Abstract
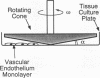
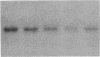
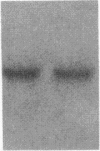
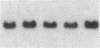
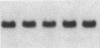
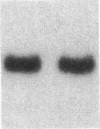
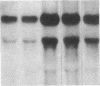
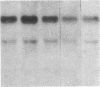
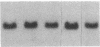
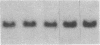
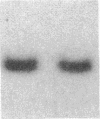
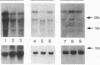
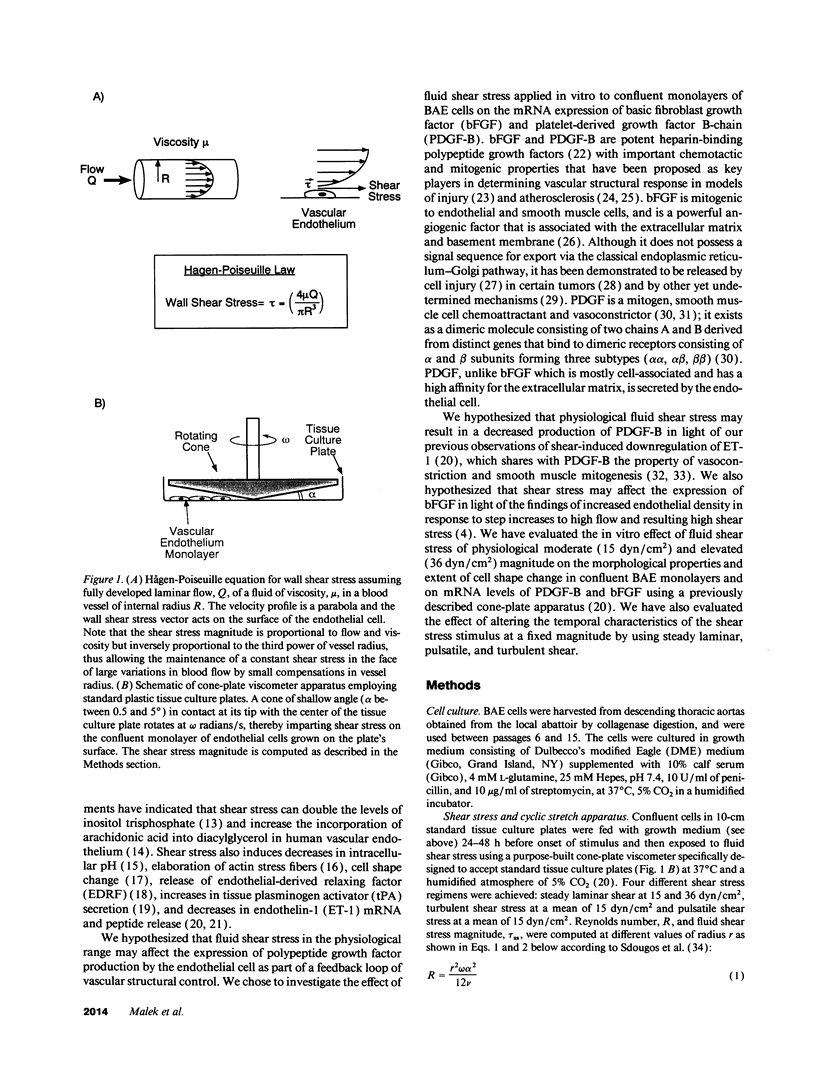
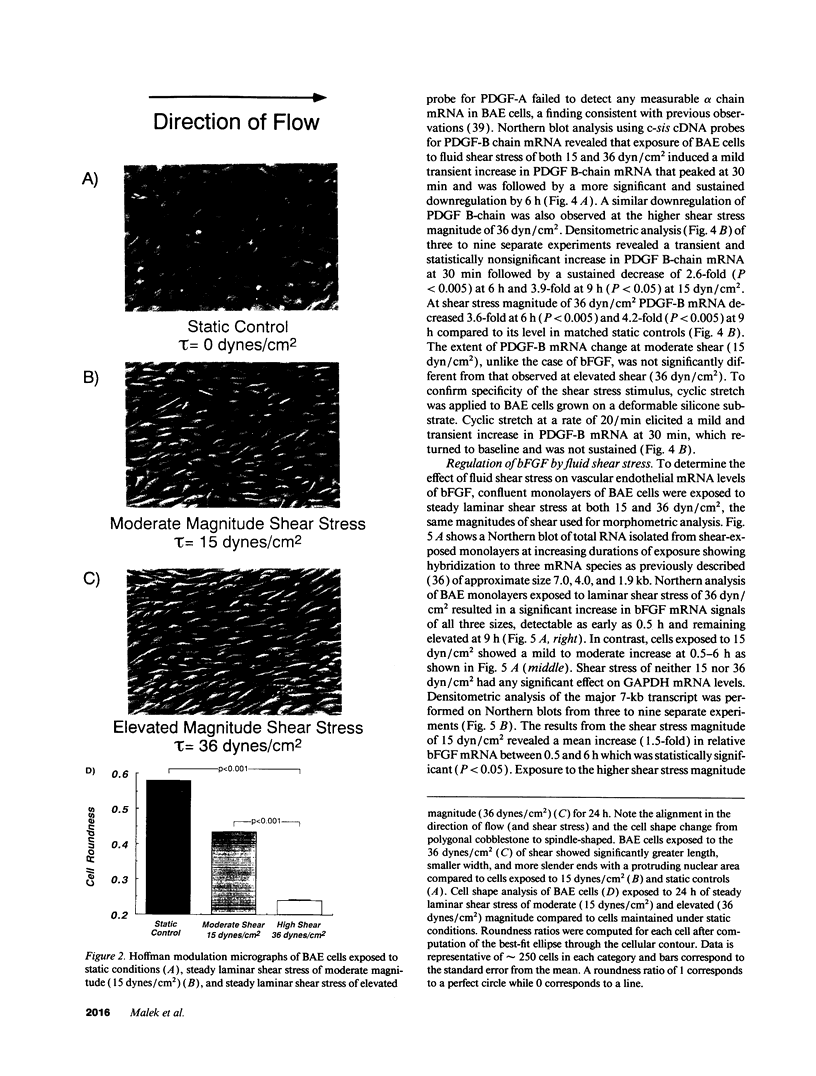
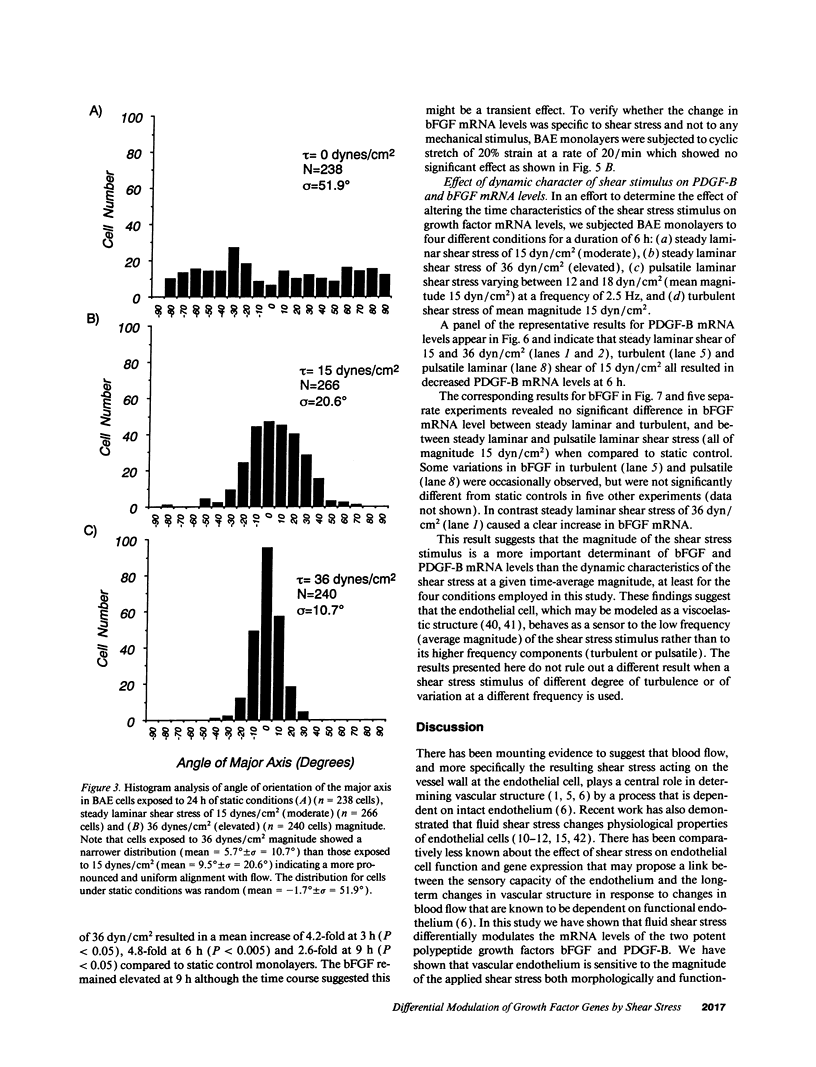
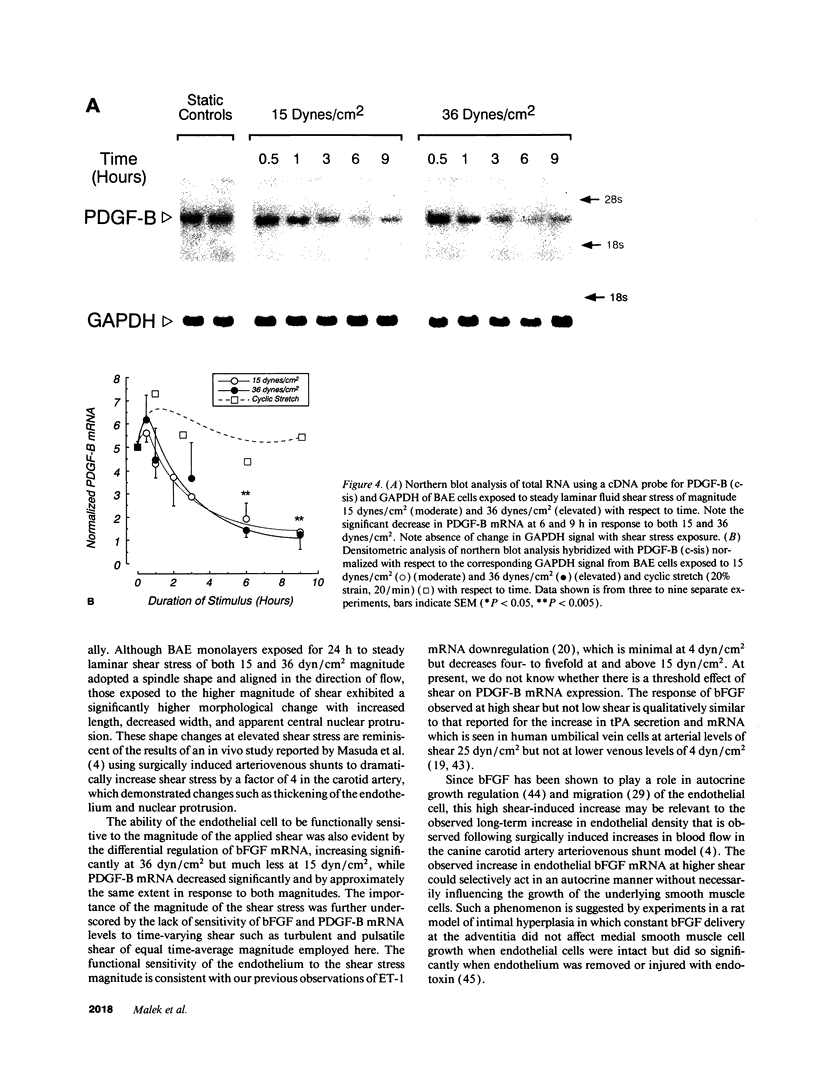
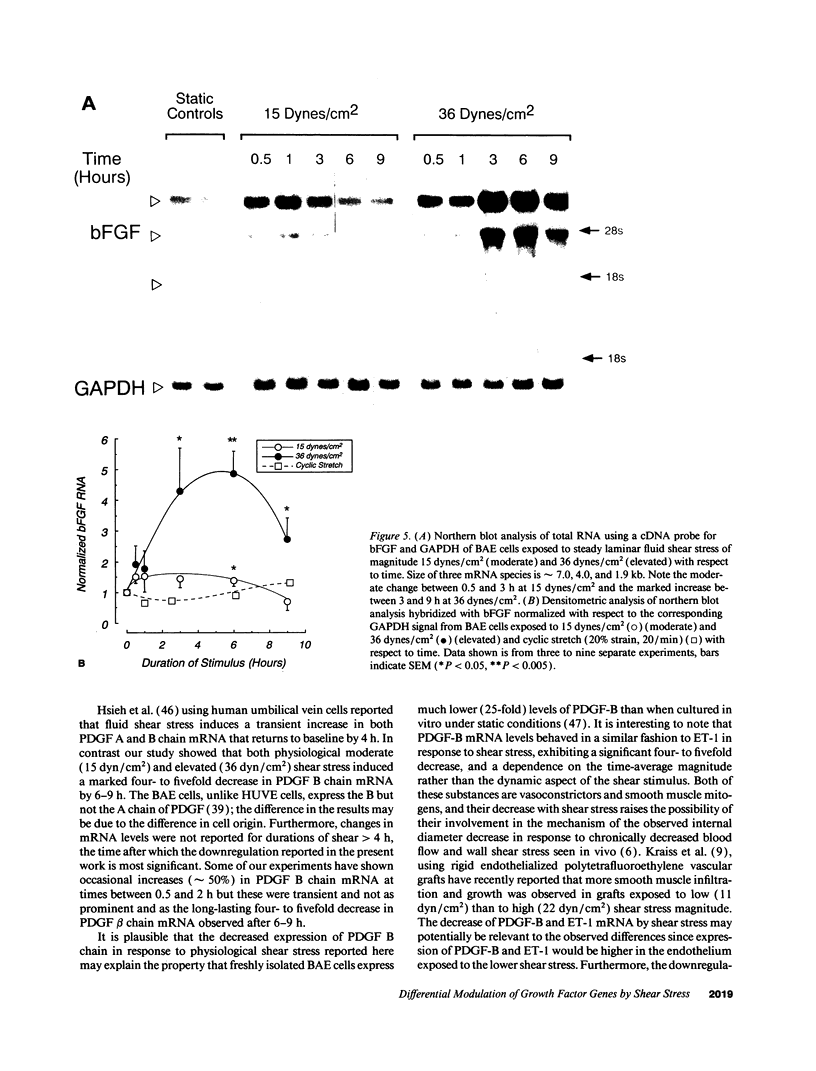
Fluid shear stress has been shown to be an important regulator of vascular structure and function through its effect on the endothelial cell. We have explored the effect of shear stress on the expression of the heparin-binding growth factors platelet-derived growth factor B chain (PDGF-B) and basic fibroblast growth factor (bFGF) in bovine aortic endothelial cells using a purpose-built cone-plate viscometer. Using morphometric analysis, we have mimicked the endothelial cell shape changes encountered in vivo in response to shear stress and correlated these with changes in gene expression. Steady laminar shear stress of 15 and 36 dyn/cm2 both resulted in endothelial cell shape change, but the higher shear stress induced greater and more uniform alignment in the direction of flow and nuclear protrusion after 24 h. Steady laminar shear stress of both 15 and 36 dyn/cm2 induced a significant 3.9- and 4.2-fold decrease, respectively, in PDGF-B mRNA at 9 h. In contrast, steady laminar shear of 15 dyn/cm2 induced a mild and transient 1.5-fold increase in bFGF mRNA while shear of 36 dyn/cm2 induced a