Abstract
AIM
To compare a test version of HFA fluticasone/salmeterol (FP/SM) combination inhaler (Neolab, UK) with the reference product Seretide (GlaxoSmithKline, UK).
METHODS
An in vitro Anderson cascade impactor was used to compare the fine particle dose (<4.7 µm). Two separate randomized cross-over studies were performed to compare the systemic bioavailability of test vs. reference (T vs. R) formulations of FP/SM 250/25 µg pMDI in healthy volunteers. In study 1 blood pharmacokinetic analysis using oral charcoal block was performed over 24 h following a single dose of four puffs via pMDI alone. In study 2 systemic bioactivity was measured following single doses of four and eight puffs via a spacer device: serum potassium (K+) to reflect SM, and overnight urinary cortisol : creatinine (OUCC) for FP. An early pharmacokinetic profile was also assessed over 120 min.
RESULTS
The in vitro fine particle dose was similar for test vs. reference pMDI alone and via spacer. The results of both studies were consistent: No significant differences between formulations were seen in terms of FP kinetics. Analysis of SM kinetics revealed superiority of the test product. No significant dose–response or difference in T : R ratio was noted for OUCC. Fall in K+ revealed a significant dose–response with a non-significant T : R ratio.
CONCLUSIONS
The in vitro fine particle dose may not predict pharmacokinetic and systemic pharmacodynamic outcomes. Single dosing studies with fluticasone/salmeterol 250/25 µg via pMDI or with spacer showed pharmacokinetic equivalence with FP, but not SM. No significant difference between formulations was seen with either adrenal suppression or hypokalaemia.
Keywords: fluticasone, salmeterol, HFA, pharmacokinetics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Seretide (GlaxoSmithKline, UK) is a combination inhaler which contains both fluticasone (FP) and salmeterol (SM).
A generic fluticasone/salmeterol (FP/SM) combination inhaler (Neolab, UK) has recently been developed.
Determination of therapeutic equivalence is essential in the development of generic products.
WHAT THIS STUDY ADDS
This paper determines that in vitro fine particle dose may not predict pharmacokinetic and systemic pharmacodynamic outcomes.
This paper shows that the generic and reference products were equivalent in terms of FP, but not SM pharmacokinetics.
No significant differences were found between generic and reference products in terms of either adrenal suppression or hypokalaemia.
Introduction
Inhalers containing a combination of long acting β-adrenoceptor agonist (LABA) and inhaled corticosteroid (ICS) are recommended in step 3 of current asthma guidelines [1, 2]. They aim to improve compliance by offering bronchoprotection whilst simultaneously treating airway inflammation. The use of combination inhalers has been shown to be superior to doubling the dose of inhaled steroid in terms of improving symptoms, lung function and decreasing exacerbations [3–6]. Seretide (GlaxoSmithKline, UK) is a combination inhaler which contains both fluticasone (ICS) and salmeterol (LABA) (reference product). It has been extensively studied in both adults and children [7, 8], and is widely marketed throughout the UK. A generic fluticasone/salmeterol (FP/SM) combination inhaler (Neolab, UK) has recently been developed (test product).
Determination of therapeutic equivalence is essential in the development of generic products. Comparative in vitro data can occasionally be used to determine equivalence provided strict criteria are satisfied. The fine particle dose (stages 3–5) measured in vitro should predict the dose of drug delivered to the lung, and hence the therapeutic efficacy of the product. Pulmonary deposition can also be measured indirectly through the analysis of serum drug concentrations in pharmacokinetic studies. Fluticasone has negligible oral bioavailability from the swallowed fraction due to 99% first pass hepatic metabolism [9, 10]. The systemic bioavailability of fluticasone is therefore entirely dependent on lung absorption [11], and hence the fine particle dose should reflect early or total bioavailability as Cmax or AUC(0,last). Salmeterol on the other hand is only subject to partial first pass metabolism and the swallowed fraction contributes to 28–36% of the total systemic bioavailability [12]. Cmax or AUC(0,30 min) should therefore best reflect early bioavailability from the lung, as it obviates the later component of gut bioavailability from the swallowed fraction. AUC(0,last) will represent bioavailability from both the lung and swallowed fractions of the drug [13], but in the presence of oral charcoal will reflect the lung only. Pharmacodynamic studies allow the comparison of systemic actions of the drug through the measurement of clinically relevant outcomes. Suppression of overnight urinary cortisol creatinine clearance (OUCC) is a sensitive surrogate for systemic bioavailability of fluticasone [14]. The peak fall in serum potassium has been shown to reflect the early lung bioavailability of salmeterol [15].
