Abstract
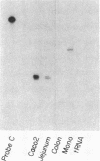
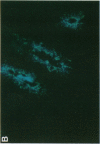
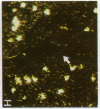
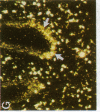
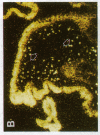
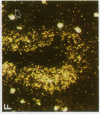
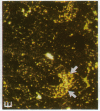
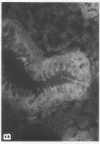
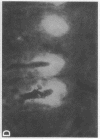
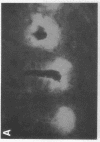
alpha 1-Antitrypsin (alpha 1-AT) is an acute phase plasma protein predominantly derived from the liver which inhibits neutrophil elastase. Previous studies have suggested that alpha 1-AT is also expressed in human enterocytes because alpha 1-AT mRNA could be detected in human jejunum by RNA blot analysis, and alpha 1-AT synthesis could be detected in a human intestinal adenocarcinoma cell line Caco2, which spontaneously differentiates into villous-like enterocytes in tissue culture. To definitively determine that the alpha 1-AT gene is expressed in human enterocytes in vivo, we examined tissue slices of human jejunum and ileum by in situ hybridization. The results demonstrate specific hybridization to enterocytes from the bases to the tips of the villi. Although there was no hybridization to enterocytes in most of the crypt epithelium, there was intense specific hybridization in one region of the crypt. Double-label immunohistochemical studies showed that alpha 1-AT and lysozyme co-localized to this region, indicating that it represented Paneth cells. Finally, there was a marked increase in hybridization to alpha 1-AT mRNA in villous enterocytes and Paneth cells in Crohn's disease. The results of this study provide definitive evidence that alpha 1-AT is expressed in human jejunal and ileal enterocytes in vivo, and show that alpha 1-AT is also a product of Paneth cells. Together with the results of other studies, these data raise the possibility that alpha 1-AT detected in fecal alpha 1-AT clearance assays for diagnosing protein-losing enteropathies is predominantly derived from sloughed enterocytes.
