and 25 mg) in 22 women. These data were combined and adapted from two previously published studies (Evans et al., 2002 and Evans & Foltin, 2006a). The bottom panel shows results for intranasal cocaine in 8 women. These data were adapted from Collins et al. (2007). Bars represent the mean + 1 SEM. * indicates a significant difference from the follicular phase.
We subsequently conducted an analogous study to assess the effects of intranasal cocaine during the follicular and luteal phases of the menstrual cycle in 8 women (Collins et al., 2007). That study tested 4 doses of intranasal cocaine (0.06, 0.34, 0.69 and 1.37 mg/kg), and each session participants received two administrations of the same dose spaced 40 minutes apart. Interestingly, as shown in the lower panel of Figure 1, there were no differences in the subjective response to intranasal cocaine administration between the luteal and follicular phases of the menstrual cycle. Previous studies with both intranasal cocaine (Lukas et al., 1996) and intravenous cocaine (Mendelson et al., 1999a) also failed to show differences in subjective effects between the luteal and follicular phases of the menstrual cycle. These findings suggest that the route of cocaine administration influences whether or not there are changes in the response to cocaine across the menstrual cycle.
Sex Differences in Subjective Response to Cocaine
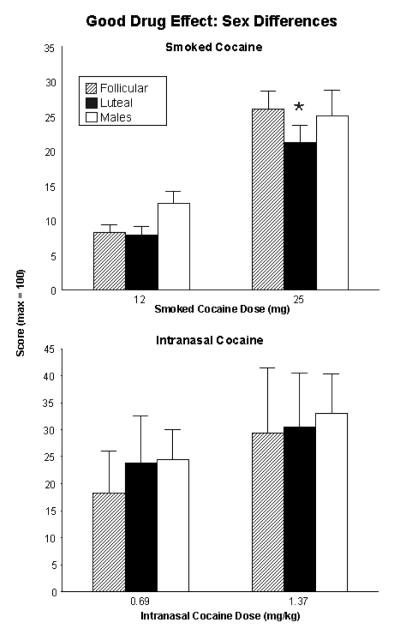
In our 2006 study (Evans and Foltin, 2006a) we also assessed sex differences in response to smoked cocaine. As expected, cocaine produced dose-related increases in positive subjective effects in both men and women. The most important finding shown in the top panel of Figure 2 (adapted from Evans and Foltin, 2006a) is that the response to cocaine was virtually identical in men and women tested in the follicular phase, indicating that women do not have an enhanced response to cocaine during the follicular phase. In contrast, the response to cocaine was reduced when women were tested in the luteal phase compared to men or their own follicular phase. These data are similar to what other studies have published with smoked cocaine (Sofuoglu et al., 1999) as well as oral d-amphetamine (White et al., 2002).
Figure 2.
Mean “Good Drug Effect” cluster scores as a function of menstrual cycle phase and sex following repeated doses of cocaine. The top panel shows results for smoked cocaine (6, 12 and 25 mg) in 11 women and 10 men; bars represent the mean + 1 SEM. These data were combined across two previously published studies (Evans et al., 2002 and Evans & Foltin, 2006a). The bottom panel shows results for intranasal cocaine in 8 women and 10 men. These data were adapted from Collins et al. (2007). Bars represent the mean + 1 SEM. * indicates a significant difference from the follicular phase.
Based on our intriguing findings showing both sex and menstrual cycle differences with smoked cocaine, we compared the effects of intranasal cocaine in women during the follicular and luteal phase of the menstrual cycle (Collins et al., 2007) to a previous study conducted in men with intranasal cocaine (Foltin and Haney, 2004). In contrast to smoked cocaine, as shown in the lower panel of Figure 2, there were no differences in the subjective response to intranasal cocaine between men and women or as a function of menstrual cycle phase.
