Abstract
Approximately 10% of employees undertake night-work which is a significant predictor of weight-gain, possibly because responses to activity and eating are altered at night. It is known that the appetite-related hormone, acylated ghrelin is suppressed after an acute bout of exercise during the day, but no researcher has explored whether evening exercise alters acylated ghrelin and other appetite-related outcomes during a subsequent night-shift. Six healthy men (mean±SD: age 30±8 yrs, body mass index 23.1±1.1 kg/m2) completed two crossover trials (control and exercise) in a random order. Participants fasted from 10:00 h, consumed a test meal at 18:00 h and then cycled at 50% peak oxygen uptake or rested between 19:00-20:00 h. Participants then completed light activities during a simulated night-shift which ended at 05:00 h. Two small isocaloric meals were consumed at 22:00 and 02:00 h. Venous blood samples were drawn via cannulation at 1-h intervals between 19:00-05:00 h for the determination of acylated ghrelin, leptin, insulin, glucose, triglyceride and non-esterified fatty acids concentrations. Perceived hunger and wrist actimetry were also recorded. During the night-shift, mean±SD acylated ghrelin concentration was 86.5±40.8 pg/ml following exercise compared with 71.7±37.7 pg/ml without prior exercise (P=0.015). Throughout the night-shift, leptin concentration was 263±242 pg/ml following exercise compared with 187±221 pg/ml without prior exercise (P=0.017). Mean levels of insulin, triglyceride, non-esterified fatty acids and wrist actimetry were also higher during the night-shift that followed exercise (P<0.05). These data indicate that prior exercise increases acylated ghrelin and leptin concentrations during a subsequent simulated night-shift. These findings differ from the known effects of exercise on acylated ghrelin and leptin during the day, and therefore have implications for energy balance during night-work.
Keywords: acylated ghrelin, leptin, hunger, exercise, night-work
Introduction
At least 10% of employees in the USA and most other developed countries participate in a shift-work schedule that involves periods of work in the evening or at night (U.S. Department of Labor – Bureau of Labor Statistics, 2005). Shift-work is a significant risk factor for weight gain (Suwazono et al., 2008), which is probably explained by behavioural-and biological-based disruption of factors involved in energy balance (Atkinson et al., 2008). For example, circadian disruption has been shown to decrease circulating concentrations of the anerexoic hormone, leptin (Scheer et al., 2009). Shift-workers also tend to ‘graze’ on food during the night-shift (Reinberg et al., 1979) when postprandial thermogenesis is lowest (Romon et al., 1993). Intuitively, a reduction in nocturnal feeding, via some intervention which can alter hunger during a night-shift, could help maintain energy balance and, therefore, benefit the long-term health of the shift-worker. One such intervention could be exercise.
Acylated ghrelin and leptin act on the hypothalamus to control energy balance by increasing (Wren et al., 2001) and decreasing (Halaas et al., 1995) preferred food intake, respectively. It is known that an acute bout of non-exhaustive exercise lasting ≤90 min transiently (up to 9 h) suppresses plasma acylated ghrelin concentration (Broom et al., 2007, 2009), but generally has little affect upon plasma/serum leptin level (Fisher et al., 2001; Hulver & Houmard, 2003; Kraemer et al., 1999; Weltman et al., 2000). These findings relate to the finding that exercise has been found to decrease hunger transiently post-exercise (Broom et al., 2007, 2009; King & Blundell, 1995; King et al., 1994; Thompson et al., 1988; Westerterp-Plantenga et al., 1997), although no change (King et al., 1997) or increases (Lluch et al., 1998, Maraki et al., 2005) in hunger have also been reported. These data indicate that acute bouts of exercise may prevent adipose tissue gain, not just via an increase in energy expenditure but in helping to regulate energy intake. Nevertheless, all previous related research has been undertaken during the hours of daylight on participants living a ‘normal’ diurnal existence. No previous researcher has examined the effects of prior evening exercise on appetite regulation when awake throughout the night. Given the knowledge that circadian rhythms exist in human metabolism (Holmbäck et al., 2002, 2003a,b; Ribeiro et al., 1998; Simon et al., 2000) and in the physiological responses to exercise (Drust et al., 2005), we question whether the exercise-mediated responses of hunger, plasma acylated ghrelin and serum leptin are different when awake and eating during a simulated night-shift compared with the findings from previous studies undertaken during the day.
Methods
Participants
In previous experiments (Broom et al., 2007, 2009), exercise has been found to mediate relatively large (26-35%) decreases in plasma acylated ghrelin concentration, which can be measured with good within-assay precision (CV=4.8-6.6%). Our primary comparison was the difference in plasma acylated ghrelin averaged over the simulated night-shift between the exercise and no exercise trials. Using the above estimations of effect size and variance, we estimated that a sample size of 6 would result in adequate statistical power (>80%) so that the predicted magnitude of difference changes would be statistically significant in our repeated-measures experiment. Therefore, we recruited 6 healthy males (mean±SD: age = 30±8 yrs, height = 178±8 cm, body mass index = 23.1±1.1 kg.m2, peak oxygen uptake = 49±7 ml.kg−1.min−1). Participants lived a conventional diurnal lifestyle prior to the experiment (nocturnal sleep of 6-8 h.day−1) and provided written informed consent to participate. None of the participants had been involved in night-work before or travelled across more than 2-3 time-zones in the previous 6 months. The study was approved by the local ethics committee and all procedures adhered to Chronobiology International guidelines (Portaluppi et al., 2008).
