Abstract
TGFβ has seemingly contradictory roles in tumor progression: it can promote metastatic invasion but also act as a tumor suppressor. Recently, two studies have used intravital imaging to unravel the role of TGFβ at different stages of the metastatic process. TGFβ promotes single cell motility which enables invasion into blood vessels. However the activation of TGFβ signaling is a transient event and is not maintained at distant sites. The downregulation of TGFβ signaling at secondary sites then permits growth of secondary tumors. In the absence of TGFβ, cells are restricted to collective movement and lymphatic spread. Here, we discuss these findings and their potential implications.
Introduction
Metastasis research has traditionally relied heavily on end-point assays that measure the overall efficiency of the process but provide little information about the intermediate steps (1). This has led to significant gaps in our understanding and much controversy. Many breast cancer metastases retain epithelial traits but there are also links between the trans-differentiation of breast cancer cells to mesenchymal cells and increased metastatic ability (2, 3). These observations have led to the proposal that a transient pulse of signaling from the tumor microenvironment may enable cancer cells to disseminate, possibly with mesenchymal characteristics (2). These cells would then revert to a more differentiated state at secondary sites.
Signaling by Transforming Growth Factor β (TGFβ) could account for transient changes in cell behavior during metastasis. Treatment of cells in culture with TGFβ1 can promote an invasive phenotype and the acquisition of mesenchymal characteristics, such as vimentin expression (4). Furthermore, elevated levels of TGFβ1 correlate with poor prognosis in breast cancer (5). However, determining whether there are transient changes in cell behavior during metastasis and whether these changes are linked to TGFβ signaling has proved difficult. This problem has been tackled by correlating live tumor imaging of TGFβ signaling with the dynamic behavior of cancer cells. Fluorescently labeled breast cancer cells (either MTLn3 or 410.4) were grown orthotopically in the mammary fat pad of mice. When the primary tumors reached ~5mm, the mice were anesthetized and the tumors imaged using intravital 2-photon microscopy. This method allowed signaling events and motile behavior of individual cells to be monitored.
Different modes of motility
Intravital imaging revealed that only a very small proportion of cells are motile within the primary tumor (between 1-5 %) (6). Cell motility is heterogeneously distributed even within highly metastatic tumors, suggesting that invasive potential is not due only to intrinsic factors. The cells that do display cell motility in the primary tumor fall into two categories: fast moving single cells, or slower moving collective chains (7). Collective motility may be similar to modes of migration used by healthy cells during development, such as branching morphogenesis or angiogenesis (8). Collective invasion of chains of tumor cells (Indian file pattern) has often been observed by pathologists. Singly moving cells escape cell-cell contacts, and move between other cells and extracellular matrix by changing their shape and squeezing between obstacles(9). In contrast, collectively moving cells maintain contacts with neighboring cells and proceed more slowly.
Cell motility in the primary tumor is just the first step of metastasis. To form secondary tumors, cancer cells must invade either the blood or lymphatic vessels surrounding the tumor to be carried to other organs. The formation of significant metastases also requires cancer cells to proliferate at their new locations. Imaging of cancer cells that had already spread to lymph nodes revealed that these cells were no longer motile. Therefore, the migratory behavior that enables escape from primary tumors is a transient characteristic of metastatic cells.
TGFβ promotes single cell motility and entry into the blood
To gain information about TGFβ signalling pathway during intravital imaging two reporter constructs were used. Firstly an EGFP-Smad2 fusion construct, which accumulates in the nucleus when TGFβ signaling is active (10), was introduced into breast cancer cells. To complement this approach, a Smad3-dependent reporter plasmid which expresses ECFP in response to TGFβ stimulation was also used (CAGA12::CFP) (11). These approaches enabled the activity of the TGFβ signaling pathway to be determined with cellular resolution in live tumor models and correlated with cell behavior.
TGFβ signaling was not evenly distributed in the primary tumor, and only a minority of the cells displayed reporter activation (Figure 1). The fast moving single cells showed high levels of TGFβ signaling. In contrast, cells moving collectively did not display TGFβ signaling (7). Thus, it seemed that activation of TGFβ signaling could influence the mode with which cells moved, ie single vs collective. The data also indicated that TGFβ signaling is not sufficient to elicit motility as many cells with high TGFβ signaling were non motile.