Role of Exogenous Hormone Administration and Response to Cocaine
Given that we were able to show modest changes in subjective responses to smoked cocaine across the menstrual cycle (Evans et al., 2002), we were interested in exploring the hormonal mechanism underlying this response. Although there was an extensive preclinical literature showing that estradiol enhanced the effects of cocaine (see reviews by Becker et al., 2001; Carroll et al., 2004; Festa and Quiñones-Jenab, 2004; Lynch et al., 2002; Mello and Mendelson, 2002; Roth et al., 2004), these findings have not been confirmed in other species. A single study in female monkeys showed that estradiol failed to alter cocaine discrimination or cocaine self-administration behavior (Mello et al., 2008). Among the few studies conducted in women, estradiol produced minimal changes in the response to another stimulant, d-amphetamine (Justice and de Wit, 2000a, 2000b; Lile et al., 2007). Further, the data from our 2002 study (Evans et al., 2002) showed that the effects of cocaine were reduced during the luteal phase compared to the follicular phase even though estradiol levels were actually greater in the luteal phase; of note, the luteal phase was also when progesterone levels were elevated. Similar effects have been observed in another study with smoked cocaine (Sofuoglu et al., 1999) and with d-amphetamine (White et al., 2002). This led us to hypothesize that progesterone may be involved in attenuating the response to cocaine, as opposed to estradiol enhancing the effects of cocaine. At the time, the effects of progesterone on the behavioral response to cocaine in rodents was inconsistent (Quiñones-Jenab et al., 2000; Russo et al., 2003; Sell et al., 2000), however there was some interesting preliminary data in humans showing that progesterone attenuated the subjective effects of smoked and intravenous cocaine in humans (Sofuoglu et al., 2002, 2004).
Therefore, we conducted a study (Evans and Foltin, 2006a) to determine if oral micronized progesterone, in doses designed to mimic midluteal progesterone levels, administered during the follicular phase would attenuate the response to cocaine compared to the normal follicular phase in 11 women. To address the role of sex differences, 10 men were included and administered progesterone during one inpatient stay and placebo during another inpatient stay. During each inpatient admission, a full dose-response function for smoked cocaine (0, 6, 12 and 25 mg cocaine base) was determined and each session participants were administered 6 doses of cocaine. Figure 3, shows that administration of progesterone during the follicular phase attenuated “Good Drug Effect” cluster scores after repeated doses of 25 mg smoked cocaine in women compared to the normal follicular phase, but progesterone did not alter the subjective response to smoked cocaine in men, despite similar plasma levels of progesterone (see Evans, 2007; data adapted from Evans and Foltin, 2006a). These results extend and confirm previous findings that oral progesterone produced small, transient decreases in the subjective response to both smoked (Sofuoglu et al., 2002) and intravenous cocaine (Sofuoglu et al., 2004). Although Sofuoglu et al. (2004) did not observe any sex differences with progesterone pretreatment, this may have been due to the small sample size (6 men and 4 women) relative to our study (Evans and Foltin, 2006a). In the only pilot treatment trial of oral progesterone conducted to date, progesterone did not reduce cocaine use in male cocaine abusers maintained on methadone (Sofuoglu et al., 2007). Taken together, these data suggest that men may not be as sensitive to the effects of progesterone.
Figure 3.
Mean “Good Drug Effect” cluster scores as a function of oral micronized progesterone or placebo pretreatment following repeated doses of smoked cocaine (12 and 25 mg) in 11 women and 10 men; bars represent the mean + 1 SEM. The data presented for women were collected during a normal follicular phase when placebo progesterone was administered and during another follicular phase when progesterone was administered. Similarly, the data presented for men were collected during a phase when placebo progesterone was administered and during a phase when progesterone was administered. These data were adapted from Evans & Foltin (2006) and also published in Evans (2007). * indicates a significant difference from the follicular phase.
Given the growing evidence that progesterone attenuates the subjective effects of cocaine, particularly in women, we are currently conducting a similar study to our 2006 study (Evans and Foltin, 2006a) to determine whether oral micronized progesterone also alters the subjective response to oral d-amphetamine in non-drug using women. Women were tested during two follicular phases and in each phase they were administered oral d-amphetamine (0, 10, 20 mg). In one follicular phase they were pretreated with 200 mg oral micronized progesterone and in another phase they were pretreated with placebo progesterone. To date a total of 14 women have completed the study and there is minimal evidence that progesterone alters the subjective effects of oral d-amphetamine (Reed et al., CPDD presentation 2009).
