Abstract
Tetraspanin CD151 is associated with laminins-binding integrins (i.e. α3β1, α6β1 and α6β4) and regulates tumour cell migration and invasion. Here we examined the role of CD151 in proliferation of mammary epithelial cells using in vitro and in vivo models. Depletion of CD151 suppressed growth of HB2 cells, a non-tumourigenic breast epithelial cell line, in 3-D extracellular matrices (ECM) and in Matrigel-based xenografts. Whilst the presence of α3β1 (but not α6 integrins) was necessary to support growth of HB2 cells in 3-D ECM, the pro-proliferative activity of CD151 did not require the direct interaction with integrins. Furthermore, depletion of CD151 potentiated formation of the internal lumen and partial restoration of polarity when HB2 cells were cultured in 3-D ECM. This correlated with a decrease in phosphorylation levels of Erk1/2 and cAkt in CD151-negative cells and increase in activation of Caspase-3. Accordingly, the number of CD151-positive colonies with internal lumen was increased by approximately five-fold when cells were cultured in the presence of MEK (U0126) and PI3-K (LY29004) inhibitors. To establish physiological relevance of pro-proliferative and morphogenetic activities of CD151 we analysed expression of this tetraspanin in ductal carcinoma in situ (DCIS), which is characterized by neoplastic proliferation of mammary epithelial cells. Strong homogeneous membrane expression of CD151 was found to be associated with high grade of DCIS (p=0.004). Taken together these results strongly suggest that CD151 complexes play a crucial role in the development of hyperproliferative diseases in the mammary gland.
Keywords: tetraspanin, integrin, CD151, breast cancer, DCIS
INTRODUCTION
Four transmembrane domain proteins of the tetraspanin superfamily are associated with integrin adhesion receptors and are known to regulate motility and invasiveness of various cell types (1). It has been proposed that tetraspanins function through a special type of microdomains on the cell surface, which are referred to as tetraspanin-enriched microdomains (TERM or tetraspanin webs) (2). It is thought that function of TERM-associated integrins (e.g. α3β1 and α6 integrins) is influenced by other proteins within TERM including cytoplasmic enzymes and adaptors (1). In addition to their motility-dependent functions tetraspanins regulate cell-cell fusion (3), trafficking and processing of the associated molecules (4) and can influence lipid composition of the plasma membranes (5).
Early studies involving anti-tetraspanin mAbs have shown that various members of the tetraspanin superfamily also function as co-stimulatory molecules in T- and B-cells (6). Co-stimulatory/pro-proliferative activities of tetraspanins were linked to their ability to interact with critical components of the T-cell receptor complex including CD4, CD8, CD25 and the others. The involvement of tetraspanins in proliferation of hematopoietic cells was confirmed more recently using various knockout models (7-10). Whilst in most of these studies underlying molecular mechanisms have not been investigated, the experiments using CD37-negative T-cells cells have suggested that this tetraspanin is involved in dephosphorylation of Lck, a Src family tyrosine kinase responsible for delivering the proliferative signal from CD3 (8).
Tetraspanin CD151 is directly associated with laminin-binding integrins (i.e. α3β1, α6β1, α6β4 and α7β1) and known to regulate cell motility (11-14). In epithelial cells it also controls “group cell migration” (15). The involvement of CD151 in proliferation of non-hematopoietic cells remains controversial. There were no obvious proliferative defects CD151-deficient mice and humans (16-19). Consistent with this deletion of CD151 did not affect proliferation of primary endothelial cells on Matrigel in vitro (13). On the other hand, proliferation of CD151-negative primary keratinocytes on a laminin substrate was impaired (20). We and others have found that whilst depletion of CD151 diminished growth of tumour cells in immunocompromised animals, cell proliferation under standard conditions was not affected (21, 22). Taken together, these results suggest one of the following possibilities: i) involvement of CD151 in proliferation may be cell type specific; ii) host microenvironment may have an important role in CD151-dependent cell proliferation; iii) the involvement of CD151 in proliferation of tumour cells under standard culturing conditions (i.e. growth on plastic) may be overshadowed by intrinsic activating mutations in genes that control cell proliferation and are found in most established cancer cell lines (e.g. Ras, B-Raf, PI3-kinase).
Mammary ductal carcinoma in situ is the non-obligate precursor of invasive breast cancer and is characterized by proliferation of neoplastic cells within the duct lumen. Here we found that the elevated expression of CD151 correlated with a more aggressive phenotype in DCIS. By knocking down expression of CD151 in HB2 cells, a non-tumourigenic mammary epithelial cell line, we found that this tetraspanin controls proliferation of cells in vivo (mouse xenografts) and in 3-D extracellular matrix (ECM). Furthermore, many of CD151-negative colonies developed internal lumens when grown in 3-D ECM. The expression of CD151 and its pro-proliferative and morphogenetic activities correlated with increased activation of Erk1/2 and c-Akt. These results strongly suggest that CD151 may play an important role in the development of hyperproliferative diseases in mammary gland and may determine behaviour of DCIS.