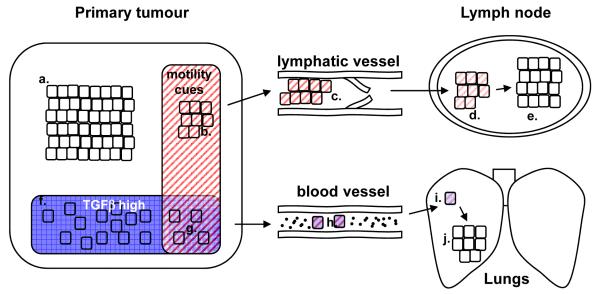
1. Combinations of signals determine metastatic behavior.
Cells receiving no signals in the primary tumor are cohesively packed and non-motile (a). Receipt of pro-motility cues (b), but not TGFβ, causes cohesive cell movement. Cohesively moving cells can enter lymphatic vessels (c). The pro-motility cues that triggered cells to leave theprimary tumor are absent in lymph nodes (d) and the signal decays cells with the result that are no longer motile (e). Alternatively, cells receive TGFβ signals (f) leading to a loss of cell-cell cohesion and reduced proliferation, but not motility. Only when pro-motility cues and TGFβ signals coincide do cells move singly (g), however proliferation remains low. Singly moving cells are able to enter the blood(h). TGFβ and the pro-motility cues that triggered cells to leave the primary tumor are absent in the lungs and the signal decays (i). The loss of anti-proliferative TGFβ signals enables cells to resume growth in the lungs ( j).
To investigate TGFβ signaling further, cells unable to respond to TGFβ signaling were generated by over-expressing a dominant negative TGFβ type II receptor (TGFβRDN). TGFβRDN expressing cells could only move cohesively and could not intravasate into blood vessels. Nonetheless, TGFβRDN cells could still spread via the lymphatics and produce large lymph node metastasis (Figure 1). In contrast, cells with hyper-active TGFβ signaling disseminated prevalently as single cells and were more able to intravasate into blood vessels compared to control cells. Thus activation of TGFβ signaling in disseminating cells determines their motility mode, i.e. single versus collective, and profoundly influences the route of metastatic spread.
TGFβ target genes linked with single cell motility
In vitro analysis showed MTLn3E breast cancer cells plated at low density grow to form distinct colonies with cell-cell junctions. Cells within each colony are constantly in motion changing positions, but they seldom move away as single cells. When TGFβ1 is added, a scattering response is observed. Cell-cell junctions are lost, stress fiber formation is increased and cells migrate as single cells leading to dispersion of the colony (7). The scattering process occurs relatively slowly (>8 hours) and requires transcriptional up-regulation of TGFβ-target genes. Depletion of Smad4, a key mediator of TGFβ regulated transcription, completely abolished the scattering response.
Microarray analysis of genes regulated by TGFβ1 was performed to identify the molecular players responsible for the switch to single cell motility. Several candidate genes were identified that are implicated in cell-cell adhesion, cell migration and invasion (see Table 1). These included Rho-family GTPases and their regulators (12). TGFβ induced the expression of RhoC, which is implicated tumor progression and metastasis in several malignancies, including breast cancer (13, 14). Other less well characterized regulators of Rho-family proteins were also found to be TGFβ-regulated: these include the atypical Rac activator NEDD9, a Rho-interacting regulator of myosin phosphatase activity M-RIP, and a FERM domain-containing Rho exchange factor Farp-1. Functional analysis revealed that RhoA, RhoC, M-RIP, Farp-1 and Nedd9 all contributed to switch to single cell motility. Depletion of RhoA, RhoC, M-RIP, and Farp-1 reduced the disassembly of cell-cell junctions following TGFβ treatment and compromised the cortical actomyosin network of singly moving cells. The contraction of this network is critical for the movement of single cells in vivo (15). In contrast, Nedd9 was important for the formation of the actin-rich protrusion at the leading edge of singly moving cells. In addition to regulators of Rho-family proteins several other TGFβ target genes were shown to play a role in single cell migration. These include EGFR and c-jun, both of which have been extensively implicated in invasion and metastasis (16, 17).
