Abstract
DJ-1 is a multifunctional protein linked to recessively inherited Parkinson’s disease (PD) due to loss of function mutations. Among its activities is antioxidant property leading to cytoprotection under oxidative stress conditions. A key effector of oxidant-induced cell death is the MAP3 kinase Apoptosis Signal Regulating Kinase 1 (ASK1) which is bound to and inhibited by thioredoxin 1 (Trx1) under basal conditions. Upon oxidative stimuli, however, ASK1 dissociates from this physiological inhibitor and is activated. In the present study, we investigated the role of DJ-1 in regulating Trx1/ASK1 interaction. Over-expression of DJ-1 suppressed ASK1 activation in response to H2O2 in a time-dependent manner. Wild-type DJ-1, but not the PD-associated L166P mutant, prevented the dissociation of ASK1 from Trx1 in response to H2O2. Among cysteine mutants of DJ-1, C46S, C53S, and C106S, only C106S failed to inhibit this dissociation implying that cysteine 106 is essential for Trx1/ASK1 regulation. Furthermore, compared to wild-type mice, DJ-1 null mouse brain homogenates and embryonic fibroblasts were more susceptible to oxidant-induced dissociation of ASK1 from Trx1, activation of the downstream kinase c-Jun N-terminal kinase, and to cell death. These findings point to yet another mechanism through which DJ-1 has anti-oxidant and cytoprotective properties by regulating the Trx1/ASK1 complex and controlling the availability of ASK1 to effect apoptosis.
Keywords: DJ-1, ASK1, Thioredoxin 1, oxidative stress, Parkinson’s disease (PD)
1. Introduction
Homozygous deletions and point mutations in the DJ-1 (PARK7) gene are associated with autosomal recessive early-onset Parkinson’s disease (PD) (Bonifati et al., 2003). DJ-1 belongs to the Thi/Pfp1 superfamily and is highly conserved from human to Escherichia coli (E. coli) (Bonifati et al., 2003; Lee et al., 2003). It is ubiquitously expressed in mammalian tissues, forms a homodimer and functions through various mechanisms including as an antioxidant, transcriptional co-activator, a protease or molecular chaperone (Olzmann et al., 2004; Shendelman et al., 2004; Taira et al., 2004; Xu et al., 2005).
Accumulating evidence suggests that DJ-1 responds to oxidative insults by shifting to a more acidic species (Mitsumoto and Nakagawa, 2001; Mitsumoto et al., 2001). This acidic shift is even detected in the brains of patients with sporadic PD (Bandopadhyay et al., 2004). DJ-1 has properties of an atypical peroxiredoxin-like peroxidase (Andres-Mateos et al., 2007). It also protects cells through up-regulation of glutathione (GSH) synthesis as well as stabilization of the antioxidant transcriptional regulator NF-E2-Related Factor 2 (Nrf2) (Clements et al., 2006; Zhou and Freed, 2005). DJ-1 deficiency leads to increased vulnerability to oxidative stress-induced cell death in culture and in animal models (Kim et al., 2005; Martinat et al., 2004; Meulener et al., 2005), whereas over-expression of wild-type (WT) DJ-1 is protective against various insults (Canet-Aviles et al., 2004; Inden et al., 2006; Junn et al., 2005; Taira et al., 2004). The PD causing L166P mutation impairs dimer formation, decreases protein stability, and disrupts its cytoprotective function (Macedo et al., 2003; Miller et al., 2003; Moore et al., 2003; Olzmann et al., 2004).
Apoptosis signal-regulating kinase 1 (ASK1) is a mitogen-activated protein (MAP) kinase kinase kinase (MAPKKK) family member that activates c-Jun N-terminal kinase (JNK) and p38 MAP kinase pathway. It is activated by various stresses including oxidative stress and Tumor Necrosis Factor alpha (TNFα) (Ichijo et al., 1997; Nishitoh et al., 1998). Over-expression of wild-type or a constitutively active ASK1 isoform induces apoptosis in various cell types (Chang et al., 1998; Ichijo et al., 1997). ASK1 plays a critical role in 6-hydroxydopamine (6-OHDA)-and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced apoptosis in cellular and animal models of PD (Karunakaran et al., 2007; Ouyang and Shen, 2006; Saeed et al., 2009). One of the molecular events that activate ASK1 is its interaction with the death protein Daxx (Chang et al., 1998). Upon stress situations, Daxx, which is a nuclear protein under basal conditions, translocates to the cytoplasm, interacts and activates ASK1 leading to cell death. Notably, DJ-1 potently blocks this death signaling pathway by interacting with Daxx in the nucleus and preventing it from gaining access to ASK1 in the cytoplasm (Junn et al., 2005). DJ-1 may also interact with ASK1 (Waak et al., 2009).
