Abstract
Targeted therapy of cancer using oncolytic viruses has generated much interest over the past few years in the light of the limited efficacy and side effects of standard cancer therapeutics for advanced disease. In 2006, the world witnessed the first government-approved oncolytic virus for the treatment of head and neck cancer. It has been known for many years that viruses have the ability to replicate in and lyse cancer cells. Although encouraging results have been demonstrated in vitro and in animal models, most oncolytic viruses have failed to impress in the clinical setting. The explanation is multifactorial, determined by the complex interactions between the tumor and its microenvironment, the virus, and the host immune response. This review focuses on discussion of the obstacles that oncolytic virotherapy faces and recent advances made to overcome them, with particular reference to adenoviruses.
Keywords: oncolytic virus, adenovirus, vaccinia virus, cancer gene, host immune response
1. Introduction
Cancer is a major cause of death globally. Although treatments for the disease have improved significantly, conventional chemotherapy or radiotherapy still have limited effects against many forms of cancer, not to mention a plethora of treatment-related side effects. This situation signifies a need for novel therapeutic strategies, and one such approach is the use of viruses. The ability of viruses to kill cancer cells has been recognized for more than a century [1]. They achieve this by a number of mechanisms, including direct lysis, apoptosis, expression of toxic proteins, autophagy and shut-down of protein synthesis, as well as the induction of anti-tumoral immunity. Although clinical trials of several naturally-occurring oncolytic viruses were started back in the 1950s, it was only in 1991 that a herpes simplex virus-1 (HSV-1) with deletion of its thymidine kinase UL23 gene became the first genetically-engineered, replication-selective oncolytic virus to be tested in the laboratory [2]. In 2005, an adenovirus (Ad) with E1B 55K gene deletion (H101(Oncorine); Shanghai Sunway Biotech, Shanghai, China) was approved in China as the world’s first oncolytic virus for head and neck cancer in combination with chemotherapy [3]. However, until now the widespread use of oncolytic virotherapy is still far from reality. Promising laboratory results have not been translated to improved clinical outcomes, and this appears to be determined by the complex interactions between the tumor and its microenvironment, the virus, and the host immunity. There are already a number of reviews on oncolytic viruses for cancer treatment but this article will focus on the obstacles facing oncolytic virotherapy, with particular reference to Ads, and the recent advances made to overcome these hurdles.
Mechanisms of tumor selectivity
The term ‘oncolytic viruses’ applies to viruses that are able to replicate specifically in and destroy tumor cells, and this property is either inherent or genetically-engineered. Inherently tumor-selective viruses can specifically target cancer by exploiting the very same cellular aberrations that occur in these cells, such as surface attachment receptors, activated Ras and Akt, and the defective interferon (IFN) pathway (Figure 1). Some viruses have been engineered with specific gene deletion – these genes are crucial for the survival of viruses in normal cells but expendable in cancer cells (Figure 2). Deletion of the gene that encodes thymidine kinase, an enzyme needed for nucleic acid metabolism, results in dependence of viruses such as HSV and vaccinia virus on cellular thymidine kinase expression, which is high in proliferating cancer cells but not in normal cells. Vaccinia also produces the vaccinia growth factor (VGF) that binds to and activates the epidermal growth factor receptor (EGFR), creating an environment that supports its replication. It follows that deletion of genes encoding for both thymidine kinase and VGF leads to further selectivity of vaccinia virus in cancers with an activated EGFR-Ras pathway [4]. Another approach in conferring tumor selectivity is to restrict virus replication by its dependence on transcriptional activities that are constitutively activated in tumor cells. This can be achieved by the insertion of a tumor-specific promoter driving the expression of a critical gene [5–11]. Others viruses either possess naturally (e.g., Coxsackievirus A21 [12] and measles virus (MV) [13]) or have been designed to have specific tropism based on the expression of cell surface receptors unique to cancer cells [14–20].
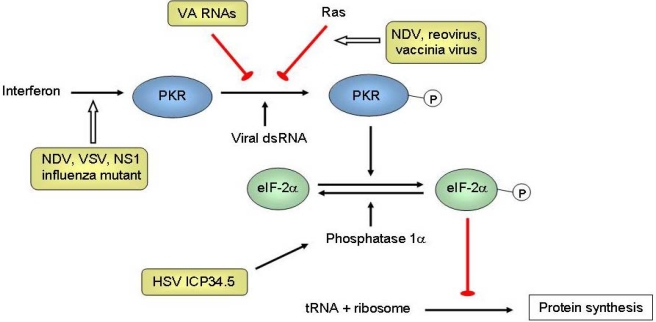
Figure 1.
Mechanisms of tumor selectivity of several oncolytic viruses. The interferon (IFN)/double-stranded RNA-activated protein kinase (PKR) pathway is a natural anti-viral defense system. IFNs produced by infected cells result in the upregulation of PKR. On binding to viral double-stranded RNA (dsRNA), PKR autophosphorylates, which in turn phosphorylates the α subunit of eIF-2. Phosphorylated eIF-2α sequesters eIF-2B, a guanine nucleotide exchange factor. Without eIF-2B, the GDP bound to eIF-2 cannot be exchanged for GTP. As a result eIF-2 is unable to bring the initiator transfer RNA (tRNA) to the 40S ribosomal subunit, and the synthesis of viral protein is inhibited. Inactivated IFN and activated Ras pathways are frequently found in cancer (the latter could inhibit PKR), and some naturally-found viruses can replicate selectively in cancer but not normal cells, including the Newcastle disease virus (NDV) [21], reovirus [22], vaccinia virus [23], and vesicular stomatitis virus (VSV) [24]. The herpes simplex virus (HSV) protein ICP34.5 interacts with cellular phosphatase 1α to dephosphorylate eIF-2α, leading to synthesis of proteins needed for virus replication. Deletion of gene that encodes for ICP34.5 (RL1) results in selective replication in tumors with a defective IFN/PKR pathway [25]. The influenza virus NS1-deleted mutant is also dependent on this defective pathway [26]. Adenoviruses normally produce virus-associated (VA) RNAs to inhibit PKR. As such, engineered VAI-deleted adenovirus (dl331) could replicate selectively in tumors with an activated Ras pathway [27]. Epstein-Barr virus (EBV) also expresses RNAs similar to VA RNAs and these can complement dl331, resulting in selectivity in EBV-associated tumors [28].
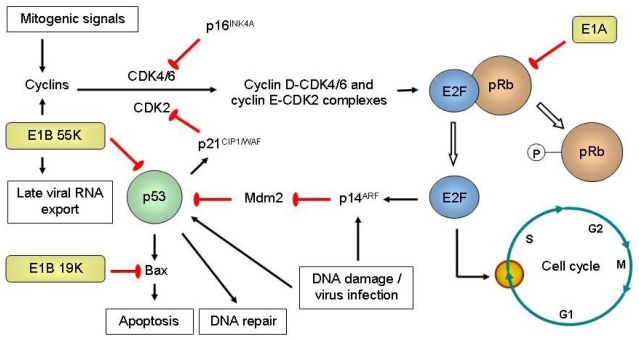
Figure 2.
Engineered replication selectivity of oncolytic adenoviruses (Ads) by deletion of the E1A, E1B 19K or E1B 55K gene. Retinoblastoma protein (pRb) is normally hypophosphorylated and binds to transcription factors of the E2F family to regulate the G1-to-S checkpoint of the cell cycle. Upon stimulation by mitogenic signals, upregulation of cyclins enables cyclin-dependent kinases (CDKs) to phosphorylate pRb, releasing E2F that leads to the expression proteins needed for DNA synthesis and thus cell cycle progression. E2F upregulates p14ARF, which inhibits Mdm2. Mdm2 normally results in p53 degradation. p53 is a transcription factor that is upregulated and activated by stress signals such as virus infection or DNA damage. It results in the expression of proteins that induce apoptosis (Bax), cell cycle arrest (p21CIP1/WAF via its inhibition of CDK2) or DNA repair. p16INK4A is a tumor suppressor that inactivates CDK4/6. The adenoviral E1A proteins bind to pRb to release E2F, so that viral DNA could be replicated. E1A also promotes the acetylation of pRb by p300/CBP, causing pRb to associate with Mdm2 to inhibit p53. Because cancer cells are often in the S phase, E1A CR2-deleted Ad5 mutant (dl922-947) could selectively replicate in and destroy replicating cancer cells but not normal resting cells [29]. E1B 19K binds to and inhibits Bax. The tumor selectivity of E1B 19K-deleted Ad2 (dl250) is due to multiple defects in the apoptotic pathways, where survival of the virus in normal cells would be limited owing to rapid apoptosis induction in the presence of tumor necrosis factor-α (TNF-α) [30]. E1B 55K interacts with the adenovirus E4 open reading frame 6 (E4orf6) protein to form an E3 ubiquitin ligase complex that targets p53 for degradation. It also induces the expression of cyclin E as well as simultaneously inhibits cellular mRNA export and promotes the export of late viral mRNAs. E1B 55K-deleted Ad could replicate in tumor selectively because of non-functioning p53 [31], cyclin E overexpression [32], and E1B 55K-independent late viral RNA export in cancer but not normal cells [33].
More recently, gene silencing by RNA interference technology has been utilized to confer tumor selectivity. MicroRNAs (miRNAs) or small interfering RNAs (siRNAs) regulate gene expression post-transcriptionally by translation block or cleavage of specific, complementary mRNA via the RNA-induced silencing complex (RISC). By inserting a complementary sequence next to a critical viral gene, it is possible to confine virus replication to tumor but not normal cells that express high levels of the corresponding miRNA. This has been demonstrated by several groups [34–38]. Gürlevik et al. [39] developed a recombinant Ad that encodes multiple RNA-interfering transcripts under the control of a p53-responsive promoter. The transcripts could effectively silence a set of critical viral genes. As p53 is a transcription factor often lost or mutated in human malignancy, this virus could therefore replicate in cancer but not normal cells where functional p53 would lead to an anti-viral RNA interference.
Optimizing oncolytic viruses for improved anti-tumoral potency
Gene-manipulated oncolytic viruses such as Ad, herpes virus and vaccinia virus are being developed as a new class of anti-tumoral agent [23,40,41]. Selective intratumoral replication of the virus may lead to improved efficacy over non-replicating agents due to the self-perpetuating nature of the treatment with virus multiplication, lysis of the infected tumor and spread to adjacent cells. One potential limitation of this approach, however, is that gene deletions resulting in tumor selectivity also frequently result in reduced oncolytic potency. For example, dl1520 (ONYX-015; Onyx Pharmaceuticals, California, USA) is an oncolytic Ad2/Ad5 hybrid with deletion of its E1B 55K and E3B genes. The E1B 55K protein is involved in p53 inhibition, viral mRNA transport and host cell protein synthesis shut-off [42] (Figure 2), whilst E3B proteins are important for immune avoidance (see below). This virus was the first engineered, replicating Ad to enter clinical trials for cancers including those of the head and neck [43–45] and pancreas [46,47]. Whilst the virus has shown good tumor selectivity and safety [48], durable objective responses with this virus as a single agent have been limited and this could be partly due to the loss of other essential functions of the E1B 55K and E3B genes. A recent finding by Thomas et al. [49] revealed that dl1520 was less efficient in lysing cells infected in the G1 phase of the cell cycle due to a reduced rate of late viral protein synthesis, and this appears to be a result of the adenoviral gene product encoded by open reading frame 1 of early region 4 (E4orf1). As such there is a need to increase the potency of these viruses by identifying mutations that result in tumor selectivity but not those that result in attenuated virus replication and oncolysis. Since the first generation of replication-selective Ads was tested in pre-clinical experiments and clinical trials, several advances have been made to improve potency by dissecting the functions of different genes of Ad.