MATERIALS AND METHODS
Cells, antibodies and reagents
All cells were grown in DMEM (Sigma) supplemented with 10% foetal bovine serum (FBS), 5μg/ml insulin, 10μg/ml hydrocortisone. Plasmids encoding constitutively active MEK1 (DD-MEK) and cAkt (myr-Akt) were provided by Dr.E.Tulchinsky and Dr.A.Eliopoulos, respectively. HB2/CD151(−) were generated after transfection of pSuperior-shRNA-CD151 (23) and subsequent cell sorting. HB2/CD151rec and HB2/CD151-QRD cell lines were established after transfections of HB2/CD151(−) cells with constructs encoding shRNA-resistant wild-type and QRD194-196→INF mutant of CD151 (21, 23). Cell lines expressing low levels of α3β1 and α6 integrins (HB2/α3β1(−) and HB2/α6(−)) were established by infecting of HB2 cells with pLVTHM-based lentivirus encoding shRNA that targets α3 or α6 integrin subunit, respectively (target sequences were: α3 – 5′-gctacatgattcagcgcaa-3′; α6 – 5′- GGTCGTGACATGTGCTCAC-3′). The mouse anti-CD151 mAb: 5C11 (24), 11B1G4 (kindly provided by Dr.L.Ashman), NCL-CD151 (Novocastra). Mouse mAb against laminin alpha5 chain (5D6) was kindly provided by Dr.K.Sekiguchi. Mouse mAb against β-actin was from Sigma; mouse mAbs against β4 integrin subunit (3E1) and laminin γ2 subunit were from Chemicon; mouse mAb against E-cadherin were from Abcam; mouse mAb against ESA was from Dako; mouse mAb to GM130 was from BD Bioscience; mouse mAb against human MUC1, keratins 18 and 19 were from Cancer Research UK; mouse mAb against human keratin 5/6 were from Dako. Rabbit polyclonal antibodies to α3 and α6 integrin were generously provided by Dr F. Watt and Dr A. Cress. Goat pAb against α3 integrin subunit was purchased from Santa Cruz. Mouse mAbs to α3- (A3-IVA5), α6- (A6-ELE) and β1- (TS2/16) integrin subunit were described previously (25-27). The rest of the antibodies used in this study were purchased from Cell Signaling Technology.
Culturing cells in 3-D Matrigel
Culturing of cells in 3-D ECM was performed as previously described (21). For morphological analyses in 3-D culture experiments representative pictures were taken using Axiovert 26 Zeiss microscope. Immunofluorescence staining in 3-D ECM was carried out as described in (28) and analysed using Zeiss LSM510 META confocal system.
Analysis of activation of FAK, PKB/cAkt and ERK1/2 in 3-D ECM
Cells were released from 3-D ECM by mechanical pippetting. Released cells were washed once with PBS and lysed directly into Laemmli sample buffer supplemented with 2mM phenylmethylsulfonyl fluoride, 10μg/ml aprotinin, 10 μg/ml leupeptin, 100μM Na3VO4, 10mM NaF, 10mM Na3P4O7. Cellular proteins were resolved on 10-12% SDS-PAGE, transferred to the nitrocellulose membrane and probed with appropriate phospho-specific or conventional Abs.
Xenograft experiments
All experiments were performed in accordance with institutional and national animal research guidelines. Cells (1×106/0.2ml Matrigel:PBS) were injected subcutaneously. Four to six animals per group were used in the experiments. The experiments were terminated 4, 8 or 14 weeks post injection.
Immunohistochemistry (IHC)
After antigen retrieval, immunostaining of paraffin sections was performed using a standard avidin-biotin-peroxidase complex (ABC) method. The sections counterstained with haematoxylin were analyzed using the Olympus DP70 camera and the Olympus DP-Soft software (Japan). Quantification of glandular structures in xenografts was performed by counting structures in 9 high power (400x) fields corresponding to 0.0625mm2. The numbers represent the average from 9 counts. For analysis of DCIS tissue samples, sections of formalin-fixed paraffin-embedded tissue were taken from 87 cases of DCIS (39 pure DCIS and 48 with associated invasion) and 14 normal breast samples and stained as described above. The use of the samples for the study was approved by Local NHS Research Ethics Committee (COREC ref 06/Q0403/182).
Analysis of staining
Staining intensity of CD151 was scored in tumour cells of DCIS cases on an individual duct basis to be able to study the relationship between pattern and expression intensity as well as grade and expression intensity. Only membranous staining was considered positive. Staining was scored as heterogeneous when the staining intensity was different in the tumour cells located at the periphery of the duct from those located at the centre. Statistical analysis of the results was done by Pearson Chi-square test with P value <0.05 considered as significant and using SPSS software version 11.
RESULTS
Increased expression of CD151 correlates with higher grade of DCIS
We have recently found that the elevated expression of CD151 correlates with the advanced stage in patients with invasive ductal carcinoma of the breast (IDC) (21).To examine whether deregulation of CD151 expression could be observed earlier during breast cancer evolution we analysed the expression of this tetraspanin in ductal carcinoma in situ (DCIS), a preinvasive form of breast cancer, which is characterised by filling of the ductal luminal space. Eighty-seven cases of DCIS (39 pure DCIS and 48 with associated invasive disease) were included in this study. In normal breast tissue CD151 is restricted to the myoepithelial cell layer, with no or very weak staining of luminal epithelial cells (Fig.1A). Since DCIS can exhibit significant heterogeneity of grade and pattern within a case the expression was analysed on a duct-by-duct basis (a total of 394 ducts). The luminal neoplastic cells in 104 ducts were negative for CD151 expression. More than two-thirds of the remaining ducts showed strong, homogeneous membrane expression of CD151 by all the DCIS cells (Fig.1A). Expression of CD151 was found to be associated with high grade of DCIS (p=0.004; Supplementary Table 1) but there was no association with disease pattern. Furthermore, the frequency of positivity of DCIS was similar in the pure cases to those with established invasion, though the pattern of staining with CD151 expressed by cells at the periphery of the duct and not centrally was more frequent in those cases with invasion.