This paper aims to present in vitro data and the results of two separate studies which compare the systemic bioavailability of test and reference formulations of FP/SM. Study 1 aimed to examine the pharmacokinetics of salmeterol and fluticasone over 24 h. Study 2 aimed to confirm the results of the first study in terms of the early pharmacokinetic profile as well as comparing the systemic pharmacodynamic profile of both drugs.
Methods
In vitro Anderson cascade impactor
Particle distribution from test and reference products was determined (with and without aerochamber plus) in a standardized fashion using an eight-stage Andersen Cascade Impactor (Copley, Nottingham, England). The impactor stages were calculated using high-performance liquid chromatography with a wavelength of detection set at 239 nm. Fine particle dose (i.e. respirable fraction) was defined as particles <4.7 µm.
Eligibility criteria and study procedures
To be eligible for randomization, participants were required to be healthy, non-smokers, aged 18–65 years, and have no history of respiratory of other disease. Subjects were excluded if they had a BMI >30 kg m−2, FEV1 <80% (predicted for age/sex/race), FEV1 : FVC ratio <70%, QTc interval >450 ms (males) and >470 ms (females), abnormal routine bloods or were taking any concomitant medications.
At their initial screening suitability was checked. All volunteers underwent a full physical examination, spirometry, electrocardiogram, evaluation of baseline biochemistry and haematology, and if deemed suitable, were randomized into the study. All participants gave written informed consent, and both studies were approved by medical research ethics committees.
Study 1 (pharmacokinetic)
This was a randomized, open-label, crossover pharmacokinetic study comparing four puffs of test and reference FP/SM (250/25) formulations via pMDI alone. Study visits were separated by a 1 week wash-out period. Blood sampling for pharmacokinetic analysis of fluticasone and salmeterol was performed using oral charcoal block at 2, 5, 10, 20, 30, 45, 60, 90 and 120 min and 3, 4, 8, 12, and 24 h after administration of study medication at each of the treatment visits.
Study 2 (pharmacokinetic/pharmacodynamic)
This was a single centre, double-blind, double-dummy, randomized, four way crossover study comparing four and eight puffs of test and reference FP/SM (250/25 µg). The study medications were administered via pMDI and optimally primed and pre-washed Aerochamber plus. Eligible subjects were randomly allocated to receive a sequence of four single treatments on four separate visits, following a crossover study design. Study visits were separated by a 1 week wash-out period [16]. Participants were required to collect 10 h overnight urine in a pre-labelled sealed container for assessment of overnight cortisol and creatinine during the night prior to, and following each study visit. During the visits subjects had an intravenous cannula inserted and were asked to remain supine for 30 min prior to administration of study drug and then for the 120 min assessment period. Optimum inhaler technique was demonstrated by the researcher at each study visit. Heart rate and blood pressure were measured and blood was taken for FP and SM pharmacokinetic analysis at 0, 5, 10, 20, 30, 45, 60, 90 and 120 min post-drug administration. A dose of three Sando K® (HK Pharma, 12 mmol K+ per tablet) was given following the 120 min blood sample to reverse and prevent any hypokalaemia in subjects.
Blood measurements
Blood samples (10 ml) for potassium were collected into heparinized labelled plastic tubes. Blood samples (10 ml) were collected for determination of salmeterol and fluticasone plasma concentrations, and immediately placed on ice. The blood samples were then centrifuged within 30 min after collection at 3000 g at 0–8°C for 10 min. Supernatant plasma was transferred into poly-propylene cryotubes (approx 1.1 ml) and stored at −70°C until analysis. Samples were analyzed using liquid chromatography with tandem mass spectrometry.
Overnight urinary cortisol clearance (OUCC)
Urinary cortisol was measured using a commercial radioimmunoassay kit (DiaSorin Ltd, Wokingham, Berkshire, UK). The intra-assay coefficient of variation was 11% and the inter-assay coefficient of variation was 14%. Urinary creatinine was measured on a Cobas-Bio auto analyzer (Roche Products, Welwyn Garden City, UK). The intra-assay and inter-assay co-efficient of variation was 3.2% and 6.4%, respectively.
Statistical analysis
The primary endpoint for both studies was the pharmacokinetic profile of both FP and SM. Maximum observed plasma drug concentration (Cmax), area under the curve (AUC), and time to maximum plasma concentration (tmax) were determined for both FP and SM. These were normalized to a dose of 1 µg prior to statistical analysis. Natural log transformed (dose-normalized) pharmacokinetic parameters were subjected to analysis of variance (anova) with terms for treatment, subject and period. The term for subject was split into terms for sequence and subject within sequence (a residual term). 95% confidence intervals (95% CI) were calculated for the difference in terms of means between the test and reference products with regards to each combination dose of FP/SM. The treatment differences and 95% CIs were back transformed to the original scale to give point estimates and 95% CI estimates for the ratios of the treatments. Equivalent pharmacokinetics of the two products at each dose level were concluded if the entire 95% CI was contained between the conventional ± 20% bioequivalence range of 0.8–1.25. tmax values for FP and SM were subjected to non-parametric analysis. 95% confidence intervals were calculated for the respective median differences using the Wilcoxon Rank-Sum (Mann-Whitney) test for paired data.