The adenoviral E1A is the earliest gene to be transcribed after virus entry into the host cell [50]. E1A normally interacts with the retinoblastoma protein (pRb) (the latter is important in regulating the G1-to-S cell-cycle checkpoint), and this pushes quiescent cells into S phase to allow for virus replication (Figure 2). Therefore, dl922–947, the mutant Ad with specific deletion of the E1A CR2 region (pRb binding site), was unable to replicate in quiescent normal cells but was able to do so in cancer cells with defective G1-to-S checkpoint. This virus has demonstrated superior anti-tumoral activity in vivo compared to dl1520 after intratumoral and intravenous injections [29], although it might also target proliferating non-malignant cells. In addition to its effect on virus release and spread [51,52], adenoviral E1B 19K is a functional homolog of Bcl-2 and is able to bind to Bax [53–55] and also prevent Fas-mediated apoptosis [56]. Replication of the mutant Ad2 with E1B 19K deletion (dl250) was significantly reduced in normal cells secondary to rapid apoptosis induction in the presence of tumor necrosis factor-α (TNF-α), whilst the opposite occurred in cancer cells due to multiple defects in the apoptotic pathways (e.g., p53 mutation, Bcl-2 overexpression) [30] (Figure 2). Virus replication, spread and anti-tumoral potency was significantly better than dl1520 and wild-type Ad2. E1B 19K-deleted Ad5-infected cancer cells also expressed lower levels of EGFR and anti-apoptotic proteins [57].
Ads also produce the virus-associated (VA) RNAs. These are RNA polymerase III transcripts that, amongst other functions, are obligatory for efficient translation of viral and cellular mRNAs by blocking the double-stranded RNA-activated protein kinase (PKR) [58,59], a natural host anti-viral defense system (Figure 1). We have shown that VAI-deleted Ad5 (dl331) was able to selectively target Epstein-Barr virus (EBV)-associated tumors such as Burkitt’s lymphoma and nasopharyngeal carcinoma [28]. This is because EBV expresses the RNAs EBER1 and EBER2, whereby EBER1 could complement dl331 to enable the synthesis of viral proteins. Interestingly, anti-tumoral efficacy in vitro and in vivo was superior to wild-type Ad5 and this might be the result of PKR-induced apoptosis, increased IFN-β production, and the adenoviral E3B gene deletion.
Gene products encoded by the adenoviral E3 region could also affect its oncolytic potency. These include the E3 11.6K (or adenovirus death protein – ADP), which facilitates late cytolysis of infected cells and release of progeny viruses [60]. Ads that overexpress ADP showed better cell lysis and spread [61,62]. The effects of E3B and E3 gp19K genes on the potency of oncolytic adenovirus will be discussed later.
Arming oncolytic viruses with therapeutic genes
The discovery of the genetic basis of malignancy has in part promoted the development of cancer gene therapy, which involves the introduction of exogenous nucleic acid to restore, express or inhibit a particular gene of interest. Viruses are at present the most efficient gene delivery system. A well-known example is Gendicine (Shenzhen SiBiono GeneTech, Shenzhen, China), an Ad5 vector encoding the human TP53 gene that was approved in 2004 by China’s State Food and Drug Administration for the treatment of head and neck cancer [63]. Although developed for safety reasons, one major shortcoming of using non-replicating vectors such as Gendicine (by virtue of its E1A gene deletion) is that infectivity is limited to only one cycle. In contrast, oncolytic viruses can replicate and spread in cancer cells resulting in longer transgene expression. Together with tumor lysis this would lead to better therapeutic efficacy. Arming oncolytic viruses with anti-cancer genes has been a major focus in cancer virotherapy, and transgenes exploited include tumor suppressor, pro-apoptotic, anti-angiogenic, “suicide”, and immunomodulatory genes.
Like Gendicine, oncolytic viruses could be armed with tumor suppressor or pro-apoptotic genes that are frequently lost in cancer. One example is by the use of p16INK4A-armed oncolytic Ad, which has shown good inhibition of gastric tumor xenografts [64]. Wang et al. [65] developed an Ad in which the E1A gene is regulated by the human telomerase reverse transcriptase (hTERT) promoter and hypoxia response element, together with p53 under the strong cytomegalovirus (CMV) promoter. This virus showed tumor selectivity with efficient p53 expression and oncolysis. Nonetheless, targeting a single gene is unlikely to have a major impact on survival, given that in cancer a large number of genetic alterations affect only a core set of signaling pathways and processes, as has been recently described for pancreatic cancer [66]. Hence there should be a move from targeting these genes individually to targeting cancer signaling pathways, such as arming oncolytic Ad with an engineered transgene that encodes transforming growth factor (TGF)-β receptor II fused with the human Fc IgG1, as studied by Hu et al. [67]. Anti-tumoral effects were observed with a replication-selective (but not replication-deficient) virus encoding this gene, highlighting the importance of virus replication. Viruses that enhance the apoptotic pathways have also been studied. Jin et al. [68] and Chen et al. [69] utilized the chimeric Ad5/35 carrying the gene encoding the TNF-related apoptosis-inducing ligand (TRAIL) to promote receptor-independent infection (see below) and apoptosis of leukemic and gastric cancer cells, respectively. Zhang et al. [70] treated pancreatic cancer cells by replacing the gene for human somatostatin receptor 2 (lost in 90% of pancreatic cancers) and introducing the gene for TRAIL by means of an oncolytic Ad, with good results in vivo. A reciprocal approach is to ablate the function of oncogenes post-transcriptionally by arming oncolytic Ad with small hairpin RNA (shRNA). Recent work includes those targeting hTERT [71], Ki-67 [72], Survivin [73], and Apollon [74], all of which have shown efficient anti-tumoral effects in vitro and in vivo.
The tumor microenvironment plays a critical role in promoting malignant cell growth and progression, as well as restricting virus spread. One important issue is tumor angiogenesis. A recent finding by Ikeda et al. [75] suggested that the replication-selective Ad OBP-301, in which the E1 genes are under the control of the hTERT promoter, could stimulate peripheral blood mononuclear cells (PBMCs) to produce IFN-γ that has anti-angiogenic properties, resulting in reduced tumor vascularity and slowed growth in immunocompetent mice. However, Kurozumi et al. [76] also showed that intratumoral treatment of rat glioma with oncolytic HSV could promote neovascularization of the residual tumor, and this was associated with a significant increase in the angiogenic factor CYR61. This could have an impact on subsequent tumor growth and the observation suggests that a combination of oncolytic virus with anti-angiogenic transgene might be needed; for this we refer the reader to our recent article for a more comprehensive review [77]. Recent work includes the use of the anti-angiogenic factors endostatin/angiostatin [78–80], interleukin-18 (IL-18) [81,82], canstatin [83], and trichostatin A [84], as well as arming viruses with genes that inhibit pro-angiogenic molecules such as IL-8 [85] and vascular endothelial growth factor (VEGF) [86,87]. Kang et al. [88] made use of a transcriptional repressor based on zinc-finger protein to target the VEGF promoter. An oncolytic Ad armed with this gene significantly reduced vessel density and tumor size of human glioblastoma xenografts in mice. The matrix metalloproteinases (MMPs) are a family of proteolytic enzymes that degrade the extracellular matrix and are essential for tumor spread and neovascularization. Oncolytic viruses armed with genes that encode MMP inhibitors have shown encouraging results in delaying tumor growth and angiogenesis [89,90].
Gene-directed prodrug activation therapy (or suicide gene therapy) involves the delivery of a gene that would lead to the expression of an enzyme, followed by the administration of a prodrug that is activated selectively by this enzyme. One example is the HSV thymidine kinase (HSV-TK)-ganciclovir method, whereby HSK-TV is able to monophosphorylate ganciclovir, which is subsequently converted by cellular kinases to the triphosphorylated forms, blocking DNA synthesis and inducing cell death. Most publications have described the use of replication-deficient viruses with this approach, but recent studies that demonstrated its efficacy using replication-selective oncolytic Ads include treatment for prostate [91], gallbladder [92], and liver [93] cancers. Alternative combinations include nitroreductase with the prodrug CB1954 (converted into an alkylating agent) [94], and cytosine deaminase (CD) with 5-fluorocytosine, which is converted into the cytotoxic and radiosensitizing 5-fluorouracil [95,96]. An Ad5 with E1B 55K deletion, ADP overexpression and CD/TK fusion gene expression is currently in a phase III trial in combination with radiotherapy for patients with prostate cancer.
The tumor environment and oncolytic viruses
Viruses are naturally larger than other anti-cancer agents such as chemicals and antibodies (for example 90 nm and 300 nm for Ad and vaccinia virus, respectively). After intratumoral injection, effective virus spread could be impaired by the extracellular matrix, areas of fibrosis and necrosis, and surrounding normal cells in the tumor bed, although Kolodkin-Gal et al. [97] found that the extracellular components collagen and mucin could restrict HSV-1 infectivity in normal colon, but these molecules were expressed in lesser amounts in colonic carcinoma, facilitating its spread. Ganesh et al. [98] studied the co-administration of the enzyme hyaluronidase with oncolytic Ads during intratumoral injection. This degraded the major constituents of the extracellular matrix, hyaluronan, resulting in enhanced virus spread in vivo. Induction of cancer cell death with an apoptosis-inducing agent prior to injection of oncolytic HSV could also produce channels for effective virus spread [99]. Elevated interstitial hydrostatic pressure as a result of fibrosis and vessel abnormalities poses another physical barrier to successful virus delivery and this effect increases with tumor volume [100]. Injected viruses could escape back through the injection site or by drainage into the circulation, resulting in reduced efficacy and increased risk of systemic toxicities. Bazan-Peregrino et al. [101] examined the retention of Ad5 in MDA-231 and ZR75.1 human breast carcinoma xenografts after intratumoral injection. For MDA-231, occlusion of injection sites with surgical adhesives and the use of small injected volumes resulted in significantly higher virus retention within the tumors. ZR75.1, however, took up more Ad than MDA-231 when identically infected, suggesting a role of tumor type in virus retention. Recently, tumor-associated stromal cells have been shown to play a role in either enhancing or reducing the efficacy of oncolytic Ads, depending on the tumor type [102]. Hypoxia, a common feature in tumor tissues, has been found to reduce the replicative and oncolytic potential of Ads despite the unaltered expression of surface receptors [103,104]. In this regard there might be a role for the development of oncolytic viruses in which replication is not attenuated by hypoxia, such as vaccinia virus [105] or HSV [106,107].
For viruses that have reached the immediate vicinity of the tumor, cellular genetic changes could prevent successful virus entry into the cells. For cellular entry of most Ads (those in subgroups A, C, D, E and F – which include the commonly used Ad5), they must first bind to the Coxsackie and adenovirus receptor (CAR) on the surface membrane via the knob portions of their fibers, followed by internalization mediated by the viral penton proteins and cellular integrins. CAR is ubiquitously expressed in epithelial cells, but its expression is often downregulated in many cancer types due to activation of the Raf-MAPK pathway [108]. Recent work has shown that the molecule leucine-rich repeat-containing protein 15 (LRRC15 or hLib), frequently overexpressed in tumor cells, could result in the redistribution of CAR away from cell surfaces, thus impeding Ad infection [109]. In contrast, most subgroup B Ads bind to CD46 [110], a receptor often upregulated in a number of tumor types, including breast, cervical, liver, lung, endometrial and hematological malignancies [111–113]. Several chimeric oncolytic Ad5 have been developed to contain the fiber tropism of subgroup B Ads and they all have shown encouraging results [68,69,114–117]. The use of intact subgroup B Ads as oncolytic agents is still under-explored but has great potential [118,119]. They have different tropism and infectivity compared to chimeric viruses [120], and are more beneficial in terms of a reduced propensity for neutralization by pre-existing antibodies (see below). Besides CD46, evidence suggests that the subgroup B Ad, Ad11, also utilizes another unidentified receptor [121,122], tentatively named ‘receptor X’ by Tuve et al. [123]. They also discovered that the other subgroup B Ads, Ad16, -21, -35 and -50 exclusively use CD46, whereas Ad3, -7 and -14 use ‘receptor X’ but not CD46. It is possible that Ad11 could infect a wider range of tumor cells and overcome receptor downregulation; the latter is a known problem with Ad35 and CD46 [124]. Strauss et al. [125] showed that Ads that utilize CAR or CD46 as primary attachment receptors failed to infect and lyse ovarian cancer cells of the epithelial phenotype, which are found in in situ tumors and tumor xenografts. These receptors are trapped in the tight junctions and therefore not accessible to the virus. However, Ads that use receptor X (Ad3, -7, -11 and -14) could induce epithelial-mesenchymal transition and result in efficient oncolysis.