Summary
The human studies to date with smoked cocaine tend to support the growing evidence from the rodent literature that the response to cocaine varies between men and women, and that these differences appear to be related to hormonal fluctuations across the menstrual cycle. In humans and monkeys, estradiol does not appear to enhance the effects of stimulants (Justice and de Wit, 2000a, 2000b; Lile et al., 2007; Mello et al., 2008). In contrast, progesterone appears to attenuate the subjective effects of cocaine and based on the few published studies in humans, this effect may be more pronounced in women. Recently, studies in rodents have also focused their attention on the role of progesterone in modulating the behavioral effects of cocaine. For instance, Feltenstein and See (2007) reported that cocaine-seeking in normally cycling female rats was reduced during proestrus when progesterone levels were the highest and cocaine-seeking was elevated during estrus, when progesterone levels were low. In a subsequent study by the same group (Feltenstein et al., 2009), progesterone administration decreased cocaine-primed seeking in female rats only during estrus. There is also converging evidence from other recent studies that progesterone decreases the behavioral effects of cocaine in female rats, including the acquisition of cocaine self-administration (Jackson et al., 2006), the escalation of cocaine self-administration (Larson et al., 2007), and the reinstatement of cocaine-seeking behavior (Anker et al., 2007). However, none of these studies assessed the effects of progesterone in male rats. Thus, across a range of species, progesterone attenuates the behavioral effects of cocaine in females, but comparable studies with males are needed to determine if this is a sex specific effect.
Cocaine Self-Administration
In some of the studies mentioned above, including the pharmacokinetic studies in monkeys, we specifically did not use a self-administration or choice procedure in order to ensure that all of the doses were administered. Studies from our laboratory have shown repeatedly that a decrease in the positive subjective effects of cocaine does not necessarily result in a decrease in actual drug taking using cocaine self-administration procedures (Haney and Spealman, 2008). Thus, even if the subjective effects of cocaine vary across the menstrual cycle or between males and females, we do not know if there would be a corresponding difference in drug taking behavior. Therefore, it is important to also assess changes in cocaine self-administration between males and females and at different phases of the menstrual cycle.
Human Studies
Our laboratory has only conducted a limited number of cocaine self-administration studies in humans that address the role of sex or menstrual cycle differences. In our early study (Evans et al., 1999), repeated self-administration of 50 mg smoked cocaine was compared between 11 men and 9 women. Participants had the opportunity to self-administer up to 6 doses of 50 mg cocaine base twice a day over 2 consecutive days for a maximum of 24 cocaine doses. Under these conditions, men and women self-administered a similar number of cocaine doses (21.7 and 21.6, respectively). In another study (Haney et al., 1998), 7 men and 5 women self-administered intravenous cocaine using a modified 7-trial progressive ratio choice procedure between cocaine (0, 8, 16, 32 mg/70 kg) and $5 tokens. Participants had two sample trials (one of the available cocaine dose and one with tokens). Participants then had 5 choice trials and following each trial the response requirement increased by 400. Figure 4 shows that the progressive ratio breakpoint increased as a function of cocaine dose in both men and women, but that the breakpoint for the highest dose of cocaine was greater in women (exploratory analysis not previously published). While these data confirm previous cocaine self-administration studies using progressive ratio schedules in rodents (e.g., Carroll et al., 2002; Hecht et al., 1999; Roberts et al., 1989), this study did not control for menstrual cycle phase and was limited by the small sample size. In contrast, no other studies in humans have shown sex differences in cocaine self-administration with either smoked (Sofuoglu et al., 1999) or intravenous cocaine (Lynch et al., 2008; Sofuoglu et al., 2004).
Figure 4.

Mean progressive-ratio breakpoint as a function of intravenous cocaine dose and sex in 5 women and 7 men; bars represent the mean + 1 SEM. These represent unpublished data from Haney et al. (1998). * indicates a significant difference between men and women at a given cocaine dose.