OUCC was log-transformed before analysis. Plasma potassium and heart rate were untransformed. The change from baseline in systemic bioactivity variables was subjected to an anova with terms for treatment, subject and period.
Both studies were designed with a sample size of 24 completed per protocol. This calculation was based on the results of a previous kinetic study by Kempsford et al. [13]
Results
In vitro Anderson cascade impactor data
In vitro Anderson cascade data revealed that test and reference products had a similar overall fine particle dose (particles <4.7 µm) for both SM and FP at a dose of 250/25 µg (Tables 1, 2). The T : R ratios for FP and SM fine particle dose, with pMDI alone, were 1.01 (90% CI 96.6, 106.2) and 1.10 (105.7, 114.9), respectively. Equivalent T : R ratios with Aerochamber plus were 1.01 (90% CI 91.1, 114.1) and 1.01 (88.1, 116.3), respectively. The use of a spacer device increased the fine particle dose delivery, but did not affect the T : R ratio. The difference between test and reference products lay within ±15% equivalence limits for both pMDI alone and with spacer.
Table 1.
Comparison of grouped stage data for the fluticasone moiety of test and reference FP/SM 250/25 µg via pMDI ± Aerochamber plus
| Conventional actuator | ‘Aerochamber plus’ | |||
|---|---|---|---|---|
| Stage/group | Test | Reference | Test | Reference |
| S0 | 3.57 | 7.91 | 0.99 | 1.36 |
| S1 | 2.97 | 7.52 | 1.81 | 2.73 |
| S2 | 6.77 | 11.78 | 6.79 | 8.08 |
| S3/5 | 77.49 | 77.09 | 101.03 | 100.68 |
| S6/7 | 2.58 | 1.50 | 3.22 | 1.77 |
| FPM | 83.68 | 82.63 | 108.11 | 106.03 |
Values given as µg. FPM, fine particle mass.
Table 2.
Comparison of grouped stage data for the salmeterol moiety of test and reference FP/SM 250/25 µg via pMDI ± Aerochamber plus
| Conventional actuator | ‘Aerochamber plus’ | |||
|---|---|---|---|---|
| Stage/group | Test | Reference | Test | Reference |
| S0 | 0.37 | 0.78 | 0.09 | 0.14 |
| S1 | 0.26 | 0.73 | 0.15 | 0.28 |
| S2 | 0.54 | 1.16 | 0.51 | 0.83 |
| S3/5 | 7.39 | 7.14 | 9.03 | 9.54 |
| S6/7 | 0.62 | 0.10 | 0.62 | 0.12 |
| FPM | 8.40 | 7.62 | 10.14 | 10.02 |
Values given as µg. FPM, fine particle mass.
Patient demographics at screening visit
Baseline demographics of the study populations are given in Table 3. In study 1, 36 subjects were screened, 31 were randomized, and 30 were analyzed (one was withdrawn after experiencing vasovagal syncope). In study 2, 40 were screened, 31 were randomized, and 28 completed the study. During the study three subjects withdrew from the study due to personal reasons and one was withdrawn due to failure to comply with the protocol. Twenty-six completed the study per protocol.
Table 3.
Screening data
| Study 1 | Study 2 | |
|---|---|---|
| Sex (male : female) | 14:17 | 17:14 |
| Age (years) | 30.60 (9.81) | 23.4 (4.81) |
| Race (Caucasian : Black : Asian) | 26:5:0 | 27:1:3 |
| Height (cm) | 171.90 (7.48) | 174.99 (9.26) |
| Weight (kg) | 74.98 (12.60) | 74.55 (11.23) |
| BMI (kg m−2) | 25.32 (3.68) | 24.0 (2.65) |
Data presented as mean (SEM) unless otherwise stated.