Cellular signaling pathways can also affect virus infectivity. Recently our group [126] has shown that certain pancreatic cancer cell lines overexpress the carcinoembryonic antigen–related cell adhesion molecule 6 (CEACAM6), which antagonizes the Src signaling pathway, downregulates cancer cell cytoskeleton proteins, and blocks Ad trafficking to the nucleus. Knockdown of CEACAM6 by siRNA significantly enhanced the anti-tumoral potency of oncolytic Ad5. For virus that has successfully entered the cell, it needs to replicate for efficient cell lysis and virus spread. The protein p21CIP1/WAF normally inhibits cyclin-dependent kinase 2 (CDK2) (Figure 2) and blocks the progression of the cell cycle from G1 to S phase. Shiina et al. [127] showed that siRNA knockdown of p21CIP1/WAF increased Ad replication and oncolysis. It was suggested that this could be due to the inhibition of SET and proliferating cell nuclear antigen (PCNA) by p21CIP1/WAF, whereby SET and PCNA normally increase viral DNA replication. In the case of vaccinia virus, recent work has suggested that cells with activated c-Jun NH2-terminal kinase (JNK) signaling cascade could activate PKR (Figure 1), thus reducing virus replication [128].
Cancer stem cells form part of the heterogenous tumor population. They not only contribute to neoplastic progression and metastasis, but also to resistance to chemotherapy and radiotherapy. Evidence has shown that oncolytic Ads are able to destroy these cells [129–131]. Zhang et al. [132] have recently demonstrated that a telomerase-specific oncolytic Ad armed with a gene that encodes the apoptotic TRAIL was able to preferentially target stem-like esophageal cancer cells and prolong the survival of mice bearing tumors composed of these cells. Whilst this is of interest, cancer stem cells only form a small subset of the tumor mass and the value of targeting them specifically will remain an issue to be resolved.
Modification of the host immune response in favor of oncolytic viruses
Most studies of oncolytic viruses have been done, by necessity, on human tumor xenografts in immunodeficient mice – far from reflective of the human condition. Unsurprisingly, data from these studies have not been predictive for clinical trial results. The effects of the host immune response on the efficacy of oncolytic viruses are complex. When stimulated, immune cells could result in virus clearance but might also induce specific and non-specific anti-tumoral activities. It appears that the innate immune response plays an important role in virus clearance, whereas T cell-mediated responses are largely responsible for the anti-tumoral effect [133–137].
For the treatment of metastatic or hematological malignancies, intravenous virus delivery could be hindered by neutralizing antibodies, complement activation, non-specific uptake by other tissues such as the liver and spleen, as well as poor virus escape from the vascular compartment (Figure 3). For Ad, adhesion to blood cells could also lead to therapeutic inhibition [138]. Numerous experiments have been done to modify the immune response in favor of virus replication and tumor lysis. One method is by using an immunosuppressive agent, such as cyclophosphamide, that has been shown to improve virus spread and anti-tumoral efficacy [139–145]. Kurozumi et al. [146] found that single doses of the angiostatic and anti-inflammatory cyclic peptide of arginine-glycine-aspartic (cRGD), given before an oncolytic HSV, resulted in reduced tumor vessel permeability, leukocyte infiltration and IFN-γ, leading to increased survival of rats with intracranial gliomas. Various data suggest that pre-existing antibodies decrease virus spread after intravenous delivery [147–149], but have a lesser effect on intratumoral injection [44,150,151]. Although antibodies could prevent possible toxicity [152], they could also reduce efficacy. Possible ways to circumvent this include plasmapheresis to deplete antibodies and the use of other viral strains with a lower prevalence of antibodies in the human population. One example is Ad11 [118,119], with a reported antibody prevalence of 10–31% compared to 45–90% for Ad5 [122,153–155]. These antibodies are mainly directed against the viral hexon proteins [156], suggesting that the use of Ad11 virion might be better than chimeric Ad5/11, where the fibers are derived from Ad11 but the rest, including hexon, belong to Ad5. A caveat to this is that for unknown reasons, Ad11 appears to induce more pro-inflammatory cytokines and chemokines than Ad5 or Ad5/11 in mice after systemic injection [120].
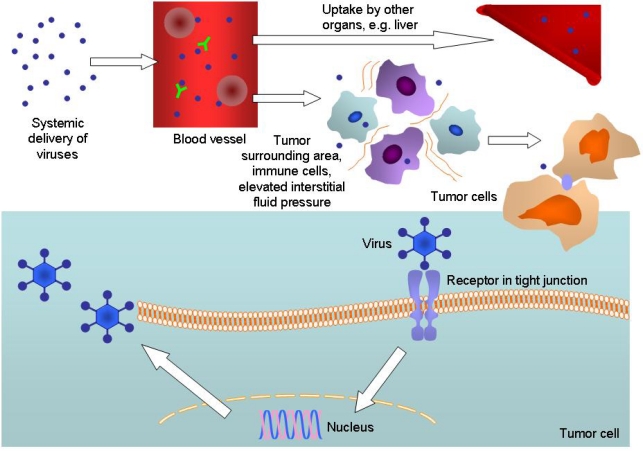
Figure 3.
Obstacles to successful delivery of oncolytic viruses to tumor cells. After intravenous injection, viruses are neutralized by pre-existing antibodies and complement activation. Adenoviruses (Ads) also interact with blood cells. Recent work has revealed that Ad5 binds to erythrocytes via the Coxsackie and adenovirus receptor (CAR) and complement receptor 1 (CR1) in the absence and presence of anti-Ad5 antibodies, respectively [178]. Sequestration into other organs and the reticuloendothelial system is a particular problem, often with resulting toxicities. From the blood stream, viruses have to pass through a mixture of extracellular matrix, cells (including normal and immune cells) and high interstitial fluid pressure before reaching the tumor. They then have to attach to the cellular receptor (often trapped in tight junction), be internalized, translocate to the nucleus, replicate, produce structural and other proteins, lyse the cell and release their progenies – some of these steps could be inhibited by factors such as the natural host immune response, hypoxic environment, soluble factors, and genetic changes in the tumor cell.
Instead of injecting naked virions, using cells as delivery vehicles could hide the viral antigen from antibodies and complements. This so-called “Trojan horse” strategy involved infecting the body’s cells in vitro and administering these cells back systemically, which would then carry the oncolytic virus to the tumor environment. Cells that have been tested include mesenchymal stem cells [157–159], monocytes [160], outgrowth endothelial cells [160], tumor cells [161–163], T cells [164–166], and dendritic cells (DCs) [165]. Ong et al. [167] showed that MV-infected T cells could facilitate tumoral delivery in low, but not high antibody concentration. Power et al. [168] tested a number of carrier cells including solid tumor and leukemic cells, and demonstrated that the efficacy of oncolytic vesicular stomatitis virus (VSV) was significantly improved compared to naked virion injection. Interestingly, Zhu et al. [169] demonstrated that mice pre-immunized with HSV exhibited reduced growth of S-180 tumor after intratumoral treatment with HSV. PBMCs from seropositive mice showed greater cytotoxicity in vitro compared to naïve mice, with higher IFN-γ induction. It is not known if this also applies to intravenous virus delivery or to other oncolytic viral species. Whilst the cell carrier approach has yielded promising data in vivo, numerous issues must be considered before clinical application, including the best cell type to use, ease of infection, tumor-targeting capabilities, protection of virus from the host immune response, virus delivery, and tumorigenicity. Recently Kangasniemi et al. [170] have demonstrated that silica gel-encapsulated Ads allowed for extended release of the viruses and slightly delayed the development of anti-Ad antibodies. This method has anti-tumoral activity, but comparison with other methods of administration was not performed.
After intravenous delivery the liver, part of the reticuloendothelial system, is the predominant site of Ad5 sequestration with significant hepatocyte transduction [171,172]. Ad5 is known to cause liver toxicity, and its use has raised some concerns after the death of Jesse Gelsinger in 1999 from Ad5-based gene therapy injected directly into the hepatic artery [173]. A landmark study by Waddington et al. [174] showed that liver transduction is mediated by interaction of the adenoviral hexon protein with the blood coagulation factor X. This provides a further rationale for the development of other Ad serotypes for oncolytic therapy, such as Ad11 and Ad35 as they bind weakly to factor X compared to Ad5 [118] or other Ad5 chimera. In CD46 transgenic mice, Ad11 persisted much longer in the circulation after intravenous delivery compared to Ad5 together with the absence of liver transduction [120,122]. As for Ad5, ways to reduce liver uptake include recent experiments performed by Barry et al. They studied the effect of Kupffer cell depletion (by pre-dosing mice with non-replicating Ad5) and warfarin treatment (to inhibit vitamin K-dependent coagulation factors) and found that this approach significantly increased the anti-tumoral effect of systemically delivered oncolytic Ad5 in nude mice [175]. Good results have also been demonstrated by coating Ad5 with high molecular weight polyethylene glycol [176] or by genetic modification of the hexon protein to ablate blood factor binding [177] for liver detargeting.
A plethora of immunostimulatory genes have been inserted into the genome of oncolytic viruses with the aim of stimulating effective anti-tumoral immune responses. Recent examples include the heat shock proteins [179,180], chemokine (C-C motif) ligand 5 (CCL5) [181], IFN [182], granulocyte macrophage colony-stimulating factor (GM-CSF) [183–185], IL-12 [186], IL-18 [81,82], and IL-24 [187,188]. Vaccinia virus normally expresses a number of type I IFN-inhibiting proteins to counteract the cellular IFN anti-viral response. Because cancer cells frequently have an inactivated IFN pathway, anti-IFN gene-deleted vaccinia could selectively replicate in these cells. Kirn et al. [189] utilized this mutant and inserted a gene that encodes IFN-β (which itself has anti-proliferative, anti-angiogenic, and immunomodulatory anti-tumoral effects), and demonstrated enhanced tumor selectivity and potency in vivo. Shashkova et al. [190] used a four-pronged approach by co-infecting cancer cells with a replicating oncolytic Ad with ADP overexpression and IFN-α expression, given together with a non-replicating virus encoding the gene for TRAIL, with impressive results. The currently used oncolytic MVs were derived from the attenuated Edmonston tag (Edmtag) strain. Significantly, they lack antagonizing activity against the host anti-viral IFN immune response, thus inhibiting virus spread. Recombinant MV encoding the measles phosphoprotein (P) gene product from wild-type MV, an IFN antagonist, has been found to exhibit reduced IFN sensitivity and better oncolytic potency in vivo [191]. A recombinant VSV vector which expresses a gene from human CMV has been found to have increased anti-tumoral activity in vivo [192]. The expressed protein inhibited the natural killer (NK) cell-activating ligand CD155, resulting in decreased accumulation of NK and NKT cells at the infected tumor site and elevated virus replication.
Antigen-specific activation and proliferation of lymphocytes are regulated by interaction of the peptide-antigen-major histocompatibility complex (MHC) with the T cell receptor, as well as both positive and negative signals from co-stimulatory molecules expressed on antigen-presenting cells (APCs). The most important of the APCs are the DCs. DCs are capable of capturing antigens secreted or shed by tumor cells and upon maturation, present the peptides to T cells. Endo et al. [193] showed that virus replication led to the production of uric acid in cancer cells, which stimulated DCs to produce IFN-γ and IL-12. IFN-γ subsequently induced the expression of the proteosome activator PA28, which functions to generate tumor antigenic peptides required for MHC class I presentation, resulting in the induction of cytotoxic T lymphocytes (CTLs) against tumor cells. Lapteva et al. [194] and Ramakrishna et al. [195] demonstrated that increased DC migration and maturation by oncolytic Ad encoding β-defensin-2 or macrophage inflammatory protein 1α (MIP-1α) and Fms-like tyrosine kinase-3 ligand (Flt3L) significantly enhanced anti-tumoral immune responses. Chuang et al. [196] used another approach whereby tumor-bearing mice were first primed with DNA encoding a highly immunogenic foreign antigen ovalbumin (OVA), followed by intratumoral injection of vaccinia virus encoding the same antigen. The DNA vaccination served to generate OVA-specific CTLs against infected cancer cells, and the virus resulted in further oncolysis. A study by Diaz et al. [135] revealed that depletion of regulatory T cells reduced the efficacy of oncolytic VSV, due to the relief of anti-viral immune response suppression. Anti-tumoral immune activity could be improved by adoptive T cell transfer or incorporation of tumor-associated antigen into the virus. Huang et al. [197] utilized an oncolytic Ad armed with IL-12 and 4-1BB ligand, and demonstrated impressive results in mice bearing B16-F10 melanoma tumors. Amongst other functions, 4-1BB ligand (expressed on DCs) enhances T cell proliferation and IL-12 promotes their differentiation. The anti-tumoral effect was even greater when the virus was given together with DCs.