Since ASK1 is a potent apoptotic molecule, it is subject to tight regulatory control. Under basal conditions, it is bound to thioredoxin 1 (Trx1), which is a key redox modulator as well as physiological inhibitor. Upon oxidative stress, however, Trx1 dissociates from ASK1 thereby activating this kinase (Saitoh et al., 1998). Considering the role of DJ-1 as an anti-oxidant and regulator of ASK1 activity, we sought to investigate additional mechanisms by which DJ-1 keeps ASK1 in check. In this study, we demonstrate that DJ-1 suppresses ASK1 activity by preventing the dissociation of Trx1 from ASK1.
2. Materials and Methods
2.1. Materials
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from Invitrogen. Tissue culture dishes were from Costar. β-Galactosidase Enzyme Assay System was from Promega. Other reagents and bicinchoninic acid (BCA) assay kit were purchased from Sigma.
2.2. Cell culture, plasmids and transfection
HEK 293T cells were maintained in DMEM supplemented with 10% FBS and transiently transfected using the calcium phosphate-mediated method. Myc-ASK1, WT and L166P mutant human DJ-1 (Junn et al., 2005) or C46S, C53S and C106S DJ-1 mutants (Junn et al., 2009) have been described previously. Glutathione-S-transferase (GST)-tagged human Trx1 has been described previously (Junn et al., 2000). β-Galactosidase activity was measured with the β-Galactosidase Enzyme Assay System to normalize for transfection efficiency.
2.3. Co-immunoprecipitation and Western blot analysis
Cells were washed in ice-cold phosphate-buffered saline (PBS) and lysed with 0.1% Nonidet P-40 containing protease and phosphatase inhibitors (Roche, Basel, Swiss). Lysates were centrifuged for 10 min at 13,000 rpm at 4°C. Supernatants were collected and normalized using β-Galactosidase assay and their protein contents were determined using a BCA assay kit. Protein samples were immunoprecipitated with agarose-conjugated anti-Myc antibody (Santa Cruz) or pulled down with glutathione sepharose 4B (GE Healthcare). Western blot analyses were performed using antibodies to peroxidase-conjugated anti-Myc (Santa Cruz) or anti-Flag (Sigma), anti-GST (GE Healthcare), phospho-ASK1 (polyclonal antibody generated with a synthetic phospho-peptide corresponding to residues surrounding Thr845), phospho-JNK (Cell signaling), total ASK1 (Santa Cruz), total JNK (Santa Cruz) and DJ-1 (691, a kind gift from Benoit I. Giasson). Specific signals were detected using an enhanced chemiluminescence (ECL) kit (PerkinElmer LAS, Inc). Quantification of immunoreactivity was performed using ImageJ (NIH, USA).
To examine the effects of DJ-1 on the endogenous interaction of ASK1 with Trx1, whole brain homogenates from WT and DJ-1 null mice were lysed in RIPA buffer (150 mM NaCl, 50 mM Tris–HCl (pH 7.5), 10 mM EDTA, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100 containing protease inhibitors and phosphatase inhibitors), treated with H2O2 for 30 min, and the lysates were immunoprecipitated with normal rabbit IgG or anti-Trx1 rabbit antibody (Santa Cruz). The samples were incubated with protein G-agarose (Roche) and analyzed by immunoblotting using anti-ASK1 (Santa Cruz) and anti-Trx1 antibodies. Aliquots of whole brain homogenates were subjected to Western blotting with anti-ASK1 or anti-DJ-1 antibodies.
2.4. Cell death assay in mouse embryonic fibroblasts
Mouse embryonic fibroblasts (MEFs) were isolated from wild-type C57BL/6 and DJ-1 null mice (a kind gift from Ted Dawson). Briefly, E13.5 embryos were minced and trypsinized with 0.1% trypsin-EDTA for 20 min. Cells were collected using a cell strainer without tissue debris and suspended in DMEM containing 10% FBS. MEFs were cultured for 3 passages at 37 °C and 5% CO2. WT and DJ-1 null MEFs were incubated with 0.1 mM H2O2 for 24 h. Cell death was assessed by measuring the release of LDH from damaged cells using Cytotoxicity Detection Kit (Roche) according to the manufacturer’s instructions. One hundred percent cell death was determined by lysing the cells with 1% Triton X-100.
2.5. Statistical analysis
All data were analyzed by GraphPad Prizm 4 software. Results are presented as means ±S.E.M. Statistical analyses were performed using Student’s t-test or ANOVA followed by Newman-Kuls test.