Table 1. TGFβ1-induced candidate genes involved in switch to singlecell motility.
| Gene name | Biological processes/ function/ pathway |
|---|---|
| RhoC | Motility, actin cytoskeleton, adhesion, force generation |
| NEDD9 | Cell shape/plasticity, cell adhesion and actin cytoskeleton |
| m-RIP (RhoIP3) | Regulation of stress fibers formation, force generation |
| FARP-1 | Rho exchange factor |
| EGFR (ErbB1) | Cell migration, adhesion, proliferation |
| c-jun | AP-1 signaling, invasion, tumor progression |
None of the genes tested completely prevented scattering in response to TGFβ when depleted in isolation. Thus TGFβ signaling causes a switch to single cells motility thorough the induction of a multi-genic transcriptional program. Interestingly, several of the TGFβ target genes identified were also shown to be over-expressed when motile cancer cells are experimentally collected from the same breast cancer model (18). This further supports the evidence that TGFβ signaling is up-regulated in motile cancer cells. However, other genes up-regulated in motile cells are not TGFβ regulated (18). This implies that additional signals or factors may be needed to make cancer cells disseminate.
Does TGFβ signaling cause a change in differentiation status?
TGFβ signaling has been implicated in promoting the mesenchymal characteristics during breast cancer invasion. Vimentin expression is a widely used marker of mesenchymal cells. Intravital imaging revealed a heterogeneous pattern of vimentin promoter::GFP reporter construct expression. Similar to the CAGA12 reporter, a greater proportion of cells moving singly were positive for vimentin expression, this probably reflects elevated TGFβ signaling. However, the increase in vimentin reporter expression was rather modest. Unlike activation of TGFβ signaling, vimentin expression was also compatible with cohesive movement. Ex vivo microarray analysis suggested that TGFβ did not drive the expression of a broad range of mesenchymal markers in MTLn3 breast cancer cells. Together these data are rather ambiguous, but they are consistent with a minor shift in the differentiation status of disseminating cells.
Imaging analysis of second model of metastasis provided much clearer insights into the differentiation status of disseminating cells. Melanoma is a type of cancer that arises from pigment producing melanocytes (19, 20). Initial attempts at intravital imaging of B16 melanoma cells using multiphoton techniques were hampered by extremely noisy fluorescence signals. These signals were only observed in melanotic B16 tumours and not amelanotic melanoma. Further in vivo and in vitro correlative light and electron microscopy analysis demonstrated that this ‘noise’ was originating from stage 3 and 4 melanosomes (21). The ability to image an intrinsic signal emanating from melanosomes enabled inferences about the differentiation status of cells in the B16 melanoma model to be made. This revealed that while both primary tumors and metastases contained many cells producing pigment, the cells in transit lacked pigment. A failure to produce pigment does not necessarily indicate a change in differentiation status. Therefore another method was sought to investigate the differentiation status of the motile melanoma cells. Brn-2/POU3F2 expression is high in migratory melanoblasts in culture and decreases as melanocytes differentiate (22). To investigate the relationship between Brn-2 expression and melanoma dissemination B16 cells were engineered to contain the Brn-2 promoter driving the expression of GFP. A clear increase in Brn-2 promoter-driven GFP expression in the motile and circulating cells was observed. This increase was largely down-regulated in cells growing in the lungs. Together these data demonstrate the melanoma cells in transit between primary and secondary sites are less differentiated.
Microarray analysis was used to learn more about the less differentiated melanoma cells ‘in transit’ between primary and secondary sites. Pigmented and non-pigmented cells were isolated from the same tumor and their gene expression profiles compared. The poorly pigmented population, which includes motile cells, had higher expression of TGFβ2. The expression of TGFβ2 can be induced by TGFβ signaling (7) and suggests that TGFβ signaling is active in cells with low pigment levels. In vitro studies confirmed that both TGFβ1 and TGFβ2 inhibited pigmentation and stimulated B16 cell motility.