The E3 region of the adenoviral genome is divided into E3A (encodes the 12.5K, 6.7K, gp19K and 11.6K proteins) and E3B (10.4K, 14.5K and 14.7K proteins) and is involved in immune response evasion and virus release from cells. Because it is dispensable, this region is frequently deleted in many adenoviral mutants to provide more space for therapeutic gene insertion, although recent work has suggested that transgene expression was higher if gene was inserted at regions other than E3, such as L3 [198]. Deletion of the whole E3B region, however, could attenuate the virus oncolytic potency by increasing macrophage infiltration and expression of TNF and IFN-γ [51,133]. Potency could be restored by selective deletion of E3 gp19K whilst retaining other E3 regions [133,199]. In addition to the inhibition of NK cell activation [200], gp19K is an endoplasmic reticulum membrane glycoprotein that inhibits the transport of MHC class I to the cell surface and delays its expression to avoid killing by CTLs [201,202]. CTL evasion is common in tumor cells and therefore the function of gp19k is redundant in these cells. Deletion of this gene, however, would ensure normal cells infected with this virus are eradicated, and in effect this confines virus replication to tumor cells.
Conclusions
The field of oncolytic virotherapy is expanding and viruses continue to hold promise as effective treatments in combination with chemotherapy or other therapeutic modalities. As continuing work is being done to improve the currently available oncolytic viruses, novel viral species are also emerging and worth exploring, for example the porcine Seneca Valley virus [203], myxoma virus [204], Sindbis virus [205], and Semliki Forest virus [206]. Viruses have unique properties in comparison to small molecular drugs. They can replicate and spread in addition to carry anti-tumoral therapeutic genes. However, during the course of evolution the human body has developed ways to overcome infection and this has imposed a significant barrier towards achieving maximum therapeutic efficacy of oncolytic viruses. Recent advances in our understanding of tumor biology and virology have helped to overcome some of these hurdles, and different groups have successfully targeted features that varied from virus delivery to altering the host immune response. It is hoped that this collective effort will finally pave way for the development of effective and safe viruses for cancer therapy.
Acknowledgments
The authors’ work is supported by Cancer Research UK, Nature Sciences Foundation of China (30530800), the Medical Research Council, Digestive Cancer Campaign and the National Institute for Health Research (Experimental Cancer Medicine Centres).
Footnotes
Competing interests
The authors declare that they have no competing interests.
References and Notes
- 1.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 2.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 3.Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 4.Thorne SH, Hwang TH, O'Gorman WE, Bartlett DL, Sei S, Kanji F, Brown C, Werier J, Cho JH, Lee DE, Wang Y, Bell J, Kirn DH. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest. 2007;117:3350–3358. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huch M, Gros A, Jose A, Gonzalez JR, Alemany R, Fillat C. Urokinase-type plasminogen activator receptor transcriptionally controlled adenoviruses eradicate pancreatic tumors and liver metastasis in mouse models. Neoplasia. 2009;11:518–528. doi: 10.1593/neo.81674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan W, Bodempudi V, Esfandyari T, Farassati F. Utilizing ras signaling pathway to direct selective replication of herpes simplex virus-1. PLoS One. 2009;4:e6514. doi: 10.1371/journal.pone.0006514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cafferata EG, Maccio DR, Lopez MV, Viale DL, Carbone C, Mazzolini G, Podhajcer OL. A novel A33 promoter-based conditionally replicative adenovirus suppresses tumor growth and eradicates hepatic metastases in human colon cancer models. Clin Cancer Res. 2009;15:3037–3049. doi: 10.1158/1078-0432.CCR-08-1161. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh JL, Lee CH, Teo ML, Lin YJ, Huang YS, Wu CL, Shiau AL. Transthyretin-driven oncolytic adenovirus suppresses tumor growth in orthotopic and ascites models of hepatocellular carcinoma. Cancer Sci. 2009;100:537–545. doi: 10.1111/j.1349-7006.2008.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakajima O, Matsunaga A, Ichimaru D, Urata Y, Fujiwara T, Kawakami K. Telomerase-specific virotherapy in an animal model of human head and neck cancer. Mol Cancer Ther. 2009;8:171–177. doi: 10.1158/1535-7163.MCT-08-0620. [DOI] [PubMed] [Google Scholar]
- 10.Doloff JC, Waxman DJ, Jounaidi Y. Human telomerase reverse transcriptase promoter-driven oncolytic adenovirus with E1B-19 kDa and E1B-55 kDa gene deletions. Hum Gene Ther. 2008;19:1383–1400. doi: 10.1089/hum.2008.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu KF, Wu CL, Huang SC, Hsieh JL, Huang YS, Chen YF, Shen MR, Chung WJ, Chou CY, Shiau AL. Conditionally replicating E1B-deleted adenovirus driven by the squamous cell carcinoma antigen 2 promoter for uterine cervical cancer therapy. Cancer Gene Ther. 2008;15:526–534. doi: 10.1038/cgt.2008.37. [DOI] [PubMed] [Google Scholar]
- 12.Shafren DR, Dorahy DJ, Ingham RA, Burns GF, Barry RD. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J Virol. 1997;71:4736–4743. doi: 10.1128/jvi.71.6.4736-4743.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson BD, Nakamura T, Russell SJ, Peng KW. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 14.Nishimoto T, Yoshida K, Miura Y, Kobayashi A, Hara H, Ohnami S, Kurisu K, Yoshida T, Aoki K. Oncolytic virus therapy for pancreatic cancer using the adenovirus library displaying random peptides on the fiber knob. Gene Ther. 2009;16:669–680. doi: 10.1038/gt.2009.1. [DOI] [PubMed] [Google Scholar]
- 15.Conner J, Braidwood L, Brown SM. A strategy for systemic delivery of the oncolytic herpes virus HSV1716: redirected tropism by antibody-binding sites incorporated on the virion surface as a glycoprotein D fusion protein. Gene Ther. 2008;15:1579–1592. doi: 10.1038/gt.2008.121. [DOI] [PubMed] [Google Scholar]
- 16.Coughlan L, Vallath S, Saha A, Flak M, McNeish IA, Vassaux G, Marshall JF, Hart IR, Thomas GJ. In vivo retargeting of adenovirus type 5 to alphavbeta6 integrin results in reduced hepatotoxicity and improved tumor uptake following systemic delivery. J Virol. 2009;83:6416–6428. doi: 10.1128/JVI.00445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes EM, Rodrigues MS, Phadke AP, Butcher LD, Starling C, Chen S, Chang D, Hernandez-Alcoceba R, Newman JT, Stone MJ, Tong AW. Antitumor activity of an oncolytic adenoviral-CD40 ligand (CD154) transgene construct in human breast cancer cells. Clin Cancer Res. 2009;15:1317–1325. doi: 10.1158/1078-0432.CCR-08-1360. [DOI] [PubMed] [Google Scholar]
- 18.Piao Y, Jiang H, Alemany R, Krasnykh V, Marini FC, Xu J, Alonso MM, Conrad CA, Aldape KD, Gomez-Manzano C, Fueyo J. Oncolytic adenovirus retargeted to Delta-EGFR induces selective antiglioma activity. Cancer Gene Ther. 2009;16:256–265. doi: 10.1038/cgt.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison J, Briggs SS, Green N, Fisher K, Subr V, Ulbrich K, Kehoe S, Seymour LW. Virotherapy of ovarian cancer with polymer-cloaked adenovirus retargeted to the epidermal growth factor receptor. Mol Ther. 2008;16:244–251. doi: 10.1038/sj.mt.6300363. [DOI] [PubMed] [Google Scholar]
- 20.Allen C, Paraskevakou G, Iankov I, Giannini C, Schroeder M, Sarkaria J, Puri RK, Russell SJ, Galanis E. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol Ther. 2008;16:1556–1564. doi: 10.1038/mt.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorence RM, Katubig BB, Reichard KW, Reyes HM, Phuangsab A, Sassetti MD, Walter RJ, Peeples ME. Complete regression of human fibrosarcoma xenografts after local Newcastle disease virus therapy. Cancer Res. 1994;54:6017–6021. [PubMed] [Google Scholar]
- 22.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 23.Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- 24.Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, Bell JC. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 25.Whitley RJ, Kern ER, Chatterjee S, Chou J, Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J Clin Invest. 1993;91:2837–2843. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muster T, Rajtarova J, Sachet M, Unger H, Fleischhacker R, Romirer I, Grassauer A, Url A, Garcia-Sastre A, Wolff K, Pehamberger H, Bergmann M. Interferon resistance promotes oncolysis by influenza virus NS1-deletion mutants. Int J Cancer. 2004;110:15–21. doi: 10.1002/ijc.20078. [DOI] [PubMed] [Google Scholar]
- 27.Cascallo M, Capella G, Mazo A, Alemany R. Ras-dependent oncolysis with an adenovirus VAI mutant. Cancer Res. 2003;63:5544–5550. [PubMed] [Google Scholar]
- 28.Wang Y, Xue SA, Hallden G, Francis J, Yuan M, Griffin BE, Lemoine NR. Virus-associated RNA I-deleted adenovirus, a potential oncolytic agent targeting EBV-associated tumors. Cancer Res. 2005;65:1523–1531. doi: 10.1158/0008-5472.CAN-04-3113. [DOI] [PubMed] [Google Scholar]
- 29.Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, Hawkins L, Kirn D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 30.Liu TC, Hallden G, Wang Y, Brooks G, Francis J, Lemoine N, Kirn D. An E1B-19 kDa gene deletion mutant adenovirus demonstrates tumor necrosis factor-enhanced cancer selectivity and enhanced oncolytic potency. Mol Ther. 2004;9:786–803. doi: 10.1016/j.ymthe.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 32.Zheng X, Rao XM, Gomez-Gutierrez JG, Hao H, McMasters KM, Zhou HS. Adenovirus E1B55K region is required to enhance cyclin E expression for efficient viral DNA replication. J Virol. 2008;82:3415–3427. doi: 10.1128/JVI.01708-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, Boyle L, Pandey K, Soria C, Kunich J, Shen Y, Habets G, Ginzinger D, McCormick F. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Ylosmaki E, Hakkarainen T, Hemminki A, Visakorpi T, Andino R, Saksela K. Generation of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific MicroRNA. J Virol. 2008;82:11009–11015. doi: 10.1128/JVI.01608-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cawood R, Chen HH, Carroll F, Bazan-Peregrino M, van Rooijen N, Seymour LW. Use of tissue-specific microRNA to control pathology of wild-type adenovirus without attenuation of its ability to kill cancer cells. PLoS Pathog. 2009;5:e1000440. doi: 10.1371/journal.ppat.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CY, Rennie PS, Jia WW. MicroRNA regulation of oncolytic herpes simplex virus-1 for selective killing of prostate cancer cells. Clin Cancer Res. 2009;15:5126–5135. doi: 10.1158/1078-0432.CCR-09-0051. [DOI] [PubMed] [Google Scholar]
- 37.Edge RE, Falls TJ, Brown CW, Lichty BD, Atkins H, Bell JC. A let-7 MicroRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication. Mol Ther. 2008;16:1437–1443. doi: 10.1038/mt.2008.130. [DOI] [PubMed] [Google Scholar]
