Abstract
Background
Urine specimens are commonly used in biomarker research. Urinary creatinine (UCr) is often used to adjust for urine analyte concentration. We aim to explore the applicability of UCr as a normalization method in a cystic fibrosis (CF) population during hospitalization.
Methods
Multiple spot urine samples were collected from CF patients hospitalized for a pulmonary exacerbation. Single spot specimens were obtained from asthmatics and healthy children for comparison. The assumptions and implications from the use of UCr as a normalization factor for urinary desmosine measurements were investigated.
Results
UCr differed significantly across disease groups and decreased significantly over time in the CF population. Differing results were obtained when contrasting normalization by UCr with specific gravity.
Conclusions
UCr levels are not completely attributable to simply variations in urine concentration. Analysis of urinary biomarker measurements should be initiated with an understanding of the relative effects of the normalization process on the results.
Keywords: Urinary biomarkers, Urinary Creatinine, Cystic Fibrosis, Normalization Factor, Specific Gravity, Pulmonary Exacerbation
Introduction
Urine specimens are commonly employed in cystic fibrosis (CF) biomarker research for the measurement of infectious and inflammatory mediators. Urine is an ideal specimen, given it can be collected non-invasively, is usually plentiful and poses minimal risk. Spot urine samples are preferred to 24-hour collections for feasibility and to avoid improper and incomplete 24-hour collection. The major disadvantage of spot urine samples is the variation in dilution effects, sample volume and the rate of urine production (1). In an attempt to adjust for this variation, urinary creatinine (UCr) concentration is most commonly used in a ratio format to normalize analyte quantification for specimen concentration. The normalization process involves dividing the concentration of the analyte of interest by the UCr concentration obtained in the same urine sample, with the result reported as the concentration of target analyte per milligram of creatinine.
Creatinine is a waste product of muscle metabolism and is excreted in urine at a relatively constant rate through glomerular filtration (2–3). It is the relative stability within an individual that makes it an attractive approach for normalization of analyte concentrations. However, the rate of creatinine excretion has been shown to vary across different patient demographics, introducing the possibility of bias in urinary biomarker measurements that use UCr for normalization (4). Creatinine excretion has been documented to increase throughout the growth period, peaking at 20–30 years of age, and decreasing thereafter, reflecting the changes in body muscle tissue mass (1). Furthermore, UCr concentrations are known to vary by gender, ethnicity and dietary protein levels (5–8). Creatinine adjustment is thought to work best when the renal elimination mechanism of the analyte is similar to the renal elimination mechanism of creatinine (2, 4, 9).
Patients with CF may have reduced creatinine levels or irregular urinary excretion of creatinine due to several biological factors, including reduced muscle mass related to nutritional difficulties, the presence of a hyper-catabolic state from chronic systemic inflammation, limited exercise capacity, and frequent pulmonary exacerbations or illness (10–12). CF patients may also have renal impairment (decreased glomerular filtration rate), potentially related to cumulative aminoglycoside use, even in the presence of a normal blood urea and serum creatinine (10–13). Given the potential variability in excretion and quantity, UCr may not be an appropriate normalizing factor to use for urinary biomarker quantification in a CF population during illness or when making comparisons with this population.
An alternative method for normalization is specific gravity (SG), which is defined as the ratio of the density of a urine specimen to the density of water. SG values increase with solute concentration. Our aim was to investigate the effect of using UCr as a normalization factor for spot urine samples in hospitalized CF patients and for comparison with samples obtained from patients with asthma and from healthy controls without lung disease. To provide further evaluation of the appropriate measure of urinary concentration, SG values will also be examined. This investigation is necessary if accurate inferences from urinary biomarker studies within the hospitalized CF population are to be achieved.
Methods
Study Population
Fifty-three patients with a confirmed diagnosis of CF (14) resulting in 63 hospital admissions were enrolled into our study of urinary desmosine concentration during pulmonary exacerbation. Patients were identified upon admission to the inpatient unit at The Children’s Hospital, Denver and were required to have ≥ 2 intravenous (IV) antibiotics administered for treatment of their exacerbation to qualify for the study. Spot urine samples from 15 control children without lung disease and 50 stable asthmatic outpatients were collected for comparison. All study protocols were approved by the Colorado Multiple Institutional Review Board and/or the National Jewish Research Hospital Institutional Review Board. Informed consent and/or assent were obtained from each of the subjects and/or their parents or guardians.