3. Results
3.1. DJ-1 represses oxidative stress-induced ASK1 activation
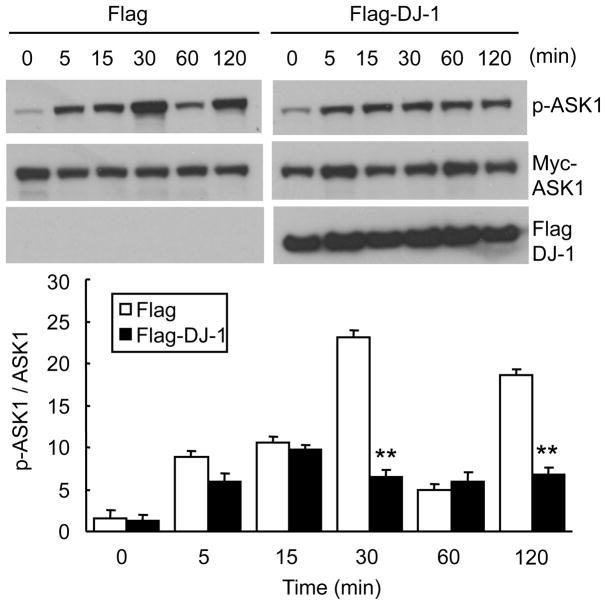
To determine if DJ-1 inhibits ASK1, we compared the extent of ASK1 activation induced by hydrogen peroxide in cells transfected with DJ-1 expression plasmid or empty control plasmid (Fig. 1). HEK293T cells were transfected with Myc-ASK1 and Flag-DJ-1 (or empty Flag vector), and incubated with 0.5 mM H2O2 for various time points. Without DJ-1 over-expression, the level of phosphorylated ASK1 (p-ASK1) increased in a time dependent manner beginning as early as 5 minutes, peaking at 30 min, declining thereafter and having a second smaller peak at 2 h, consistent with previous reports using other cell types (Kutuzov et al., 2005; Morita et al., 2001). On the other hand, in the presence of DJ-1 expression plasmid, the increase in p-ASK1 levels following H2O2 exposure was blunted without distinct peaks (Fig. 1), suggesting that DJ-1 suppresses ASK1 activation by oxidative stress.
Fig. 1. DJ-1 represses ASK1 activation.
HEK293T cells were transfected with Myc-ASK1 along with Flag-DJ-1 or empty Flag vector. Thirty six h later, cells were incubated with 0.5 mM H2O2 for the indicated times. Cell lysates were subjected to Western blot analysis with anti-p-ASK1, anti-Myc and anti-Flag antibodies. For each sample, the relative intensity of p-ASK1 was normalized to total Myc-ASK1 using NIH ImageJ. Data represent means±S.E.M. of three independent experiments. ** p<0.01 compared to empty Flag vector-transfected cells by Student t-test.
3.2. DJ-1 inhibits the dissociation of Trx1 from ASK1
Oxidative stimuli lead to the dissociation of the inhibitor Trx1 from ASK1 (Noguchi et al., 2005; Saitoh et al., 1998). Based on the role of DJ-1 as an antioxidant, we hypothesized that DJ-1 can regulate H2O2-induced dissociation of Trx1 from ASK1. We, therefore, investigated the association of Trx1/ASK1 complex and its regulation by oxidative stress in transfected cells. HEK293T cells were transfected with GST-Trx1 and Myc-ASK-1 in the presence or absence of Flag-DJ-1 and incubated with 0.5 mM H2O2 for 30 min. Subsequently, the interaction between ASK1 and Trx1 was determined by immunoprecipitation using Myc antibody followed by Western blotting with anti-GST. Without oxidative stress, bound to Trx1 equally in the absence or presence of DJ-1 transfection (compare lanes 2 in Fig. 2A). Upon H2O2 treatment, the interaction between ASK1 and Trx1 dramatically in the absence of DJ-1 (Fig. 2A lane 2 vs 3), but this dissociation was prevented in the presence of DJ-1 (Fig. 2A lane 3 vs lane 5). The ability of DJ-1 to inhibit dissociation of Trx1 from ASK1 was also tested by the reverse experiment using GST-down of Trx1 yielding similar results (Fig. 2B). The diminished interaction between and Trx1 due to H2O2 (Fig. 2B lane 1 vs 2) is not evident in the presence of DJ-1 (Fig. 2B lane 3 vs 4). These results indicate that DJ-1 prevents H2O2-induced dissociation of from Trx1.
Fig. 2. DJ-1 inhibits the dissociation of Trx1 from ASK.