- 38.Kelly EJ, Hadac EM, Greiner S, Russell SJ. Engineering microRNA responsiveness to decrease virus pathogenicity. Nat Med. 2008;14:1278–1283. doi: 10.1038/nm.1776. [DOI] [PubMed] [Google Scholar]
- 39.Gurlevik E, Woller N, Schache P, Malek NP, Wirth TC, Zender L, Manns MP, Kubicka S, Kuhnel F. p53-dependent antiviral RNA-interference facilitates tumor-selective viral replication. Nucleic Acids Res. 2009;37:e84. doi: 10.1093/nar/gkp374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cody JJ, Douglas JT. Armed replicating adenoviruses for cancer virotherapy. Cancer Gene Ther. 2009;16:473–488. doi: 10.1038/cgt.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benencia F, Coukos G. Biological therapy with oncolytic herpesvirus. Adv Exp Med Biol. 2008;622:221–233. doi: 10.1007/978-0-387-68969-2_18. [DOI] [PubMed] [Google Scholar]
- 42.Blackford AN, Grand RJ. Adenovirus E1B 55-kilodalton protein: multiple roles in viral infection and cell transformation. J Virol. 2009;83:4000–4012. doi: 10.1128/JVI.02417-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganly I, Kirn D, Eckhardt G, Rodriguez GI, Soutar DS, Otto R, Robertson AG, Park O, Gulley ML, Heise C, Von Hoff DD, Kaye SB. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- 44.Nemunaitis J, Ganly I, Khuri F, Arseneau J, Kuhn J, McCarty T, Landers S, Maples P, Romel L, Randlev B, Reid T, Kaye S, Kirn D. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60:6359–6366. [PubMed] [Google Scholar]
- 45.Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, Kuhn J, McCarty T, Landers S, Blackburn A, Romel L, Randlev B, Kaye S, Kirn D. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 46.Mulvihill S, Warren R, Venook A, Adler A, Randlev B, Heise C, Kirn D. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther. 2001;8:308–315. doi: 10.1038/sj.gt.3301398. [DOI] [PubMed] [Google Scholar]
- 47.Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555–561. [PubMed] [Google Scholar]
- 48.Nemunaitis J, Cunningham C, Buchanan A, Blackburn A, Edelman G, Maples P, Netto G, Tong A, Randlev B, Olson S, Kirn D. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 2001;8:746–759. doi: 10.1038/sj.gt.3301424. [DOI] [PubMed] [Google Scholar]
- 49.Thomas MA, Broughton RS, Goodrum FD, Ornelles DA. E4orf1 limits the oncolytic potential of the E1B-55K deletion mutant adenovirus. J Virol. 2009;83:2406–2416. doi: 10.1128/JVI.01972-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frisch SM, Mymryk JS. Adenovirus-5 E1A: paradox and paradigm. Nat Rev Mol Cell Biol. 2002;3:441–452. doi: 10.1038/nrm827. [DOI] [PubMed] [Google Scholar]
- 51.Liu TC, Wang Y, Hallden G, Brooks G, Francis J, Lemoine NR, Kirn D. Functional interactions of antiapoptotic proteins and tumor necrosis factor in the context of a replication-competent adenovirus. Gene Ther. 2005;12:1333–1346. doi: 10.1038/sj.gt.3302555. [DOI] [PubMed] [Google Scholar]
- 52.Subramanian T, Vijayalingam S, Chinnadurai G. Genetic identification of adenovirus type 5 genes that influence viral spread. J Virol. 2006;80:2000–2012. doi: 10.1128/JVI.80.4.2000-2012.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han J, Modha D, White E. Interaction of E1B 19K with Bax is required to block Bax-induced loss of mitochondrial membrane potential and apoptosis. Oncogene. 1998;17:2993–3005. doi: 10.1038/sj.onc.1202215. [DOI] [PubMed] [Google Scholar]
- 54.Han J, Sabbatini P, Perez D, Rao L, Modha D, White E. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev. 1996;10:461–477. doi: 10.1101/gad.10.4.461. [DOI] [PubMed] [Google Scholar]
- 55.Sundararajan R, White E. E1B 19K blocks Bax oligomerization and tumor necrosis factor alpha-mediated apoptosis. J Virol. 2001;75:7506–7516. doi: 10.1128/JVI.75.16.7506-7516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez D, White E. E1B 19K inhibits Fas-mediated apoptosis through FADD-dependent sequestration of FLICE. J Cell Biol. 1998;141:1255–1266. doi: 10.1083/jcb.141.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vijayalingam S, Subramanian T, Ryerse J, Varvares M, Chinnadurai G. Down-regulation of multiple cell survival proteins in head and neck cancer cells by an apoptogenic mutant of adenovirus type 5. Virology. 2009;392:62–72. doi: 10.1016/j.virol.2009.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schneider RJ, Safer B, Munemitsu SM, Samuel CE, Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci USA. 1985;82:4321–4325. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider RJ, Weinberger C, Shenk T. Adenovirus VAI RNA facilitates the initiation of translation in virus-infected cells. Cell. 1984;37:291–298. doi: 10.1016/0092-8674(84)90325-8. [DOI] [PubMed] [Google Scholar]
- 60.Tollefson AE, Ryerse JS, Scaria A, Hermiston TW, Wold WS. The E3-11.6-kDa adenovirus death protein (ADP) is required for efficient cell death: characterization of cells infected with adp mutants. Virology. 1996;220:152–162. doi: 10.1006/viro.1996.0295. [DOI] [PubMed] [Google Scholar]
- 61.Zou A, Atencio I, Huang WM, Horn M, Ramachandra M. Overexpression of adenovirus E3-11.6K protein induces cell killing by both caspase-dependent and caspase-independent mechanisms. Virology. 2004;326:240–249. doi: 10.1016/j.virol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 62.Doronin K, Toth K, Kuppuswamy M, Krajcsi P, Tollefson AE, Wold WS. Overexpression of the ADP (E3-11.6K) protein increases cell lysis and spread of adenovirus. Virology. 2003;305:378–387. doi: 10.1006/viro.2002.1772. [DOI] [PubMed] [Google Scholar]
- 63.Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005;16:1016–1027. doi: 10.1089/hum.2005.16.1016. [DOI] [PubMed] [Google Scholar]
- 64.Ma J, He X, Wang W, Huang Y, Chen L, Cong W, Gu J, Hu H, Shi J, Li L, Su C. E2F promoter-regulated oncolytic adenovirus with p16 gene induces cell apoptosis and exerts antitumor effect on gastric cancer. Dig Dis Sci. 2009;54:1425–1431. doi: 10.1007/s10620-008-0543-0. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, Su C, Cao H, Li K, Chen J, Jiang L, Zhang Q, Wu X, Jia X, Liu Y, Wang W, Liu X, Wu M, Qian Q. A novel triple-regulated oncolytic adenovirus carrying p53 gene exerts potent antitumor efficacy on common human solid cancers. Mol Cancer Ther. 2008;7:1598–1603. doi: 10.1158/1535-7163.MCT-07-2429. [DOI] [PubMed] [Google Scholar]
- 66.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu Z, Robbins JS, Pister A, Zafar MB, Zhang ZW, Gupta J, Lee KJ, Neuman K, Yun CO, Guise T, Seth P. A modified hTERT promoter-directed oncolytic adenovirus replication with concurrent inhibition of TGFbeta signaling for breast cancer therapy. Cancer Gene Ther. 2009 doi: 10.1038/cgt.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin J, Liu H, Yang C, Li G, Liu X, Qian Q, Qian W. Effective gene-viral therapy of leukemia by a new fiber chimeric oncolytic adenovirus expressing TRAIL: in vitro and in vivo evaluation. Mol Cancer Ther. 2009;8:1387. doi: 10.1158/1535-7163.MCT-08-0962. [DOI] [PubMed] [Google Scholar]
- 69.Chen L, Chen D, Gong M, Na M, Li L, Wu H, Jiang L, Qian Y, Fang G, Xue X. Concomitant use of Ad5/35 chimeric oncolytic adenovirus with TRAIL gene and taxol produces synergistic cytotoxicity in gastric cancer cells. Cancer Lett. 2009;284:141–148. doi: 10.1016/j.canlet.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Z, Huang Y, Newman K, Gu J, Zhang X, Wu H, Zhao M, Xianyu Z, Liu X. Reexpression of human somatostatin receptor gene 2 gene mediated by oncolytic adenovirus increases antitumor activity of tumor necrosis factor-related apoptosis-inducing ligand against pancreatic cancer. Clin Cancer Res. 2009;15:5154–5160. doi: 10.1158/1078-0432.CCR-09-0025. [DOI] [PubMed] [Google Scholar]
- 71.Zheng JN, Pei DS, Sun FH, Zhang BF, Liu XY, Gu JF, Liu YH, Hu XL, Mao LJ, Wen RM, Liu JJ, Li W. Inhibition of renal cancer cell growth by oncolytic adenovirus armed short hairpin RNA targeting hTERT gene. Cancer Biol Ther. 2009;8:84–91. doi: 10.4161/cbt.8.1.7204. [DOI] [PubMed] [Google Scholar]
- 72.Zheng JN, Pei DS, Mao LJ, Liu XY, Mei DD, Zhang BF, Shi Z, Wen RM, Sun XQ. Inhibition of renal cancer cell growth in vitro and in vivo with oncolytic adenovirus armed short hairpin RNA targeting Ki-67 encoding mRNA. Cancer Gene Ther. 2009;16:20–32. doi: 10.1038/cgt.2008.61. [DOI] [PubMed] [Google Scholar]
- 73.Shen W, Wang CY, Wang XH, Fu ZX. Oncolytic adenovirus mediated Survivin knockdown by RNA interference suppresses human colorectal carcinoma growth in vitro and in vivo. J Exp Clin Cancer Res. 2009;28:81. doi: 10.1186/1756-9966-28-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chu L, Gu J, Sun L, Qian Q, Qian C, Liu X. Oncolytic adenovirus-mediated shRNA against Apollon inhibits tumor cell growth and enhances antitumor effect of 5-fluorouracil. Gene Ther. 2008;15:484–494. doi: 10.1038/gt.2008.6. [DOI] [PubMed] [Google Scholar]
- 75.Ikeda Y, Kojima T, Kuroda S, Endo Y, Sakai R, Hioki M, Kishimoto H, Uno F, Kagawa S, Watanabe Y, Hashimoto Y, Urata Y, Tanaka N, Fujiwara T. A novel antiangiogenic effect for telomerase-specific virotherapy through host immune system. J Immunol. 2009;182:1763–1769. doi: 10.4049/jimmunol.182.3.1763. [DOI] [PubMed] [Google Scholar]
- 76.Kurozumi K, Hardcastle J, Thakur R, Shroll J, Nowicki M, Otsuki A, Chiocca EA, Kaur B. Oncolytic HSV-1 infection of tumors induces angiogenesis and upregulates CYR61. Mol Ther. 2008;16:1382–1391. doi: 10.1038/mt.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tysome JR, Lemoine NR, Wang Y. Combination of anti-angiogenic therapy and virotherapy: arming the oncolytic viruses with anti-angiogenic genes. Curr Opin Mol Ther. 2010 in press. [PubMed] [Google Scholar]
- 78.Tysome JR, Briat A, Alusi G, Cao F, Gao D, Yu J, Wang P, Yang S, Dong Z, Wang S, Deng L, Francis J, Timiryasova T, Fodor I, Lemoine NR, Wang Y. Lister strain of vaccinia virus armed with endostatin-angiostatin fusion gene as a novel therapeutic agent for human pancreatic cancer. Gene Ther. 2009;16:1223–1233. doi: 10.1038/gt.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su C, Na M, Chen J, Wang X, Liu Y, Wang W, Zhang Q, Li L, Long J, Liu X, Wu M, Fan X, Qian Q. Gene-viral cancer therapy using dual-regulated oncolytic adenovirus with antiangiogenesis gene for increased efficacy. Mol Cancer Res. 2008;6:568–575. doi: 10.1158/1541-7786.MCR-07-0073. [DOI] [PubMed] [Google Scholar]
- 80.Fang L, Pu YY, Hu XC, Sun LJ, Luo HM, Pan SK, Gu JZ, Cao XR, Su CQ. Antiangiogenesis gene armed tumor-targeting adenovirus yields multiple antitumor activities in human HCC xenografts in nude mice. Hepatol Res. 2009 doi: 10.1111/j.1872-034X.2009.00580.x. [DOI] [PubMed] [Google Scholar]
- 81.Zheng JN, Pei DS, Mao LJ, Liu XY, Sun FH, Zhang BF, Liu YQ, Liu JJ, Li W, Han D. Oncolytic adenovirus expressing interleukin-18 induces significant antitumor effects against melanoma in mice through inhibition of angiogenesis. Cancer Gene Ther. 2010;17:28–36. doi: 10.1038/cgt.2009.38. [DOI] [PubMed] [Google Scholar]