Study Design
This was a prospective cohort study of CF patients hospitalized for a pulmonary exacerbation. All patients received standard-of-care therapies including airway clearance, nutritional support and IV antibiotics. Standard doses of IV antibiotics were used and blood levels monitored where appropriate. An attempt to collect three urine samples from each CF patient was made, with the first targeted around 72 hours from admission, the second an interim sample and a third, final sample prior to hospital discharge. Urine samples were centrifuged before freezing at −80°C prior to shipment for analysis. Healthy control and asthmatic children provided a one-time spot urine sample for analysis as per IRB-approved specimen collection protocols.
Laboratory Assays
Urinary Analysis
The analyte of interest for this study was desmosine, a breakdown product of elastin and a major component of the extracellular matrix of the lung. Urinary desmosine concentration is thought to be reflective of lung injury (15). The analyte concentration was measured in all urine samples using a radioimmunoassay (RIA) performed by Elastin Products, Inc. (Owensville, MO, USA) (16–19). UCr was also measured by Elastin Products, Inc. using creatinine assay reagents from Sigma Diagnostics (St. Louis, MO, USA). SG was measured in the CF and control urine samples by a total solids refractometer at The Children’s Hospital, Denver (American Optical Corp., Scientific Instrument Div., Buffalo, NY).
Statistical Analyses
The UCr and analyte values were log (base 10) transformed. Two-sample t-tests and chi-squared tests were used to compare means and percentages, respectively, across disease groups. A simple linear regression was used to test the association across disease groups, age and gender. To estimate the change over time within the CF population, a repeated measures means model with a random subject effect was fit. Normalization of the analyte concentrations was performed using linear regression models where the adjustment for UCr or SG was achieved by including them as covariates, as this is the preferred method of adjustment (20–23). After graphical inspection of the associations between the analyte and UCr and SG, a quadratic association was assumed for UCr and a linear association was assumed for SG. The least square means from these models were used to assess statistical significance across disease groups and over time in the CF patients. All analyses were performed using SAS version 9.2 software (SAS Institute Inc.:Cary, NC, 2008).
Results
Patient Demographics
Hospitalized CF patients were significantly younger (p < 0.01) than the asthma group and slightly older than the healthy control group (Table 1). The gender distributions did not differ significantly across groups; however, there were noticeably more females in the control group compared to the other two disease groups (Table 1). Fifty-eight (89%) of hospital admissions for CF pulmonary exacerbation involved the use of intravenous aminoglycosides and the mean FEV1 percent predicted in the CF population upon admission was 66% (Standard error (SE): 3.9%).
Table 1.
Patient demographics across disease groups
| Mean (SE) or No. (%) | Hospitalized CF patients (n = 63) | Asthma patients (n = 50) | Healthy Controls (n = 15) |
|---|---|---|---|
| Age1 | 15 (0.9) | 20 (0.3) | 13 (1.6) |
| Female | 39 (62%) | 26 (52%) | 12 (80%) |
Indicates p-value comparing corresponding medians or percents between hospitalized CF patients and asthma patients was less than 0.05
Urinary Analyses
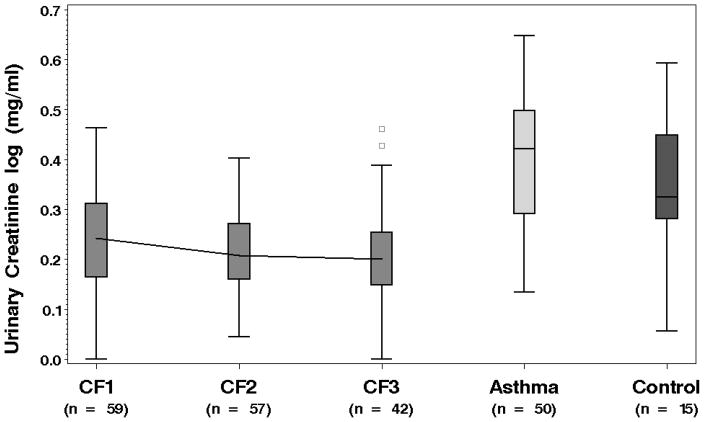
The log transformed urinary creatinine values in the CF group (mean (SE): 0.25 (.01)) were significantly lower compared to both the healthy controls and the asthma group, with means and (SEs) of 0.34 (.04) and 0.40 (0.14), respectively (both p-values < .01). These distributions are displayed in Figure 1. In addition to the differences across disease groups, UCr levels decreased significantly over time during hospitalization in the CF patients (p = 0.04). To provide further evaluation, SG was also assessed in the CF and healthy control samples. No significant differences in SG were observed between the CF and healthy controls (means and (SEs): 1.020 (.001) versus 1.017 (.002) ). Table 2 provides the least square means and 95% confidence intervals (CI) for UCr and SG at the three time points. UCr in the CF population decreased by 23.1% between measurements collected from admission to discharge (p < 0.01). There was no corresponding decrease observed over time in SG.