significant 4.8-fold increase at 6 h of shear which remained at 2.9-fold at 9 h. Pulsatile and turbulent shear stress showed the same effect as steady laminar shear stress (all at 15 dyn/cm2 time-average magnitude) on PDGF-B and bFGF mRNA content. Cyclic stretch (20% strain, 20/min) of cells grown on silicone substrate did not significantly affect either PDGF-B or bFGF mRNA levels. These results suggest that expression of each peptide growth factor gene is differentially regulated by fluid shear stress in the vascular endothelial cell. These results may have implications on vascular structure and function in response to hemodynamic forces and present a model for the study of transduction of mechanical stimuli into altered gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Adams D. S. Mechanisms of cell shape change: the cytomechanics of cellular response to chemical environment and mechanical loading. J Cell Biol. 1992 Apr;117(1):83–93. doi: 10.1083/jcb.117.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura T., Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res. 1990 Apr;66(4):1045–1066. doi: 10.1161/01.res.66.4.1045. [DOI] [PubMed] [Google Scholar]
- Barrett T. B., Benditt E. P. Platelet-derived growth factor gene expression in human atherosclerotic plaques and normal artery wall. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2810–2814. doi: 10.1073/pnas.85.8.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T. B., Gajdusek C. M., Schwartz S. M., McDougall J. K., Benditt E. P. Expression of the sis gene by endothelial cells in culture and in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6772–6774. doi: 10.1073/pnas.81.21.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk B. C., Alexander R. W., Brock T. A., Gimbrone M. A., Jr, Webb R. C. Vasoconstriction: a new activity for platelet-derived growth factor. Science. 