Preliminary measurements
Before the first laboratory visit, participants refrained from exercise as well as consumption of alcohol and caffeine for 24 h. Participants were instructed to consume 5 ml.kg−1 body mass of water, and nothing else, in the 2 h before arrival. After height and body mass were measured (Seca Ltd, Birmingham, UK), participants completed a continuous incremental test on a cycle ergometer (Ergo_bike, 8000 TRS, Daum Electronics, Fürth, Germany) to determine their peak oxygen uptake (v̇o2peak), which was measured with an automated gas analyzer (MetaMax, Cortex Biophysik, Leipzig, Germany). The first stage of the v̇o2peak protocol consisted of cycling at 100 Watts for 2 min. Exercise intensity was then increased by 25 Watts every 2 min until the participants reached volitional fatigue. Immediately before exercise began, heart rate was measured using short-range radio telemetry (Polar S610i; Polar Electro Oy, Kempele, Finland) and was continuously monitored and recorded at 2 min intervals, and at the point of exhaustion. Participants were deemed to have reached v̇o2peak if one or more of the following criteria were met: a plateau in v̇o2 (<2.1 ml.kg−1.min−1), a respiratory exchange ratio of 1.15 or above, and/or a heart rate within 10 beats.min−1 of a participant’s age-predicted maximum heart rate.
Experimental procedures
Approximately one week after undertaking the v̇o2peak test, participants performed the first of two experimental trials which were administered in a random order and were separated by ≥7 days. Before both trials, participants abstained from exercise and the consumption of alcohol and caffeine for ≥48 h. Participants were instructed to sleep between 23:00 and 07:00 h the night before each trial. On arrival at the laboratory, participants did not report any atypical sleep characteristics (e.g., number of nocturnal awakenings and perceived sleep latency) during this pre-test night. The stipulated bedtimes were within ± 1 h of the participants ‘normal’ times of retiring and rising. Both trials began at 19:00 h and continued until 05:00 h in a laboratory where mean (±SD) ambient temperature was 22°C (±0.3), relative humidity was 47% (±4) and light intensity was 150 lux. Participants weighed and recorded their food and drink intake, using a diary, during the two days that preceded their first trial, and replicated it before their second trial.
On the day of each trial, participants reported to the laboratory at 17:30 h, after an 7.5 h fast (this was confirmed by questioning the participant about their food and drink intake in the previous 7.5 h). Thereafter, participants promptly adopted a semi-supine position and a cannula was inserted into an antecubital vein. Before both trials began, participants consumed a test meal at 18:00 h and consumed it within 15 min, then rested quietly. At the start of the exercise trial, between 19:00-20:00 h, participants cycled at 50% v̇o2peak (individual absolute power outputs ranged from 130-170 W) on the ergometer described above. Participants completed self-paced mental and physical work at 21:00-21:05 h, 00:00-00:05 h and 05:00-05:05 h in order to simulate activities that may occur during a night-shift. At 22:00 and 02:00 h participants consumed two isocaloric test meals. Participants consumed 100 ml of water at 18:00 h and thereafter at 1 h intervals until 05:00 h. At all other times when participants were not completing tasks, eating or providing blood samples, they rested quietly (e.g., sat watching television). The control trial was identical to the intervention trial, except participants rested (e.g., sat whilst reading) quietly instead of exercising between 19:00 and 20:00 h.
Test meals
The test meal consumed at 18:00 h has been utilized previously (Broom et al., 2007). The meal consisted of a sandwich (Cheddar cheese, mayonnaise and butter), crisps, a chocolate bar and milk-shake. The meal provided 1.47 g carbohydrate, 0.34 g protein, 0.81 g fat and 60 kJ per kg body mass. The test meals consumed at 22:00 and 02:00 h were identical to the abovementioned meal in content, but were halved in portion size. This feeding schedule was chosen to replicate typical food intake of night-workers (Reinberg et al., 1979; Reeves et al., 2004).
Perceived hunger and activity
Perceived hunger was measured using a validated scale (Broom et al., 2009), ranging from 0 (not hungry) to 15 (very hungry). Perceived hunger was recorded at 1-h intervals between 19:00 and 05:00 h. Participant’s activity was recorded via an accelerometer (Actiwatch AW4; Cambridge Neurotechnology Ltd, Cambridge, UK), attached at the wrist. Data were recorded at 19:00 h until 05:00 h using 10 sec epocs.
