Abstract
The update of the Study of Tamoxifen and Raloxifene (STAR) by Vogel et al. (beginning on p. XXX in this issue of the journal) highlights the overall importance of long-term follow-up of cancer prevention trials, which need long follow-up to fully determine agent risks and benefits. Biomarkers (e.g., reduced cervical intraepithelial neoplasia 3 after human papillomavirus vaccination) can provide an early indication of efficacy but almost never replace the cancer endpoint in determining an agent’s ultimate utility. Long follow-up also is important to fully determine preventive benefit, as illustrated by the tamoxifen trials, where only 60% as many treated women were needed to prevent one cancer at 10 years as at approximately 5 years, the time of the early reports.
Carcinogenesis is a long process, often spanning 50 years, and so the need for long-term follow-up of preventive interventions is particularly great. Even interventions aimed at the later stages of this process may require long-term follow-up to establish their full benefit, especially if they also affect early precursor stages which would take a decade or more to become cancer. Equally emphasizing the need for long-term follow-up, the occurrence of late side effects has a greater impact on prevention than would be seen for adjuvant treatment because prevention is focused on individuals with a long estimated life expectancy.
Many of these issues are well illustrated by the activities in breast-cancer prevention, where a number of large trials have explored a range of preventive agents. These trials are discussed in detail below, but the principles they reflect apply to a much broader range of activities.
For example, the early results of vaccination against the human papillomavirus (HPV) suggest almost complete protection against cancers caused by the two cancer-associated types of HPV (16 and 18) in the vaccine, if vaccination is provided before infection occurs (1). The current evidence, however, is based on intermediate precursor endpoints [either persistent viral infection or the appearance of precursor cervical intraepithelial neoplasia– (CIN)]. These endpoints are likely to predict a subsequent reduction of cancers, but direct verification is highly desirable and likely to take at least another 10–15 years (2). Furthermore, the vaccine is thought to be effective for a lifetime, but data currently exist for at most 8 years (3). Only long-term follow-up will provide evidence of continuing protection against both the two HPV types in the vaccine and, even more speculatively, against the closely related HPV types for which early follow-up showed cross-protection (4, 5), which may not be so long-enduring. Pharmaceutical companies and other funding agencies are understandably reluctant for various reasons to commit substantial resources to continue long-term follow-up in randomized trials, but simple passive surveillance conducted via screening and cancer registries provides a cost-effective option. Because of the widespread introduction of HPV vaccination, long-term protection also should be demonstrable on a population basis, again using passive surveillance via cancer and screening registries. For this passive approach to be viable, however, it is essential to have a registry of all vaccinated girls. The United Kingdom offers an excellent opportunity to create such a comprehensive registry since vaccine coverage has been very high (2) and national screening and cancer registries exist. Further opportunities for population-wide assessments exist in Scandinavian countries (because of their comprehensive national registration systems) and in the program to monitor vaccine efficacy in the state of New Mexico (6), where lower population coverage may provide more information on efficacy as a paradoxical result of a larger comparison group of unvaccinated women.
Early results of vaccine studies were based on persistent infection and high-grade CIN, which are biomarkers thought to predict subsequent cancer. Such biomarkers are essential for expediting progress in identifying new preventive agents since they are amenable to relatively short-term evaluations, whereas evaluation of the cancer endpoint requires several years. Although development of these biomarkers must be a major component of preventive work, they do need validation as reliable surrogates for the desired effect on the final endpoint, and this validation requires long-term evaluation. This evaluation could happen within the defined period of a large, long-term trial, or during long-term follow-up of shorter-term trials. Even when a trial has specified a predefined surrogate biomarker-endpoint, the strength of the findings can be increased if primary endpoints are evaluated during post-trial, long-term follow-up, especially if it is blinded.
