Abstract
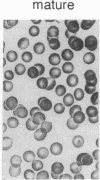
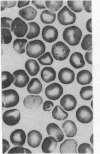
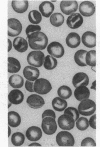
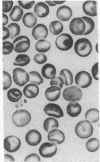
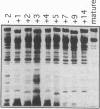
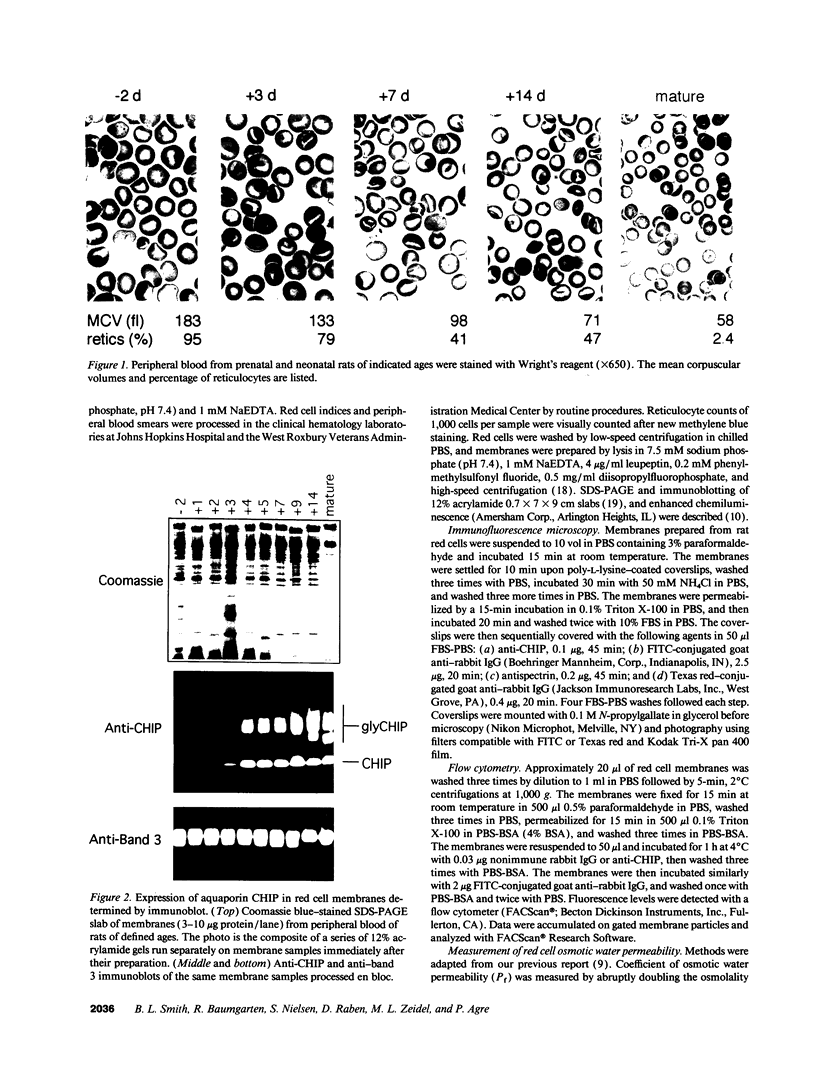
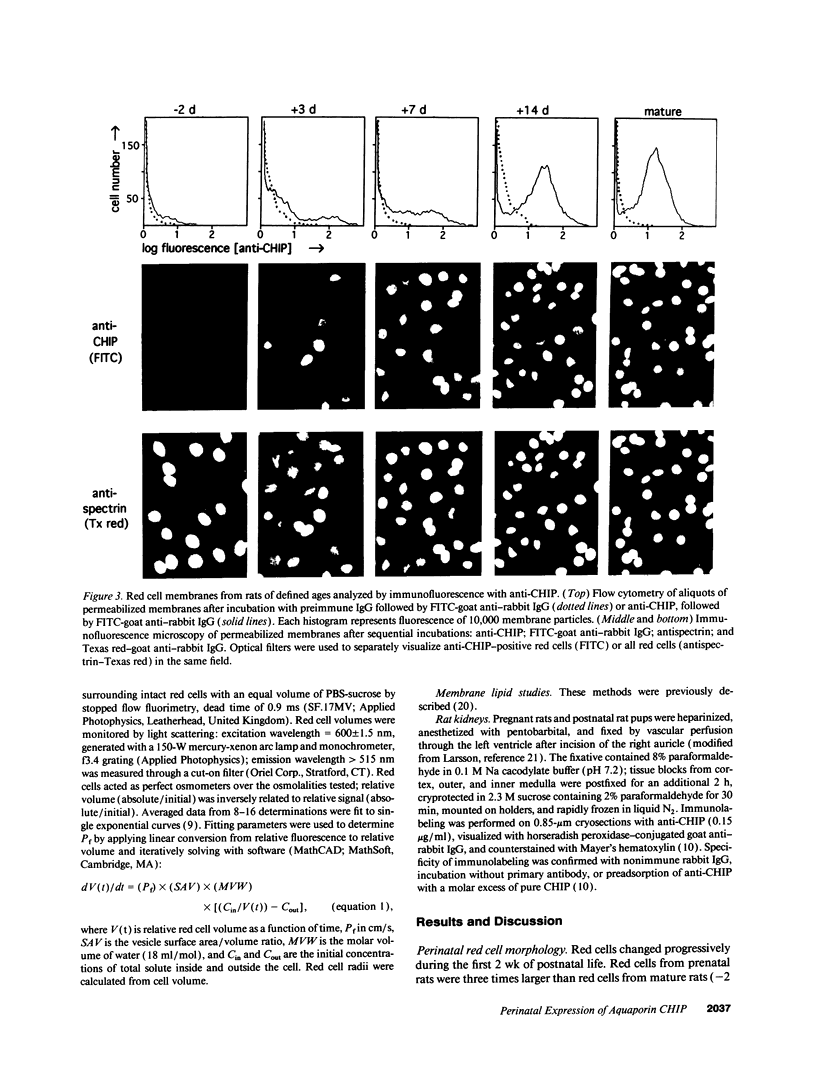
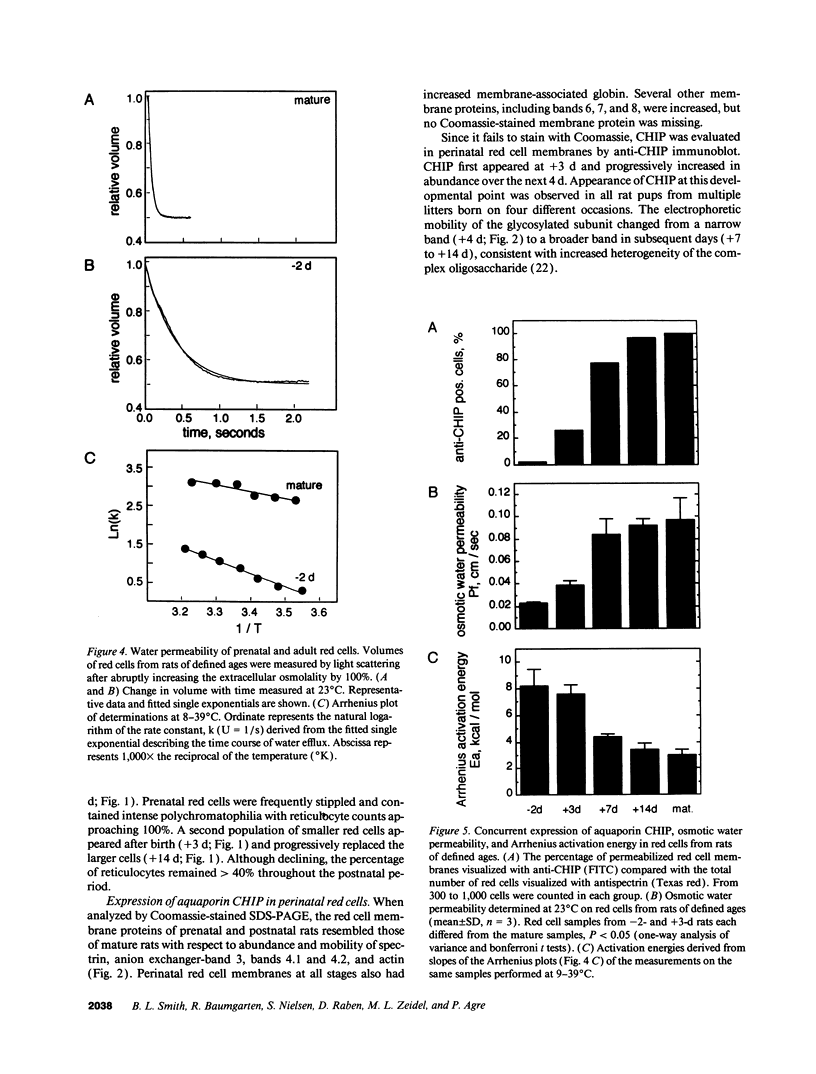
Major phenotypic changes occur in red cell membranes during the perinatal period, but the underlying molecular explanations remain poorly defined. Aquaporin CHIP, the major erythroid and renal water channel, was studied in perinatal rats using affinity-purified anti-CHIP IgG for immunoblotting, flow cytometry, and immunofluorescence microscopy. CHIP was not detected in prenatal red cells but was first identified in circulating red cells on the third postnatal day. Most circulating red cells were positive for CHIP by the seventh postnatal day, and this proportion rose to nearly 100% by the 14th day. The ontogeny of red cell CHIP correlated directly with acquisition of osmotic water permeability and inversely with Arrhenius activation energy. Only minor alterations in the composition of red cell membrane lipids occurred at this time. Immunohistochemical analysis of perinatal kidneys demonstrated a major induction of CHIP in renal proximal tubules and descending thin limbs at birth, coincident with the development of renal concentration mechanisms. Therefore, water channels are unnecessary for oxygen delivery or survival in the prenatal circulation, however CHIP may confer red cells with the ability to rehydrate rapidly after traversing the renal medulla, which becomes hypertonic after birth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTON T. C., BROWN D. A. WATER PERMEABILITY OF THE FETAL ERYTHROCYTE. J Gen Physiol. 1964 May;47:839–849. doi: 10.1085/jgp.47.5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. Proteins involved in membrane--cytoskeleton association in human erythrocytes: spectrin, ankyrin, and band 3. Methods Enzymol. 1983;96:313–324. doi: 10.1016/s0076-6879(83)96029-9. [DOI] [PubMed] [Google Scholar]