The phenotypic stability of well-differentiated and poorly-differentiated melanoma cells was investigated. When either un-pigmented tumor cells or Brn2 promoter ‘high’ cells were purified and re-injected into mice the resulting tumors contained differentiated cells. This confirms that less differentiated cells can subsequently differentiate. However, the conversion from more differentiated to less differentiated cells was very infrequent. Thus aspects of the differentiation hierarchy remain in a model of melanoma metastasis. It would be tempting to speculate that the less differentiated cells may have cancer stem cell properties. However, melanoma cells exhibited the same tumorigenic potential regardless of their differentiation status. This is consistent with other findings regarding the lack of distinct stem cell populations in melanoma (23).
These findings could explain why not all breast cancer cells with activated TGFβ signaling are motile. If only less differentiated cells are able to become motile then cancer dissemination would result only when TGFβ signals were received by an intrinsically less differentiated cell. High TGFβ signaling in well differentiated cells would not lead to cell motility. Alternatively, it might be that TGFβ needs to act in concert with additional extrinsic cues (Figure 1). Indeed several lines of evidence suggest that the coincidence of TGFβ and EGF signaling in breast cancer triggers single cell dissemination.
TGFβ from the primary tumor can explain the transient nature of TGFβ signaling
TGFβ signaling is heterogeneous within primary breast tumors. Although TGFβ signaling is high in motile cells, TGFβ signaling is not maintained once cells reach secondary sites and form metastases. One explanation is that the major source of this cytokine and other pro-motility cues are host cells in the primary tumor. After cells escape the primary tumor, they are no longer near the source of the signals. This would lead to a decline in TGFβ signaling and ultimately cells would stop migrating and switch to a proliferative phenotype forming secondary tumors. This model would also allow for different cells within the primary tumor to be exposed to different amounts of TGFβ depending on their particular microenvironment.
Efficient metastasis requires transient switching of TGFβ signaling
TGFβ signaling clearly provides an advantage at the early stages of hematogenous metastasis by promoting single cell motility and intravasation into blood vessels. However, persistent TGFβ signaling appears to be detrimental to the overall metastatic spread to the lungs (7). Cells with constitutive TGFβ signaling form fewer lung metastases than control cells despite their increased intravasation capabilities. This result remains even if cells are injected directly in tail vein, thus avoiding the intravasation step. In contrast, when MLTn3 cells are pulsed with a burst of TGFβ ligand prior to injection they are favored for lung colonization (24). These data can be explained if prolonged TGFβ signaling plays a tumor suppressive role, promoting a pro-apoptotic and/or anti-proliferative effect in the cancer cells. In agreement with this idea, TGFβ reduces the growth of MTLn3E cells in soft agar. Our results suggest that the highest metastatic efficiency is achieved when TGFβ signaling in disseminating cells is transient; high as cancer cells escape the primary tumor and intravasate, but then low as cells reach a secondary site and begin to proliferate. There may also tissue-specific influences on the role of TGFβ signaling in metastasis. For example, bone metastasis may actually require continued TGFβ signaling to stimulate osteoclast activity (25).
Previous literature has suggested that mutations or down-regulation in key components of the TGFβ signaling pathway would be required for cancer progression to occur, so that anti-proliferative responses would be selectively lost but pro-motility effects maintained (26). Mutations in TGFβ signal transducing molecules are frequently observed in tumors of gastro-intestinal tract but are rare in breast cancer and melanoma (26). The data discussed here suggest that selective loss of anti-proliferative responses is not necessary because TGFβ signaling is only switched on transiently and in a minority of cells. Although cancer cells ‘in transit’ are less proliferative (27), the transient nature of the TGFβ signal enables proliferation to resume at secondary sites (7). The therapeutic implication of these finding are complex: direct blockade of TGFβ signaling may hinder further hematogenous spread to distant sites while at the same time boosting growth of both primary tumor and those metastatic cells that have already disseminated at the time of patient presentation. Thus a strategy targeting directly the TGFβ regulated genes responsible for single cell motility/ increased intravasation may be preferred.