To date, we have assessed changes in cocaine self-administration across the menstrual cycle in one study (unpublished data). In that study, 10 women were tested during the follicular and luteal phases of the menstrual cycle. Each phase, participants had the opportunity to self-administer up to 5 doses of smoked cocaine (0, 12, 25 and 50 mg) on separate sessions. Each session they were given $25 from their study earnings; following the free sample dose, participants were given 5 opportunities to purchase another dose of cocaine, at a cost of $5 per choice. Figure 5 shows that there were no differences in smoked cocaine self-administration between the follicular and luteal phases of the menstrual cycle. This study also evaluated the effects of oral micronized progesterone pretreatment during the follicular phase and despite decreasing some positive subjective effects, progesterone did not alter cocaine self-administration (data not shown). Progesterone administration also failed to decrease intravenous cocaine self-administration (Sofuoglu et al., 2004) and maintenance on progesterone did not reduce cocaine use among male methadone patients seeking treatment for their cocaine use (Sofuoglu et al., 2007).
Figure 5.
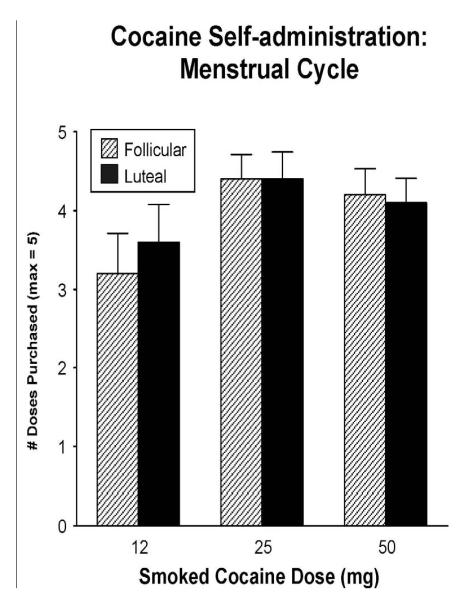
Mean number of smoked cocaine doses purchased as a function of cocaine dose and menstrual cycle phase in 10 women (unpublished data). Bars represent the mean + 1 SEM.
Monkey Studies
As mentioned before, non-human primates provide a unique opportunity to carefully evaluate changes across the menstrual cycle. To this end, we conducted a study to assess intravenous cocaine self-administration using a progressive ratio schedule in female rhesus monkeys. For cocaine self-administration, monkeys were fitted with a chronic indwelling catheter (Access Technologies, Skokie, IL) that terminated in a subcutaneous vascular access port (Wojnicki et al., 1994). For self-administration sessions, monkeys were guided from their home cage and seated into custom-built primate chairs, moved to the workstation, and connected to the infusion apparatus for the 2.5-hour experimental sessions. The reinforcing efficacy of intravenous cocaine was determined in 5 female rhesus monkeys across multiple menstrual cycles using a progressive ratio procedure (Hodos, 1961). The progressive ratio procedure entails having the animals make progressively more responses on a lever in order to get each subsequent dose of drug. A step size multiplier of 1.40 was used, such that the response requirement increased by 140 percent after each cocaine delivery. Therefore, if a monkey had an initial response requirement of 180 lever pulls, the progression of responses for subsequent trials was 252, 352, …up to 2656 for the last trial. Monkeys were allowed to self-administer up to 10 doses and each session lasted 2.5 hours. To ensure that cocaine did not disrupt the menstrual cycle (Mello et al., 1997; Potter et al., 1998), sessions were conducted 4 days per week and relatively low doses of cocaine were used (0.0125, 0.025 and 0.05 mg/kg/injection). The progressive ratio breakpoint (the highest ratio completed) and the number of doses self-administered were determined for each session. In this study, monkeys had different initial response requirements (ranging from 100 to 180), therefore data are presented as the number of doses self-administered rather than the progressive ratio breakpoint. Monkeys were tested on a given dose for 3-5 menstrual cycles. To verify ovulatory menstrual cycles and determine menstrual cycle phase, at least one day each week, a blood sample was drawn to determine levels of estradiol and progesterone.