HEK293T cells were transfected with the indicated expression plasmids and incubated with 0.5 mM H2O2 for 30 min. (A) Cell lysates were immunoprecipitated with anti-Myc and Western blot analysis was performed with anti-GST and anti-Myc. The relative intensity of GST-Trx1 was normalized to Myc-ASK1 (B) GST-pull down experiment. GST-Trx1 was pulled down with glutathione-4B-sepharose beads, and Western blots performed using anti-Myc and anti-GST. Data shown are representative from three independent experiments. The relative density of Myc-ASK1 was normalized to GST-Trx1. Data represent means±S.E.M. * p<0.01.
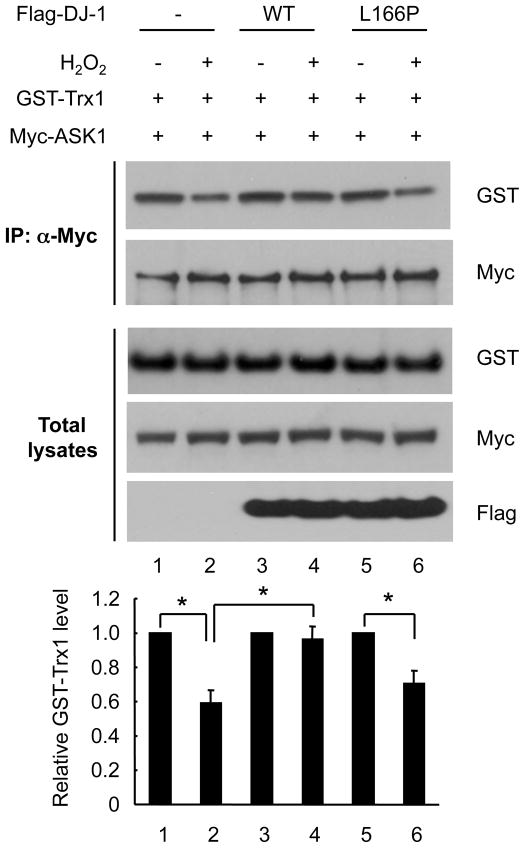
3.3. L166P mutant DJ-1 fails to regulate Trx1/ASK1 interaction
The disease associated L166P mutation in DJ-1 disrupts DJ-1 protein dimerization, resulting in lower steady state levels and loss of cytoprotective function (Moore et al., 2003; Olzmann et al., 2004). We examined whether this mutant can still regulate Trx1/ASK1 dissociation. HEK293T cells were transfected with Myc-ASK1 and GST-Trx1 in the presence or absence of WT Flag-DJ-1 or L166P mutant DJ-1. Because of instability of this mutant, we used three times as much mutant construct as compared to the WT construct to attain comparable expression levels. Western blot analysis of immunoprecipitates with anti-Myc showed that the decreased band intensity due to H2O2 treatment (Fig. 3 lanes 1 and 2) was prevented in the presence of WT DJ-1 (lanes 3 and 4) but not in the presence of L166P mutant DJ-1 (Fig. 3 lanes 5 and 6). These results suggest that the non-functional DJ-1 mutant cannot inhibit the dissociation of Trx1/ASK1 upon oxidative stress.
Fig. 3. L166P mutant DJ-1 fails to regulate Trx1/ASK1 interaction.
HEK293T cells were transfected for 36 h with GST-Trx1, Myc-ASK1, and 1 μg of WT Flag-DJ-1, or 3 μg of L166P mutant Flag-DJ-1. Cells were exposed to 0.5 mM H2O2 for 30 min, followed by immunoprecipitation with anti-Myc. Western blot analysis was performed with anti-GST and anti-Myc. The relative intensity of GST-Trx1 was normalized to Myc-ASK1. Data are shown as means±S.E.M. of three independent experiments. * p<0.01.