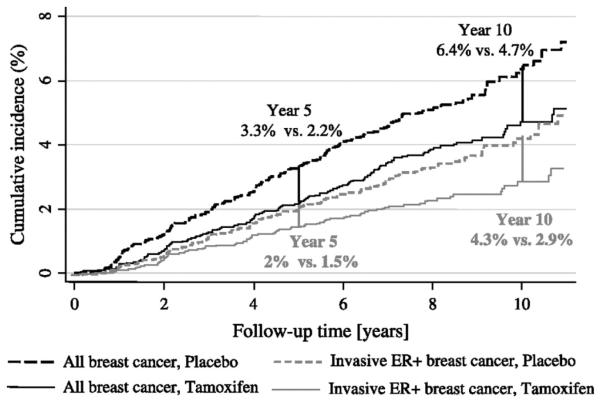
Long-term follow-up also is needed to identify the full absolute benefit of a new agent, which is crucial for accurately identifying the number needed to treat to prevent one cancer (NNT), which has become a key cost-benefit parameter. The importance of this issue is best illustrated by the trials of tamoxifen for the prevention of breast cancer. Early results from an overview of all the prevention trials (7) indicated a 38% relative reduction in incidence (versus placebo) after 5 years of tamoxifen treatment. Subsequent follow-up of the International Breast Cancer Intervention Study I (IBIS-I) and Royal Marsden study, however, found that a further benefit of the same relative order of magnitude exists several years after treatment has been completed (Fig. 1; refs. 8, 9). Thus for ER-positive invasive tumors, the cumulative incidence in IBIS-I was reduced after 5 years of follow-up from 2.0% in the placebo arm to 1.5% in the tamoxifen arm, but after 10 years the values were 4.3% versus 2.9%, respectively, indicating an almost 3-fold lager effect (from 0.5% to 1.4%). In the Royal Marsden trial (which gave tamoxifen for 8 years), there was virtually no difference in invasive ER-positive cancers at 5 years (2.5% versus 2.4%), but clear reductions at 10 years (6.2% versus 5.2%) and even larger differences at 15 years (9.6% versus 7.5%). Similar results appear to have occurred in the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial [also called the Breast Cancer Prevention Trial (BCPT)], although they are difficult to interpret because of the unblinding and subsequent use of tamoxifen in the placebo arm (10). However, an additional benefit that persisted long term after treatment was completed was not seen in the Italian trial even though this trial was kept blinded for 10 years of follow-up (11). The collective data suggest that the NNT (to prevent one cancer) after five years is about 95 (the ratio 1:95 = 1.05% absolute benefit), but the NNT is reduced to 56 (1.77% absolute benefit) after 10 years, making tamoxifen prophylaxis about 70% more effective for 10-year versus 5-year outcomes. Furthermore, available evidence shows no diminution of effectiveness at the 10-year point, and so if the benefit continues beyond 10 years, the cost-benefit ratio will be even more favorable. These findings are consistent with the possibility that tamoxifen has beneficial effects on undetectable precursor changes, which would not manifest clinically as cancer for 5–10 years or even longer. Also, most side effects do not persist after treatment is completed (8), except possibly an increased risk of endometrial cancer, which may continue longer (12). It is also important to document fully any late side effects, but the available evidence suggests that benefit/risk ratios for tamoxifen will be substantially underestimated if only 5 years of follow-up data are used.
Fig. 1.
One-minus Kaplan-Meier curves of the cumulative incidence rates for all breast cancers (invasive and noninvasive) and invasive estrogen-receptor-positive (ER+) breast cancers according to treatment arm in the International Breast Cancer Intervention Study I (IBIS-I). Differences in absolute breast-cancer risk at 5 and 10 years are also given. The figure originally appeared in ref. 8 and is reproduced here by permission of Oxford University Press.
As reported by Vogel et al. in this issue of the journal (13), additional follow-up of the Study of Tamoxifen and Raloxifene (STAR) comparing tamoxifen with raloxifene, now extended from the original median 47 months to 81 months, also demonstrates the value of long-term follow-up. The updated STAR results indicate that raloxifene became less effective than tamoxifen in preventing invasive breast cancer and grew closer to tamoxifen in preventing noninvasive breast cancer over time (versus the initial results reported in 2006), and that in both cases the efficacy of raloxifene is about 75% of tamoxifen’s. Furthermore, the anticipated lower risk of endometrial cancer is now clearly established, and fewer thromboembolic events and other gynecologic complications with raloxifene continued. These findings, plus long-term findings of IBIS-I and the Marsden study, will have a major impact on the choice of agent for risk reduction in certain postmenopausal women, where tamoxifen now has a much stronger indication after long-term follow-up than it did after 5 years.