Study 1 results
Analysis of pharmacokinetic data for FP revealed no significant difference between products. The geometric mean (95% CI) Cmax was 192.5 pg ml−1 (166.1, 218.9) for test, and 210.9 pg ml−1 (177.9, 243.9) for reference FP/SM. The test : reference (T : R) ratio for Cmax was 0.91 (95% CI 0.80, 1.04). The geometric mean (95% CI) AUC(0,last) was 1764.4 pg ml−1 h (1539.3, 1989.5) for test and 1682.6 pg ml−1 h (1386.7, 1978.5) for reference, making the T : R ratio for AUC(0,last) 1.05 (95% CI 0.91, 1.21). The 95% CI for T : R ratios for FP included unity indicating no significant difference between products. In addition, the entire CIs were contained within ±20% equivalence limits (i.e. ratio of 0.8–1.25). The median tmax values (range) for test and reference were 1.25 h (0.08–3.00) and 1.5 h (0.5–2.03), respectively.
The geometric mean (95% CI) Cmax for SM was 491.3 pg ml−1 (418.2, 565.2) for test and 343.7 pg ml−1 (276.2, 411.2) for reference, making the T : R ratio 1.43 (1.25, 1.63). Analysis of AUC(0,last) revealed a geometric mean (95% CI) of 365.9 pg ml−1 h (277.6, 454.2) for test and 287.3 pg ml−1 h (218.1, 356.5) for reference, giving a T : R ratio of 1.29 (1.11, 1.49). The lower CI for the T : R exceeded unity and the upper CI was greater than 1.25, indicating that the test product had significantly higher systemic exposure than the reference product. The median (range) tmax for SM was 0.08 h (0.03–0.17) for test and 0.08 h (0.03–0.10) for reference.
Study 2 results
Pharmacokinetics
There was significant log linear dose separation between four and eight puffs with all pharmacokinetic outcomes for FP and SM for both test and reference products (see Table 4). The mean (95% CI) T : R ratio for FP at four puffs was 1.11 (0.96, 1.29) for Cmax, and 1.07 (0.92, 1.25) for AUC(0,120 min). The mean (95% CI) T : R ratio for Cmax and AUC(0,120 min) at eight puffs was 0.93 (0.80, 1.07) and 0.94 (0.80, 1.10), respectively. These results did not show any significant differences between formulations at either dose as the CIs all included unity. Equivalence within the 20% limits of 0.8–1.25 was demonstrated, aside from Cmax at four puffs where the upper CI >1.25. The median tmax (range) for T vs. R was 120.0 min (45–121) vs. 120.0 min (10–125) at four puffs and 120.0 min (90–126) vs. 120.0 min (61–125) at eight puffs.
Table 4.
Dose–response of pharmacokinetic variables in study 2
| Test FP/SM (250/25 µg) | Reference FP/SM (250/25 µg) | |||||
|---|---|---|---|---|---|---|
| Fluticasone | Four puffs | Eight puffs | Ratio | Four puffs | Eight puffs | Ratio |
| Cmax (pg ml−1) | 301.8 | 538.6 | 0.56 | 273.3 | 579.1 | 0.46 |
| (254.5, 349.1) | (442.9, 634.3) | (0.48, 0.65) | (233.2, 313.4) | (476.2, 681.9) | (0.40, 0.56) | |
| AUC(0,120 min) (pg ml−1 h) | 24 588.5 | 44 557.0 | 0.55 | 23 143.6 | 47 149.2 | 0.48 |
| (20 545.6, 28 631.4) | (37 409.0, 51 704.9) | (0.47, 0.64) | (19 673.4, 26 613.7) | (39 253.5, 55 044.8) | (0.41, 0.56) | |
| tmax (min)* | 120.0 | 120.0 | −0.5 | 120.0 | 120.0 | −0.5 |
| (45–121) | (90–126) | (−15.0–0.0) | (10–125) | (61–125) | (−15.0–3.0) | |
| AUC(0,30 min) (pg ml−1 h) | 3 628.3 | 6 348.7 | 0.58 | 3 161.6 | 5 718.9 | 0.54 |
| (3 004.8, 4 251.7) | (5 511.5, 7 185.8) | (0.47, 0.71) | (2 704.0, 3 619.3) | (4 646.0, 6 791.8) | (0.44, 0.66) | |
| AUC(0,60 min) (pg ml−1 h) | 9 222.8 | 16 746.1 | 0.55 | 8 612.0 | 16 756.8 | 0.50 |