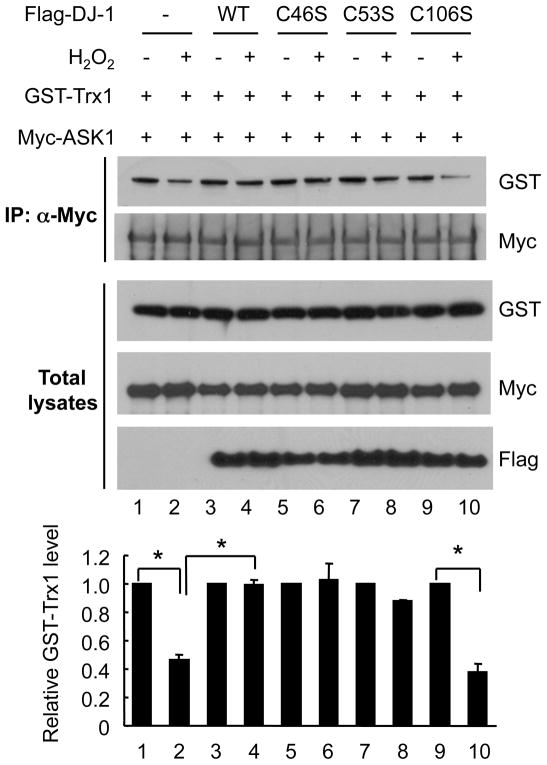
3.4. Cysteine 106 residue of DJ-1 is important in the regulation of Trx1/ASK1 complex
The human DJ-1 protein has three cysteine (Cys) residues. A number of previous studies have shown that C106 is the most reactive and functionally important residue in carrying out the anti-oxidant function of DJ-1 (Canet-Aviles et al., 2004; Meulener et al., 2006; Taira et al., 2004), with some reports suggesting that C53 and C46 also play a redox-dependent function (Shendelman et al., 2004). Therefore, we tested whether these Cys residues are involved in the regulation of the Trx1/ASK1 complex. We used three Cys mutants, C46S, C53S, and C106S and examined their impact on oxidant-induced dissociation of ASK1 from Trx1. Without H2O2, ASK1 bound to Trx1 to a similar extent in the presence of all DJ-1 constructs as in vector control transfected cells (Fig. 4). Like WT DJ-1, C46S and C53S mutants retained the interaction between ASK1 and Trx1 despite H2O2 challenge (lane 3 vs 4, lane 5 vs 6 and lane 7 vs 8). In contrast, C106S mutant could not prevent the dissociation of ASK1 from Trx1 upon oxidant stress (lane 9 vs 10). These data demonstrate that cysteine 106 of DJ-1 is important in preventing H2O2-induced dissociation of Trx1 from ASK1.
Fig. 4. DJ-1 cysteine 106 mutant fails to regulate Trx1/ASK1 interaction.
HEK293T cells were transfected for 36 h with GST-Trx1, Myc-ASK1, along with WT Flag-DJ-1, or cysteine mutants C46S, C53S, or C106S Flag-DJ-1. Cells were incubated with 0.5 mM H2O2 for 30 min, followed by immunoprecipitation with anti-Myc. Western blot analysis was performed with anti-GST and anti-Myc. As Flag Western of total lysates shows, C46S mutant has lower expression than the other constructs, as reported previously (Junn et al., 2009; Waak et al., 2009). The results shown are representative from three independent experiments. The relative intensity of GST-Trx1 was normalized to Myc-ASK1. Data are shown as means±S.E.M. * p<0.001.
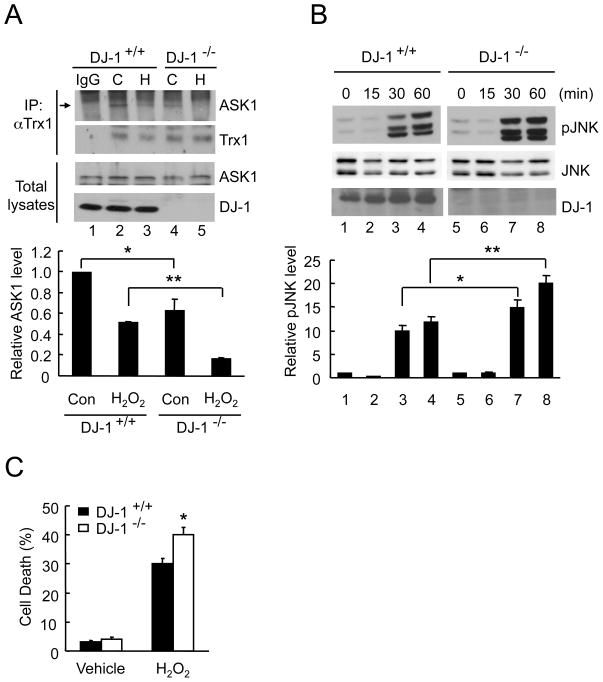
3.5. Loss of DJ-1 promotes oxidative stress-induced dissociation of ASK1/Trx1, JNK activation and cell death
To examine the effect of DJ-1 on the endogenous binding of ASK1/Trx1, whole brain homogenates from WT and DJ-1 null mice were incubated with 0.1 mM H2O2 for 30 min and immunoprecipitated with anti-Trx1 antibody. Western blotting was performed with anti-ASK1 antibody. Upon H2O2 challenge, ASK1/Trx1 dissociated more readily in DJ-1 null brains than in WT brains (Fig. 5A, lanes 3 and 5). In addition, even under basal conditions in the absence of H2O2, ASK1/Trx1 binding was less intense in DJ-1 null brain homogenates compared to WT brains (Fig. 5A, lanes 2 and 4) suggesting that this interaction is more labile in the absence of DJ-1..
Fig. 5. DJ-1 deletion promotes the dissociation of ASK1/Trx1 and cell death.