1986 Apr 4;232(4746):87–90. doi: 10.1126/science.3485309. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins T., Pober J. S., Gimbrone M. A., Jr, Hammacher A., Betsholtz C., Westermark B., Heldin C. H. Cultured human endothelial cells express platelet-derived growth factor A chain. Am J Pathol. 1987 Jan;126(1):7–12. [PMC free article] [PubMed] [Google Scholar]
- Cooke J. P., Stamler J., Andon N., Davies P. F., McKinley G., Loscalzo J. Flow stimulates endothelial cells to release a nitrovasodilator that is potentiated by reduced thiol. Am J Physiol. 1990 Sep;259(3 Pt 2):H804–H812. doi: 10.1152/ajpheart.1990.259.3.H804. [DOI] [PubMed] [Google Scholar]
- Devare S. G., Reddy E. P., Law J. D., Robbins K. C., Aaronson S. A. Nucleotide sequence of the simian sarcoma virus genome: demonstration that its acquired cellular sequences encode the transforming gene product p28sis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):731–735. doi: 10.1073/pnas.80.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond S. L., Eskin S. G., McIntire L. V. Fluid flow stimulates tissue plasminogen activator secretion by cultured human endothelial cells. Science. 1989 Mar 17;243(4897):1483–1485. doi: 10.1126/science.2467379. [DOI] [PubMed] [Google Scholar]
- Diamond S. L., Sharefkin J. B., Dieffenbach C., Frasier-Scott K., McIntire L. V., Eskin S. G. Tissue plasminogen activator messenger RNA levels increase in cultured human endothelial cells exposed to laminar shear stress. J Cell Physiol. 1990 May;143(2):364–371. doi: 10.1002/jcp.1041430222. [DOI] [PubMed] [Google Scholar]
- Dull R. O., Davies P. F. Flow modulation of agonist (ATP)-response (Ca2+) coupling in vascular endothelial cells. Am J Physiol. 1991 Jul;261(1 Pt 2):H149–H154. doi: 10.1152/ajpheart.1991.261.1.H149. [DOI] [PubMed] [Google Scholar]