Figure 6 shows the mean number of cocaine doses self-administered by the monkeys as a function of cocaine dose and day of the menstrual cycle. Overall, there was a dose-related increase in cocaine self-administration, but particularly at the two highest doses (0.025 and 0.05 mg/kg/injection) there were no differences as a function of day across the menstrual cycle. While there was more variability with the lowest dose of cocaine, this was not related to menstrual cycle day, but more likely a result of the variability associated with low cocaine doses and the fact that only three monkeys were tested with this dose. Figure 7 summarizes the data by collapsing days of the menstrual cycle by phase, showing more clearly the absence of any changes in cocaine self-administration as a function of menstrual cycle phase. Mello et al. (2007) conducted a similar study in cynomolgus monkeys (3 females and 2 males per dose), also using a progressive ratio schedule. Although that study used a different species of non-human primate, a lower progressive ratio schedule, and lower unit doses of cocaine, Mello et al. (2007) also found no consistent changes in the progressive-ratio breakpoints as a function of menstrual cycle phase. However, at the lowest cocaine dose available (0.0032 mg/kg/inj), the progressive ratio breakpoint was higher during the follicular phases compared to the luteal phases. Further, Mello et al. (2007) showed clear sex differences, with female monkeys having higher progressive ratio breakpoints than male monkeys at all cocaine doses tested. Unfortunately, we did not test male rhesus monkeys, but sex differences observed in the study by Mello et al. (2007) are consistent with previous studies conducted in rodents showing that female rats have higher progressive ratio breakpoints for cocaine than male rats (e.g., Carroll et al., 2002; Hecht et al., 1999; Roberts et al., 1989).
Figure 6.
Mean number of intravenous cocaine doses self-administered as a function of cocaine dose and day of the menstrual cycle in 3 (0.0125 mg/kg/inj dose) or 5 female rhesus monkeys (unpublished data). Monkeys were tested for three to five menstrual cycles at each cocaine dose. Symbols represent the mean ± 1 SEM.
Figure 7.
Mean number of intravenous cocaine doses self-administered as a function of cocaine dose and menstrual cycle phase in 5 female rhesus monkeys. Early follicular: days 1-5; Mid-follicular: days 6-10; Mid-luteal: days 19-24; Late luteal: days 25-29. Monkeys were tested for three to five menstrual cycles at each cocaine dose. Bars represent the mean + 1 SEM.
Summary
At this time relatively few studies have investigated sex differences and/or menstrual cycle effects on cocaine self-administration in humans and non-human primates, therefore any interpretations are speculative at this time. To date, only one study in humans (Haney et al., 1998, unpublished analysis) and one study in cynomolgus monkeys have demonstrated sex differences in cocaine self-administration and there do not appear to be any changes in cocaine self-administration as a function of menstrual cycle phase in either humans or non-human primates. Thus, in contrast to the robust findings in rodents, the data in humans and non-human primates indicate that there may be only limited differences in cocaine self-administration as a function of menstrual cycle phase, particularly at the higher doses, suggesting that these differences may not be clinically relevant at the doses routinely abused by humans. However, to directly address this important question we are currently conducting a pilot treatment trial to determine whether progesterone treatment will delay cocaine relapse and cocaine use in cocaine-dependent women.
Implications
Taken together, there is converging evidence that across a range of species the behavioral effects of cocaine 1) differ between males and females, 2) differ across the menstrual cycle (or estrus cycle in rodents), 3) can be attenuated by progesterone (at least in females), and 4) do not appear to be related to differences in cocaine pharmacokinetics. Despite the progress made thus far, much more data is still needed to fill in the existing gaps with respect to the possible mechanisms underlying these sex and hormonal influences and the translational validity across the results from rodents, non-human primates and humans. For instance, studies in both humans and non-human primates need to more adequately address sex differences and the role that estradiol and progesterone play in modulating cocaine self-administration since many of the studies have not always included males. Correspondingly, studies in male rodents are needed to determine the effects of progesterone administration on cocaine. In addition, route and dose of cocaine need to be considered. With humans, the most consistent sex and menstrual cycle differences have been shown with smoked cocaine, but not with intranasal or intravenous cocaine. In addition, in some instances, the subjective response to low doses of cocaine or low-dose cocaine self-administration can be modulated, but hormonal influences are less evident with higher doses of cocaine in both humans and non-human primates (also see rodent study by Cain et al., 2004). So while the data with rodents are quite compelling, these findings may not correspond to the clinical situation. In fact, in humans the noted sex and menstrual cycle differences in response to cocaine are less pronounced than in other species. Regardless, the growing evidence that progesterone decreases the response to cocaine is encouraging despite the one unsuccessful in treatment trial in men (Sofuoglu et al., 2007).