Figure 1.
Boxplots displaying the distribution of urinary creatinine in each group. Urinary creatinine is significantly lower in the CF pulmonary exacerbation group compared to the other populations.
Table 2.
Least square mean values for UCr and SG at each of three collection times in hospitalized CF patients
| Least square means (95% CI) | Initial | Interim | Final |
|---|---|---|---|
| SG | 1.019 (1.018, 1.021) | 1.020 (1.018, 1.021) | 1.019 (1.017, 1.021) |
| Log CR | 0.26 (0.24, 0.29) | 0.22 (0.19, 0.24) | 0.20 (0.17, 0.23) |
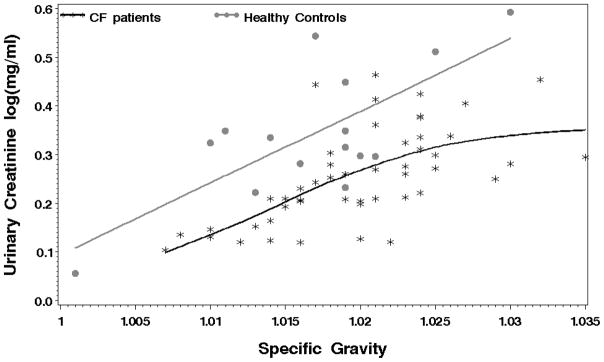
The differences in log UCr across the three disease groups remain significant (p < .01) even after adjusting for the disparities in the age and gender distributions. The inclusion of these three factors in a simple linear regression explained 26.6% of the variability observed in UCr values. There was an increase in UCr values with increasing age (Parameter estimate (SE) = .003 (.002)) and females had lower UCr values on average (Parameter estimate (SE) = −0.03 (.02)) compared to males. There was no discernable difference in the UCr levels in the CF group between those given aminoglycosides versus those that were not at any of the three time points nor in the differences between admission and discharge. In a separate regression, which excluded the asthma group, SG measurements explained 29.0% of the UCr variability. The association between UCr and SG are visibly different, although not significantly, between the CF patients and the healthy control group (Figure 2).
Figure 2.
Relationship between UCr and SG in CF patients (black stars) and healthy controls (grey dots). The association in the CF patients is shifted downwards compared to the associations in the healthy controls.
To investigate the effects of UCr normalization, the analyte hypotheses of interest were tested by calculating the differences in least square means from several mixed models using variations of normalization factors. The results for three comparisons of interest are displayed in Table 3 and consist of contrasts; between CF and healthy controls, between CF and asthma patients and over time in the CF patients. There is a significant elevation in the analyte levels in the CF urine samples compared to both the healthy controls and the asthma patients after adjusting for UCr. This difference is less pronounced, however, when adjustment for SG or no adjustment is made. A significant decrease in the analyte levels over time in the CF patients is observed when adjustment for SG or no adjustment is made, whereas with an UCr adjustment, the decrease is only slightly detectable. In addition to investigating the normalization factors separately, they were also considered jointly, when both UCr and SG were adjusted for as covariates in the model, both contributed significantly to the determination of the analyte levels. Moreover, the analyte levels were significantly elevated in the CF group compared to controls and the decrease over time was more pronounced than when UCr was considered alone.
Table 3.