Full text
PDF

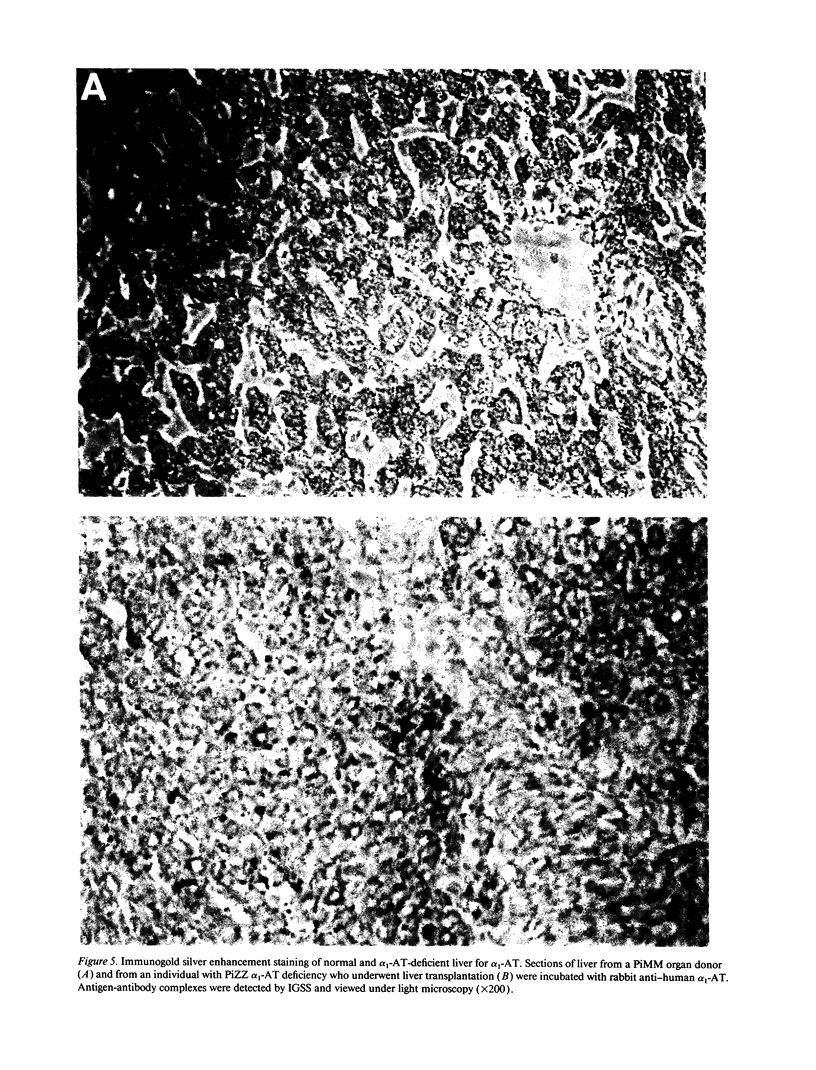
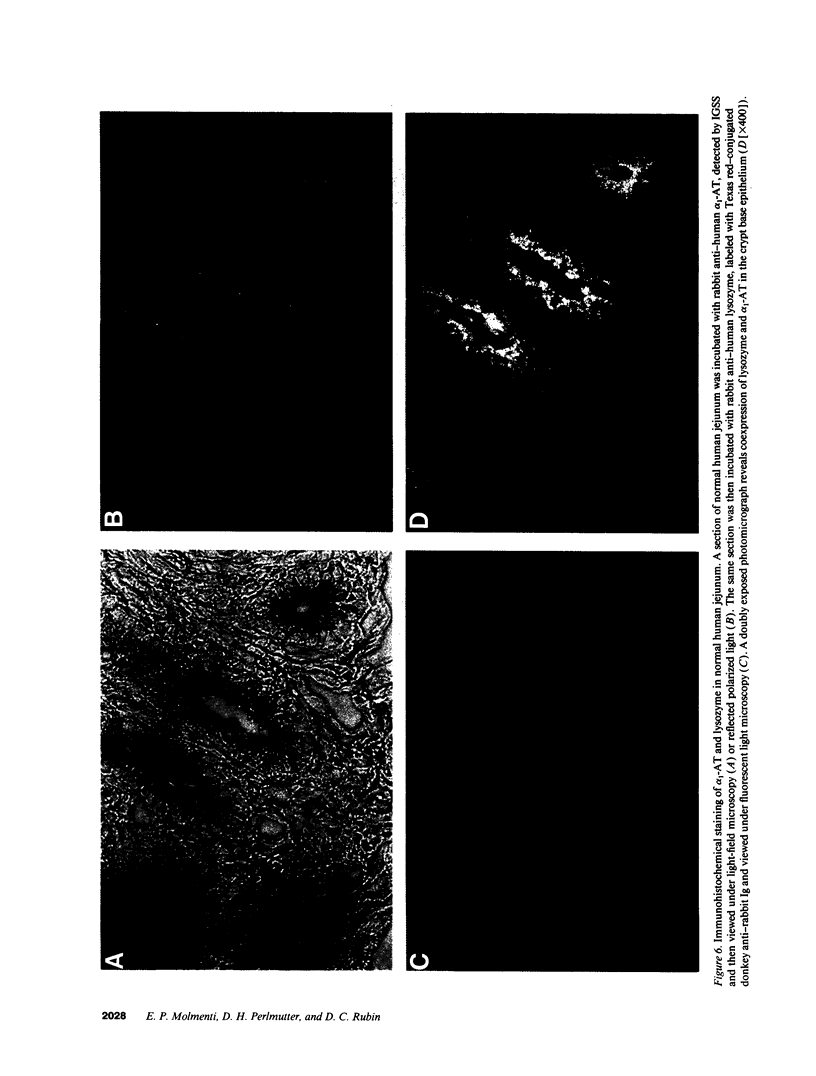


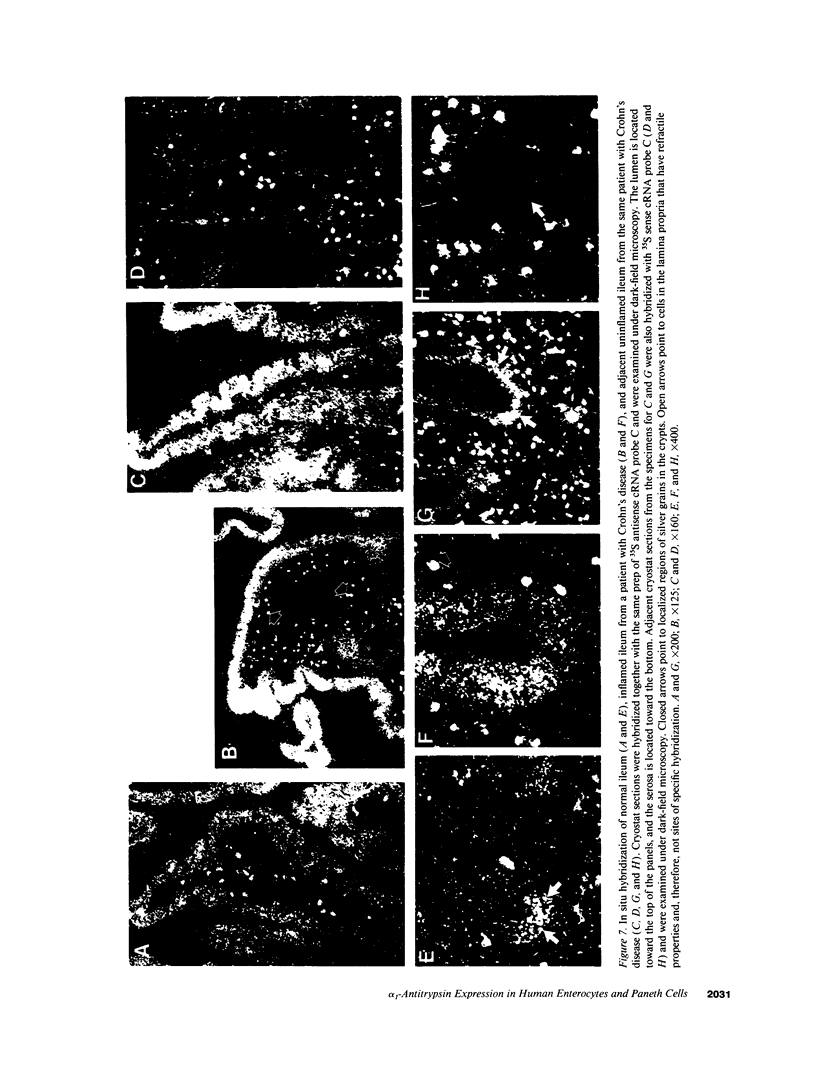
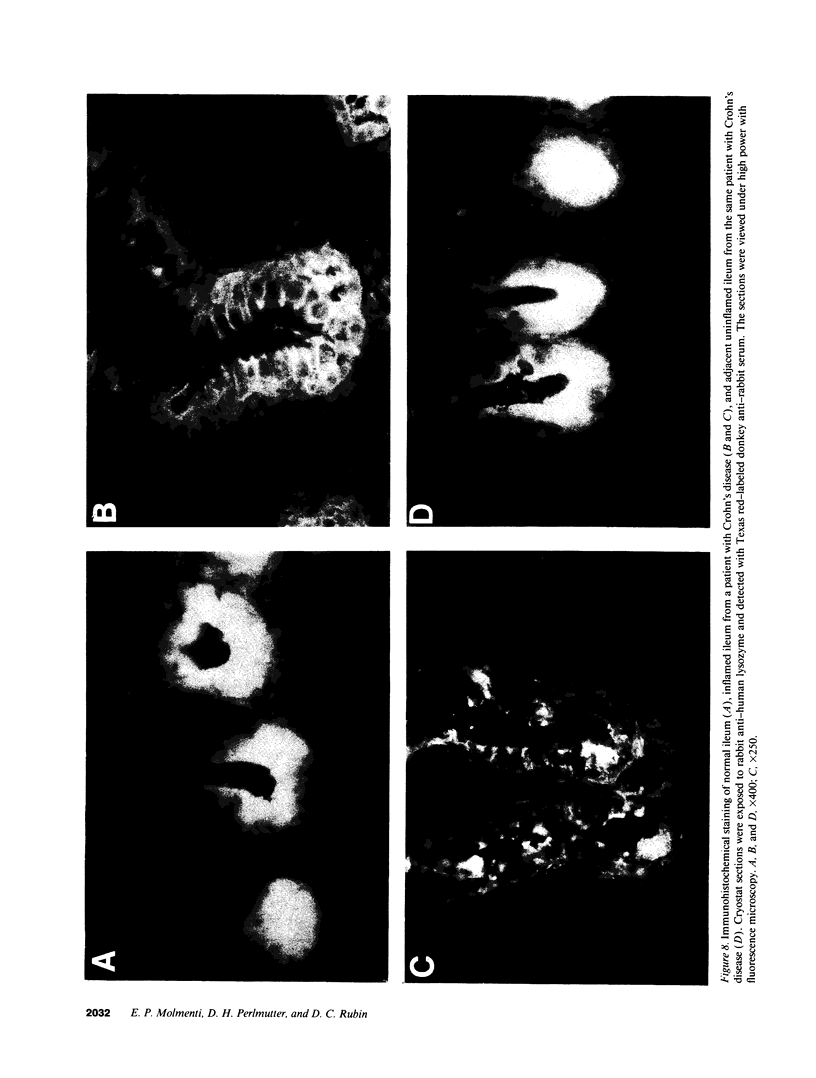


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrenstedt O., Knutson L., Nilsson B., Nilsson-Ekdahl K., Odlind B., Hällgren R. Enhanced local production of complement components in the small intestines of patients with Crohn's disease. N Engl J Med. 1990 May 10;322(19):1345–1349. doi: 10.1056/NEJM199005103221903. [DOI] [PubMed] [Google Scholar]
- Alper C. A., Raum D., Awdeh Z. L., Petersen B. H., Taylor P. D., Starzl T. E. Studies of hepatic synthesis in vivo of plasma proteins, including orosomucoid, transferrin, alpha 1-antitrypsin, C8, and factor B. Clin Immunol Immunopathol. 1980 May;16(1):84–89. doi: 10.1016/0090-1229(80)90169-5. [DOI] [PubMed] [Google Scholar]
- Barbey-Morel C., Pierce J. A., Campbell E. J., Perlmutter D. H. Lipopolysaccharide modulates the expression of alpha 1 proteinase inhibitor and other serine proteinase inhibitors in human monocytes and macrophages. J Exp Med. 1987 Oct 1;166(4):1041–1054. doi: 10.1084/jem.166.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J. A., Rogers B. B., Sifers R. N., Hawkins H. K., Finegold M. J., Woo S. L. Multiple tissues express alpha 1-antitrypsin in transgenic mice and man. J Clin Invest. 1988 Jul;82(1):26–36. doi: 10.1172/JCI113580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn S. M., Roth K. A., Birkenmeier E. H., Gordon J. I. Temporal and spatial patterns of transgene expression in aging adult mice provide insights about the origins, organization, and differentiation of the intestinal epithelium. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1034–1038. doi: 10.1073/pnas.88.