Clearly, one important lesson from this research is that laboratory studies that involve the administration of stimulants should control for menstrual cycle and include males to address any potential sex differences. Lastly, more attention needs to be paid to potential sex differences in patients seeking treatment for stimulants in clinical settings. In light of the existing data, clinical trials for stimulant abuse should strive to include women, and track the menstrual cycle or hormonal status of women to determine if these hormonal fluctuations alter drug use and/or treatment outcome. The smoking cessation field has already shown that nicotine withdrawal and different nicotine treatments vary across the menstrual cycle in women (e.g., Allen et al., 2000). Further, sex differences in the success of smoking cessation rates and type of treatment (e.g., Borrelli et al., 2004; King et al., 2006) have been observed, suggesting that women need different treatments than men. Similarly, the results of this review indicate that treatment interventions for stimulant abuse or dependence need to attend to sex differences and target treatment strategies specifically for women.
Acknowledgments
This manuscript was supported by Grants No. DA-08105 (Principal Investigator: Richard W. Foltin), DA-009114 (Principal Investigator: Suzette M. Evans) and DA12675 (Principal Investigator: Suzette M. Evans) from the National Institute on Drug Abuse and grant No. MOI-RR-00645 from the National Institutes of Health. For some of the studies conducted by Evans and colleagues, oral micronized progesterone and matching placebo capsules were generously provided by the Women’s International Pharmacy (Madison, WI). The authors have no financial disclosures or conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen SS, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine Tobacco Res. 2000;2:231–241. doi: 10.1080/14622200050147493. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Glidden LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp. Clin. Psychopharmacol. 2007;15:472–480. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Annals New York Academy Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Papandonatos G, Spring B, Hitsman B, Niaura R. Experimenter-defined quit dates for smoking cessation: adherence improves outcomes for women but not for men. Addiction. 2004;99:378–385. doi: 10.1111/j.1360-0443.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Vaughn SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM. Effects of sex and gonadectomy on cocaine metabolism in the rat. J. Pharmacol. Exp. Ther. 1999;290:1316–1323. [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentrations of LH, FSH, prolactin, progesterone and estradiol-17ß throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Cain SB, Bowen CA, Yu G, Zuzga Dd., Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004;29:929–942. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol. Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Campbell UC, Lynch WJ, Dess NK. Influence of estrogen in the acquisition of intravenously self-administration in rats selectively bred for differenential saccarhin intake: phenotype and sex differences. Psychopharmacology. 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Vandenbergh JG. Effect of chronic cocaine on reproduction in female house mice. Pharmacol. Biochem. Behav. 1994;48:909–913. doi: 10.1016/0091-3057(94)90199-6. [DOI] [PubMed] [Google Scholar]
- Collins SL, Evans SM, Foltin RW, Haney M. Intranasal cocaine in humans: effects of sex and menstrual cycle. Pharmacol. Biochem. Behav. 2007;86:117–124. doi: 10.1016/j.pbb.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM. The role of estradiol and progesterone in modulating the subjective effects of stimulants in humans. Exp. Clin. Psychopharmacology. 2007;15:418–426. doi: 10.1037/1064-1297.15.5.418. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Pharmacokinetics of intravenous cocaine across the menstrual cycle in rhesus monkeys. Neuropsychopharmacology. 2004;29:1889–1900. doi: 10.1038/sj.npp.1300486. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006a;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Pharmacokinetics of repeated doses of intravenous cocaine across the menstrual cycle in rhesus monkeys. Pharmacol. Biochem. Behav. 2006b;83:56–66. doi: 10.1016/j.pbb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Fischman MW, Foltin RW. Limited sex differences in response to binge smoked cocaine use in humans. Neuropsychopharmacology. 1999;21:445–454. doi: 10.1016/S0893-133X(98)00120-1. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 2009;34:343–352. doi: 10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa ED, Quiñones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm. Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quiñones-Jenab V. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology. 2004;46:672–687. doi: 10.1016/j.neuropharm.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Intranasal cocaine in humans: acute tolerance, cardiovascular, and subjective effects. Pharmacol. Biochem. Behav. 2004;78:93–101. doi: 10.1016/j.pbb.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Haney M, Foltin RW, Fischman MW. Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacology. 1998;137:15–24. doi: 10.1007/s002130050588. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev. Psychobiol. 1999;35:136–145. [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 2008;92:208–216. doi: 10.1016/j.drugalcdep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Justice AJH, de Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol. Biochem. Behav. 2000a;66:509–515. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Justice AJH, de Wit H. Acute effects of estradiol pretreatment on the response to d-amphetamine in women. Neuroendocrinology. 2000b;71:51–59. doi: 10.1159/000054520. [DOI] [PubMed] [Google Scholar]
- King TS, Canez MS, Gaskill S, Javors MA, Schenken RS. Chronic cocaine disruption of estrous cyclicity in the rat: Dose-dependent effects. J. Pharmacol. Exp. Ther. 1993;264:29–34. [PubMed] [Google Scholar]
- King A, de Wit H, Riley RC, Cao D, Niaura R, Hatsukami D. Efficacy of naltrexone in smoking cessation: a preliminary study and an examination of sex differences. Nicotine Tobacco Res. 2006;8:671–682. doi: 10.1080/14622200600789767. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, Oliveto AH, Price LH. Gender differences in response to intranasal cocaine administration to humans. Biological Psychiatry. 1996;39:147–148. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp. Clin. Psychopharmacol. 2007;15:461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kendall SL, Babalonis S, Martin CA, Kelly TH. Evaluation of estradiol administration on the discriminative-stimulus and subject-rated effects of d-amphetamine in healthy pre-menopausal women. Pharmacol. Biochem. Behav. 2007;87:258–266. doi: 10.1016/j.pbb.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Hundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology. 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kalayasiri R, Sughondhabirom A, Pittman B, Coric V, Morgan PT, Malison RT. Subjective responses and cardiovascular effects of self-administered cocaine in cocaine-abusing men and women. Addict. Biol. 2008;13:403–410. doi: 10.1111/j.1369-1600.2008.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth MN, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers- implications for treatment and prognosis. Am. J. Addict. 1999;8:300–311. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- Mello NK. Wetherington CL, Roman AB, editors. Cocaine abuse and reproductive function in women. U.S. Government Printing Office; Washington, DC: Drug Addiction Research and the Health of Women. 1998:131–149.
- Mello NK, Bowen CA, Mendelson JH. Comparison of plasma cocaine levels during a “binge” pattern of cocaine administration in male and female rhesus monkeys. Psychopharmacology. 2002;164:19–26. doi: 10.1007/s00213-002-1188-x. [DOI] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Mendelson JH. Sex and menstrual cycle effects on progressive ratio measures of cocaine self-administration in cynomolgus monkeys. Neuropsychopharmacology. 2007;32:1956–1966. doi: 10.1038/sj.npp.1301314. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Cocaine, hormones, and behavior: Clinical and preclinical studies. Horm. Brain Behav. 2002;5:665–745. [Google Scholar]
- Mello NK, Mendelson JH, Kelly M, Bowen C. The effects of cocaine on basal and human chorionic gonadotropin-stimulated ovarian steroid hormones in female rhesus monkeys. J. Pharmacol. Exp. Ther. 2000;294:1137–1145. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kelly M, Diaz-Migoyo N, Sholar JW. The effects of chronic cocaine self-administration on the menstrual cycle in rhesus monkeys. J. Pharmacol. Exp. Ther. 1997;281:70–83. [PubMed] [Google Scholar]
- Mello NK, Negus SS, Knudson IM, Kelly M, Mendelson JH. Effects of estradiol on cocaine self-administration and cocaine discrimination by female rhesus monkeys. Neuropsychopharmacology. 2008;33:783–795. doi: 10.1038/sj.npp.1301451. [DOI] [PubMed] [Google Scholar]