Comparison of analyte results across normalization methods
| Differences in Least square means (95% CI) | Increase in CF patients compared to healthy controls | Increase in CF patients compared to asthma subject | change from admission to discharge in CF patients |
|---|---|---|---|
| Unadjusted analyte log(pmol/ml) | 0.12 (−0.13, 0.37) | −0.08 (−0.26, 0.09) | −0.18 (−0.36, −0.01) |
| UCr regression adjusted analyte log(pmol/ml) | 0.43 (0.30, 0.56) | 0.25 (0.13, 0.36 ) | −.006 (−0.09, 0.08) |
| SG regression adjusted analyte log(pmol/ml) UCr & SG regression | 0.05 (−0.08, 0.18) | ---- | −0.14 (−0.23, −0.04) |
| adjusted analyte log(pmol/ml) | 0.21 (0.08, 0.34) | ---- | −0.07 (−0.15, 0.01) |
Values in bold indicate differences with p-values < 0.05
Discussion
Creatinine is thought to be excreted at a normal and constant rate in healthy individuals and it is this assumption that makes UCr an appealing factor for normalization (2–3). This study sought to explore the use of urinary creatinine as an acceptable method of specimen normalization for spot urine samples collected during a CF pulmonary exacerbation. We showed that UCr levels varied significantly across disease groups and over time in hospitalized CF patients. These differences could not be fully explained by variations in age and gender distributions across groups, nor by deviations in urine concentration, as measured by SG. Most importantly, depending on the normalization method used, we obtained varying results for our comparisons of interest. In summation, the SG adjustment yielded similar results to those obtained with no adjustment and the UCr adjustment differed widely from both SG and no adjustment. This is likely due to the fact that UCr levels cannot be completely attributable to variations in urine concentration. Adjusting for urine concentration is similar to quantifying a source of random noise across all samples, whereas the UCr values are likely adjusting for other factors that might be specific to a group of subjects, such as renal function or reduced muscle mass. It remains unclear, however, whether it is necessary or appropriate to adjust for these extraneous factors.
Heavner et al (2) showed that creatinine regression adjusted biomarker values correlated well with the 24-hour urinary biomarker values. This correlation, however, indicates that the paired UCr adjustment can be used to approximate the 24-hour value relatively well within an individual but does not necessarily mean that the UCr adjusted value can be used to accurately compare concentrations across individuals. UCr has similarly been found to be a problematic normalization factor both within certain populations (2, 4, 24) and when used to compare biomarker measurements across different demographic groups with known variability in UCr (2–4, 25). Previous studies have likewise shown decreased UCr levels from 24-hour urine samples in the stable CF population compared to age-matched control patients (25–26) as well as decreased renal function with aminoglycoside use (10–13, 27–28). This, in addition to the association with SG observed here, suggests that the lower UCr values in the hospitalized CF population are likely the result of lower creatinine production and impaired renal function rather than lower specimen concentration. Unless it is known that the creatinine level is directly relational to the analyte level (i.e., as in the case where the analyte is cleared similarly to creatinine, in which case renal function becomes relevant), these differences will bias the results when comparing UDes across groups while using UCr as a normalization factor (5).
This study focused on the implications of the choice of normalization, specifically, within a hospitalized CF population. It is not uncommon for urinary analyte concentrations to be normalized to urinary creatinine concentrations in a CF population (19, 26, 29–30). However, special consideration is warranted to determine the appropriate correction factor and whether corrections are needed for features such as renal function or differences in patient demographics. Analysis of urinary measurements should be initiated with a conscientious understanding of the relative effects of the normalization process on the results. The future of urinary biomarker measurement and translation into clinical practice depends on our ability to make accurate measurements of potential biomarkers.
Acknowledgments
This work was supported by the Cystic Fibrosis Foundation (CFF LAGUNA06A0) and the National Institutes of Health (NIH 1UO1HL081335-01, UL1 RR025780 and M01RR00069). The authors did not receive writing assistance for this manuscript. The study sponsors did not have any role in the study design; in the collection, analysis and interpretation of the data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Footnotes
Data from this manuscript has previously been presented in poster form at the North American Cystic Fibrosis Conference, Orlando, FL; October 2008.
CONFLICT OF INTEREST
Dr. Wagner’s conflict of interest statement is as follows: Dr. Wagner has no conflicts of interest to disclose.