Figure 1.

Expression of CD151 in DCIS and characterization of HB2 xenografts. A. In normal breast the staining is limited to the myoepithelial-basement membrane interface (top left panel, arrows), with sparing of the luminal cells. In DCIS staining was either homogeneously positive (top right panel), with strong membrane staining in all the neoplastic luminal cells, or hetererogeneous (bottom panels), with strong membrane staining of neoplastic cells at the periphery of the duct, but lack of CD151 expression in the cells towards the centre of the ducts, particularly seen as the cells started to form small lumen. B, Cells were injected subcutaneously into nude mice as described in Materials and Methods and xenografts were grown for 8 weeks. Shown morphology (H&E staining) of representative glandular structures.
Characterization of HB2 cellular aggregates in vivo and in 3-D ECM
To examine whether CD151 can contribute to the proliferative phenotype associated with the development of DCIS, we used HB2 cells as a model system (29). HB2 cells express various markers of luminal cells (30) and, therefore, they are more closely resemble DCIS (more than 90% of which exhibit luminal phenotype (31)), than a more widely used DCIS.com cell model, which have marker profile of myoepithelial cells (32). When injected into nude mice HB2 cells formed glandular-like structures of ~30-70 μm in diameter 8 weeks after the injection (Fig.1B) These structures were of different degree of morphogenic organization with most of the structures having appeared as cysts with a single layer of epithelium. None of the xenografts have developed into a tumour mass and most of them regressed by 12-14 weeks (results are not shown). Immunohistochemical analysis has shown that CD151 and α3β1 were abundant at cell-cell contacts (Supplementary Fig.1). When cultured in vitro in 3-D ECM, HB2 cells formed compact round colonies with well-defined contours (Supplementary Fig.2A). Further characterisation of these structures has shown that in contrast to HB2 xenografts most of the colonies in 3-D ECM did not develop internal lumens even after prolonged culturing. Immunofluorescent staining of 10-day structures revealed that cells inside colonies formed tight cell-cell contacts (as indicated by staining with anti-E-cadherin and anti-ESA mAbs) (Supplementary Fig.2B). We found that α3β1 integrin was also abundant at the cell-cell contacts (Supplementary Fig.2B).
Depletion of CD151 suppresses growth of HB2 xenografts and proliferation of the cells in 3-D ECM
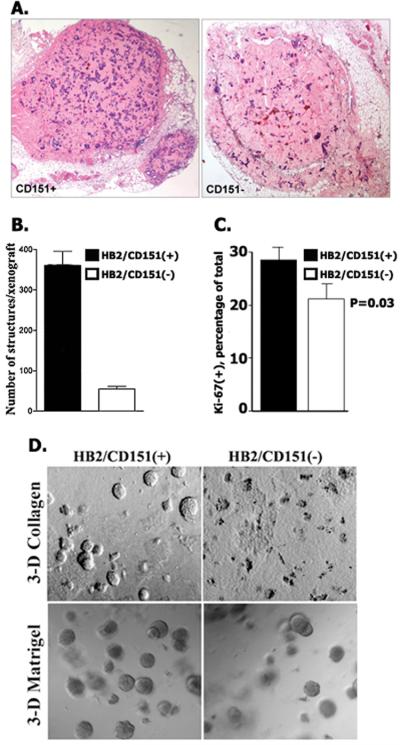
To establish the role of CD151 in growth of HB2 cells in vivo and in 3-D ECM we have generated HB2/CD151(−) cells in which expression of this tetraspanin was decreased by >90% (Supplementary Fig.3). Similar to the control HB2/CD151(−) cells formed glandular structures when grown as subcutaneous xenografts in nude mice. However, detailed quantification of xenografts has revealed that the total number of glandular structures formed by CD151-negative cells was ~7-fold lower when compared with HB2/CD151(+) cells (Fig.2A,B). Accordingly, we found that Ki-67 index was higher in CD151(+) xenografts (Fig.2C). In contrast, there was no apparent difference between HB2/CD151(+) and HB2/CD151(−) xenografts when sections were stained with the antibody recognising activated Caspase-3 (not shown). These results suggested that differences in growth of HB2/CD151(+) and HB2/CD151(−) cells in vivo are due to the effect of CD151 on cell proliferation rather than apoptosis. There were also differences in the total number of CD151–positive and CD151–negative colonies when cells were cultured in 3-D ECM (Fig. 2D). Importantly, when expression of CD151 protein was reconstituted in HB2/CD151(−) (HB2/CD151rec cells), the “CD151-negative phenotypes” reverted to that of the control cells (Supplementary Fig.4). Taken together, these results showed that tetraspanin CD151 controls proliferative potential of mammary epithelial cells in vivo and in 3-D ECM.
Figure 2.
The effect of CD151 depletion on growth of HB2 cells in vivo and in 3-D ECM. A. Sections of representative HB2/CD151(+) and HB2/CD151(−) xenografts (8 weeks). B. Quantification of structures formed by CD151-positive and CD151-negative HB2 cells in vivo. Shown means of the number of structures. Error bars represent SEM. C. Ki-67 labelling index. Shown results of quantification of Ki-67- immunopositive cells (average from 10 high power fields (x400) ~ 500 cells /sample). D. HB2/CD151(+) and HB2/CD151(−) cells were grown for 10 days in 3-D collagen (top panel) or 3-D Matrigel (bottom panel). Shown representative fields.
