Abstract
AIM
To assess the bioavailability and pharmacokinetics of CAT-354, an anti-IL-13 human monoclonal IgG4 antibody, following subcutaneous (s.c.) and intravenous (i.v.) administration.
METHODS
This was a single-dose, randomized, open-label, parallel-group bioavailability study. Healthy male subjects aged 20–54 years were randomly assigned to one of three dose groups (n= 10/group) to receive CAT-354: 150 mg i.v.; 150 mg s.c. or 300 mg s.c. (two 150 mg injections). Serum pharmacokinetics, adverse events (AEs), vital signs, electrocardiograms and laboratory parameters were assessed.
RESULTS
CAT-354 showed bioavailability of 62% and 60% after 150 mg and 300 mg s.c. doses, respectively, and linear pharmacokinetics over the dose range tested. Peak serum concentrations in the s.c. groups occurred after 3–9 (median 5) days, with a mean elimination half-life of 19.2 ± 3.1 days (150 mg) and 19.4 ± 3.59 days (300 mg) after s.c. and 21.4 ± 2.46 days after i.v. administration. Volume of distribution at steady state (Vss) was 4960 ± 1440 ml kg−1 after i.v. (slightly greater than plasma volume). Average apparent clearances (CL/F) were 292 ± 82.3 and 307 ± 109 ml day−1 after 150 and 300 mg s.c., respectively; systemic CL of 188 ± 84.0 ml day−1 after i.v. dosing was consistent with endogenous IgG and reticuloendothelial elimination. No severe or serious AEs occurred. Among 40 reported AEs, 25 were headache, sinus disorders/respiratory symptoms and changes in body temperature perception.
CONCLUSIONS
CAT-354 exhibited bioavailability of approximately 60% when given s.c. to healthy male subjects.
Keywords: antibody, asthma, bioavailability, CAT-354, half-life, IL-13
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
The safety profile and effective dose of CAT-354 have been determined with intravenous (i.v.) administration, but no information is available on subcutaneous (s.c.) administration.
WHAT THIS STUDY ADDS
This study characterized the pharmacokinetics of CAT-354 following s.c. administration of 150 mg and 300 mg doses, and indicated a bioavailability of approximately 60%.
Introduction
Asthma is characterized by airway hyper-responsiveness (AHR), airway inflammation and underlying structural changes to the airways. Almost 30% of the study population in the Gaining Optimal Asthma controL (GOAL) study failed to maintain control despite regular use of high-dose fluticasone and salmeterol [1]. Omalizumab, a humanized monoclonal antibody that binds to immunoglobulin E (IgE), has been shown to decrease asthma exacerbations and corticosteroid requirements and reduce hospitalizations and emergency room visits in moderate-to-severe allergic asthmatics [2]. Despite these benefits, however, approximately 40% of allergic asthmatics failed to have an optimal clinical response [2]. This suggests that treatment with corticosteroids or IgE pathway blockade may not be sufficient to control some asthmatics and indicates that there is clearly an unmet need for the effective and safe treatment of asthma.
The pathogenesis of asthma involves cytokines produced by the T-helper-2 (TH-2) cells that express interleukin (IL)-4, IL-5 and IL-13 [3, 4]. IL-13 receptors are expressed on a multitude of cell types, and the action of IL-13 on these cells is to promote the acute inflammatory process, AHR and underlying structural changes to the airways [5]. In animal models, IL-13 has been shown to be both necessary and sufficient to induce a phenotype that closely resembles allergic asthma [6].
Elevated IL-13 production has been detected in the airways and sputum of patients with asthma [7, 8], and mast cell-derived IL-13 (as well as IL-4) has been demonstrated in airway smooth muscle of patients with asthma [5, 9]. Animal model data show that IL-13 causes AHR, eosinophilic inflammation and mucus hypersecretion [6, 10–13]. Taken together, these findings suggest an important role for IL-13 in the pathophysiology of asthma. Therefore, antagonizing the function of IL-13 may offer therapeutic benefit to patients with asthma.
CAT-354 is a human monoclonal IgG4 antibody which has been shown to neutralize IL-13 in both the murine airway [14] and human lung mast cells [15]. Phase 1 data in subjects with mild-to-moderate asthma have demonstrated safety and tolerability profiles that support continued clinical development of CAT-354 [16]. However, all human studies to date have utilized an i.v. infusion of CAT-354 over a period of 30 min. CAT-354 has demonstrated good bioavailability, linear pharmacokinetics over the dose range tested and an acceptable safety profile and tolerability after i.v. administration in another phase 1 study in patients with mild-to-moderate asthma [16]. Simpler injection protocols of s.c. administration may offer patients advantages in ease of use.