- Edelman E. R., Nugent M. A., Smith L. T., Karnovsky M. J. Basic fibroblast growth factor enhances the coupling of intimal hyperplasia and proliferation of vasa vasorum in injured rat arteries. J Clin Invest. 1992 Feb;89(2):465–473. doi: 10.1172/JCI115607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke R. P., Gräfe M., Schnittler H., Seiffge D., Mittermayer C., Drenckhahn D. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature. 1984 Feb 16;307(5952):648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- Goldsmith H. L., Turitto V. T. Rheological aspects of thrombosis and haemostasis: basic principles and applications. ICTH-Report--Subcommittee on Rheology of the International Committee on Thrombosis and Haemostasis. Thromb Haemost. 1986 Jun 30;55(3):415–435. [PubMed] [Google Scholar]
- Herman I. M., Brant A. M., Warty V. S., Bonaccorso J., Klein E. C., Kormos R. L., Borovetz H. S. Hemodynamics and the vascular endothelial cytoskeleton. J Cell Biol. 1987 Jul;105(1):291–302. doi: 10.1083/jcb.105.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H. J., Li N. Q., Frangos J. A. Shear stress increases endothelial platelet-derived growth factor mRNA levels. Am J Physiol. 1991 Feb;260(2 Pt 2):H642–H646. doi: 10.1152/ajpheart.1991.260.2.H642. [DOI] [PubMed] [Google Scholar]
- Jawien A., Bowen-Pope D. F., Lindner V., Schwartz S. M., Clowes A. W. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992 Feb;89(2):507–511. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A., Bukhari R., Togawa T. Adaptive regulation of wall shear stress optimizing vascular tree function. Bull Math Biol. 1984;46(1):127–137. doi: 10.1007/BF02463726. [DOI] [PubMed] [Google Scholar]