The role of CD151 associated integrins in 3-D ECM growth of HB2 cells
It has been postulated that in non-hematopoietic cells CD151 functions via integrins (33, 34). To examine whether the pro-proliferative activity of CD151 is dependent on its association with integrins we analysed behaviour of HB2/CD151-QRD cells. These cells express QRD194-196→ INF mutant of CD151 (CD151-QRD), which is unable to interact with laminin-binding integrins directly (35). Interestingly, although expression of the CD151-QRD mutant in CD151-negative cells has effectively restored their colony-forming potential in 3-D ECM, HB2/CD151-QRD aggregates appeared distinctly different from either CD151(+) or CD151(−) colonies. In contrast to typically compact structures, a large proportion of the HB2/CD151-QRD colonies appeared as aggregates of loosely associated cells (Fig.3). These experiments suggested that i) the pro-proliferative activity of CD151 does not depend on its association with integrins; ii) the formation of compact colonies in 3-D ECM relies on the CD151-integrin complexes. Integrins α3β1, α6β1 and α6β4 are principal partners of CD151 in epithelial cells. For further assessment of the roles played by CD151-associated integrins we analysed growth of HB2 cells deficient in expression of either α3β1 or α6β1/α6β4 (HB2/α3β1(−) and HB2/α6(−) cells, respectively). Whilst the ability of HB2/α3β1(−) cells to grow in 3-D ECM was severely compromised, depletion of α6(−) integrins had no apparent effect on the plating efficiency and morphology of the colonies formed by HB2 cells (Fig.3). Although these results were somewhat surprising given an important role of α6 integrins in regulating growth of epithelial cells in Matrigel (36, 37), we found that HB2 cells deposited laminin-332 at the perimeter of growing colonies (see below). Thus, it is likely that in the absence of α6 integrins HB2 cells fully rely on the interaction between α3β1 integrin and cell-produced laminin-332. Collectively, these results indicated that 1) proliferative and morphogenetic activities of CD151 are dependent on α3β1; 2) α6β1/4 – laminin-332 interactions can't compensate for the absence of α3β1 in 3-D ECM.
Figure 3.
The role of CD151-associated integrins in pro-proliferative and morphogenetic activities of the tetraspanin. A. Morphological appearance of colonies formed by indicated HB2 sublines grown for 10 days in 3-D Matrigel. Insets show magnified images of representative colonies. Note, rare colonies formed by HB2/α3β1(−) cells have the appearance of the CD151(+) aggregates. B. Western blots showing expression of CD151 and integrin subunits in cells used in the panel A.
Depletion of CD151 induces lumen formation in 3-D ECM cultures of HB2 cells
We noticed that in spite of apparent deficiency in their ability to grow in 3-D ECM, a large proportion of CD151-negative colonies which did grow, develop internal lumens (Fig.4A). Formation of the lumen in CD151-negative colonies also correlated with the appearance of apoptotic cells inside the colonies and increased activation of Caspase-3 (Fig.4B). In other experiments we found that activation of Caspase-3 in cells denied attachment was more pronounced in the absence of CD151 (Fig.4C). These data strongly suggest that CD151 regulates lumen formation through signalling pathways linking integrins with activation of Caspase 3. Thus, we examined the effect of CD151 depletion on distribution of the associated integrins in cells cultured in 3-D ECM. Lumen formation in HB2/CD151(−) cells correlated with re-distribution of α3β1 integrin from cell-cell contacts to the peripheral, cell-ECM interface (Fig.5A). By contrast, depletion of CD151 had no effect on localisation of α6 integrins. The redistribution of integrins in CD151-depleted cells towards the cell-ECM interface was specific as both E-cadherin and ESA retained their localization in cell-cell contacts (Fig.5A). Next we investigated whether CD151-dependent relocalisation of α3β1 can be linked to changes in secretion and/or deposition of Ln-332 or Ln-511/Ln-521, known laminin substrates for this integrin. We found that both cell lines secreted small quantities Ln-332 which was deposited at the cell-ECM interface (Fig.5A); Ln-511/Ln-521 was not produced by these cells as judged by lack of immunostaining with mAb to α5 laminin subunit (results are not shown). The membrane matrix metalloproteinase MT1-MMP/MMP14 is known to control α3β1-mediated interactions with Ln-332 (38). Importantly, CD151 is co-precipitated with MT1-MMP/MMP14 and regulates its subcellular distribution (39). We found that removing of CD151 did not affect distribution of MT1-MMP/MMP14 in 3-D cultures: weak MT1-MMP/MMP14 staining was diffusely spread over the cell surface and in cytoplasm with no apparent differences observed between the HB2/CD151(+) and HB2/CD151(−) colonies (Fig.5A). We also found that depletion of CD151 did not affect total levels of LN-332 and MT1-MMP/MMP14 in cells grown in 3-D Matrigel (not shown). Having considered all these results we concluded that it is unlikely that redistribution of α3β1 integrin in CD151-depleted cells is driven by deposition (or reorganisation) of its ECM ligands. Depletion of CD151 can change organisation of actin filaments at cell-cell junctions in cells grown in 2-D (15). Therefore, it was plausible to suggest that relocalization of α3β1 might have been caused by changes in actin cytoskeleton. However, we found that distribution of actin filaments was not affected by CD151 removal when cells were cultured in 3-D Matrigel (Fig.5B). Finally, more restricted and polarised distribution of α3β1 in HB2/CD151(−) cells prompted us to analyse whether depletion of CD151 has a more general effect on cell polarity. Thus, we examine position of Golgi in cells grown in 3-D Matrigel and distribution of MUC-1, a well-established apical marker. In most HB2/CD151(−) cells Golgi was positioned between the nucleus and surface facing the lumen (Fig.5B). By contrast, distribution of the Golgi marker GM130 was more random in HB2/CD151(+) colonies (Figure 5B). In spite of such a pronounced effect of CD151 depletion on Golgi we found no evidence for apical localisation of MUC1 in HB2/CD151(−) colonies (Fig.5B). In fact, in both CD151(−) and CD151(+) colonies the most prominent staining was concentrated at the cell-ECM (i.e. basal) interface. Taken together these results indicated that there was a partial restoration of the polarised phenotype in CD151-depeleted cells.