The primary objective of this single-dose study in healthy male subjects was to assess the bioavailability of single-dose administration of CAT-354 by s.c. and i.v. routes at two s.c. dose levels (150 mg and 300 mg) in comparison with that following a single 150 mg i.v. dose. Additional objectives were to compare other measures of the pharmacokinetics of CAT-354 and to assess the tolerability, safety and immunogenicity profiles of these single i.v. and s.c. doses of CAT-354 in these subjects.
Compartmental pharmacokinetic modelling was also conducted to characterize further the absorption kinetics and define pharmacokinetic parameters to support future modelling and simulation of repeat-dose s.c. regimens.
Methods
Subject eligibility
This study was conducted at a single centre in the United States, MDS Pharma Services, Lincoln, NE. The study was conducted in accordance with the ethical principles set forth in the Declaration of Helsinki, the International Conference on Harmonisation Guidance for Good Clinical Practice and the US Code of Federal Regulations (21 CFR). The study protocol and informed consent documents were approved by the MDS Pharma Services (US) Inc. Institutional Review Board prior to subject enrolment.
Males aged 19–55 years with a body mass index (BMI) between 18 and 30 kg m−2, inclusive, who had no clinically significant abnormality on clinical examination, medical history, electrocardiogram (ECG), clinical chemistry, haematology or urinalysis results (negative screen for drug abuse and alcohol use was also required) were eligible to participate in this study.
Subjects were excluded from participation if they had experienced any acute illness in the 2 weeks before study day 0 (study visit 2) or any active concomitant disease (including psychological disorders), had donated blood or had significant loss of blood within 56 days (or donated plasma within 7 days) of study day 0, had a positive test for or received treatment for hepatitis B, hepatitis C, HIV and/or other immunodeficiency disorders, or had a history of receiving any medication that might have had carry-over effects into this study. Specifically, subjects who had previously received any monoclonal antibody or similar related protein that might have sensitized them to CAT-354 were excluded. In addition, subjects could not have participated in another investigational product study within 3 months of study day 0 (or five half-lives of the previously administered investigational product, whichever was longer).
Study design
This was a phase 1, single-dose, randomized, open-label, parallel-group bioavailability and pharmacokinetic study of CAT-354 in healthy male subjects. The study design is summarized in Figure 1.
Figure 1.
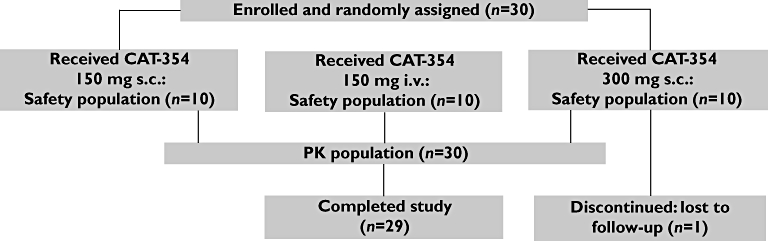
Flowchart of subject assignment and treatment through the study
Following confirmation of eligibility and completion of a 14-day screening phase, subjects were randomly assigned at the second study visit to one of three dose groups: 150 mg CAT-354 given i.v. (one 30-min infusion), 150 mg CAT-354 given s.c. (one injection into the thigh) or 300 mg CAT-354 given s.c. (one injection of 150 mg given into the anterior aspect of one thigh immediately followed by a second injection of 150 mg into the anterior aspect of the contralateral thigh). Doses of the assigned treatment were administered at visit 2, following screening and randomization. Subjects remained in the study for assessments of safety characteristics and blood sampling for pharmacokinetics and immunogenicity until at least day 56 post-dose.
CAT-354 was manufactured on behalf of MedImmune under current good manufacturing practice, supplied by MedImmune in identifiable bulk packs containing medication vials and labelled in accordance with good clinical practice and any other local regulatory requirements. CAT-354 was formulated at a nominal concentration of 150 mg ml−1 in an acetate buffer (pH 5.5). It was presented as a sterile, opalescent solution in 2-ml clear glass vials, each containing a nominal volume of 1 ml.