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie M. G., Fok K. F., Kato J., Moore R. J., Hamra F. K., Duffin K. L., Smith C. E. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsen S. L., Parsons J. A., Taylor T. D. Ultrastructural immunocytochemical localization of lysozyme in the Paneth cells of man. J Histochem Cytochem. 1974 Jun;22(6):401–413. doi: 10.1177/22.6.401. [DOI] [PubMed] [Google Scholar]
- Geyer G. Lysozyme in Paneth cell secretions. Acta Histochem. 1973;45(1):126–132. [PubMed] [Google Scholar]
- Gordon J. I. Intestinal epithelial differentiation: new insights from chimeric and transgenic mice. J Cell Biol. 1989 Apr;108(4):1187–1194. doi: 10.1083/jcb.108.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill B. B., Tinghitella T., Hillemeier C., Gryboski J. Increased intestinal clearance of alpha 1-antitrypsin in patients with alpha 1-antitrypsin deficiency. J Pediatr Gastroenterol Nutr. 1983;2(1):95–98. doi: 10.1097/00005176-198302010-00010. [DOI] [PubMed] [Google Scholar]
- Hafeez W., Ciliberto G., Perlmutter D. H. Constitutive and modulated expression of the human alpha 1 antitrypsin gene. Different transcriptional initiation sites used in three different cell types. J Clin Invest. 1992 Apr;89(4):1214–1222. doi: 10.1172/JCI115705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood J. M., Koep L. J., Peters R. L., Schröter G. P., Weil R., 3rd, Redeker A. G., Starzl T. E. Liver transplantation for advanced liver disease with alpha-1-antitrypsin deficiency. N Engl J Med. 1980 Jan 31;302(5):272–275. doi: 10.1056/NEJM198001313020505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshav S., Lawson L., Chung L. P., Stein M., Perry V. H., Gordon S. Tumor necrosis factor mRNA localized to Paneth cells of normal murine intestinal epithelium by in situ hybridization. J Exp Med. 1990 Jan 1;171(1):327–332. doi: 10.1084/jem.171.