Figure 4.
Depletion of CD151 induces lumen formation in colonies formed by HB2 cells in 3-Matrigel. HB2/CD151(+) and HB2/CD151(−) cells were cultured in 3-D Matrigel for 10 days. A. The lumen formation was analysed by the confocal microscopy as described in Materials and Methods. Nuclei were stained with Hoechst 33342. Proportion of the colonies with lumen/partial lumen was quantified in 3 independent experiments with 60-80 colonies assessed in each of the experiments. Error bars represent SEM. B. Activation of Caspase-3 in HB2/CD151(+) and HB2/CD151(−) cells cultured 3-D Matrigel. Colonies were fixed with paraformaldehyde and stained with antibodies to active Caspase-3 (upper panel). Scale bar represents 50μm. Western blot analysis of caspase-3 activation in cultures of HB2/CD151(+) and HB2/CD151(−) cells in 3-D Matrigel (lower panel). C. Depletion of CD151 potentiates detachment-induced activation of Caspase-3. HB2/CD151(+) and HB2/151(−) cells were detached and kept in suspension over poly-HEMA – coated surface in serum-free DMEM for 24 or 48 hours. Proteins were resolved by 12% SDS-PAGE and presence of activated Caspase-3 (and actin) was visualised using Western blotting. Lysates from cells attached to plastic (and incubated in serum-free DMEM) were used as controls (lanes 1 and 2).
Figure 5.
Depletion of CD151 causes redistribution of α3β1 integrin and apical relocalisation of Golgi. Cells were grown in Matrigel for 10 days. The colonies were fixed with paraformaldehyde and stained with indicated mAb. In B. actin filaments were visualised using Alexa 594- conjugated phalloidin and nuclei were stained with DAPI. Note fragmented nuclei inside HB2/CD151(−) colony. Shown representative images. Scale bars represent 50μm.
Pro-proliferative function of CD151 in 3-D Matrigel is linked to activation of Erk1/2 and c-Akt
To establish which of the signalling pathways may be relevant to pro-proliferative and morphogenetic activities of CD151 we compared activation of various integrin-dependent signalling pathways in HB2/CD151(+) and HB2/CD151(−) cells grown in 3-D ECM. Deficiency in CD151 correlated with the decrease in the levels of phosphorylated Erk1/2 (Fig.6A). The effect of CD151 depletion was more pronounced at days 4 and 5 after the embedding. We also observed decrease in the level of active c-Akt in HB2/CD151(−) cells. To examine whether decrease in the levels of active ERK1/2 and c-Akt were sufficient to inhibit growth in 3-D ECM and change the phenotypic pattern of colonies, we cultured the control HB2/CD151(+) cells in the presence of U0126 and LY29004, widely used inhibitors of Erk1/2 and PI3-K/c-Akt signalling pathways, respectively. These experiments showed that both chemicals had a strong inhibitory effect on proliferation of the cells in 3-D Matrigel (Fig6B). Collectively, these results showed that the pro-proliferative function of CD151 in 3-D ECM is dependent on activation of Erk1/2 and c-Akt.
Figure 6.
Depletion of CD151 attenuates activation of Erk1/2 and c-Akt in HB2 cells. A. Analysis of signalling pathways in CD151(+) and CD151(−) cells grown in 3-D Matrigel for indicated times. Shown results of one of three independent experiments. B. Inhibition of c-Akt and Erk1/2 signalling pathway suppresses proliferation of HB2/CD151(+) cells in 3-D Matrigel. Cells were cultured in 3-D Matrigel for 10 days in the presence of 10μM U0126 (middle panel) or 10μM LY29004 (bottom panel). Shown images of representative fields. C. HB2 cells were grown in Matrigel for 10 days in the presence of 10μM U0126 (top panel) or 10ηM LY29004 (bottom panel). Nuclei were stained with Hoechst 33342. Shown images of representative fields.
Importantly, growing HB2/CD151(+) cells in the presence of Erk1/2 or PI3-K inhibitors facilitated lumen formation in ~50% of the colonies (Fig.6C). Conversely, when constitutively active MEK and cAkt were expressed in HB2/CD151(−) cells the effect of CD151 depletion on lumen formation was reversed (Supplementary Fig.5). These results provided further evidence for the role of CD151-dependent activation of PI3-K and Erk1/2 in 3-D ECM growth and lumen formation via Caspase-3. A slight discordance between the kinetics of CD151-dependent activation of Caspase-3 and decrease in phosphorylation of cAkt and Erk1/2 suggests the contribution of additional pathways leading to Caspase-3 cleavage (e.g. involvement of PKA, Ca+2 and/or PKCδ (40-42)).