Blood sampling
In both trials, and after participants had been in a semi-supine position for at least 10 min, blood samples (10 ml) were collected into syringes at 19:00 h (immediately before the exercise) and thereafter at 1 h intervals until 05:00 h, via a cannula placed in an antecubital vein. At 20:00 h, in both trials, (i.e., at the end of exercise in the intervention trial) blood was collected immediately after participants had adopted the semi-supine position. In order to maintain cannula patency, 5 ml of non-heparinised saline was flushed through the system after each sampling point. Moreover, 2-3 ml of blood/saline was drawn off at the beginning of each sampling point to prevent a high concentration of saline being present in our samples.
At each sampling point, blood was immediately dispensed into serum separator tubes, pre-cooled lithium heparin and EDTA (some of which contained p-hydroxymercuribenzoic acid to prevent the degradation of acylated ghrelin) tubes. Lithium heparin and EDTA (not containing p-hydroxymercuribenzoic acid) tubes were placed in a refrigerated centrifuge (4°C) within 15 min of collection, and spun at 4000 revs/min for 15 min. Serum separator tubes, after standing at room temperature for 30-35 min were placed into a centrifuge and spun, as detailed above. Immediately after collection, EDTA tubes containing p-hydroxymercuribenzoic acid were placed into a refrigerated centrifuge (4°C) and spun for 10 min at 3500 revs.min−1. The resultant supernatant was dispensed into a microtube, and 1 M hydrochloric acid was then added (100 uL per mL of plasma) and the sample was subsequently spun for 5 min at 3500 revs.min−1 in a refrigerated centrifuge (4°C). Immediately after centrifuging, plasma/serum supernatants were dispensed into microtubes and stored at −80°C for later analysis. Acylated ghrelin and non-esterified fatty acids (NEFA) concentrations were determined from plasma derived from EDTA tubes. Leptin and insulin levels were measured in serum. Glucose and triglyceride concentrations were determined from plasma derived from lithium heparin tubes. At each sampling point, hemoglobin concentraion and hematocrit percentage were determined in order to estimate plasma volume changes (Dill & Costill, 1974).
Biochemistry
Plasma acylated ghrelin concentrations were determined by ELISA (SPI BIO, Montigny le Bretonneux, France) with within- and inter-assay CVs of 14.0% and 1.7%, respectively. Serum leptin and insulin concentrations were determined by Muliplexing, and had within- and inter-assay CVs of 59.9%, 57.6%, 6.6% and 7.1%, respectively. Plasma concentrations of glucose, triglyceride and non-esterified fatty acids were determined via enzymatic, colorimetric methods (Randox Laboratories LTD, Cumlin, Northern Ireland) with within-assay variations of 1.3%, 1.3% and 3.6%. Accuracy was monitored with quality control sera (Randox Laboratories LTD, Cumlin, Northern Ireland) and samples from each participant were analysed in the same batch to prevent inter-assay variation.
Statistical analysis
Paired-samples t-tests or Wilcoxon signed ranked tests (if the paired differences did not follow a Guassian distribution) were used to determine if baseline values were significantly different between trials. No significant differences at baseline were found for all but one of the outcome variables studied (P≥0.17). Therefore, the post-exercise data for hormones, metabolites and activity counts were analyzed with two factor (trial x time) repeated measures linear mixed models (Cnaan et al., 1997). The P-value for the baseline differences in mean hunger rating approached significance (P=0.081). Therefore, an analysis of covariance using baseline values from both trials as covariates was used to analyse this particular outcome variable (Vickers & Altman, 2001). Within-subjects correlations (Bland & Altman, 1995) were calculated to explore relations between the changes in the different variables during the exercise trial over time. Plasma volume changes were not significantly different between trials, thus unadjusted values for metabolites and hormones were analysed and are reported. All statistical procedures were performed via SPSS for Windows version 15 (SPSS, Inc., Chicago, USA). Descriptive data are presented as means±SD, unless otherwise stated.
Results
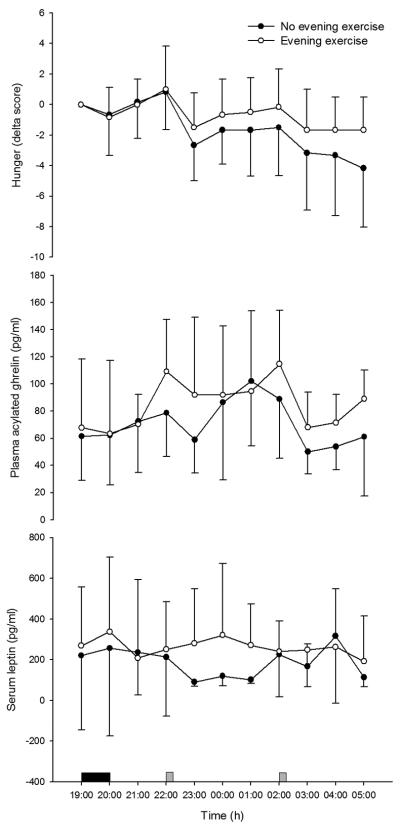
Mean plasma acylated ghrelin concentration was 86.5±40.8 pg/ml during the night-shift (20:00-05:00) after exercise compared with 71.7±37.7 pg/ml in the control trial (95% confidence interval for trial main effect = 2.9 to 26.7 pg/ml, P=0.015; Figure 1A). During the night-shift, serum leptin concentration was also significantly higher in the exercise (263±242 pg/ml) than control trial (187±221 pg/ml; P=0.017; Figure 1B). No significant interactions between trial and time were found for acylated ghrelin and leptin (P>0.05).