| (7 722.7, 10 722.8) | (14 461.6, 19 030.6) | (0.46, 0.66) | (7 368.6, 9 855.4) | (39 253.5, 19 481.6) | (0.42, 0.60) | |
| Salmeterol | Four puffs | Eight puffs | Ratio | Four puffs | Eight puffs | Ratio |
| Cmax (pg ml−1) | 12 227.5 | 2 215.0 | 0.56 | 765.0 | 1 792.9 | 0.42 |
| (10 106.5, 14 348.5) | (1 818.8, 2 611.2) | (0.47, 0.66) | (646.6–883.5) | (1 436.7–2 149.2) | (0.35, 0.50) | |
| AUC(0,120 min) (pg ml−1 h) | 36 260.4 | 69 251.7 | 0.52 | 22 390.3 | 55 809.4 | 0.40 |
| (30 540.1, 41 980.7) | (59 065.6, 79 437.8) | (0.45, 0.61) | (19 203.1, 25 577.5) | (46 889.4, 64 729.4) | (0.34, 0.46) | |
| tmax (min)* | 5.00 | 8.50 | −2.0 | 5.00 | 5.50 | −0.5 |
| (4–10) | (5–16) | (−2.5–0.0) | (4–12) | (2–20) | (−2.5–0.0) | |
| AUC(0,30 min) (pg ml−1 h) | 20 242.7 | 36 841.6 | 0.55 | 11 950.5 | 28 879.4 | 0.41 |
| (16 849.8, 23 635.5) | (30 938.2, 42 745.0) | (0.47, 0.64) | (10 206.0, 13 695.0) | (23 833.6, 33 925.3) | (0.35, 0.48) | |
| AUC(0,60 min) (pg ml−1 h) | 27 545.9 | 51 426.1 | 0.53 | 16 522.4 | 40 503.5 | 0.40 |
| (23 078.9, 32 012.8) | (43 745.7, 59 106.4) | (0.46, 0.62) | (14 152.7, 18 892.0) | (33 800.3, 47 206.7) | (0.35, 0.47) | |
All data presented as geometric mean with 95% confidence intervals unless otherwise indicated.
Presented as median (range). ‘Ratio’ is the mean ratio of four puffs vs. eight puffs, presented with 95% CIs.
Pharmacokinetic analysis of SM at four puffs revealed a mean (95% CI) T : R ratio of 1.62 (1.36, 1.94) for Cmax and 1.62 (1.39, 1.89) for AUC(0,30 min). At eight puffs the mean (95% CI) T : R ratios were 1.22 (1.02, 1.46) and 1.24 (1.06,1.44), respectively. The lower CI for the T : R ratio exceeded unity and the upper CI was greater than 1.25, indicating that the test product had significantly higher systemic exposure than the reference product. The median tmax (range) for T vs. R was 5.0 min (4–10) vs. 5.0 min (4–12) at four puffs and 8.5 min (5–16) vs. 5.5 min (2–20) at eight puffs.
Pharmacodynamics/Safety data
Analysis of OUCC for the FP moiety revealed no significant dose separation for either product: The geometric mean fold ratio (95% CI) in OUCC for four puffs vs. eight puffs was 0.88 (0.51, 1.52) with test and 0.71 (0.41, 1.23) with reference. The mean (95% CI) T : R ratio was 1.48 (0.86, 2.55) at four puffs and 1.19 (0.69, 2.06) at eight puffs (Table 5). The 95% CIs included unity confirming that there were no significant differences at either dose (Figure 1).
Table 5.
T : R ratio for PK outcomes in study 2
| T : R ratio (95% CI) | ||
|---|---|---|
| Four puffs | Eight puffs | |
| Fluticasone | ||
| Cmax | 1.11 (0.96, 1.29) | 0.93 (0.80, 1.07) |
| AUC(0,120 min) | 1.07 (0.92, 1.25) | 0.94 (0.80, 1.10) |
| OUCC | 1.48 (0.86, 2.55) | 1.19 (0.69, 2.06) |
| Salmeterol | ||
| Cmax | 1.62 (1.36, 1.94) | 1.22 (1.02, 1.46) |
| AUC(0,30 min) | 1.62 (1.39, 1.89) | 1.24 (1.06, 1.44) |
All data presented as test : reference ratio (95% CI).
Figure 1.
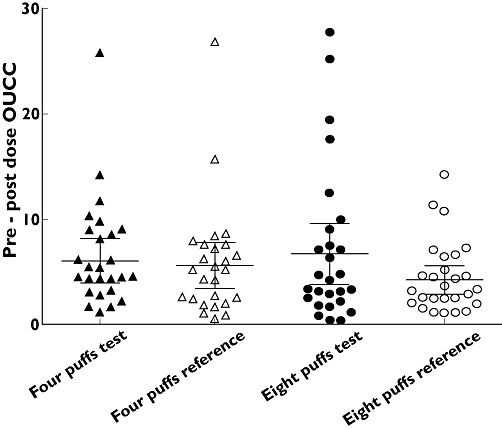
Comparison of pre-post overnight urinary cortisol : creatinine (OUCC) ratio. Data presented with mean (95% CI)
Analysis of maximal potassium drop revealed significant dose separation. The mean (95% CI) difference in fall between four puffs and eight puffs was 0.29 mmol l−1 (0.18, 0.40) with test and 0.27 mmol l−1 (0.17, 0.38) with reference FP/SM. The mean difference in maximum fall and AUC for maximum change are detailed in Table 6.