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P., Povey S., Lovell-Badge R. H. Widespread expression of human alpha 1-antitrypsin in transgenic mice revealed by in situ hybridization. Genes Dev. 1989 Jan;3(1):16–25. doi: 10.1101/gad.3.1.16. [DOI] [PubMed] [Google Scholar]
- Lechene de la Porte P., Lafont H., Lombardo D. Immunocytochemical localization of pancreatic carboxylic ester hydrolase in human paneth cells. Histochemistry. 1986;86(2):211–214. doi: 10.1007/BF00493390. [DOI] [PubMed] [Google Scholar]
- Mason D. Y., Taylor C. R. The distribution of muramidase (lysozyme) in human tissues. J Clin Pathol. 1975 Feb;28(2):124–132. doi: 10.1136/jcp.28.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molmenti E. P., Ziambaras T., Perlmutter D. H. Evidence for an acute phase response in human intestinal epithelial cells. J Biol Chem. 1993 Jul 5;268(19):14116–14124. [PubMed] [Google Scholar]
- Ouellette A. J., Greco R. M., James M., Frederick D., Naftilan J., Fallon J. T. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989 May;108(5):1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer P. E., DeLellis R. A., Wolfe H. J. Immunohistochemistry of liver in alpha1-antitrypsin deficiency. A comparative study. Am J Clin Pathol. 1974 Sep;62(3):350–354. doi: 10.1093/ajcp/62.3.350. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Cole F. S., Kilbridge P., Rossing T. H., Colten H. R. Expression of the alpha 1-proteinase inhibitor gene in human monocytes and macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(3):795–799. doi: 10.1073/pnas.82.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Daniels J. D., Auerbach H. S., De Schryver-Kecskemeti K., Winter H. S., Alpers D. H. The alpha 1-antitrypsin gene is expressed in a human intestinal epithelial cell line. J Biol Chem. 1989 Jun 5;264(16):9485–9490. [PubMed] [Google Scholar]