- Biemesderfer D., Dekan G., Aronson P. S., Farquhar M. G. Assembly of distinctive coated pit and microvillar microdomains in the renal brush border. Am J Physiol. 1992 Jan;262(1 Pt 2):F55–F67. doi: 10.1152/ajprenal.1992.262.1.F55. [DOI] [PubMed] [Google Scholar]
- Bondy C., Chin E., Smith B. L., Preston G. M., Agre P. Developmental gene expression and tissue distribution of the CHIP28 water-channel protein. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4500–4504. doi: 10.1073/pnas.90.10.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin F. C., Gallois Y., Rapin D., Meskar A., Chabaud J. J., Vicariot M., Ménez J. F. Impaired fetal erythrocytes' filterability: relationship with cell size, membrane fluidity, and membrane lipid composition. Blood. 1992 Apr 15;79(8):2148–2153. [PubMed] [Google Scholar]
- Deen P. M., Dempster J. A., Wieringa B., Van Os C. H. Isolation of a cDNA for rat CHIP28 water channel: high mRNA expression in kidney cortex and inner medulla. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1267–1273. doi: 10.1016/0006-291x(92)91368-z. [DOI] [PubMed] [Google Scholar]
- Denker B. M., Smith B. L., Kuhajda F. P., Agre P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem. 1988 Oct 25;263(30):15634–15642. [PubMed] [Google Scholar]
- Doering T. L., Pessin M. S., Hoff E. F., Hart G. W., Raben D. M., Englund P. T. Trypanosome metabolism of myristate, the fatty acid required for the variant surface glycoprotein membrane anchor. J Biol Chem. 1993 May 5;268(13):9215–9222. [PubMed] [Google Scholar]
- Fukuda M., Dell A., Fukuda M. N. Structure of fetal lactosaminoglycan. The carbohydrate moiety of Band 3 isolated from human umbilical cord erythrocytes. J Biol Chem. 1984 Apr 25;259(8):4782–4791. [PubMed] [Google Scholar]
- Fushimi K., Uchida S., Hara Y., Hirata Y., Marumo F., Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993 Feb 11;361(6412):549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanahan A., Williams J. B., Sanders L. K., Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992 Sep;12(9):3919–3929. doi: 10.1128/mcb.12.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L. Effects of different fixatives on the ultrastructure of the developing proximal tubule in the rat kidney. J Ultrastruct Res. 1975 Apr;51(1):140–151. doi: 10.1016/s0022-5320(75)80014-1. [DOI] [PubMed] [Google Scholar]