Figure 1.
Circulating acylated ghrelin (A) and leptin (B) concentrations and hunger (C) during exercise and control trials (mean±SD). Solid rectangle, cycling; grey square, test meal consumption.
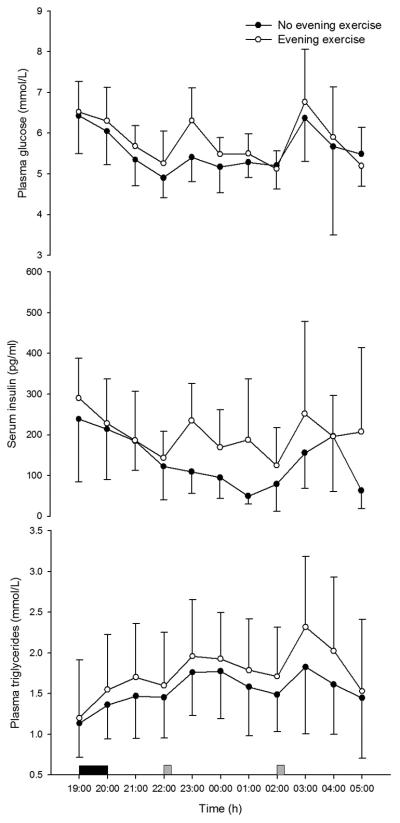
Throughout the night-shift, serum insulin concentration was significantly higher when preceded by exercise (193±127 pg/ml) compared with rest (128±98 pg/ml; P=0.001; Figure 2A) and plasma triglyceride concentration was also significantly higher in the exercise trial (1.8±0.7 mmol/L) than control (1.6±0.6 mmol/L; P=0.004; Figure 2B). Similarly, NEFA concentration was significantly higher when preceded by exercise (0.36±0.16 mmol/L) compared with rest (0.26±0.14 mmol/L; P<0.0005). Plasma glucose was 5.8±0.9 mmol/L during night-work in the exercise trial and 5.5±1.0 mmol/L in the rest trial, the differences between trials not reaching statistical significance (P=0.090; Figure 2C).
Figure 2.
Circulating insulin (A) and triglyceride (B) and glucose (C) concentrations during exercise and control trials (mean±SD). Solid rectangle, cycling; grey square, test meal consumption.
Night-shift activity counts were significantly higher in the exercise (263±139) than control trial (178±104; P<0.0005). Mean hunger during the night-shift generally reduced as meals were ingested throughout the night-shift (Figure 1C), but a significant interaction between trial and time was found (P=0.015). With the baseline values of hunger added as covariates, hunger was found to decrease more rapidly over time during the night-shift when no even exercise had been undertaken. There was no significant effect of trial on hunger (P=0.120; Figure 1C). Within-subjects correlation coefficients of 0.24 (P=0.078; df=100), −0.27 (P=0.043; df=100) and −0.31 (P=0.022; df=100) were found between hunger scores and plasma concentrations of acylated ghrelin, triglyceride and NEFA, respectively. No significant correlations were found between hunger and the other outcomes (P≥0.133; df=100).
Discussion
No previous researcher has published data regarding appetite regulation during nocturnal waking after a bout of prior exercise in the evening. Our novel findings are that an early-evening bout of exercise mediated higher values of circulating concentrations of acylated ghrelin and leptin during a subsequent night-shift compared to no prior exercise. These findings contrast with those from similar investigations undertaken during the daytime which have shown that exercise suppresses acylated ghrelin level (Broom et al., 2007, 2009), but has no effect upon leptin concentrations (Weltman et al., 2000). This indicates that time of day is an important factor to consider in the exploration of relationships between exercise and the abovementioned regulators of appetite.
It is known that intravenous infusions of glucose (Nakagawa et al., 2002), insulin (Möhlig et al., 2002) and NEFA (Gormsen et al., 2006) suppress total circulating ghrelin levels. In our study, there was no significant difference in plasma glucose concentration between trials, although circulating levels of insulin and NEFA were found to be higher nocturnally after prior exercise. These findings offer evidence that the relationships between these metabolic- and appetite-related outcomes change at night, when energy intake is controlled. Growth hormone may suppress circulating concentrations of ghrelin, this suggestion derives from studies which have shown that acromegalic patients have lower concentrations of ghrelin than normal individuals (Cappiello et al., 2002). Post-exercise (approximately 2 h after) suppression of growth hormone is greater in evening than morning (Kanaley et al., 2001). This finding may explain why we found an increase, rather than a decrease in plasma acylated ghrelin level as reported in daytime studies (Broom et al., 2007, 2009). Ghrelin secretion is also known to be regulated by somatostatin, melatonin, glucagon, parasympathetic nervous activity and thyroid hormones (van der Lely et al., 2004). Melatonin might be a focus of further study, given its known role in the circadian system and the fact that it is responsive to exercise (Atkinson et al., 2003).