Dr. Accurso’s conflict of interest statement is as follows: Dr. Accurso has received grant support from the National Institutes of Health and the Cystic Fibrosis Foundation. He has served as a consultant to Inspire Pharmaceuticals, Inc. in the last three years. He has participated in industry studies through the Cystic Fibrosis Foundation Therapeutic Development Network (Gilead Sciences, Inc.; Targeted Genetics, Inc.; PTC Therapeutics, Inc.; Vertex Pharmaceuticals, Inc.; Altus Biologics, Inc.; Digestive Care, Inc.; KalobBios Pharmaceuticals, Inc.)
Dr. Laguna’s conflict of interest statement is as follows: Dr. Laguna has received grant support from the American Thoracic Society and the Cystic Fibrosis Foundation. She has provided an educational symposium sponsored by Hill-Rom.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin MD, Woods JS, Leroux BG, Rue T, Derouen TA, Leitao J, et al. Longitudinal urinary creatinine excretion values among preadolescents and adolescents. Transl Res. 2008 Jan;151(1):51–6. doi: 10.1016/j.trsl.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Heavner DL, Morgan WT, Sears SB, Richardson JD, Byrd GD, Ogden MW. Effect of creatinine and specific gravity normalization techniques on xenobiotic biomarkers in smokers’ spot and 24-h urines. J Pharm Biomed Anal. 2006 Mar 3;40(4):928–42. doi: 10.1016/j.jpba.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Suwazono Y, Akesson A, Alfven T, Jarup L, Vahter M. Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers. 2005 Mar-Jun;10(2–3):117–26. doi: 10.1080/13547500500159001. [DOI] [PubMed] [Google Scholar]
- 4.Alessio L, Berlin A, Dell’Orto A, Toffoletto F, Ghezzi I. Reliability of urinary creatinine as a parameter used to adjust values of urinary biological indicators. Int Arch Occup Environ Health. 1985;55(2):99–106. doi: 10.1007/BF00378371. [DOI] [PubMed] [Google Scholar]
- 5.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005 Feb;113(2):192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neubert A, Remer T. The impact of dietary protein intake on urinary creatinine excretion in a healthy pediatric population. J Pediatr. 1998 Nov;133(5):655–9. doi: 10.1016/s0022-3476(98)70107-6. [DOI] [PubMed] [Google Scholar]
- 7.Penner SB, Bernstein KN, Fine A. Changes in endogenous creatinine clearance in man on a controlled protein diet: effect of route of administration of a protein load. Clin Invest Med. 1990 Oct;13(5):233–6. [PubMed] [Google Scholar]
- 8.Worsfold M, Davie MW, Haddaway MJ. Age-related changes in body composition, hydroxyproline, and creatinine excretion in normal women. Calcif Tissue Int. 1999 Jan;64(1):40. doi: 10.1007/s002239900576. [DOI] [PubMed] [Google Scholar]
- 9.Jatlow P, McKee S, O’Malley SS. Correction of urine cotinine concentrations for creatinine excretion: is it useful? Clin Chem. 2003 Nov;49(11):1932–4. doi: 10.1373/clinchem.2003.023374. [DOI] [PubMed] [Google Scholar]
- 10.Al-Aloul M, Jackson M, Bell G, Ledson M, Walshaw M. Comparison of methods of assessment of renal function in cystic fibrosis (CF) patients. J Cyst Fibros. 2007 Jan;6(1):41–7. doi: 10.1016/j.jcf.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Al-Aloul M, Miller H, Alapati S, Stockton PA, Ledson MJ, Walshaw MJ. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatr Pulmonol. 2005 Jan;39(1):15–20. doi: 10.1002/ppul.20138. [DOI] [PubMed] [Google Scholar]
- 12.Tan KH, Mulheran M, Knox AJ, Smyth AR. Aminoglycoside prescribing and surveillance in cystic fibrosis. Am J Respir Crit Care Med. 2003 Mar 15;167(6):819–23. doi: 10.1164/rccm.200109-012CC. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Salgado C, Lopez-Hernandez FJ, Lopez-Novoa JM. Glomerular nephrotoxicity of aminoglycosides. Toxicol Appl Pharmacol. 2007 Aug 15;223(1):86–98. doi: 10.1016/j.taap.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr. 1998 Apr;132(4):589–95. doi: 10.1016/s0022-3476(98)70344-0. [DOI] [PubMed] [Google Scholar]