- Perlmutter D. H., Kay R. M., Cole F. S., Rossing T. H., Van Thiel D., Colten H. R. The cellular defect in alpha 1-proteinase inhibitor (alpha 1-PI) deficiency is expressed in human monocytes and in Xenopus oocytes injected with human liver mRNA. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6918–6921. doi: 10.1073/pnas.82.20.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H. The cellular basis for liver injury in alpha 1-antitrypsin deficiency. Hepatology. 1991 Jan;13(1):172–185. [PubMed] [Google Scholar]
- Potten C. S., Loeffler M. A comprehensive model of the crypts of the small intestine of the mouse provides insight into the mechanisms of cell migration and the proliferation hierarchy. J Theor Biol. 1987 Aug 21;127(4):381–391. doi: 10.1016/s0022-5193(87)80136-4. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990 Dec;110(4):1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Roth K. A., Gordon J. I. Spatial differentiation of the intestinal epithelium: analysis of enteroendocrine cells containing immunoreactive serotonin, secretin, and substance P in normal and transgenic mice. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6408–6412. doi: 10.1073/pnas.87.16.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth K. A., Hertz J. M., Gordon J. I. Mapping enteroendocrine cell populations in transgenic mice reveals an unexpected degree of complexity in cellular differentiation within the gastrointestinal tract. J Cell Biol. 1990 May;110(5):1791–1801. doi: 10.1083/jcb.110.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. C., Roth K. A., Birkenmeier E. H., Gordon J. I. Epithelial cell differentiation in normal and transgenic mouse intestinal isografts. J Cell Biol. 1991 Jun;113(5):1183–1192. doi: 10.1083/jcb.113.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. C. Spatial analysis of transcriptional activation in fetal rat jejunal and ileal gut epithelium. Am J Physiol. 1992 Dec;263(6 Pt 1):G853–G863. doi: 10.1152/ajpgi.1992.263.6.G853. [DOI] [PubMed] [Google Scholar]