Study procedures
Pharmacokinetic and immunogenicity assessments
Venous blood samples for quantitation of CAT-354 in serum were obtained before dosing, at the end of infusion (∼30 min) or immediately following injection(s), at 30 min (± 10 min), 1 h (± 10 min), 3, 8 and 24 h (± 30 min), and at 3, 5, 7, 9, 14, 21, 28, 35, 42 and 56 days post-infusion stop/injection. Additional blood samples were collected from all subjects before dosing and at day 56 for CAT-354 immunogenicity determination. The total blood volume drawn from an individual subject for all assessments during the study was less than 100 ml. Each serum sample was divided into three aliquots and stored at −80°C until shipped to MedImmune for analysis.
Pharmacokinetic assay
The concentration of CAT-354 in the serum samples was determined using a qualified immunoassay performed on the Gyrolab assay platform (Gyros AB, Uppsala, Sweden) as follows: biotinylated CAT-375 (antibody against the idiotypic region of CAT-354) was captured onto streptavidin-coated columns of the Gyros compact disc. Prediluted samples and CAT-354 standards were flowed through the columns. CAT-354 that bound to the immobilized CAT-375 was quantified with a sheep anti-human IgG4 antibody labelled with Alexa Fluor® 647 (Invitrogen Corp., Carlsbad, CA) and detected by fluorescence. The CAT-375 anti-idiotype antibody only binds to free CAT-354 (i.e. CAT-354 where one or both variable regions are not bound to serum IL-13); therefore, by design the assay only detects free CAT-354, and gives results equivalent to using IL-13 antigen capture. This method exhibits accuracy of ≤25% absolute relative error and precision of ≤20% coefficient of variation (CV).
Immunogenicity assays
The anti-drug antibody (ADA) screening assay was a validated, homogeneous, drug-tolerant, double-bridging immunoassay with detection by electrochemiluminescence (ECL). All samples, including positive and negative controls, were treated with 300 mm acetic acid for 1 h at room temperature, and then neutralized by 1 m Tris-HCl. The acid-treated samples, biotinylated CAT-354, and ruthenium-conjugated CAT-354 were mixed with equal volumes to form an immunocomplex. The ADA-bridged immunocomplex was incubated at 2–8°C overnight. Streptavidin-coated standard MA 2400 96 plates were blocked with MSD Blocker A buffer (Blocker A) for at least 1 h at room temperature. The immunocomplex was captured by the streptavidin-coated standard MSD plate. The ECL signal was captured with an MSD Sector Imager 2400 (all MSD assay components supplied by Meso-Scale Discovery, Gaithersburg, MD). CAT-375 prepared in 100% pooled normal human serum at a concentration of 250 ng ml−1 was used as the assay positive control; 100% pooled normal human serum served as the assay negative control. The assay cut point signal-to-background ratio was 1.4 with a 1% false positive rate at the cut point. The assay limit of detection was 244 ng ml−1 in 100% pooled normal human serum with 1% false positive rate and 5% false negative rate at the limit-of-detection concentration [17]. The assay was drug tolerant, and could detect 244 ng ml−1 and 488 ng ml−1 of CAT-375 in the presence of 1 µg ml−1 and 10 µg ml−1 CAT-354, respectively.
The confirmatory ADA assay was a validated immunoassay conducted using the same immunoassay format as the screening assay. Samples that were positive in the screening assay were pre-incubated with and without 300 µg ml−1 CAT-354. Assay positive controls were 250 ng ml−1 of CAT-375 with and without 300 µg ml−1 CAT-354 in 100% pooled normal human serum; 100% pooled normal human serum with and without 300 µg ml−1 CAT-354 was used as the assay negative control. The confirmatory cut point was calculated based on the percent inhibition of signal by CAT-354 pre-incubated with 50 normal human serum samples and 10 asthma patient serum samples. With a 1.0% false-positive error rate, the confirmatory cut point was 38.8%. Samples with a percent inhibition greater than or equal to the cut point would be confirmed positive for the presence of ADA to CAT-354.
Noncompartmental analysis
Pharmacokinetic parameters for CAT-354 in serum were estimated using noncompartmental methods with WinNonlin® (version 5.2) software (Pharsight Corporation, St Louis, MO). WinNonlin® noncompartmental models 202 and 200 with default settings were used to analyze the i.v. and s.c. serum pharmacokinetic data, respectively. Pharmacokinetic modelling and simulation were performed using the NONMEM software program, Version VI, Level 1.0 (Regents of the University of California, Oakland, CA).