- 15.Starcher BC. Lung Elastin and Matrix. Chest. 2000;117:229S–34S. doi: 10.1378/chest.117.5_suppl_1.229s-a. [DOI] [PubMed] [Google Scholar]
- 16.Starcher B. A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal Biochem. 2001 May 1;292(1):125–9. doi: 10.1006/abio.2001.5050. [DOI] [PubMed] [Google Scholar]
- 17.Starcher B, Conrad M. A role for neutrophil elastase in the progression of solar elastosis. Connect Tissue Res. 1995;31(2):133–40. doi: 10.3109/03008209509028401. [DOI] [PubMed] [Google Scholar]
- 18.King GS, Mohan VS, Starcher BC. Radioimmunoassay for desmosine. Connect Tissue Res. 1980;7(4):263–7. doi: 10.3109/03008208009152362. [DOI] [PubMed] [Google Scholar]
- 19.Starcher B, Green M, Scott M. Measurement of urinary desmosine as an indicator of acute pulmonary disease. Respiration. 1995;62(5):252–7. doi: 10.1159/000196458. [DOI] [PubMed] [Google Scholar]
- 20.Kronmal RA. Spurious Correlation and the Fallacy of the Ratio Standard Revisited. J R Statist Soc A. 1993;156:379–92. [Google Scholar]
- 21.Liu Y, Schutz RW. Statistical validity of using ratio variables in human kinetics research. Res Q Exerc Sport. 2003 Sep;74(3):226–35. doi: 10.1080/02701367.2003.10609087. [DOI] [PubMed] [Google Scholar]
- 22.Tanner JM. Fallacy of per-weight and per-surface area standards, and their relation to spurious correlation. J Appl Physiol. 1949 Jul;2(1):1–15. doi: 10.1152/jappl.1949.2.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB. Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord. 1995 Sep;19(9):644–52. [PubMed] [Google Scholar]
- 24.Carrieri M, Trevisan A, Bartolucci GB. Adjustment to concentration-dilution of spot urine samples: correlation between specific gravity and creatinine. Int Arch Occup Environ Health. 2001 Jan;74(1):63–7. doi: 10.1007/s004200000190. [DOI] [PubMed] [Google Scholar]
- 25.Guman-Wignot TM, Kaufman J, Holsclaw DS, Jr, Schmoyer IR, Alhadeff JA. Analysis and HPLC fractionation of urine from patients with cystic fibrosis, chronic lung diseases and normal controls. Clin Biochem. 1989 Oct;22(5):377–83. doi: 10.1016/s0009-9120(89)80036-0. [DOI] [PubMed] [Google Scholar]
- 26.Bode DC, Pagani ED, Cumiskey WR, von Roemeling R, Hamel L, Silver PJ. Comparison of urinary desmosine excretion in patients with chronic obstructive pulmonary disease or cystic fibrosis. Pulm Pharmacol Ther. 2000;13(4):175–80. doi: 10.1006/pupt.2000.0245. [DOI] [PubMed] [Google Scholar]
- 27.Etherington C, Bosomworth M, Clifton I, Peckham DG, Conway SP. Measurement of urinary N-acetyl-b-D-glucosaminidase in adult patients with cystic fibrosis: before, during and after treatment with intravenous antibiotics. J Cyst Fibros. 2007 Jan;6(1):67–73. doi: 10.1016/j.jcf.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Halacova M, Kotaska K, Kukacka J, Vavrova V, Kuzelova M, Ticha J, et al. Serum cystatin C level for better assessment of glomerular filtration rate in cystic fibrosis patients treated by amikacin. J Clin Pharm Ther. 2008 Aug;33(4):409–17. doi: 10.1111/j.1365-2710.2008.00932.x. [DOI] [PubMed] [Google Scholar]
- 29.Downey DG, Martin SL, Dempster M, Moore JE, Keogan MT, Starcher B, et al. The relationship of clinical and inflammatory markers to outcome in stable patients with cystic fibrosis. Pediatr Pulmonol. 2007 Mar;42(3):216–20. doi: 10.1002/ppul.20553. [DOI] [PubMed] [Google Scholar]
- 30.Stone PJ, Konstan MW, Berger M, Dorkin HL, Franzblau C, Snider GL. Elastin and collagen degradation products in urine of patients with cystic fibrosis. Am J Respir Crit Care Med. 1995 Jul;152(1):157–62. doi: 10.1164/ajrccm.152.1.7599816. [DOI] [PubMed] [Google Scholar]