Researchers have reported that acute intravenous infusion of insulin increases circulating leptin concentrations (Malmström et al., 1996; Utriainen et al., 1996), whereas others have observed no effect (Dagogo-Jack et al., 1996; Ryan & Elahi, 1996). In our study, the exercise-mediated increase in nocturnal serum insulin concentration occurred concurrently with increased serum leptin level. Intravenous infusion of milk (98% triglycerides) significantly induces leptin resistance at the blood-brain barrier (Banks et al., 2004). In our study, triglyceride concentration was significantly higher during the night-shift when preceded by evening exercise than rest. Besides this finding having significant health implications itself (Gill, 2004), it may explain why leptin concentration were higher in the exercise than control trial. Circulating leptin levels are affected by factors other than insulin and triglycerides, such as glucocorticoids and the sympathetic nervous system (Trayhurn et al., 1998). A commonly known glucocorticoids is cortisol, this hormone is known to increase after exercise (Kanaley et al., 2001) and increases circulating levels of leptin (Dagogo-Jack et al., 2005; Laferrère et al., 2006). Therefore, in this study, it is possible that evening exercise mediated an increase night-shift cortisol levels which in turn increased circulating levels of leptin. Peak post-exercise serum cortisol response is dependent upon time of day, with a greater response from baseline occurring in the eveing compared to morning (Kanaley et al., 2001). This dirunal variation may explain why we observed an increase in post-exercise leptin level rather than no effect reported in many day time investigations (Fisher et al., 2001; Weltman et al., 2000).
The nocturnal level of plasma acylated ghrelin was approximately 21% higher during the night-shift that followed exercise compared with control. Circulating concentrations of the anorectic hormones leptin and insulin (Woods et al., 1984) were also higher after exercise. Therefore, we found little evidence of a reciprocal relationship between ghrelin and leptin in our study on post-exercise nocturnal responses. Hunger was found to decrease more rapidly during the night-shift when no evening exercise had been undertaken, which may suggest the exercise-modulated increase in leptin (a hormone which decreases preferred food intake) was more influential on the hunger ratings than the increase in acylated ghrelin. However, no significant association between hunger and leptin concentration was found. Nevertheless, it is noted that despite our attempts to control for baseline differences in hunger with an ANCOVA model, two of the participants remained relatively hungry after consuming the test meal at 18:00 h in the non-exercise trial. Consequently, this may have accentuated the decrease in hunger after eating the test meal at 22:00 h in the no exercise condition. It seems that the effects of exercise on hunger are also inconsistent with daytime studies, with increases (Lluch et al., 1998, Maraki et al., 2005) and decreases (Broom et al., 2007, 2009; King & Blundell, 1995; King et al., 1994; Thompson et al., 1988; Westerterp-Plantenga et al., 1997) in hunger being reported after exercise. The exercise intensity used in our study was lower than that chosen by other investigators who have reported post-exercise hunger suppression, and this could be an important factor. Nevertheless, we deemed it important to select a moderate rather than a high exercise intensity that was feasible for night-workers to adopt. Furthermore, it should also be noted that our study was not powered to detect between trials differences in regard to hunger and therefore related inferences should be viewed with caution.
There are several limitations to our study, firstly in terms of the generalizability of our data to real shift-workers. We administered high-fat, energy dense meals in keeping with the previous daytime-based studies on the acylated ghrelin responses to exercise (Broom et al., 2007; 2009). Although some shift-workers tend to eat high-fat, energy dense foods on the night-shift, it is possible that some shift-workers consume meals with different macronutrient and energy content, and therefore our results may not be applicable to all shift-workers. Our participants were not permanent night-workers. Therefore our findings may have been different if we had been able to study participants over a number of night-shifts. Nevertheless, Folkard (2008) concluded that very few (<3%) fixed night-workers exhibit full circadian adjustment and therefore our findings may be indeed relevant to these permanent workers as wells as those who switch from days to nights frequently.
Under conditions of controlled diet but only partially-controlled activity, we found that prior exercise increased wrist activity measured during the night-shift. Therefore, it is unclear whether, in real shift-work circumstances, the overall increase in energy expenditure modulated by an exercise bout may or may not cause a negative energy balance when night-workers are completely free to choose their diet. Future researchers into this question might measure energy expenditure via more valid methods (e.g., wearing an accelerometer on the waist rather than the wrist) throughout both trials under conditions of ‘free-living’ whilst measuring food intake during the night-shift.
As in almost all exercise-related experiments of this type, it was extremely difficult to blind participants as to which experimental trial they were undertaking before arriving to the laboratory. Thus, it is possible that the expectation of exercising may have influenced our hunger vaules, but it unlikely to have impacted upon the biological blood-borne data. A future study might involve a comparison of different exercise intensities which may help reduce such an expectancy effect.