(A) Brain homogenates from WT and DJ-1 null mice were exposed to 0.1 mM H2O2 (H) or buffer (C) for 30 min, followed by immunoprecipitation with anti-Trx1 or normal rabbit IgG. Western blot analysis was performed with anti-ASK1, anti-Trx1, and anti-DJ-1 antibodies. The relative intensity of ASK1 was normalized to Trx1. Data represent means±S.E.M. of three independent experiments. * p<0.05, ** p<0.01. (B) MEFs isolated from WT and DJ-1 null mice were challenged with 0.5 mM H2O2 for the indicated times. Western blot analysis was performed with anti-pJNK, anti-JNK, or anti-DJ-1. The relative intensity of pJNK was normalized to JNK. Data shown are means±S.E.M. of three independent experiments. * p<0.05, ** p<0.01. (C) WT and DJ-1 null MEFs were incubated with 0.1 mM H2O2 for 24 h, and cell death was measured by LDH assay. Data represent means±S.E.M (n=6). * p<0.05 compared to WT H2O2-treated cells.
ASK1 activation results in the activation of its downstream effectors including JNK. To verify whether DJ-1 is required for inhibiting H2O2-induced JNK activation, we compared the induction of phosphorylated JNK in WT and in DJ-1 null MEFs. Compared to WT MEFs, JNK activation following exposure to 0.5 mM H2O2 for up to 1 h was more pronounced in DJ-1 null MEFs (Fig. 5B). We also examined the sensitivity of DJ-1 null MEFs to oxidant-induced cell death (Fig. 5C). WT and DJ-1 null MEFs were incubated with 0.1 mM H2O2 for 24 h and cell death was quantified by LDH assay. DJ-1 null MEFs had greater degree of LDH release following oxidant challenge compared to WT MEFs indicating susceptibility with loss of DJ-1 expression. These findings indicate that DJ-1 plays a protective role in oxidative stress-induced JNK activation and cell death.
4. Discussion
This study demonstrates a new mechanism by which DJ-1 protects against oxidative stress. By inhibiting oxidant induced dissociation between the physiological redox regulator Trx1 and the apoptotic kinase ASK1, DJ-1 acts as a potent inhibitor of ASK1 activation. The PD-associated point mutant L166P loses this protective function, and Cys 106 is required for this effect. Thus, DJ-1 is critical for regulating Trx1/ASK1 interaction in response to oxidative stress.
ASK1 is activated by various stresses such as oxidative stress, ER stress, and TNFα. Under basal conditions, ASK1 forms a large molecular mass complex, the ASK1 signalosome, which comprises not only ASK1 homo-oligomerization but also negative regulators such as Trx1, phosphatase 2C epsilon and 14-3-3 (Goldman et al., 2004; Saito et al., 2007; Saitoh et al., 1998). Upon oxidative stress, ASK1 dissociates from negative regulators leading to its activation and subsequent cell death (Goldman et al., 2004; Saito et al., 2007; Saitoh et al., 1998). Trx1 has two Cys residues, C32 and C35, which are involved in sensing the redox state. Reduced Trx1 binds to ASK1 and inhibits its kinase activity in unstressed cells. On the other hand, oxidized Trx1 dissociates from ASK1 resulting in ASK1 activation (Saitoh et al., 1998). Previous reports suggest that antioxidants such as N-acetyl-L-cysteine (NAC) or propyl gallate suppress this dissociation of the Trx1/ASK1 complex (Noguchi et al., 2005; Saitoh et al., 1998). Since DJ-1 itself has three Cys residues sensitive to oxidative stress, we investigated if it is also a physiological inhibitor of Trx1/ASK1 interaction as a reducing agent. WT DJ-1, but not L166P mutant, prevented oxidant-induced dissociation of ASK1 from Trx1. The L166P mutant is not only less stable than WT DJ-1 and incapable of dimer formation, it also forms high molecular-weight complexes with other proteins (Macedo et al., 2003; Moore et al., 2003; Olzmann et al., 2004), resulting in loss of DJ-1 function. Thus, the dimeric structure of DJ-1 appears to correlate with Trx1/ASK1 regulation under oxidative stress conditions. It is conceivable that DJ-1 scavenges ROS through self-oxidation, thus, minimizing dissociation of the complex. Mass spectrometric studies have shown that DJ-1 oxidizes, leading to the acidic pI shift, and functions as an atypical peroxiredoxin-like peroxidase that scavenges ROS (Andres-Mateos et al., 2007; Canet-Aviles et al., 2004). Among three Cys mutants of DJ-1, only C106S failed to inhibit the dissociation between Trx1 and ASK1, suggesting that Cys 106 of DJ-1 is important in regulating the Trx1/ASK1 complex in response to H2O2. The role of Cys106 oxidation in inhibiting ASK1 activation has also been shown in DJ-1 null MEFs (Waak et al., 2009).