DISCUSSION
In this report we described a new cellular model for mammary ductal carcinoma in situ (DCIS) and showed that tetraspanin CD151 is likely to play an important role in the development of this disease. Specifically, we found that i) elevated expression of CD151 regulates of proliferation and controls morphogenetic behaviour of non-tumourigenic mammary epithelial cells both in vivo and in 3-D ECM; 2) removal of CD151 facilitates formation of the internal lumen by cells cultured in 3-D ECM and their partial polarization; 3) CD151 controls behaviour of cells in 3-D ECM via activation of ERK1/2 and cAkt. The physiological relevance of these findings is emphasised by our observation that increased expression of CD151 is associated with development of DCIS, a pre-invasive form of breast cancer characterised by proliferation of neoplastic cells filling the lumen of ducts.
It has been previously shown that various biological activities of CD151 in non-hematopoietic cells are dependent on its associated integrin partners (35, 21). Here, we found for the first time that at least one of the CD151-dependent functions in non-tumourigenic mammary epithelial cells (i.e. its pro-proliferative activity) does not require a direct contact of the tetraspanin with integrin molecules. Yet, the presence of fully functional CD151 and α6 integrins was not sufficient to allow proliferation of α3β1-negative cells in 3-D ECM. This suggests that CD151 functions as a co-stimulatory molecule. Importantly, our report and an earlier study, which was focussed on tetraspanin CD81 in T-cells (43), have shown that co-stimulatory activities of tetraspanins are linked to activation of Erk1/2. Thus, there may exist a common mediator of tetraspanin-dependent activation of Erk1/2, which seems to be utilised in the context of signalling pathways activated by various tetraspanin-associated receptors.
In addition to its role in cell proliferation CD151 (or rather CD151-α3β1 complex) controls cell-cell interactions. Importantly, this became apparent only when cells were cultured in 3-D ECM. Although, earlier and more recent data have shown that CD151 regulates cell-cell interaction via E-cadherin (15, 44), we found that distribution of E-cadherin or its association with actin cytoskeleton in HB2 cells were not affected in HB2/CD151(−) cells (Fig.5A and not shown). Furthermore, we were unable to co-precipitate E-cadherin with either CD151 or α3β1 integrin. Thus, it appears that the effect of CD151 on intercellular adhesion in mammary epithelial cells is likely to be mediated by a non-cadherin receptor. Among the possible candidates are EpCAM and one of the claudins, both of which are known to associate with tetraspanins and function as cell-cell adhesion molecules (45).
Our data point to a previously unknown function of CD151 as a potential regulator of epithelial cell polarity. In this regard, it has been reported that CD151 regulates the activity of cdc42 (13, 44), a key player in establishing and maintenance of epithelial cell polarity (46). Therefore, it is possible that CD151 depletion results in refining GTP·CDC42. GDP·CDC42 balance in HB2 cells thus allowing to establish polarity in 3-D ECM. Furthermore, attenuation of Erk1/2 – dependent signalling observed in HB2/CD151(−) cells may also facilitate their polarization (47). It is important to emphasise that despite the prominent effect of CD151 removal on repositioning Golgi, polarization of HB2 cells in 3-D Matrigel was incomplete. Indeed, we showed that MUC1 was predominantly delivered to the basal surface irrespectively of CD151 expression (Fig5B). Furthermore, we found that various tight junction proteins (e.g. occludin, claudins and ZO-1) were distributed along the entire cell-cell contact membranes in HB2/CD151(−) colonies (not shown). Hence, CD151 depletion restores only certain features of polarised epithelial cells. Nevertheless, it is tempting to speculate that overexpression of CD151 in mammary tumour cells can contribute to changes in cell polarity, one of the early events in the development of DCIS.
The fact that the elevated expression CD151 is specifically associated with high grade forms of DCIS (which is characterised by a high level of proliferation and inhibition of apoptosis), further supports the physiological relevance of our findings linking function of CD151 with proliferation of HB2 cells in 3-D ECM. In normal breast, expression of CD151 in the ducts is restricted to the myoepithelial cell layer with luminal cells either being completely negative or weakly positive. Therefore, one may consider that the up-regulation of CD151 expression in some forms of DCIS could simply reflect a transition from the luminal to basal/myoepithelial phenotypes (48, 49). However, basal-like DCIS accounts for only 5-10% of DCIS, whereas up-regulation of CD151 is evident in >70% of ducts with DCIS in our study. This suggests that dysregulated expression of CD151 is not related to differentiation but is more likely to be a driver of the high proliferation seen in this high-grade disease. Although this study was specifically focused on pure DCIS, in some of the selected mixed DCIS cases (i.e. which included an invasive component) we observed that the expression of CD151 was either reduced or lost in the invasive tumour cells (results are not shown). Whilst the significance of this observation remains to be investigated, these data seem to indicate that the major function of CD151 in DCIS is to stimulate intraductal proliferation of cancerous cells.
In conclusion, we found that CD151 plays a critical role in controlling proliferation of non-malignant mammary epithelial cells. The up-regulation of CD151 in a majority of DCIS, and its significant association with high grade DCIS supports the idea that CD151 is involved in driving proliferation of epithelial cells in the early stages of breast cancer. It will be of interest to determine at what point in disease evolution CD151 becomes up-regulated, and to address this future studies will analyse earlier pre-cursor lesions including atypical ductal hyperplasia and usual type hyperplasia.
Supplementary Material
Acknowledgement
We are very grateful to all our colleagues for their generous gifts of the reagents that were used in this study. This work was supported by the CR UK grant C1322/A5705 (to F.B.).