- 82.Zheng JN, Pei DS, Sun FH, Liu XY, Mao LJ, Zhang BF, Wen RM, Xu W, Shi Z, Liu JJ, Li W. Potent antitumor efficacy of interleukin-18 delivered by conditionally replicative adenovirus vector in renal cell carcinoma-bearing nude mice via inhibition of angiogenesis. Cancer Biol Ther. 2009;8:599–606. doi: 10.4161/cbt.8.7.7914. [DOI] [PubMed] [Google Scholar]
- 83.He XP, Su CQ, Wang XH, Pan X, Tu ZX, Gong YF, Gao J, Liao Z, Jin J, Wu HY, Man XH, Li ZS. E1B-55kD-deleted oncolytic adenovirus armed with canstatin gene yields an enhanced anti-tumor efficacy on pancreatic cancer. Cancer Lett. 2009;285:89–98. doi: 10.1016/j.canlet.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 84.Liu TC, Castelo-Branco P, Rabkin SD, Martuza RL. Trichostatin A and oncolytic HSV combination therapy shows enhanced antitumoral and antiangiogenic effects. Mol Ther. 2008;16:1041–1047. doi: 10.1038/mt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoo JY, Kim JH, Kim J, Huang JH, Zhang SN, Kang YA, Kim H, Yun CO. Short hairpin RNA-expressing oncolytic adenovirus-mediated inhibition of IL-8: effects on antiangiogenesis and tumor growth inhibition. Gene Ther. 2008;15:635–651. doi: 10.1038/gt.2008.3. [DOI] [PubMed] [Google Scholar]
- 86.Frentzen A, Yu YA, Chen N, Zhang Q, Weibel S, Raab V, Szalay AA. Anti-VEGF single-chain antibody GLAF-1 encoded by oncolytic vaccinia virus significantly enhances antitumor therapy. Proc Natl Acad Sci USA. 2009;106:12915–12920. doi: 10.1073/pnas.0900660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guse K, Diaconu I, Rajecki M, Sloniecka M, Hakkarainen T, Ristimaki A, Kanerva A, Pesonen S, Hemminki A. Ad5/3-9HIF-Delta24-VEGFR-1-Ig, an infectivity enhanced, dual-targeted and antiangiogenic oncolytic adenovirus for kidney cancer treatment. Gene Ther. 2009;16:1009–1020. doi: 10.1038/gt.2009.56. [DOI] [PubMed] [Google Scholar]
- 88.Kang YA, Shin HC, Yoo JY, Kim JH, Kim JS, Yun CO. Novel cancer antiangiotherapy using the VEGF promoter-targeted artificial zinc-finger protein and oncolytic adenovirus. Mol Ther. 2008;16:1033–1040. doi: 10.1038/mt.2008.63. [DOI] [PubMed] [Google Scholar]
- 89.McNally LR, Rosenthal EL, Zhang W, Buchsbaum DJ. Therapy of head and neck squamous cell carcinoma with replicative adenovirus expressing tissue inhibitor of metalloproteinase-2 and chemoradiation. Cancer Gene Ther. 2009;16:246–255. doi: 10.1038/cgt.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mahller YY, Vaikunth SS, Ripberger MC, Baird WH, Saeki Y, Cancelas JA, Crombleholme TM, Cripe TP. Tissue inhibitor of metalloproteinase-3 via oncolytic herpesvirus inhibits tumor growth and vascular progenitors. Cancer Res. 2008;68:1170–1179. doi: 10.1158/0008-5472.CAN-07-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahn M, Lee SJ, Li X, Jimenez JA, Zhang YP, Bae KH, Mohammadi Y, Kao C, Gardner TA. Enhanced combined tumor-specific oncolysis and suicide gene therapy for prostate cancer using M6 promoter. Cancer Gene Ther. 2009;16:73–82. doi: 10.1038/cgt.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fukuda K, Abei M, Ugai H, Kawashima R, Seo E, Wakayama M, Murata T, Endo S, Hamada H, Hyodo I, Yokoyama KK. E1A, E1B double-restricted replicative adenovirus at low dose greatly augments tumor-specific suicide gene therapy for gallbladder cancer. Cancer Gene Ther. 2009;16:126–136. doi: 10.1038/cgt.2008.67. [DOI] [PubMed] [Google Scholar]
- 93.Zheng FQ, Xu Y, Yang RJ, Wu B, Tan XH, Qin YD, Zhang QW. Combination effect of oncolytic adenovirus therapy and herpes simplex virus thymidine kinase/ganciclovir in hepatic carcinoma animal models. Acta Pharmacol Sin. 2009;30:617–627. doi: 10.1038/aps.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Braidwood L, Dunn PD, Hardy S, Evans TR, Brown SM. Antitumor activity of a selectively replication competent herpes simplex virus (HSV) with enzyme prodrug therapy. Anticancer Res. 2009;29:2159–2166. [PubMed] [Google Scholar]
- 95.Chalikonda S, Kivlen MH, O’Malley ME, Eric Dong XD, McCart JA, Gorry MC, Yin XY, Brown CK, Zeh HJ, 3rd, Guo ZS, Bartlett DL. Oncolytic virotherapy for ovarian carcinomatosis using a replication-selective vaccinia virus armed with a yeast cytosine deaminase gene. Cancer Gene Ther. 2008;15:115–125. doi: 10.1038/sj.cgt.7701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Foloppe J, Kintz J, Futin N, Findeli A, Cordier P, Schlesinger Y, Hoffmann C, Tosch C, Balloul JM, Erbs P. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Ther. 2008;15:1361–1371. doi: 10.1038/gt.2008.82. [DOI] [PubMed] [Google Scholar]
- 97.Kolodkin-Gal D, Zamir G, Edden Y, Pikarsky E, Pikarsky A, Haim H, Haviv YS, Panet A. Herpes simplex virus type 1 preferentially targets human colon carcinoma: role of extracellular matrix. J Virol. 2008;82:999–1010. doi: 10.1128/JVI.01769-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ganesh S, Gonzalez-Edick M, Gibbons D, Van Roey M, Jooss K. Intratumoral coadministration of hyaluronidase enzyme and oncolytic adenoviruses enhances virus potency in metastatic tumor models. Clin Cancer Res. 2008;14:3933–3941. doi: 10.1158/1078-0432.CCR-07-4732. [DOI] [PubMed] [Google Scholar]
- 99.Nagano S, Perentes JY, Jain RK, Boucher Y. Cancer cell death enhances the penetration and efficacy of oncolytic herpes simplex virus in tumors. Cancer Res. 2008;68:3795–3802. doi: 10.1158/0008-5472.CAN-07-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 101.Bazan-Peregrino M, Carlisle RC, Purdie L, Seymour LW. Factors influencing retention of adenovirus within tumours following direct intratumoural injection. Gene Ther. 2008;15:688–694. doi: 10.1038/gt.2008.2. [DOI] [PubMed] [Google Scholar]
- 102.Lopez MV, Viale DL, Cafferata EG, Bravo AI, Carbone C, Gould D, Chernajovsky Y, Podhajcer OL. Tumor associated stromal cells play a critical role on the outcome of the oncolytic efficacy of conditionally replicative adenoviruses. PLoS One. 2009;4:e5119. doi: 10.1371/journal.pone.0005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shen BH, Hermiston TW. Effect of hypoxia on Ad5 infection, transgene expression and replication. Gene Ther. 2005;12:902–910. doi: 10.1038/sj.gt.3302448. [DOI] [PubMed] [Google Scholar]
- 104.Shen BH, Bauzon M, Hermiston TW. The effect of hypoxia on the uptake, replication and lytic potential of group B adenovirus type 3 (Ad3) and type 11p (Ad11p) Gene Ther. 2006;13:986–990. doi: 10.1038/sj.gt.3302736. [DOI] [PubMed] [Google Scholar]
- 105.Hiley CT, Yuan M, Lemoine NR, Wang Y. Lister strain vaccinia virus, a potential therapeutic vector for hypoxic tumours. Gene Ther. 2009 doi: 10.1038/gt.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aghi MK, Liu TC, Rabkin S, Martuza RL. Hypoxia enhances the replication of oncolytic herpes simplex virus. Mol Ther. 2009;17:51–56. doi: 10.1038/mt.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fasullo M, Burch AD, Britton A. Hypoxia enhances the replication of oncolytic herpes simplex virus in p53- breast cancer cells. Cell Cycle. 2009;8:2194–2197. doi: 10.4161/cc.8.14.8934. [DOI] [PubMed] [Google Scholar]
- 108.Anders M, Christian C, McMahon M, McCormick F, Korn WM. Inhibition of the Raf/MEK/ERK pathway up-regulates expression of the coxsackievirus and adenovirus receptor in cancer cells. Cancer Res. 2003;63:2088–2095. [PubMed] [Google Scholar]
- 109.O’Prey J, Wilkinson S, Ryan KM. Tumor antigen LRRC15 impedes adenoviral infection: implications for virus-based cancer therapy. J Virol. 2008;82:5933–5939. doi: 10.1128/JVI.02273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 111.Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40:109–123. doi: 10.1016/s0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 112.Kinugasa N, Higashi T, Nouso K, Nakatsukasa H, Kobayashi Y, Ishizaki M, Toshikuni N, Yoshida K, Uematsu S, Tsuji T. Expression of membrane cofactor protein (MCP, CD46) in human liver diseases. Br J Cancer. 1999;80:1820–1825. doi: 10.1038/sj.bjc.6690604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murray KP, Mathure S, Kaul R, Khan S, Carson LF, Twiggs LB, Martens MG, Kaul A. Expression of complement regulatory proteins-CD 35, CD 46, CD 55, and CD 59-in benign and malignant endometrial tissue. Gynecol Oncol. 2000;76:176–182. doi: 10.1006/gyno.1999.5614. [DOI] [PubMed] [Google Scholar]
- 114.Nandi S, Ulasov IV, Rolle CE, Han Y, Lesniak MS. A chimeric adenovirus with an Ad 3 fiber knob modification augments glioma virotherapy. J Gene Med. 2009;11:1005–1011. doi: 10.1002/jgm.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ganesh S, Gonzalez-Edick M, Gibbons D, Ge Y, VanRoey M, Robinson M, Jooss K. Combination therapy with radiation or cisplatin enhances the potency of Ad5/35 chimeric oncolytic adenovirus in a preclinical model of head and neck cancer. Cancer Gene Ther. 2009;16:383–392. doi: 10.1038/cgt.2008.90. [DOI] [PubMed] [Google Scholar]
- 116.Wang G, Li G, Liu H, Yang C, Yang X, Jin J, Liu X, Qian Q, Qian W. E1B 55-kDa deleted, Ad5/F35 fiber chimeric adenovirus, a potential oncolytic agent for B-lymphocytic malignancies. J Gene Med. 2009;11:477–485. doi: 10.1002/jgm.1326. [DOI] [PubMed] [Google Scholar]
- 117.Zhu ZB, Lu B, Park M, Makhija SK, Numnum TM, Kendrick JE, Wang M, Tsuruta Y, Fisher P, Alvarez RD, Zhou F, Siegal GP, Wu H, Curiel DT. Development of an optimized conditionally replicative adenoviral agent for ovarian cancer. Int J Oncol. 2008;32:1179–1188. doi: 10.3892/ijo_32_6_1179. [DOI] [PubMed] [Google Scholar]
- 118.Shashkova EV, May SM, Barry MA. Characterization of human adenovirus serotypes 5, 6, 11, and 35 as anticancer agents. Virology. 2009;394:311–320. doi: 10.1016/j.virol.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sandberg L, Papareddy P, Silver J, Bergh A, Mei YF. Replication-competent Ad11p vector (RCAd11p) efficiently transduces and replicates in hormone-refractory metastatic prostate cancer cells. Hum Gene Ther. 2009;20:361–373. doi: 10.1089/hum.2007.124. [DOI] [PubMed] [Google Scholar]
- 120.Stone D, Liu Y, Li ZY, Tuve S, Strauss R, Lieber A. Comparison of adenoviruses from species B, C, E, and F after intravenous delivery. Mol Ther. 2007;15:2146–2153. doi: 10.1038/sj.mt.6300319. [DOI] [PubMed] [Google Scholar]
- 121.Yu L, Shimozato O, Li Q, Kawamura K, Ma G, Namba M, Ogawa T, Kaiho I, Tagawa M. Adenovirus type 5 substituted with type 11 or 35 fiber structure increases its infectivity to human cells enabling dual gene transfer in CD46-dependent and -independent manners. Anticancer Res. 2007;27:2311–2316. [PubMed] [Google Scholar]
- 122.Stone D, Ni S, Li ZY, Gaggar A, DiPaolo N, Feng Q, Sandig V, Lieber A. Development and assessment of human adenovirus type 11 as a gene transfer vector. J Virol. 2005;79:5090–5104. doi: 10.1128/JVI.79.8.5090-5104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tuve S, Wang H, Ware C, Liu Y, Gaggar A, Bernt K, Shayakhmetov D, Li Z, Strauss R, Stone D, Lieber A. A new group B adenovirus receptor is expressed at high levels on human stem and tumor cells. J Virol. 2006;80:12109–12120. doi: 10.1128/JVI.01370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sakurai F, Akitomo K, Kawabata K, Hayakawa T, Mizuguchi H. Downregulation of human CD46 by adenovirus serotype 35 vectors. Gene Ther. 2007;14:912–919. doi: 10.1038/sj.gt.3302946. [DOI] [PubMed] [Google Scholar]