- Mello NK, Sarnyai Z, Mendelson JH, Drieze JM, Kelly M. Acute effects of cocaine on anterior pituitary hormones in male and female rhesus monkeys. J. Pharmacol. Exp. Ther. 1993;266:804–811. [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999a;21:294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Negus SS. Effects of luteinizing hormone-releasing hormone on plasma cocaine levels in rhesus monkeys. J. Pharmacol. Exp. Ther. 1999b;289:791–799. [PubMed] [Google Scholar]
- Najavits LM, Lester KM. Gender differences in cocaine dependence. Drug Alcohol Depend. 2008;97:190–194. doi: 10.1016/j.drugalcdep.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyomchai T, Akhavan A, Festa ED, Lin S-N, Lamm L, Foltz R, Quiñones-Jenab V. Estrogen and progesterone affect cocaine pharmacokinetics in female rats. Brain Res. Bull. 2006;68:310–314. doi: 10.1016/j.brainresbull.2005.09.001. [DOI] [PubMed] [Google Scholar]
- O’Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000-2001. Neuropsychopharmacology. 2005;30:1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- Potter DA, Luther MF, Eddy CA, Siler-Khodr TM, King TS, Schenken RS. Low-dose follicular-phase cocaine administration disrupts menstrual cycle and ovarian cyclicity in rhesus monkeys. J. Soc. Gynecol. Invest. 1999;6:88–94. doi: 10.1016/s1071-5576(98)00054-9. [DOI] [PubMed] [Google Scholar]
- Quiñones-Jenab V, Perrotti LI, Mc Monagle J, Ho A, Kreek MJ. Ovarian hormone replacement affects cocaine-induced behaviors in ovariectomized female rats. Pharmacol. Biochem. Behav. 2000;67:417–422. doi: 10.1016/s0091-3057(00)00381-6. [DOI] [PubMed] [Google Scholar]
- Reed SC, Levin FR, Evans SM. The effects of progesterone pretreatment on the response to oral d-amphetamine: Impulsivity, mood and performance. Poster presented at the 2009 Annual College on Problems of Drug Dependence; Reno, NV. 2009. [Google Scholar]
- Roberts DCS, Bennett SAL, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci. Biobehav. Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraisch M, Jenab S, Quiñones-Jenab V. Gonadal hormones differentially modulate cocaine-induced place preference in male and female rats. Neuroscience. 2003;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Saady JJ, Bowman ER, Aceto MD. Cocaine, ecgonine methyl ester, and benzoylecgonine plasma profiles in rhesus monkeys. J. Anal. Toxicol. 1995;19:571–575. doi: 10.1093/jat/19.7.571. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results for the 2007 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; Rockville, MD: 2008. (NSDUH Series H-34). DHHS Publication No. SMA 08-4343. [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral responses to cocaine in female rats. J. Pharmacol. Exp. Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- Shimizu K. Reproductive hormones and ovarian cycle in macaques. J. Mamm. Ova. Res. 2008;25:122–126. [Google Scholar]
- Sinha R, Rounsaville BJ. Sex differences in depressed substance abusers. J. Clin. Psychiatry. 2002;63:616–627. doi: 10.4088/jcp.v63n0715. [DOI] [PubMed] [Google Scholar]
- Siqueland L, Crits-Christoph P, Gallop R, Barber JP, Griffin ML, Thase ME, Daley DR, Blaine J, Connolly MB, Gladis M. Retention in psychosocial treatment of cocaine dependence: predictors and impact of outcome. Am. J. Addict. 2002;11:24–40. doi: 10.1080/10550490252801611. [DOI] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gondadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Neuroendocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol. Biochem. Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp. Clin. Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol. Biochem. Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Gonzalez G, Gonsai K, Oliveto A, Kosten TR. Progesterone effects on cocaine use in cocaine users maintained on methadone: A randomized, double-blind clinical trial. Exp. Clin. Psychopharmacol. 2007;15:453–460. doi: 10.1037/1064-1297.15.5.453. [DOI] [PubMed] [Google Scholar]
- Van Haaren F, Garcea M, Anderson K, Tebbett I. Cocaine and benzoylecgonine in serum microsamples of intact and gonadectomized male and female wistar rats. Pharmacol. Biochem. Behav. 1997;58:421–424. doi: 10.1016/s0091-3057(97)00294-3. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol. Biochem. Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Wojnicki FH, Bacher JD, Glowa JR. Use of subcutaneous vascular access ports in rhesus monkeys. Lab. Anim. Sci. 1994;44:491–494. [PubMed] [Google Scholar]
- Yosida T. Similarities and differences in reproductive endocrinology between non-human primates and humans. Cong. Anom. 1999;39:209–222. [Google Scholar]