Reference List
- 1.Yanez-Mo M, Barreiro O, Gordon-Alonso M, et al. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19:434–46. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Charrin S, Le NF, Silvie O, et al. Lateral organization of membrane proteins: tetraspanins spin their web. Biochem J. 2009;420:133–54. doi: 10.1042/BJ20082422. [DOI] [PubMed] [Google Scholar]
- 3.Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397–422. doi: 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- 4.Berditchevski F, Odintsova E. Tetraspanins as regulators of protein trafficking. Traffic. 2007;8:89–96. doi: 10.1111/j.1600-0854.2006.00515.x. [DOI] [PubMed] [Google Scholar]
- 5.Odintsova E, Butters TD, Monti E, et al. Gangliosides play an important role in the organisation of CD82-enriched microdomains. Biochem J. 2006;400:315–25. doi: 10.1042/BJ20060259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005;5:136–48. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- 7.Sheng KC, van Spriel AB, Gartlan KH, et al. Tetraspanins CD37 and CD151 differentially regulate Ag presentation and T-cell co-stimulation by DC. Eur J Immunol. 2009;39:50–5. doi: 10.1002/eji.200838798. [DOI] [PubMed] [Google Scholar]
- 8.van Spriel AB, Puls KL, Sofi M, et al. A regulatory role for CD37 in T cell proliferation. J Immunol. 2004;172:2953–61. doi: 10.4049/jimmunol.172.5.2953. [DOI] [PubMed] [Google Scholar]
- 9.Tarrant JM, Groom J, Metcalf D, et al. The absence of Tssc6, a member of the tetraspanin superfamily, does not affect lymphoid development but enhances in vitro T-cell proliferative responses. Mol Cell Biol. 2002;22:5006–18. doi: 10.1128/MCB.22.14.5006-5018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng J, Dekruyff RH, Freeman GJ, et al. Critical role of CD81 in cognate T-B cell interactions leading to Th2 responses. Int Immunol. 2002;14:513–23. doi: 10.1093/intimm/14.5.513. [DOI] [PubMed] [Google Scholar]
- 11.Berditchevski F, Odintsova E, Sawada S, et al. Expression of the palmitoylation-deficient CD151 weakens the association of alpha 3 beta 1 integrin with the tetraspanin-enriched microdomains and affects integrin-dependent signaling. J Biol Chem. 2002;277:36991–7000. doi: 10.1074/jbc.M205265200. [DOI] [PubMed] [Google Scholar]
- 12.Sawada S, Yoshimoto M, Odintsova E, et al. The tetraspanin CD151 functions as a negative regulator in the adhesion-dependent activation of Ras. J Biol Chem. 2003;278:26323–6. doi: 10.1074/jbc.C300210200. [DOI] [PubMed] [Google Scholar]
- 13.Takeda Y, Kazarov AR, Butterfield CE, et al. Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro. Blood. 2007;109:1524–32. doi: 10.1182/blood-2006-08-041970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada M, Sumida Y, Fujibayashi A, et al. The tetraspanin CD151 regulates cell morphology and intracellular signaling on laminin-511. FEBS J. 2008;275:3335–51. doi: 10.1111/j.1742-4658.2008.06481.x. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JL, Winterwood N, DeMali KA, et al. Tetraspanin CD151 regulates RhoA activation and the dynamic stability of carcinoma cell-cell contacts. J Cell Sci. 2009;122:2263–73. doi: 10.1242/jcs.045997. [DOI] [PubMed] [Google Scholar]
- 16.Wright MD, Geary SM, Fitter S, et al. Characterization of mice lacking the tetraspanin superfamily member CD151. Mol Cell Biol. 2004;24:5978–88. doi: 10.1128/MCB.24.13.5978-5988.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ang J, Lijovic M, Ashman LK, et al. CD151 protein expression predicts the clinical outcome of low-grade primary prostate cancer better than histologic grading: a new prognostic indicator? Cancer Epidemiol Biomarkers Prev. 2004;13:1717–21. [PubMed] [Google Scholar]
- 18.Karamatic C,V, Burton N, Kagan A, et al. CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood. 2004;104:2217–23. doi: 10.1182/blood-2004-04-1512. [DOI] [PubMed] [Google Scholar]
- 19.Karamatic C,V, Poole J, Long S, et al. Two MER2-negative individuals with the same novel CD151 mutation and evidence for clinical significance of anti-MER2. Transfusion. 2008;48:1912–6. doi: 10.1111/j.1537-2995.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- 20.Geary SM, Cowin AJ, Copeland B, et al. The role of the tetraspanin CD151 in primary keratinocyte and fibroblast functions: implications for wound healing. Exp Cell Res. 2008;314:2165–75. doi: 10.1016/j.yexcr.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Sadej R, Romanska H, Baldwin G, et al. CD151 regulates tumorigenesis by modulating the communication between tumor cells and endothelium. Mol Cancer Res. 2009;7:787–98. doi: 10.1158/1541-7786.MCR-08-0574. [DOI] [PubMed] [Google Scholar]
- 22.Yang XH, Richardson AL, Torres-Arzayus MI, et al. CD151 accelerates breast cancer by regulating alpha 6 integrin function, signaling, and molecular organization. Cancer Res. 2008;68:3204–13. doi: 10.1158/0008-5472.CAN-07-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldwin G, Novitskaya V, Sadej R, et al. Tetraspanin cd151 regulates glycosylation of alpha3beta1 integrin. J Biol Chem. 2008;283:35445–54. doi: 10.1074/jbc.M806394200. [DOI] [PubMed] [Google Scholar]
- 24.Berditchevski F, Chang S, Bodorova J, et al. Generation of monoclonal antibodies to integrin-associated proteins. Evidence that alpha3beta1 complexes with EMMPRIN/basigin/OX47/M6. J Biol Chem. 1997;272:29174–80. doi: 10.1074/jbc.272.46.29174. [DOI] [PubMed] [Google Scholar]