- Satoh Y., Ishikawa K., Tanaka H., Oomori Y., Ono K. Immunohistochemical observations of lysozyme in the Paneth cells of specific-pathogen-free and germ-free mice. Acta Histochem. 1988;83(2):185–188. doi: 10.1016/S0065-1281(88)80055-2. [DOI] [PubMed] [Google Scholar]
- Schmidt G. H., Wilkinson M. M., Ponder B. A. Cell migration pathway in the intestinal epithelium: an in situ marker system using mouse aggregation chimeras. Cell. 1985 Feb;40(2):425–429. doi: 10.1016/0092-8674(85)90156-4. [DOI] [PubMed] [Google Scholar]
- Schmidt G. H., Winton D. J., Ponder B. A. Development of the pattern of cell renewal in the crypt-villus unit of chimaeric mouse small intestine. Development. 1988 Aug;103(4):785–790. doi: 10.1242/dev.103.4.785. [DOI] [PubMed] [Google Scholar]
- Schulz S., Green C. K., Yuen P. S., Garbers D. L. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990 Nov 30;63(5):941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- Strygler B., Nicar M. J., Santangelo W. C., Porter J. L., Fordtran J. S. Alpha 1-antitrypsin excretion in stool in normal subjects and in patients with gastrointestinal disorders. Gastroenterology. 1990 Nov;99(5):1380–1387. doi: 10.1016/0016-5085(90)91165-3. [DOI] [PubMed] [Google Scholar]
- Winton D. J., Ponder B. A. Stem-cell organization in mouse small intestine. Proc Biol Sci. 1990 Jul 23;241(1300):13–18. doi: 10.1098/rspb.1990.0059. [DOI] [PubMed] [Google Scholar]
- de Sauvage F. J., Keshav S., Kuang W. J., Gillett N., Henzel W., Goeddel D. V. Precursor structure, expression, and tissue distribution of human guanylin. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9089–9093. doi: 10.1073/pnas.89.19.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]