The dissociation of ASK1 from Trx1 is required for the recruitment of adaptor proteins such as Daxx or TRAF2 to ASK1 and for ASK1 homo-oligomerization to promote its activation under stress conditions (Liu et al., 2000; Song and Lee, 2003). The present findings suggest that cytoplasmic DJ-1 functions as a negative regulator of the Trx1/ASK1 complex through preventing the dissociation of Trx1 from ASK1 in response to oxidative stress. In parallel, nuclear localized DJ-1 binds to Daxx and prevents its oxidant-induced translocation to the cytoplasm and activation of ASK1 (Junn et al., 2005). Thus, we propose a dual mechanism by which DJ-1 can keep ASK1 activation in check resulting to cytoprotection.
Acknowledgments
The authors wish to thank Benoit I. Giasson of University of Pennsylvania for providing DJ-1 antibody and Ted Dawson of Johns Hopkins University for providing DJ-1 null mice. This study is funded by National Institutes of Health grants NS053517, NS059869 to M.M.M., the American Parkinson Disease Association and the Foundation of UMDNJ to E.J. M.M.M. is the William Dow Lovett Professor of Neurology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci U S A. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandopadhyay R, Kingsbury AE, Cookson MR, Reid AR, Evans IM, Hope AD, Pittman AM, Lashley T, Canet-Aviles R, Miller DW, McLendon C, Strand C, Leonard AJ, Abou-Sleiman PM, Healy DG, Ariga H, Wood NW, de Silva R, Revesz T, Hardy JA, Lees AJ. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Nishitoh H, Yang X, Ichijo H, Baltimore D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science. 1998;281:1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman EH, Chen L, Fu H. Activation of apoptosis signal-regulating kinase 1 by reactive oxygen species through dephosphorylation at serine 967 and 14-3-3 dissociation. J Biol Chem. 2004;279:10442–10449. doi: 10.1074/jbc.M311129200. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Inden M, Taira T, Kitamura Y, Yanagida T, Tsuchiya D, Takata K, Yanagisawa D, Nishimura K, Taniguchi T, Kiso Y, Yoshimoto K, Agatsuma T, Koide-Yoshida S, Iguchi-Ariga SM, Shimohama S, Ariga H. PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson’s disease rat model. Neurobiol Dis. 2006;24:144–158. doi: 10.1016/j.nbd.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, Kim DK, Lee KW, Han PL, Rhee SG, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000;164:6287–6295. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- Junn E, Jang WH, Zhao X, Jeong BS, Mouradian MM. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res. 2009;87:123–129. doi: 10.1002/jnr.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junn E, Taniguchi H, Jeong BS, Zhao X, Ichijo H, Mouradian MM. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc Natl Acad Sci U S A. 2005;102:9691–9696. doi: 10.1073/pnas.0409635102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunakaran S, Diwakar L, Saeed U, Agarwal V, Ramakrishnan S, Iyengar S, Ravindranath V. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson’s disease: protection by alpha-lipoic acid. FASEB J. 2007;21:2226–2236. doi: 10.1096/fj.06-7580com. [DOI] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutuzov MA, Andreeva AV, Voyno-Yasenetskaya TA. Regulation of apoptosis signal-regulating kinase 1 (ASK1) by polyamine levels via protein phosphatase 5. J Biol Chem. 2005;280:25388–25395. doi: 10.1074/jbc.M413202200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Kim SJ, Kim IK, Ko J, Jeong CS, Kim GH, Park C, Kang SO, Suh PG, Lee HS, Cha SS. Crystal structures of human DJ-1 and Escherichia coli Hsp31, which share an evolutionarily conserved domain. J Biol Chem. 2003;278:44552–44559. doi: 10.1074/jbc.M304517200. [DOI] [PubMed] [Google Scholar]
- Liu H, Nishitoh H, Ichijo H, Kyriakis JM. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol. 2000;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo MG, Anar B, Bronner IF, Cannella M, Squitieri F, Bonifati V, Hoogeveen A, Heutink P, Rizzu P. The DJ-1L166P mutant protein associated with early onset Parkinson’s disease is unstable and forms higher-order protein complexes. Hum Mol Genet. 2003;12:2807–2816. doi: 10.1093/hmg/ddg304. [DOI] [PubMed] [Google Scholar]