- 25.Hemler ME, Ware CF, Strominger JL. Characterization of a novel differentiation antigen complex recognized by a monoclonal antibody (A-1A5): unique activation-specific molecular forms on stimulated T cells. J Immunol. 1983;131:334–40. [PubMed] [Google Scholar]
- 26.Tachibana I, Bodorova J, Berditchevski F, et al. NAG-2, a novel transmembrane-4 superfamily (TM4SF) protein that complexes with integrins and other TM4SF proteins. J Biol Chem. 1997;272:29181–9. doi: 10.1074/jbc.272.46.29181. [DOI] [PubMed] [Google Scholar]
- 27.Weitzman JB, Pasqualini R, Takada Y, et al. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading and homotypic cell aggregation. J Biol Chem. 1993;268:8651–7. [PubMed] [Google Scholar]
- 28.Weaver VM, Lelievre S, Lakins JN, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–16. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berdichevsky F, Alford D, D'Souza B, et al. Branching morphogenesis of human mammary epithelial cells in collagen gels. J Cell Sci. 1994;107:3357–68. doi: 10.1242/jcs.107.12.3557. [DOI] [PubMed] [Google Scholar]
- 30.Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83:249–89. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 31.Meijnen P, Peterse JL, Antonini N, et al. Immunohistochemical categorisation of ductal carcinoma in situ of the breast. Br J Cancer. 2008;98:137–42. doi: 10.1038/sj.bjc.6604112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu M, Yao J, Carroll DK, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–11. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 34.Stipp CS. Laminin-binding integrins and their tetraspanin partners as potential antimetastatic targets. Expert Rev Mol Med. 2010;12:e3. doi: 10.1017/S1462399409001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kazarov AR, Yang X, Stipp CS, et al. An extracellular site on tetraspanin CD151 determines alpha 3 and alpha 6 integrin-dependent cellular morphology. J Cell Biol. 2002;158:1299–309. doi: 10.1083/jcb.200204056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weaver VM, Lelievre S, Lakins JN, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–16. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bello-DeOcampo D, Kleinman HK, Deocampo ND, et al. Laminin-1 and alpha6beta1 integrin regulate acinar morphogenesis of normal and malignant human prostate epithelial cells. Prostate. 2001;46:142–53. doi: 10.1002/1097-0045(20010201)46:2<142::aid-pros1018>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 38.Hintermann E, Quaranta V. Epithelial cell motility on laminin-5: regulation by matrix assembly, proteolysis, integrins and erbB receptors. Matrix Biol. 2004;23:75–85. doi: 10.1016/j.matbio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Yanez-Mo M, Barreiro O, Gonzalo P, et al. MT1-MMP collagenolytic activity is regulated through association with tetraspanin CD151 in primary endothelial cells. Blood. 2008;112:3217–26. doi: 10.1182/blood-2008-02-139394. [DOI] [PubMed] [Google Scholar]
- 40.van Raam BJ, Drewniak A, Groenewold V, et al. Granulocyte colony-stimulating factor delays neutrophil apoptosis by inhibition of calpains upstream of caspase-3. Blood. 2008;112:2046–54. doi: 10.1182/blood-2008-04-149575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, Zambon AC, Vranizan K, et al. Gene expression signatures of cAMP/protein kinase A (PKA)-promoted, mitochondrial-dependent apoptosis. Comparative analysis of wild-type and cAMP-deathless S49 lymphoma cells. J Biol Chem. 2008;283:4304–13. doi: 10.1074/jbc.M708673200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaul S, Kanthasamy A, Kitazawa M, et al. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur J Neurosci. 2003;18:1387–401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- 43.Tardif MR, Tremblay MJ. Tetraspanin CD81 provides a costimulatory signal resulting in increased human immunodeficiency virus type 1 gene expression in primary CD4+ T lymphocytes through NF-kappaB, NFAT, and AP-1 transduction pathways. J Virol. 2005;79:4316–28. doi: 10.1128/JVI.79.7.4316-4328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shigeta M, Sanzen N, Ozawa M, et al. CD151 regulates epithelial cell-cell adhesion through PKC- and Cdc42-dependent actin cytoskeletal reorganization. J Cell Biol. 2003;163:165–76. doi: 10.1083/jcb.200301075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le NF, Zoller M. The tumor antigen EpCAM: tetraspanins and the tight junction protein claudin-7, new partners, new functions. Front Biosci. 2008;13:5847–65. doi: 10.2741/3121. [DOI] [PubMed] [Google Scholar]
- 46.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 47.Lawrence MC, Jivan A, Shao C, et al. The roles of MAPKs in disease. Cell Res. 2008;18:436–42. doi: 10.1038/cr.2008.37. [DOI] [PubMed] [Google Scholar]
- 48.Bryan BB, Schnitt SJ, Collins LC. Ductal carcinoma in situ with basal-like phenotype: a possible precursor to invasive basal-like breast cancer. Mod Pathol. 2006;19:617–21. doi: 10.1038/modpathol.3800570. [DOI] [PubMed] [Google Scholar]
- 49.Livasy CA, Perou CM, Karaca G, et al. Identification of a basal-like subtype of breast ductal carcinoma in situ. Hum Pathol. 2007;38:197–204. doi: 10.1016/j.humpath.2006.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.