Our experiment is novel, it involved participation in exercise as well as rigorous longitudinal physiological monitoring (including cannulation) when awake during the night. Therefore, only a relatively small sample of young non-shift-working men participated. Nevertheless, we found relatively large and statistically significant effects of prior exercise on our primary outcome (i.e. main effect of trial on plasma acylated ghrelin concentration) and some secondary outcomes, and so a type II error and any associated concerns about statistical power are not as relevant to our primary and many secondary findings. However, it is acknowledged that our study could have been underpowered to detect significant interactions between trial and time for some of our outcome variables. Statistical power is relevant only in terms of a type II error, i.e. when differences between trials were not significant. Statistical significance is also an indicator that effect sizes are large relative to error variance. The error variance in our repeated measures study is defined by the test-retest coefficient of variation (rather than the between-subjects variability indicated by the error bars in our Figures). The CVs were relatively small for most of our outcomes.
Finally, it is recognised that exercise may induce a phase-shift to some circadian rhythms, such as melatonin and core body temperature (Atkinson et al., 2007). It is possible that evening exercise caused a phase-shift in our outcome variables and this could explain our findings. However, we found it difficult to recruit participants in a multi-day study so that enough data for circadian rhythm description were obtained. Any future attempt at this would need to carefully control for the masking effects of being awake at night.
Conclusion
No previous researcher has examined the effects of prior exercise on appetite regulation when participants are awake at night. We researched this situation given its application to a change from day to night-work. We found that a prior bout of early evening exercise has no effect on mean hunger scores but increases circulating levels of acylated ghrelin and leptin during a simulated night-shift compared with no prior exercise. In previous research, participants exercised during the hours of daylight (usually mid-morning) and contrasting results were generally reported. These data indicate that time of day is an important factor to consider in the exploration of relationships between exercise, metabolism and appetite in order to explain why shift-work is a risk factor for long-term weight-gain. Although our findings indicate no effect (i.e., no benefit or harm) of evening exercise upon night-shift hunger, it is possible that evening exercise is beneficial regard to other health related factors (e.g., blood pressure). Thus, night-workers should still be encouraged to undertake evening exercise.
Acknowledgements
This research was funded by the National Prevention Research Initiative (http://www.npri.org.uk) with support from the following organisations: British Heart Foundation; Cancer Research UK; Chief Scientist Office, Scottish Government Health Directorate; Department of Health; Diabetes UK; Economic and Social Research Council; Health & Social Care Research & Development Office for Northern Ireland; Medical Research Council; Welsh Assembly Government; and World Cancer Research Fund. We also thank Laura Sutton and Robert Kennett for their assistance during data collection.
References
- Atkinson G, Drust B, Reilly T, Waterhouse J. The relevance of melatonin to sports medicine and science. Sports Med. 2003;33:809–831. doi: 10.2165/00007256-200333110-00003. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Edwards B, Reilly T, Waterhouse J. Exercise as a synchroniser of human circadian rhythms: an update and discussion of the methodological problems. Eur. J. Appl. Physiol. 2007;99:331–341. doi: 10.1007/s00421-006-0361-z. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Fullick, Grindey C, MacLaren D. Exercise, energy balance and the shift worker. Sports Med. 2008;38:671–685. doi: 10.2165/00007256-200838080-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Moreley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 1— correlation within subjects. BMJ. 1995;310:446. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom DR, Batterham RL, King JA, Stensel DJ. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, peptide YY in healthy males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R29–35. doi: 10.1152/ajpregu.90706.2008. [DOI] [PubMed] [Google Scholar]
- Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. J. Appl. Physiol. 2007;102:2165–2171. doi: 10.1152/japplphysiol.00759.2006. [DOI] [PubMed] [Google Scholar]