- Macey R. I., Yousef L. W. Osmotic stability of red cells in renal circulation requires rapid urea transport. Am J Physiol. 1988 May;254(5 Pt 1):C669–C674. doi: 10.1152/ajpcell.1988.254.5.C669. [DOI] [PubMed] [Google Scholar]
- Matovcik L. M., Mentzer W. C. The membrane of the human neonatal red cell. Clin Haematol. 1985 Feb;14(1):203–221. [PubMed] [Google Scholar]
- Moon C., Preston G. M., Griffin C. A., Jabs E. W., Agre P. The human aquaporin-CHIP gene. Structure, organization, and chromosomal localization. J Biol Chem. 1993 Jul 25;268(21):15772–15778. [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Knepper M. A., Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol. 1993 Jan;120(2):371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. M., Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. M., Carroll T. P., Guggino W. B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992 Apr 17;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Rane S., Aperia A., Eneroth P., Lundin S. Development of urinary concentrating capacity in weaning rats. Pediatr Res. 1985 May;19(5):472–475. doi: 10.1203/00006450-198505000-00013. [DOI] [PubMed] [Google Scholar]
- Smith B. L., Agre P. Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem. 1991 Apr 5;266(10):6407–6415. [PubMed] [Google Scholar]
- Solomon A. K., Chasan B., Dix J. A., Lukacovic M. F., Toon M. R., Verkman A. S. The aqueous pore in the red cell membrane: band 3 as a channel for anions, cations, nonelectrolytes, and water. Ann N Y Acad Sci. 1983;414:97–124. doi: 10.1111/j.1749-6632.1983.tb31678.x. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Ambudkar S. V., Smith B. L., Agre P. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry. 1992 Aug 25;31(33):7436–7440. doi: 10.1021/bi00148a002. [DOI] [PubMed] [Google Scholar]