Concluding remarks
The use of intravital imaging has provided important insights into the behavior of cancer cells in transit from primary to secondary locations. We have learnt how different modes of migration can affect the ability of cancer cells to enter blood or lymphatic vessels. Collectively moving cells are restricted to lymphatic spread. TGFβ signaling switches cells to single cell motility and thereby enables entry into the blood. Hematogenous metastasis also requires that TGFβ signaling is switched off at secondary sites to enable proliferation. However, there is still much to discover. The source of TGFβ signaling remains unclear. Also, TGFβ signaling alone can not account for every aspect of metastasis. In future it will be exciting to uncover how the interplay between TGFβ and other signals determines the dissemination of cancer cells.
Acknowledgements
SG was funded by the Breast Cancer Campaign (12May05), ES and SP were funded by Cancer Research UK. We apologize to colleagues whose work we were unable to cite due to space restrictions.
References
- 1.Sahai E. Illuminating the metastatic process. Nat Rev Cancer. 2007;7:737–49. doi: 10.1038/nrc2229. [DOI] [PubMed] [Google Scholar]
- 2.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Van’t Veer LJ, Weigelt B. Road map to metastasis. Nat Med. 2003;9:999–1000. doi: 10.1038/nm0803-999b. [DOI] [PubMed] [Google Scholar]
- 4.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–74. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 5.Leivonen SK, Kahari VM. Transforming growth factor-beta signaling in cancer invasion and metastasis. Int J Cancer. 2007;121:2119–24. doi: 10.1002/ijc.23113. [DOI] [PubMed] [Google Scholar]
- 6.Wyckoff JB, Jones JG, Condeelis JS, Segall JE. A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 2000;60:2504–11. [PubMed] [Google Scholar]
- 7.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–96. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–57. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 9.Pinner S, Sahai E. Imaging amoeboid cancer cell motility in vivo. J Microsc. 2008;231:441–5. doi: 10.1111/j.1365-2818.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmierer B, Hill CS. Kinetic analysis of Smad nucleocytoplasmic shuttling reveals a mechanism for transforming growth factor beta-dependent nuclear accumulation of Smads. Mol Cell Biol. 2005;25:9845–58. doi: 10.1128/MCB.25.22.9845-9858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. Embo J. 1998;17:3091–100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 13.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–5. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 14.Fritz G, Brachetti C, Bahlmann F, Schmidt M, Kaina B. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br J Cancer. 2002;87:635–44. doi: 10.1038/sj.bjc.6600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16:1515–23. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 16.Ozanne BW, McGarry L, Spence HJ, et al. Transcriptional regulation of cell invasion: AP-1 regulation of a multigenic invasion programme. Eur J Cancer. 2000;36:1640–8. doi: 10.1016/s0959-8049(00)00175-1. [DOI] [PubMed] [Google Scholar]
- 17.Xue C, Wyckoff J, Liang F, et al. Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Res. 2006;66:192–7. doi: 10.1158/0008-5472.CAN-05-1242. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Goswami S, Lapidus K, et al. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–94. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- 19.Gaggioli C, Sahai E. Melanoma invasion - current knowledge and future directions. Pigment Cell Res. 2007;20:161–72. doi: 10.1111/j.1600-0749.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 20.Haass NK, Smalley KS, Li L, Herlyn M. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res. 2005;18:150–9. doi: 10.1111/j.1600-0749.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 21.Pinner S, Jordan P, Sharrock K, et al. Intravital imaging reveals transient changes in pigment production and Brn2 expression during metastatic melanoma dissemination. Cancer Res. 2009;69:7969–77. doi: 10.1158/0008-5472.CAN-09-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisen T, Easty DJ, Bennett DC, Goding CR. The POU domain transcription factor Brn-2: elevated expression in malignant melanoma and regulation of melanocyte-specific gene expression. Oncogene. 1995;11:2157–64. [PubMed] [Google Scholar]
- 23.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welch DR, Fabra A, Nakajima M. Transforming growth factor beta stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc Natl Acad Sci U S A. 1990;87:7678–82. doi: 10.1073/pnas.87.19.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang Y, He W, Tulley S, et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci U S A. 2005;102:13909–14. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–9. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 27.Goswami S, Wang W, Wyckoff JB, Condeelis JS. Breast cancer cells isolated by chemotaxis from primary tumors show increased survival and resistance to chemotherapy. Cancer Res. 2004;64:7664–7. doi: 10.1158/0008-5472.CAN-04-2027. [DOI] [PubMed] [Google Scholar]