Long-term follow-up of the NSABP P-1 trial and several adjuvant breast-cancer trials has been compromised by early reporting and forced unblinding based on examination of a single primary endpoint. The late Sir Richard Doll advised against unblinding trials early to report a benefit unless the results were clear enough to lead to a consensus change in clinical practice. Of course this argument does not apply to early stopping due to toxicity or a futility analysis. A clinical consensus change clearly has not happened for tamoxifen prophylaxis, and I think it would be useful if Sir Richard’s approach was adopted more widely, in lieu of relying on rigid decision rules based on a single endpoint. As used here, “early” refers to unblinding before gaining sufficient information for a consensus change, whether the unblinding occurs before (as with the BCPT [P-1]) or at the completion of the planned full duration of study. Risks and benefits need to be fully evaluated in making any unblinding decisions. The non-North American tamoxifen prevention trials have taken this approach and remained largely blinded for at least 10 years. Thus, they are more able to provide important information on long-term risks and benefits, which has tipped the balance more favourably toward use of tamoxifen, especially in high-risk premenopausal women. In IBIS-I we have regularly informed women of the developing results of our trial and others, but have indicated the remaining uncertainties, and we sought reconsent to continue blinded follow-up. This effort has been very successful, and blinded follow-up to 10 years was achieved in approximately 90% of randomized women. We also allow IBIS-I women who remain blinded and have been off IBIS-I medication (tamoxifen or placebo) for at least 5 years to join the ongoing IBIS-II trial of anastrozole versus placebo. This more-demanding approach to early unblinding in order to declare a success clearly also involves cultural, political, and societal issues needing to be addressed on a wide front.
In summary, preventive interventions have long-term consequences, and so long-term follow-up is essential. Approaches which limit early unblinding to situations where clinical benefit has been clearly established or where harms clearly outweigh any potential benefits should be more widely adopted.
Footnotes
Disclosure of Potential Conflicts of Interest No conflicts of interest were disclosed.
References
- 1.Schiller JT, Castellsagué X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26:K53–62. doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in England. Br J Cancer. 2010:1–7. doi: 10.1038/sj.bjc.6605528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowhani-Rahbar A, Mao C, Alvarez FB, et al. Long-term efficacy of a prophylactic human papillomavirus type 16 vaccine. Presented at the 25th International Papillomavirus Conference: Clinical & Educational Workshop; Malmö, Sweden. 2009 May 8–14; Abstract O-01.03. [Google Scholar]
- 4.Paavonen J, Naud P, Salmerón J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 5.Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis. 2009;199:926–35. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler C. HPV OUTCOMES: PREVENTION EPIDEMIOLOGY AND SURVEILLANCE (HOPES) University of New Mexico grant: http://www.recovery.gov/Transparency/RecipientReportedData/pages/RecipientProjectSummary508.aspx?AwardIdSur=55453&AwardType=Grants.
- 7.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J, Forbes JF, Sestak I, et al. Long-Term Results of Tamoxifen Prophylaxis for Breast Cancer – 96-Month Follow-up of the Randomized IBIS-I Trial. J Natl Cancer Inst. 2007;99:272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 9.Powles TJ, Ashley S, Tidy A, et al. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–90. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Constantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 11.Veronesi U, Maisonneuve P, Rotmensz N, et al. Italian Tamoxifen Study Group Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. 2007;99:727–37. doi: 10.1093/jnci/djk154. [DOI] [PubMed] [Google Scholar]
- 12.Swerdlow AJ, Jones ME. Tamoxifen treatment for breast cancer and risk of endometrial cancer: a case-control study. J Natl Cancer Inst. 2005;97:375–84. doi: 10.1093/jnci/dji057. [DOI] [PubMed] [Google Scholar]
- 13.Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing Breast Cancer. Cancer Prev Res. 2010;3:XXX. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]