Table 6.
Fall in serum potassium and increase in heart rate
| Four puffs | Eight puffs | |||||
|---|---|---|---|---|---|---|
| Test FP/SM | Reference FP/SM | Mean difference* | Test FP/SM | Reference FP/SM | Mean difference* | |
| Potassium | ||||||
| Maximum fall | −0.30 | −0.23 | −0.07 | −0.59 | −0.50 | −0.09 |
| (mmol l−1) | (0.22) | (0.21) | (−0.18, 0.04) | (0.26) | (0.27) | (0.20, 0.02) |
| AUC for maximum fall | −15.25 | −7.42 | −8.49 | −42.94 | −33.51 | −9.19 |
| (mmol l−1 h) | (20.12) | (30.24) | (−21.36, 4.39) | (26.86) | (29.55) | (−21.93, 3.55) |
| Heart rate | ||||||
| Maximum increase | 20.0 | 15.8 | 4.2 | 28.9 | 22.6 | 6.4 |
| (beats min−1) | (9.2) | (7.7) | (0.8, 9.2) | (11.2) | (11.1) | (1.4, 11.4) |
| AUC for maximum increase | 1086.3 | 559.4 | 526.0 | 2256.6 | 1456.5 | 809.4 |
| (beats min−1 h) | (869.2) | (774.2) | (−18.0, 1070.1) | (1185.3) | (1365.8) | (270.8, 1348.1) |
All data presented as means (SD) unless otherwise indicated.
Arithmetic mean (95% CI) difference between test and reference FP/SM.
The maximum heart rate response also showed significant dose separation between four and eight puffs: for test product a mean (95% CI) increase of 9.2 (4.2, 14.2) beats min−1; for reference, 7.0 bearts min−1 (2.0, 12.0). The mean differences between test and reference products at four and eight puffs in terms of maximum change and AUC for maximum change can be seen in Table 6.
Discussion
The results from study 1, which evaluated pharmacokinetics with pMDI alone, demonstrated that the test and reference products were equivalent in terms of FP bioavailability with four puffs of 250/25 µg (Figure 2). SM bioavailability on the other hand, did not show equivalence, as there was significantly higher exposure associated with the test product compared with the reference product (Figure 3). This result was somewhat unexpected given that in vitro Anderson cascade data had shown a similar fine particle dose deposition for both moieties with pMDI alone and pMDI plus spacer. The second study was therefore carried out to confirm the results of the first study, whilst providing additional systemic safety data. The results of study 2 were consistent with those of study 1, indicating that the SM moiety of the test product produced significantly higher systemic exposure than the reference product, along with equivalent systemic exposure for FP.
Figure 2.

Comparison of geometric mean AUC(0,120 min) of FP for test and reference FP/SM at four puffs and eight puffs. Data shown as geometric mean and 95% CIs. Test FP/SM (□); Reference FP/SM ( )
)
FIgure 3.
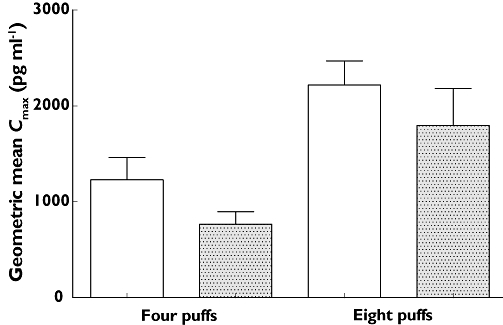
Comparison of geometric mean Cmax of SM for test and reference FP/SM at four puffs and eight puffs. Data given as geometric mean and 95% CIs. Test FP/SM (□); Reference FP/SM ( )
)
Whilst systemic superiority of the test product, in terms of pharmacokinetics, could be viewed negatively, it should be borne in mind that no significant difference was seen in systemic β2-mediated adverse effects (maximal fall in potassium), even at four times the recommended single dose via an optimally prepared spacer (eight puffs). It is also important to point out that the potassium response demonstrated assay sensitivity as there was evidence of significant dose separation, accompanied by a relatively narrow 95% CI. The mean difference in heart rate response was only 6 beats min−1, which would not be considered clinically relevant in terms of increasing propensity to cardiac arrhythmias. It should also be taken into consideration that any difference in adverse systemic effects will be significantly reduced by chronic dosing compared with single dosing as a consequence of down regulation of β2-receptors and associated desensitization of response [17–20]. However we recognise that airway pharmacodynamic studies are required to evaluate the β-adrenoceptor agonist differences further.