- Cappiello V, Ronchi C, Morpurgo PS, Epaminonda P, Arosio M, Beck-Peccoz P, Spada A. Circulating ghrelin levels in basal conditions and during glucose tolerance test in acromegalic patients. Eur. J. Endocrinol. 2002;147:189–194. doi: 10.1530/eje.0.1470189. [DOI] [PubMed] [Google Scholar]
- Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat. Med. 1997;16:2349–2380. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Dagogo-Jack S, Fanelli C, Paramore D, Brothers J, Landt M. Plasma leptin and insulin relationships in obese and nonobese humans. Diabetes. 1996;45:695–698. doi: 10.2337/diab.45.5.695. [DOI] [PubMed] [Google Scholar]
- Dagogo-Jack S, Tykodi G, Umamaheswaran I. Inhibition of cortisol biosynthesis decreases circulating leptin levels in obese humans. J. Clin. Endocrinol. Metab. 2005;90:5333–5335. doi: 10.1210/jc.2005-0803. [DOI] [PubMed] [Google Scholar]
- Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247. [DOI] [PubMed] [Google Scholar]
- Drust B, Waterhouse J, Atkinson G, Edwards B, Reilly T. Circadian rhythms in sports performance—an update. Chronobiol. Int. 2005;22:21–44. doi: 10.1081/cbi-200041039. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Van Pelt RE, Zinder O, Landt M, Kohrt WM. Acute exercise effect on postabsorptive serum leptin. J. Appl. Physiol. 2001;91:680–686. doi: 10.1152/jappl.2001.91.2.680. [DOI] [PubMed] [Google Scholar]
- Folkard S. Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol. Int. 2008;25:215–224. doi: 10.1080/07420520802106835. [DOI] [PubMed] [Google Scholar]
- Gill J. Exercise and postprandial lipid metabolism – an analysis of the current evidence. European Journal of Lipid Science and Technology. 2004;106:110–121. [Google Scholar]
- Gormsen LC, Gjedsted J, Gjedde S, Vestergaard ET, Christiansen JS, Jørgensen JO, Nielsen S, Møller N. Free fatty acids decrease circulating ghrelin concentrations in humans. Eur. J. Endocrinol. 2006;154:667–673. doi: 10.1530/eje.1.02146. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Holmbäck U, Forslund A, Forslund J, Hambraeus L, Lennernäs M, Lowden A, Stridsberg M, Åkerstedt T. Metabolic responses to nocturnal eating in men are affected by sources of dietary energy. J. Nutr. 2002;132:1892–1899. doi: 10.1093/jn/132.7.1892. [DOI] [PubMed] [Google Scholar]
- Holmbäck U, Forslund A, Lowden A, Forslund J, Åkerstedt T, Lennernäs M, Hambraeus L, Stridsberg M. Endocrine responses to nocturnal eating – possible implications for night work. Eur. J. Nutr. 2003a;42:75–83. doi: 10.1007/s00394-003-0386-6. [DOI] [PubMed] [Google Scholar]
- Holmbäck U, Lowden A, Åkerfeldt T, Lennernäs M, Hambraeus L, Forslund J, Åkerstedt T, Stridsberg M, Forslund A. The human body may buffer small differences in meal size and timing during 24-h wake period provided energy balance is maintained. J. Nutr. 2003b:2748–2755. doi: 10.1093/jn/133.9.2748. [DOI] [PubMed] [Google Scholar]
- Hulver MW, Houmard JA. Plasma leptin and exercise: recent findings. Sports Med. 2003;33:473–482. doi: 10.2165/00007256-200333070-00001. [DOI] [PubMed] [Google Scholar]
- Kanaley JA, Weltman JY, Pieper KS, Weltman A, Hartman ML. Cortisol and growth hormone responses to exercise at different times of day. J. Clin. Endocrinol. Metab. 2001;86:2881–2889. doi: 10.1210/jcem.86.6.7566. [DOI] [PubMed] [Google Scholar]
- King NA, Blundell JE. High-fat foods overcome the energy expenditure induced by high-intensity cycling or running. Eur. J. Clin. Nutr. 1995;49:114–123. [PubMed] [Google Scholar]
- King NA, Burley VJ, Blundell JE. Exercise-induced suppression of appetite: effects on food intake and implications for energy balance. Eur. J. Clin. Nutr. 1994;48:715–724. [PubMed] [Google Scholar]
- King NA, Lluch A, Stubbs RJ, Blundell JE. High dose exercise does not increase hunger or energy intake in free living males. Eur. J. Clin. Nutr. 1997;51:478–483. doi: 10.1038/sj.ejcn.1600432. [DOI] [PubMed] [Google Scholar]
- Kraemer RR, Johnson LG, Haltom R, Kraemer GR, Hebert EP, Gimpel T, Castracane VD. Serum leptin concentrations in response to acute exercise in postmenopausal women with and without hormone replacement therapy. Proc. Soc. Exp. Bio. Med. 1999;221:171–177. doi: 10.1046/j.1525-1373.1999.d01-72.x. [DOI] [PubMed] [Google Scholar]
- Laferrère B, Abraham C, Awad M, Jean-Baptiste S, Hart AB, Garcia-Lorda P, Kokkoris P, Russell CD. Inhibiting endogenous cortisol blunts the meal-entrained rise in serum leptin. J. Clin. Endocrinol. Metab. 2006;91:2232–2238. doi: 10.1210/jc.2005-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluch A, King NA, Blundell JE. Exercise in dietary restrained women: no effect on energy intake but change in hedonic ratings. Eur. J. Clin. Nutr. 1998;52:300–307. doi: 10.1038/sj.ejcn.1600555. [DOI] [PubMed] [Google Scholar]
- Malmström R, Taskinen MR, Karonen SL, Yki-Järvinen H. Insulin increases plasma leptin concentrations in normal subjects and patients with NIDDM. Diabetologia. 1996;39:993–996. doi: 10.1007/BF00403921. [DOI] [PubMed] [Google Scholar]