- Kandel J., Bossy-Wetzel E., Radvanyi F., Klagsbrun M., Folkman J., Hanahan D. Neovascularization is associated with a switch to the export of bFGF in the multistep development of fibrosarcoma. Cell. 1991 Sep 20;66(6):1095–1104. doi: 10.1016/0092-8674(91)90033-u. [DOI] [PubMed] [Google Scholar]
- Kraiss L. W., Kirkman T. R., Kohler T. R., Zierler B., Clowes A. W. Shear stress regulates smooth muscle proliferation and neointimal thickening in porous polytetrafluoroethylene grafts. Arterioscler Thromb. 1991 Nov-Dec;11(6):1844–1852. doi: 10.1161/01.atv.11.6.1844. [DOI] [PubMed] [Google Scholar]
- LaBarbera M. Principles of design of fluid transport systems in zoology. Science. 1990 Aug 31;249(4972):992–1000. doi: 10.1126/science.2396104. [DOI] [PubMed] [Google Scholar]
- Langille B. L., O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986 Jan 24;231(4736):405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- Libby P., Warner S. J., Salomon R. N., Birinyi L. K. Production of platelet-derived growth factor-like mitogen by smooth-muscle cells from human atheroma. N Engl J Med. 1988 Jun 9;318(23):1493–1498. doi: 10.1056/NEJM198806093182303. [DOI] [PubMed] [Google Scholar]
- Malek A. M., Greene A. L., Izumo S. Regulation of endothelin 1 gene by fluid shear stress is transcriptionally mediated and independent of protein kinase C and cAMP. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5999–6003. doi: 10.1073/pnas.90.13.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek A., Izumo S. Physiological fluid shear stress causes downregulation of endothelin-1 mRNA in bovine aortic endothelium. Am J Physiol. 1992 Aug;263(2 Pt 1):C389–C396. doi: 10.1152/ajpcell.1992.263.2.C389. [DOI] [PubMed] [Google Scholar]