The apparent discrepancy between Cmax and peak potassium fall is relatively difficult to explain, as the former has previously been shown to predict the latter with salbutamol [21]. This apparent disconnect has previously been reported by Kempsford et al. who compared single (50 µg) doses of HFA and chlorofluorocarbon (CFC) SM and found a 2.5 fold (95% CI 1.92, 3.32) difference in Cmax, but no significant difference in potassium (0.08 mmol l−1 (95% CI −0.02, 0.18)). In study 2 the fold difference at in Cmax at four puffs (i.e. 100 µg SM) was significant at 1.55 fold (95% CI 1.29, 1.86), whilst the difference in potassium was non-significant at 0.07 mmol l−1 (95% CI −0.04, 0.18).
The results of both studies were essentially consistent in terms of FP pharmacokinetics. In study 2 whilst the mean T : R ratios of Cmax and AUC(0,120 min) were close to unity at four puffs, the 95% CI were outside the pre-determined 20% equivalence limits of 0.8, 1.25 (0.96, 1.29 and 0.92, 1.25, respectively). However, equivalence was clearly demonstrated at eight puffs for both Cmax and AUC(0,120 min). This was substantiated by the results of study 1, where both T : R ratios were within the pre-determined equivalence limits of ±20%.
Suppression of OUCC is a well established surrogate marker of FP bioavailability, which has previously been found to correlate closely with plasma FP concentrations. In study 2 the change in OUCC following four puffs of the test product was 32% higher than the reference product, at eight puffs the difference was 16%. Neither of these differences was statistically different, as reflected by the wide 95% CI for the T : R ratio. However, these results were unexpected, as analysis of pharmacokinetic data in both studies had demonstrated equivalence in terms of FP bioavailability. This may simply be due to the study being underpowered to evaluate OUCC. However an understanding of the particular pharmacokinetic properties of FP could offer an alternative explanation. FP is a highly lipophilic compound which partitions preferably into fat soluble tissue stores, giving it a large volume of distribution, but at the same time exhibiting relatively low plasma concentrations. Thus, if the test product had higher overall systemic bioavailability, partition into fat stores could lead to increased suppression of OUCC with relatively little difference in plasma concentrations of FP. An alternative explanation is that the data for OUCC showed a large degree of intra-subject variance, which resulted in lack of dose separation and wide 95% CIs for the T : R ratios.
The results of this study raise some interesting points with regards to the procedure for establishing therapeutic equivalence between new generic and innovator products. We have demonstrated that in vitro data do not necessarily predict the pharmacokinetics or systemic pharmacodynamics of the products. Furthermore, pharmacokinetic analysis of plasma FP concentrations may not adequately account for the overall bioavailability of the drug, especially if the drug being examined partitions preferably into fat stores. Whilst establishing equivalence in terms of pulmonary deposition is important, the essential factor is whether the products are clinically equivalent in terms of efficacy and safety. A product which does not demonstrate bioequivalence in pharmacokinetic studies may still be acceptable to patients and clinicians provided there is no clinical difference between the drugs.
In conclusion, the results of both studies demonstrate that generic and innovator HFA formulations of FP/SM are clinically interchangeable following single dose administration. The SM moiety of the test product had higher systemic exposure; however, this did not result in any clinically meaningful difference in β2-adrenoceptor response, even after a single dose of eight puffs via an optimally prepared spacer. The results of this study highlight the potential pitfalls of extrapolating in vitro data for fine particle dose to a clinical setting for in vivo pharmacokinetic and pharmacodynamic outcomes.
Competing interests
Both studies were funded by Neolab Ltd.
REFERENCES
- 1.Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2007. Available at http://www.ginasthma.org (last accessed.
- 2.British Guideline on the management of asthma. BTS/SIGN guidelines revised edition 2008. Available at http://www.brit-thoracic.org.uk (last accessed.