- Maraki M, Tsofliou F, Pitsiladis YP, Malkova D, Mutrie N, Higgins S. Acute effects of a single exercise class on appetite, energy intake and mood. Is there a time of day effect? Appetite. 2005;45:272–278. doi: 10.1016/j.appet.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Möhlig M, Spranger J, Otto B, Ristow M, Tschöp M, Pfeiffer AF. Euglycemic hyperinsulinemia, but not lipid infusion, decreases circulating ghrelin levels in humans. J. Endocrinol. Invest. 2002;25:RC36–38. doi: 10.1007/BF03344062. [DOI] [PubMed] [Google Scholar]
- Nakagawa E, Nagaya N, Okumura H, Enomoto M, Oya H, Ono F, Hosoda H, Kojima M, Kangawa K. Hyperglycaemia suppresses the secretion of ghrelin, a novel growth-hormone-releasing peptide: responses to the intravenous and oral administration of glucose. Clin. Sci. (Lond.) 2002;103:325–8. doi: 10.1042/cs1030325. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Touitou Y, Smolensky MH. Ethical and methodological, standards for laboratory and medical biological rhythm research. Chronobiol. Int. 2008;25:996–1016. doi: 10.1080/07420520802544530. [DOI] [PubMed] [Google Scholar]
- Reinberg A, Migraine C, Apfelbaum M, Brigant L, Ghata J, Vieux N, Laporte A. Circadian and ultradian rhythms in the feeding behaviour and nutrient intakes of oil refinery operators with shift-work every 3-4 days. Diabete. Metab. 1979;5:33–41. [PubMed] [Google Scholar]
- Reeves SL, Newling-Ward E, Gissane C. The effect of shift-work on food intake and eating habits. Nutrition & Food Science. 2004;34:216–221. [Google Scholar]
- Ribeiro DC, Hampton SM, Morgan L, Deacon S, Arendt J. Altered postprandial hormone and metabolic responses in a simulated shift work environment. J. Endocrinol. 1998;158:305–10. doi: 10.1677/joe.0.1580305. [DOI] [PubMed] [Google Scholar]
- Romon M, Edme JL, Boulenguez C, Lescroart JL, Frimat P. Circadian variation of diet-induced thermogenesis. Am. J. Clin. Nutr. 1993;57:476–480. doi: 10.1093/ajcn/57.4.476. [DOI] [PubMed] [Google Scholar]
- Ryan AS, Elahi D. The effects of acute hyperglycemia and hyperinsulinemia on plasma leptin levels: its relationships with body fat, visceral adiposity, and age in women. J. Clin. Endocrinol. Metab. 1996;81:4433–4438. doi: 10.1210/jcem.81.12.8954055. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U.S.A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Weibel L, Brandenberger G. Twenty-four-hour rhythms of plasma glucose and insulin secretion rate in regular night workers. Am. J. Physiol. Endocrinol. Metab. 2000;278:E413–420. doi: 10.1152/ajpendo.2000.278.3.E413. [DOI] [PubMed] [Google Scholar]
- Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, Kobayashi E, Kido T, Nogawa K. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity. 2008;16:1887–1893. doi: 10.1038/oby.2008.298. [DOI] [PubMed] [Google Scholar]
- Thompson DA, Wolfe LA, Eikelboom R. Acute effects of exercise intensity on appetite in young men. Med. Sci. Sports. Exerc. 1988;20:222–227. doi: 10.1249/00005768-198806000-00002. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Hoggard N, Mercer JG, Rayner DV. Hormonal and neuroendocrinology regulation of energy balance – the role of leptin. Arch. Tierernahr. 1998;51:177–185. doi: 10.1080/17450399809381917. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Labor – Bureau of Labor Statistics Workers on flexible and shift schedules in May 2004. 2005. http://www.bls.gov/news.release/pdf/flex.pdf. Accessed 5 April 2009.
- Utriainen T, Malmström R, Mäkimattila S, Yki-Järvinen H. Supraphysiological hyperinsulinemia increases plasma leptin concentrations after 4 h in normal subjects. Diabetes. 1996;45:1364–1366. doi: 10.2337/diab.45.10.1364. [DOI] [PubMed] [Google Scholar]
- van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr. Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- Vickers AJ, Altman DG. Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltman A, Pritzlaff CJ, Wideman L, Considline RV, Fryburg DA, Gutgesell ME, Hartman ML, Veldhuis JD. Intensity of acute exercise does not affect serum leptin concentrations in young men. Med. Sci. Sports. Exerc. 2000;32:1556–1561. doi: 10.1097/00005768-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga MS, Verwegen CR, Ijedema MJ, Wijckmans NE, Saris WH. Acute effects of exercise or sauna on appetite in obese and nonobese men. Physiol. Behav. 1997;62:1345–1354. doi: 10.1016/s0031-9384(97)00353-3. [DOI] [PubMed] [Google Scholar]
- Woods SC, Stein LJ, McKay LD, Porte D., Jr. Suppression of food intake by intravenous nutrients and insulin in the baboon. Am. J. Physiol. 1984;247:R393–401. doi: 10.1152/ajpregu.1984.247.2.R393. [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001;86:5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]