- Masuda H., Kawamura K., Tohda K., Shozawa T., Sageshima M., Kamiya A. Increase in endothelial cell density before artery enlargement in flow-loaded canine carotid artery. Arteriosclerosis. 1989 Nov-Dec;9(6):812–823. doi: 10.1161/01.atv.9.6.812. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Muthukrishnan L., Warder E., D'Amore P. A. Growth factors are released by mechanically wounded endothelial cells. J Cell Biol. 1989 Aug;109(2):811–822. doi: 10.1083/jcb.109.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercola M., Wang C. Y., Kelly J., Brownlee C., Jackson-Grusby L., Stiles C., Bowen-Pope D. Selective expression of PDGF A and its receptor during early mouse embryogenesis. Dev Biol. 1990 Mar;138(1):114–122. doi: 10.1016/0012-1606(90)90181-h. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Morimoto T., Rifkin D. B. Basic fibroblast growth factor released by single, isolated cells stimulates their migration in an autocrine manner. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11007–11011. doi: 10.1073/pnas.88.24.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollert M. U., Eskin S. G., McIntire L. V. Shear stress increases inositol trisphosphate levels in human endothelial cells. Biochem Biophys Res Commun. 1990 Jul 16;170(1):281–287. doi: 10.1016/0006-291x(90)91271-s. [DOI] [PubMed] [Google Scholar]
- Nollert M. U., Hall E. R., Eskin S. G., McIntire L. V. The effect of shear stress on the uptake and metabolism of arachidonic acid by human endothelial cells. Biochim Biophys Acta. 1989 Sep 11;1005(1):72–78. doi: 10.1016/0005-2760(89)90033-7. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Clapham D. E., Davies P. F. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988 Jan 14;331(6152):168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Remuzzi A., Dewey C. F., Jr, Davies P. F., Gimbrone M. A., Jr Orientation of endothelial cells in shear fields in vitro. Biorheology. 