- Martinat C, Shendelman S, Jonason A, Leete T, Beal MF, Yang L, Floss T, Abeliovich A. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES- derived cell model of primary Parkinsonism. PLoS Biol. 2004;2:e327. doi: 10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulener M, Whitworth AJ, Armstrong-Gold CE, Rizzu P, Heutink P, Wes PD, Pallanck LJ, Bonini NM. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- Meulener MC, Xu K, Thomson L, Ischiropoulos H, Bonini NM. Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging. Proc Natl Acad Sci U S A. 2006;103:12517–12522. doi: 10.1073/pnas.0601891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DW, Ahmad R, Hague S, Baptista MJ, Canet-Aviles R, McLendon C, Carter DM, Zhu PP, Stadler J, Chandran J, Klinefelter GR, Blackstone C, Cookson MR. L166P mutant DJ-1, causative for recessive Parkinson’s disease, is degraded through the ubiquitin-proteasome system. J Biol Chem. 2003;278:36588–36595. doi: 10.1074/jbc.M304272200. [DOI] [PubMed] [Google Scholar]
- Mitsumoto A, Nakagawa Y. DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Radic Res. 2001;35:885–893. doi: 10.1080/10715760100301381. [DOI] [PubMed] [Google Scholar]
- Mitsumoto A, Nakagawa Y, Takeuchi A, Okawa K, Iwamatsu A, Takanezawa Y. Oxidized forms of peroxiredoxins and DJ-1 on two-dimensional gels increased in response to sublethal levels of paraquat. Free Radic Res. 2001;35:301–310. doi: 10.1080/10715760100300831. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Zhang L, Dawson TM, Dawson VL. A missense mutation (L166P) in DJ-1, linked to familial Parkinson’s disease, confers reduced protein stability and impairs homo-oligomerization. J Neurochem. 2003;87:1558–1567. doi: 10.1111/j.1471-4159.2003.02265.x. [DOI] [PubMed] [Google Scholar]
- Morita K, Saitoh M, Tobiume K, Matsuura H, Enomoto S, Nishitoh H, Ichijo H. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 2001;20:6028–6036. doi: 10.1093/emboj/20.21.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, Miyazono K, Ichijo H. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell. 1998;2:389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Takeda K, Matsuzawa A, Saegusa K, Nakano H, Gohda J, Inoue J, Ichijo H. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J Biol Chem. 2005;280:37033–37040. doi: 10.1074/jbc.M506771200. [DOI] [PubMed] [Google Scholar]
- Olzmann JA, Brown K, Wilkinson KD, Rees HD, Huai Q, Ke H, Levey AI, Li L, Chin LS. Familial Parkinson’s disease-associated L166P mutation disrupts DJ-1 protein folding and function. J Biol Chem. 2004;279:8506–8515. doi: 10.1074/jbc.M311017200. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Shen X. Critical role of ASK1 in the 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J Neurochem. 2006;97:234–244. doi: 10.1111/j.1471-4159.2006.03730.x. [DOI] [PubMed] [Google Scholar]
- Saeed U, Karunakaran S, Meka DP, Koumar RC, Ramakrishnan S, Joshi SD, Nidadavolu P, Ravindranath V. Redox activated MAP kinase death signaling cascade initiated by ASK1 is not activated in female mice following MPTP: novel mechanism of neuroprotection. Neurotox Res. 2009;16:116–126. doi: 10.1007/s12640-009-9058-5. [DOI] [PubMed] [Google Scholar]
- Saito J, Toriumi S, Awano K, Ichijo H, Sasaki K, Kobayashi T, Tamura S. Regulation of apoptosis signal-regulating kinase 1 by protein phosphatase 2Cepsilon. Biochem J. 2007;405:591–596. doi: 10.1042/BJ20070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Lee YJ. Role of the ASK1-SEK1-JNK1-HIPK1 signal in Daxx trafficking and ASK1 oligomerization. J Biol Chem. 2003;278:47245–47252. doi: 10.1074/jbc.M213201200. [DOI] [PubMed] [Google Scholar]
- Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waak J, Weber SS, Gorner K, Schall C, Ichijo H, Stehle T, Kahle PJ. Oxidizable Residues Mediating Protein Stability and Cytoprotective Interaction of DJ-1 with Apoptosis Signal-regulating Kinase 1. J Biol Chem. 2009;284:14245–14257. doi: 10.1074/jbc.M806902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhong N, Wang H, Elias JE, Kim CY, Woldman I, Pifl C, Gygi SP, Geula C, Yankner BA. The Parkinson’s disease-associated DJ-1 protein is a transcriptional co-activator that protects against neuronal apoptosis. Hum Mol Genet. 2005;14:1231–1241. doi: 10.1093/hmg/ddi134. [DOI] [PubMed] [Google Scholar]
- Zhou W, Freed CR. DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T alpha-synuclein toxicity. J Biol Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]