- 125.Strauss R, Sova P, Liu Y, Li ZY, Tuve S, Pritchard D, Brinkkoetter P, Moller T, Wildner O, Pesonen S, Hemminki A, Urban N, Drescher C, Lieber A. Epithelial phenotype confers resistance of ovarian cancer cells to oncolytic adenoviruses. Cancer Res. 2009;69:5115–5125. doi: 10.1158/0008-5472.CAN-09-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Y, Gangeswaran R, Zhao X, Wang P, Tysome J, Bhakta V, Yuan M, Chikkanna-Gowda CP, Jiang G, Gao D, Cao F, Francis J, Yu J, Liu K, Yang H, Zhang Y, Zang W, Chelala C, Dong Z, Lemoine N. CEACAM6 attenuates adenovirus infection by antagonizing viral trafficking in cancer cells. J Clin Invest. 2009;119:1604–1615. doi: 10.1172/JCI37905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shiina M, Lacher MD, Christian C, Korn WM. RNA interference-mediated knockdown of p21(WAF1) enhances anti-tumor cell activity of oncolytic adenoviruses. Cancer Gene Ther. 2009;16:810–819. doi: 10.1038/cgt.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu W, Hofstetter W, Guo W, Li H, Pataer A, Peng HH, Guo ZS, Bartlett DL, Lin A, Swisher SG, Fang B. JNK-deficiency enhanced oncolytic vaccinia virus replication and blocked activation of double-stranded RNA-dependent protein kinase. Cancer Gene Ther. 2008;15:616–624. doi: 10.1038/cgt.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jiang H, Gomez-Manzano C, Aoki H, Alonso MM, Kondo S, McCormick F, Xu J, Kondo Y, Bekele BN, Colman H, Lang FF, Fueyo J. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst. 2007;99:1410–1414. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- 130.Eriksson M, Guse K, Bauerschmitz G, Virkkunen P, Tarkkanen M, Tanner M, Hakkarainen T, Kanerva A, Desmond RA, Pesonen S, Hemminki A. Oncolytic adenoviruses kill breast cancer initiating CD44+CD24-/low cells. Mol Ther. 2007;15:2088–2093. doi: 10.1038/sj.mt.6300300. [DOI] [PubMed] [Google Scholar]
- 131.Mahller YY, Williams JP, Baird WH, Mitton B, Grossheim J, Saeki Y, Cancelas JA, Ratner N, Cripe TP. Neuroblastoma cell lines contain pluripotent tumor initiating cells that are susceptible to a targeted oncolytic virus. PLoS One. 2009;4:e4235. doi: 10.1371/journal.pone.0004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang X, Komaki R, Wang L, Fang B, Chang JY. Treatment of radioresistant stem-like esophageal cancer cells by an apoptotic gene-armed, telomerase-specific oncolytic adenovirus. Clin Cancer Res. 2008;14:2813–2823. doi: 10.1158/1078-0432.CCR-07-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Y, Hallden G, Hill R, Anand A, Liu TC, Francis J, Brooks G, Lemoine N, Kirn D. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat Biotechnol. 2003;21:1328–1335. doi: 10.1038/nbt887. [DOI] [PubMed] [Google Scholar]
- 134.Wakimoto H, Johnson PR, Knipe DM, Chiocca EA. Effects of innate immunity on herpes simplex virus and its ability to kill tumor cells. Gene Ther. 2003;10:983–990. doi: 10.1038/sj.gt.3302038. [DOI] [PubMed] [Google Scholar]
- 135.Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, Valdes M, Barber G, Vile RG. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 136.Worgall S, Wolff G, Falck-Pedersen E, Crystal RG. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 137.Zamarin D, Martinez-Sobrido L, Kelly K, Mansour M, Sheng G, Vigil A, Garcia-Sastre A, Palese P, Fong Y. Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses. Mol Ther. 2009;17:697–706. doi: 10.1038/mt.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lyons M, Onion D, Green NK, Aslan K, Rajaratnam R, Bazan-Peregrino M, Phipps S, Hale S, Mautner V, Seymour LW, Fisher KD. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol Ther. 2006;14:118–128. doi: 10.1016/j.ymthe.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 139.Wakimoto H, Fulci G, Tyminski E, Chiocca EA. Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide's enhancement of viral oncolysis. Gene Ther. 2004;11:214–223. doi: 10.1038/sj.gt.3302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, Kaur B, Louis DN, Weissleder R, Caligiuri MA, Chiocca EA. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, Thompson J, Selby P, de Bono J, Melcher A, Pandha H, Coffey M, Vile R, Harrington K. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li H, Zeng Z, Fu X, Zhang X. Coadministration of a herpes simplex virus-2 based oncolytic virus and cyclophosphamide produces a synergistic antitumor effect and enhances tumor-specific immune responses. Cancer Res. 2007;67:7850–7855. doi: 10.1158/0008-5472.CAN-07-1087. [DOI] [PubMed] [Google Scholar]
- 143.Ungerechts G, Springfeld C, Frenzke ME, Lampe J, Parker WB, Sorscher EJ, Cattaneo R. An immunocompetent murine model for oncolysis with an armed and targeted measles virus. Mol Ther. 2007;15:1991–1997. doi: 10.1038/sj.mt.6300291. [DOI] [PubMed] [Google Scholar]
- 144.Hirasawa K, Nishikawa SG, Norman KL, Coffey MC, Thompson BG, Yoon CS, Waisman DM, Lee PW. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63:348–353. [PubMed] [Google Scholar]
- 145.Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips NJ, Wold WS. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther. 2008;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kurozumi K, Hardcastle J, Thakur R, Yang M, Christoforidis G, Fulci G, Hochberg FH, Weissleder R, Carson W, Chiocca EA, Kaur B. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J Natl Cancer Inst. 2007;99:1768–1781. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 147.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, Harsh GRt, Louis DN, Bartus RT, Hochberg FH, Chiocca EA. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 148.Chen Y, Yu DC, Charlton D, Henderson DR. Pre-existent adenovirus antibody inhibits systemic toxicity and antitumor activity of CN706 in the nude mouse LNCaP xenograft model: implications and proposals for human therapy. Hum Gene Ther. 2000;11:1553–1567. doi: 10.1089/10430340050083289. [DOI] [PubMed] [Google Scholar]
- 149.Tsai V, Johnson DE, Rahman A, Wen SF, LaFace D, Philopena J, Nery J, Zepeda M, Maneval DC, Demers GW, Ralston R. Impact of human neutralizing antibodies on antitumor efficacy of an oncolytic adenovirus in a murine model. Clin Cancer Res. 2004;10:7199–7206. doi: 10.1158/1078-0432.CCR-04-0765. [DOI] [PubMed] [Google Scholar]
- 150.Herrlinger U, Kramm CM, Aboody-Guterman KS, Silver JS, Ikeda K, Johnston KM, Pechan PA, Barth RF, Finkelstein D, Chiocca EA, Louis DN, Breakefield XO. Pre-existing herpes simplex virus 1 (HSV-1) immunity decreases, but does not abolish, gene transfer to experimental brain tumors by a HSV-1 vector. Gene Ther. 1998;5:809–819. doi: 10.1038/sj.gt.3300643. [DOI] [PubMed] [Google Scholar]
- 151.Dhar D, Spencer JF, Toth K, Wold WS. Effect of preexisting immunity on oncolytic adenovirus vector INGN 007 antitumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. J Virol. 2009;83:2130–2139. doi: 10.1128/JVI.02127-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dhar D, Spencer JF, Toth K, Wold WS. Pre-existing immunity and passive immunity to adenovirus 5 prevents toxicity caused by an oncolytic adenovirus vector in the Syrian hamster model. Mol Ther. 2009;17:1724–1732. doi: 10.1038/mt.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kostense S, Koudstaal W, Sprangers M, Weverling GJ, Penders G, Helmus N, Vogels R, Bakker M, Berkhout B, Havenga M, Goudsmit J. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. Aids. 2004;18:1213–1216. doi: 10.1097/00002030-200405210-00019. [DOI] [PubMed] [Google Scholar]
- 154.Holterman L, Vogels R, van der Vlugt R, Sieuwerts M, Grimbergen J, Kaspers J, Geelen E, van der Helm E, Lemckert A, Gillissen G, Verhaagh S, Custers J, Zuijdgeest D, Berkhout B, Bakker M, Quax P, Goudsmit J, Havenga M. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: low seroprevalence and non-cross-reactivity with Ad5. J Virol. 2004;78:13207–13215. doi: 10.1128/JVI.78.23.13207-13215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Seshidhar Reddy P, Ganesh S, Limbach MP, Brann T, Pinkstaff A, Kaloss M, Kaleko M, Connelly S. Development of adenovirus serotype 35 as a gene transfer vector. Virology. 2003;311:384–393. doi: 10.1016/s0042-6822(03)00161-2. [DOI] [PubMed] [Google Scholar]
- 156.Sumida SM, Truitt DM, Lemckert AA, Vogels R, Custers JH, Addo MM, Lockman S, Peter T, Peyerl FW, Kishko MG, Jackson SS, Gorgone DA, Lifton MA, Essex M, Walker BD, Goudsmit J, Havenga MJ, Barouch DH. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol. 2005;174:7179–7185. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- 157.Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 158.Hakkarainen T, Sarkioja M, Lehenkari P, Miettinen S, Ylikomi T, Suuronen R, Desmond RA, Kanerva A, Hemminki A. Human mesenchymal stem cells lack tumor tropism but enhance the antitumor activity of oncolytic adenoviruses in orthotopic lung and breast tumors. Hum Gene Ther. 2007;18:627–641. doi: 10.1089/hum.2007.034. [DOI] [PubMed] [Google Scholar]
- 159.Sonabend AM, Ulasov IV, Tyler MA, Rivera AA, Mathis JM, Lesniak MS. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26:831–841. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 160.Iankov ID, Blechacz B, Liu C, Schmeckpeper JD, Tarara JE, Federspiel MJ, Caplice N, Russell SJ. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol Ther. 2007;15:114–122. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
- 161.Munguia A, Ota T, Miest T, Russell SJ. Cell carriers to deliver oncolytic viruses to sites of myeloma tumor growth. Gene Ther. 2008;15:797–806. doi: 10.1038/gt.2008.45. [DOI] [PubMed] [Google Scholar]
- 162.Coukos G, Makrigiannakis A, Kang EH, Caparelli D, Benjamin I, Kaiser LR, Rubin SC, Albelda SM, Molnar-Kimber KL. Use of carrier cells to deliver a replication-selective herpes simplex virus-1 mutant for the intraperitoneal therapy of epithelial ovarian cancer. Clin Cancer Res. 1999;5:1523–1537. [PubMed] [Google Scholar]
- 163.Raykov Z, Balboni G, Aprahamian M, Rommelaere J. Carrier cell-mediated delivery of oncolytic parvoviruses for targeting metastases. Int J Cancer. 2004;109:742–749. doi: 10.1002/ijc.20013. [DOI] [PubMed] [Google Scholar]
- 164.Qiao J, Kottke T, Willmon C, Galivo F, Wongthida P, Diaz RM, Thompson J, Ryno P, Barber GN, Chester J, Selby P, Harrington K, Melcher A, Vile RG. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat Med. 2008;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- 165.Ilett EJ, Prestwich RJ, Kottke T, Errington F, Thompson JM, Harrington KJ, Pandha HS, Coffey M, Selby PJ, Vile RG, Melcher AA. Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity. Gene Ther. 2009;16:689–699. doi: 10.1038/gt.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pfirschke C, Schirrmacher V. Cross-infection of tumor cells by contact with T lymphocytes loaded with Newcastle disease virus. Int J Oncol. 2009;34:951–962. doi: 10.3892/ijo_00000221. [DOI] [PubMed] [Google Scholar]