- 3.Masoli M. Moderate dose inhaled corticosteroids plus salmeterol versus higher doses of inhaled corticosteroids in symptomatic asthma. Thorax. 2005;60:730–4. doi: 10.1136/thx.2004.039180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med. 1996;153:1481–8. doi: 10.1164/ajrccm.153.5.8630590. [DOI] [PubMed] [Google Scholar]
- 5.Nelson HS, Chapman KR, Pyke SD, Johnson M, Pritchard JN. Enhanced synergy between fluticasone propionate and salmeterol inhaled from a single inhaler versus separate inhalers. J Allergy Clin Immunol. 2003;112:29–36. doi: 10.1067/mai.2003.1558. [DOI] [PubMed] [Google Scholar]
- 6.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma Control study. Am J Respir Crit Care Med. 2004;170:836–44. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 7.Busse W, Koenig SM, Oppenheimer J, Sahn SA, Yancey SW, Reilly D, Edwards LD, Dorinsky PM. Steroid-sparing effects of fluticasone propionate 100 ug and salmeterol 50 ug administered twice daily in a single product in patients previously controlled with fluticasone propionate 250 ug administered twice daily. J Allergy Clin Immunol. 2003;111:57–65. doi: 10.1067/mai.2003.38. [DOI] [PubMed] [Google Scholar]
- 8.Van den Berg NJ, Ossip MS, Hederos CA, Anttila H, Ribeiro BL, Davies PI. Salmeterol/fluticasone propionate (50/100 microg) in combination in a Diskus inhaler (Seretide) is effective and safe in children with asthma. Pediatr Pulmonol. 2000;30:97–105. doi: 10.1002/1099-0496(200008)30:2<97::aid-ppul4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Dempsey OJ, Wilson AM, Coutie WJR, Lipworth BJ. Evaluation of the effect of a large volume spacer on the systemic bioactivity of fluticasone propionate metered-dose inhaler. Chest. 1999;116:935–40. doi: 10.1378/chest.116.4.935. [DOI] [PubMed] [Google Scholar]
- 10.Dempsey OJ, Coutie WJR, Wilson AM, Williams P, Lipworth BJ. Evaluation of the buccal component of systemic absorption with inhaled fluticasone propionate. Thorax. 1999;54:614–17. doi: 10.1136/thx.54.7.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorsson L, Dahlstrom K, Edsbacker S, Kallen A, Paulson J, Wiren JE. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1997;43:155–61. doi: 10.1046/j.1365-2125.1997.d01-1425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett JA, Harrison TW, Tattersfield AE. The contribution of the swallowed fraction of an inhaled dose of salmeterol to it systemic effects. Eur Respir J. 1999;13:445–8. doi: 10.1183/09031936.99.13244599. [DOI] [PubMed] [Google Scholar]
- 13.Kempsford R, Handel M, Mehta R, De Silva M, Daley-Yates P. Comparison of the systemic pharmacodynamic effects and pharmacokinetics of salmeterol delivered by CFC propellant and non-CFC propellant metered dose inhalers in healthy subjects. Respir Med. 2005;99(Suppl. A):S11–9. doi: 10.1016/j.rmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Lipworth BJ. Adrenal suppression with inhaled corticosteroid. Ann Allergy Asthma Immunol. 2001;87:359–61. doi: 10.1016/S1081-1206(10)62914-6. [DOI] [PubMed] [Google Scholar]
- 15.Nair A, Clearie K, Menzies D, Meldrum K, McFarlane L, Lipworth BJ. A novel breath-actuated integrated vortex spacer device increases relative lung bioavailability of fluticasone/salmeterol in combination. Pulm Pharmacol Ther. 2009;22:305–10. doi: 10.1016/j.pupt.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Wilson AM, Sims EJ, Lipworth BJ. Dose-response with fluticasone propionate on adrenocortical activity and recovery of basal and stimulated responses after stopping treatment. Clin Endocrinol. 1999;50:329–35. doi: 10.1046/j.1365-2265.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 17.Newnham DM, McDevitt DG, Lipworth BJ. Bronchodilator subsensitivity after chronic dosing with eformoterol in patients with asthma. Am J Med. 1994;97:29–37. doi: 10.1016/0002-9343(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 18.Newnham DM, Grove A, McDevitt DG, Lipworth BJ. Subsensitivity of bronchodilator and systemic B2 adrenoceptor responses after regular twice daily treatment with eformoterol dry powder in asthmatic patients. Thorax. 1995;50:497–504. doi: 10.1136/thx.50.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan KS, Grove A, McLean A, Gnosspelius Y, Hall IP, Lipworth BJ. Systemic corticosteriod rapidly reverses bronchodilator subsensitivity induced by formoterol in asthmatic patients. Am J Respir Crit Care Med. 1997;156:28–35. doi: 10.1164/ajrccm.156.1.9610113. [DOI] [PubMed] [Google Scholar]
- 20.Grove A, Lipworth BJ. Bronchodilator subsensitivity to salbutamol after twice daily salmeterol in asthmatic patients. Lancet. 1995;346:201–6. doi: 10.1016/s0140-6736(95)91265-7. [DOI] [PubMed] [Google Scholar]
- 21.Fowler SJ, Lipworth BJ. Pharmacokinetics and systemic B2-adrenoceptor-mediated responses to inhaled salbutamol. Br J Clin Pharmacol. 2001;51:359–62. doi: 10.1046/j.1365-2125.2001.01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