1984;21(4):617–630. doi: 10.3233/bir-1984-21419. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Botelho L. H. Endothelins. FASEB J. 1991 Sep;5(12):2713–2720. doi: 10.1096/fasebj.5.12.1916094. [DOI] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987 Jan 15;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Sharefkin J. B., Diamond S. L., Eskin S. G., McIntire L. V., Dieffenbach C. W. Fluid flow decreases preproendothelin mRNA levels and suppresses endothelin-1 peptide release in cultured human endothelial cells. J Vasc Surg. 1991 Jul;14(1):1–9. [PubMed] [Google Scholar]
- Shen J., Luscinskas F. W., Connolly A., Dewey C. F., Jr, Gimbrone M. A., Jr Fluid shear stress modulates cytosolic free calcium in vascular endothelial cells. Am J Physiol. 1992 Feb;262(2 Pt 1):C384–C390. doi: 10.1152/ajpcell.1992.262.2.C384. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Fridman R., Sullivan R., Sasse J., Klagsbrun M. Aortic endothelial cells synthesize basic fibroblast growth factor which remains cell associated and platelet-derived growth factor-like protein which is secreted. J Cell Physiol. 1987 Jun;131(3):402–408. doi: 10.1002/jcp.1041310312. [DOI] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Masaki T. Molecular biology and biochemistry of the endothelins. Trends Pharmacol Sci. 1989 Sep;10(9):374–378. doi: 10.1016/0165-6147(89)90011-4. [DOI] [PubMed] [Google Scholar]
- Zarins C. K., Giddens D. P., Bharadvaj B. K., Sottiurai V. S., Mabon R. F., Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983 Oct;53(4):502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- Ziegelstein R. C., Cheng L., Capogrossi M. C. Flow-dependent cytosolic acidification of vascular endothelial cells. Science. 1992 Oct 23;258(5082):656–659. doi: 10.1126/science.1329207. [DOI] [PubMed] [Google Scholar]