- 167.Ong HT, Hasegawa K, Dietz AB, Russell SJ, Peng KW. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 2007;14:324–333. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- 168.Power AT, Wang J, Falls TJ, Paterson JM, Parato KA, Lichty BD, Stojdl DF, Forsyth PA, Atkins H, Bell JC. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther. 2007;15:123–130. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- 169.Zhu H, Su Y, Zhou S, Xiao W, Ling W, Hu B, Liu Y, Qi Y. Immune analysis on mtHSV mediated tumor therapy in HSV-1 seropositive mice. Cancer Biol Ther. 2007;6:724–731. doi: 10.4161/cbt.6.5.3953. [DOI] [PubMed] [Google Scholar]
- 170.Kangasniemi L, Koskinen M, Jokinen M, Toriseva M, Ala-Aho R, Kahari VM, Jalonen H, Yla-Herttuala S, Moilanen H, Stenman UH, Diaconu I, Kanerva A, Pesonen S, Hakkarainen T, Hemminki A. Extended release of adenovirus from silica implants in vitro and in vivo. Gene Ther. 2009;16:103–110. doi: 10.1038/gt.2008.142. [DOI] [PubMed] [Google Scholar]
- 171.Huard J, Lochmuller H, Acsadi G, Jani A, Massie B, Karpati G. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 1995;2:107–115. [PubMed] [Google Scholar]
- 172.Alemany R, Suzuki K, Curiel DT. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- 173.Hollon T. Researchers and regulators reflect on first gene therapy death. Nat Med. 2000;6:6. doi: 10.1038/71545. [DOI] [PubMed] [Google Scholar]
- 174.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L, Custers J, Morita T, Francischetti IM, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Havenga MJ, Nicklin SA, Baker AH. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 175.Shashkova EV, Doronin K, Senac JS, Barry MA. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 2008;68:5896–5904. doi: 10.1158/0008-5472.CAN-08-0488. [DOI] [PubMed] [Google Scholar]
- 176.Doronin K, Shashkova EV, May SM, Hofherr SE, Barry MA. Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Hum Gene Ther. 2009;20:975–988. doi: 10.1089/hum.2009.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shashkova EV, May SM, Doronin K, Barry MA. Expanded anticancer therapeutic window of hexon-modified oncolytic adenovirus. Mol Ther. 2009;17:2121–2130. doi: 10.1038/mt.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Carlisle RC, Di Y, Cerny AM, Sonnen AF, Sim RB, Green NK, Subr V, Ulbrich K, Gilbert RJ, Fisher KD, Finberg RW, Seymour LW. Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1. Blood. 2009;113:1909–1918. doi: 10.1182/blood-2008-09-178459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li JL, Liu HL, Zhang XR, Xu JP, Hu WK, Liang M, Chen SY, Hu F, Chu DT. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. Gene Ther. 2009;16:376–382. doi: 10.1038/gt.2008.179. [DOI] [PubMed] [Google Scholar]
- 180.Ren Z, Ye X, Fang C, Lu Q, Zhao Y, Liu F, Liang M, Hu F, Chen HZ. Intratumor injection of oncolytic adenovirus expressing HSP70 prolonged survival in melanoma B16 bearing mice by enhanced immune response. Cancer Biol Ther. 2008;7:191–195. doi: 10.4161/cbt.7.2.5254. [DOI] [PubMed] [Google Scholar]
- 181.Lapteva N, Aldrich M, Weksberg D, Rollins L, Goltsova T, Chen SY, Huang XF. Targeting the intratumoral dendritic cells by the oncolytic adenoviral vaccine expressing RANTES elicits potent antitumor immunity. J Immunother. 2009;32:145–156. doi: 10.1097/CJI.0b013e318193d31e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Willmon CL, Saloura V, Fridlender ZG, Wongthida P, Diaz RM, Thompson J, Kottke T, Federspiel M, Barber G, Albelda SM, Vile RG. Expression of IFN-beta enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma. Cancer Res. 2009;69:7713–7720. doi: 10.1158/0008-5472.CAN-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lei N, Shen FB, Chang JH, Wang L, Li H, Yang C, Li J, Yu DC. An oncolytic adenovirus expressing granulocyte macrophage colony-stimulating factor shows improved specificity and efficacy for treating human solid tumors. Cancer Gene Ther. 2009;16:33–43. doi: 10.1038/cgt.2008.46. [DOI] [PubMed] [Google Scholar]
- 184.Lee JH, Roh MS, Lee YK, Kim MK, Han JY, Park BH, Trown P, Kirn DH, Hwang TH. Oncolytic and immunostimulatory efficacy of a targeted oncolytic poxvirus expressing human GM-CSF following intravenous administration in a rabbit tumor model. Cancer Gene Ther. 2009 doi: 10.1038/cgt.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chang J, Zhao X, Wu X, Guo Y, Guo H, Cao J, Lou D, Yu D, Li J. A Phase I study of KH901, a conditionally replicating granulocyte-macrophage colony-stimulating factor: armed oncolytic adenovirus for the treatment of head and neck cancers. Cancer Biol Ther. 2009;8:676–682. doi: 10.4161/cbt.8.8.7913. [DOI] [PubMed] [Google Scholar]
- 186.Bortolanza S, Bunuales M, Otano I, Gonzalez-Aseguinolaza G, Ortiz-de-Solorzano C, Perez D, Prieto J, Hernandez-Alcoceba R. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters. Mol Ther. 2009;17:614–622. doi: 10.1038/mt.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang KJ, Wang YG, Cao X, Zhong SY, Wei RC, Wu YM, Yue XT, Li GC, Liu XY. Potent antitumor effect of interleukin-24 gene in the survivin promoter and retinoblastoma double-regulated oncolytic adenovirus. Hum Gene Ther. 2009;20:818–830. doi: 10.1089/hum.2008.205. [DOI] [PubMed] [Google Scholar]
- 188.Luo J, Xia Q, Zhang R, Lv C, Zhang W, Wang Y, Cui Q, Liu L, Cai R, Qian C. Treatment of cancer with a novel dual-targeted conditionally replicative adenovirus armed with mda-7/IL-24 gene. Clin Cancer Res. 2008;14:2450–2457. doi: 10.1158/1078-0432.CCR-07-4596. [DOI] [PubMed] [Google Scholar]
- 189.Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shashkova EV, Kuppuswamy MN, Wold WS, Doronin K. Anticancer activity of oncolytic adenovirus vector armed with IFN-alpha and ADP is enhanced by pharmacologically controlled expression of TRAIL. Cancer Gene Ther. 2008;15:61–72. doi: 10.1038/sj.cgt.7701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Haralambieva I, Iankov I, Hasegawa K, Harvey M, Russell SJ, Peng KW. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol Ther. 2007;15:588–597. doi: 10.1038/sj.mt.6300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Altomonte J, Wu L, Meseck M, Chen L, Ebert O, Garcia-Sastre A, Fallon J, Mandeli J, Woo SL. Enhanced oncolytic potency of vesicular stomatitis virus through vector-mediated inhibition of NK and NKT cells. Cancer Gene Ther. 2009;16:266–278. doi: 10.1038/cgt.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Endo Y, Sakai R, Ouchi M, Onimatsu H, Hioki M, Kagawa S, Uno F, Watanabe Y, Urata Y, Tanaka N, Fujiwara T. Virus-mediated oncolysis induces danger signal and stimulates cytotoxic T-lymphocyte activity via proteasome activator upregulation. Oncogene. 2008;27:2375–2381. doi: 10.1038/sj.onc.1210884. [DOI] [PubMed] [Google Scholar]
- 194.Lapteva N, Aldrich M, Rollins L, Ren W, Goltsova T, Chen SY, Huang XF. Attraction and activation of dendritic cells at the site of tumor elicits potent antitumor immunity. Mol Ther. 2009;17:1626–1636. doi: 10.1038/mt.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ramakrishna E, Woller N, Mundt B, Knocke S, Gurlevik E, Saborowski M, Malek N, Manns MP, Wirth T, Kuhnel F, Kubicka S. Antitumoral immune response by recruitment and expansion of dendritic cells in tumors infected with telomerase-dependent oncolytic viruses. Cancer Res. 2009;69:1448–1458. doi: 10.1158/0008-5472.CAN-08-1160. [DOI] [PubMed] [Google Scholar]
- 196.Chuang CM, Monie A, Wu A, Pai SI, Hung CF. Combination of viral oncolysis and tumor-specific immunity to control established tumors. Clin Cancer Res. 2009;15:4581–4588. doi: 10.1158/1078-0432.CCR-08-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Huang JH, Zhang SN, Choi KJ, Choi IK, Kim JH, Lee M, Kim H, Yun CO. Therapeutic and tumor-specific immunity induced by combination of dendritic cells and oncolytic adenovirus expressing IL-12 and 4-1BBL. Mol Ther. 2009 doi: 10.1038/mt.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Robinson M, Ge Y, Ko D, Yendluri S, Laflamme G, Hawkins L, Jooss K. Comparison of the E3 and L3 regions for arming oncolytic adenoviruses to achieve a high level of tumor-specific transgene expression. Cancer Gene Ther. 2008;15:9–17. doi: 10.1038/sj.cgt.7701093. [DOI] [PubMed] [Google Scholar]
- 199.Bortolanza S, Bunuales M, Alzuguren P, Lamas O, Aldabe R, Prieto J, Hernandez-Alcoceba R. Deletion of the E3-6.7K/gp19K region reduces the persistence of wild-type adenovirus in a permissive tumor model in Syrian hamsters. Cancer Gene Ther. 2009;16:703–712. doi: 10.1038/cgt.2009.12. [DOI] [PubMed] [Google Scholar]
- 200.McSharry BP, Burgert HG, Owen DP, Stanton RJ, Prod'homme V, Sester M, Koebernick K, Groh V, Spies T, Cox S, Little AM, Wang EC, Tomasec P, Wilkinson GW. Adenovirus E3/19K promotes evasion of NK cell recognition by intracellular sequestration of the NKG2D ligands major histocompatibility complex class I chain-related proteins A and B. J Virol. 2008;82:4585–4594. doi: 10.1128/JVI.02251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bennett EM, Bennink JR, Yewdell JW, Brodsky FM. Cutting edge: adenovirus E19 has two mechanisms for affecting class I MHC expression. J Immunol. 1999;162:5049–5052. [PubMed] [Google Scholar]
- 202.Hermiston TW, Tripp RA, Sparer T, Gooding LR, Wold WS. Deletion mutation analysis of the adenovirus type 2 E3-gp19K protein: identification of sequences within the endoplasmic reticulum lumenal domain that are required for class I antigen binding and protection from adenovirus-specific cytotoxic T lymphocytes. J Virol. 1993;67:5289–5298. doi: 10.1128/jvi.67.9.5289-5298.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Reddy PS, Burroughs KD, Hales LM, Ganesh S, Jones BH, Idamakanti N, Hay C, Li SS, Skele KL, Vasko AJ, Yang J, Watkins DN, Rudin CM, Hallenbeck PL. Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J Natl Cancer Inst. 2007;99:1623–1633. doi: 10.1093/jnci/djm198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lun X, Yang W, Alain T, Shi ZQ, Muzik H, Barrett JW, McFadden G, Bell J, Hamilton MG, Senger DL, Forsyth PA. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 2005;65:9982–9990. doi: 10.1158/0008-5472.CAN-05-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Saito K, Uzawa K, Kasamatsu A, Shinozuka K, Sakuma K, Yamatoji M, Shiiba M, Shino Y, Shirasawa H, Tanzawa H. Oncolytic activity of Sindbis virus in human oral squamous carcinoma cells. Br J Cancer. 2009;101:684–690. doi: 10.1038/sj.bjc.6605209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vaha-Koskela MJ, Kallio JP, Jansson LC, Heikkila JE, Zakhartchenko VA, Kallajoki MA, Kahari VM, Hinkkanen AE. Oncolytic capacity of attenuated replicative semliki forest virus in human melanoma xenografts in severe combined immunodeficient mice. Cancer Res. 2006;66:7185–7194. doi: 10.1158/0008-5472.CAN-05-2214. [DOI] [PubMed] [Google Scholar]