The AUC(0,τ) where τ= 56 days, was estimated by the linear and log-linear trapezoidal rules. The AUC(0,∞) was estimated by extrapolating the concentration–time curve from time zero to infinity. After i.v. or s.c. administration, the maximum observed concentration (Cmax) and time of Cmax (tmax) were recorded as observed. Systemic clearance (CL) after i.v. dosing and apparent clearance (CL/F) after s.c. dosing were calculated as Dose/AUC(0,∞). Volume of distribution at steady state (Vss) after i.v. dosing was estimated by the formula Vss= mean residence time from time 0 to infinity (MRT(0,∞)) CL, where MRT(0,∞) = AUCM(0,∞) (area under the moment curve)/AUC(0,∞). The elimination rate constant, λz, was determined by least-squares regression of the log-transformed concentration data using the terminal phase, identified by inspection. The elimination half-life was calculated as: 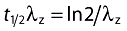 .
.
Compartmental modelling
All pharmacokinetic data were fitted simultaneously using the first-order conditional estimation plus interaction (between inter-/intra-individual and residual variability) method. Data processing and graphical analyses were done using S-PLUS (version 7.0, Insightful/TIBCO, Palo Alto, CA).
A population pharmacokinetic model that involved a linear, two-compartmental design was developed to characterize the disposition of CAT-354. During model development, various model structures (one and two compartments; one, two and three absorption compartments; with or without absorption lag time) were sequentially evaluated. The final model was selected based on comparison in diagnostic plots and the objective function values between different structural models. In the final model, for s.c. groups, the absorption was described with two absorption compartments, one of which had an absorption lag time. Parameters that characterize the fraction of the dose that enters into both absorption compartments were also included into the final model. The whole model was described by the following equations:
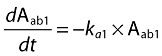 |
(1) |
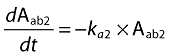 |
(2) |
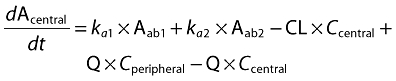 |
(3) |
 |
(4) |
Aab1 and Aab2 represent the amount of CAT-354 present in two separate absorption compartments (ab1 and ab2) after s.c. administration. Fractions of CAT-354 absorbed from both compartments are set as: Fraction Abs1 + Fraction Abs2= 1. An absorption lag time (tlag) in ab2 was also incorporated to describe the absorption delay. Acentral, Aperipheral, Ccentral and Cperipheral represent the amount and concentration in both central and peripheral compartments, respectively. The concentrations in the central and peripheral compartments were calculated by dividing the amounts in the central and peripheral compartments by the central volume of distribution, Vcentral, and the peripheral volume of distribution, Vperipheral, respectively. CL and Q represent systemic clearance and distributional clearance between central and peripheral compartments.
Interindividual variability for the fixed-effect parameters was modelled using an exponential error term:
| (5) |
where θi is the ith individual's variable, θ is the typical value of the population parameter, and ηi represents the individual-specific random effects for the ith individual parameter symmetrically distributed with zero mean and variance ω2. A combination of proportional and additive models was used to characterize the residual error variability:
| (6) |
where Yij represents the jth observation for the ith subject, F is the predicted concentration and εij1 and εij2 represent the proportional and additive residual error, respectively.
To evaluate the model results, visual predictive checks were performed to simulate the pharmacokinetic profiles using the parameter estimates generated from the original data. The 5th, 50th and 95th quantiles were calculated from the simulated profiles for the predictive checks and were compared with the raw data to allow assessment of model predictability.
Safety assessments
The safety of subjects in this study was monitored through ongoing assessments of local tolerability, vital signs, clinical laboratory tests, physical examinations, 12-lead ECGs, adverse events (AEs) and serious adverse events (SAEs), and CAT-354 immunogenicity testing. All subjects who were randomly assigned to treatment and received CAT-354 were included in the safety population.
Safety observations were recorded from the time informed consent was obtained until 56 days post-dose. Venous blood samples were obtained for haematology and biochemistry testing at screening and at visits 5, 8, 10 and 12 (termination). Vital signs were assessed at screening and at visits 2, 5, 8, 10 and 12. ECGs were performed at screening and at visits 2 and 12.
All AEs and SAEs were described using MedDRA (Medical Dictionary for Regulatory Activities) version 11.0 and graded by severity (mild, moderate, severe) and attributional relationship to CAT-354 (none, remote, possible, probable, definite).
Statistical analysis
The number of subjects studied was based on the desire to obtain adequate pharmacokinetic data whilst exposing as few healthy males as possible to CAT-354 and study procedures. A total of 24 evaluable subjects completing the study (8 per group) was considered sufficient to provide adequate information.
Log-transformed dose-normalized AUC(0,∞) (AUC(0, ∞)/D) was analyzed using WinNonlin® (version 5.2) software with an analysis of variance (anova) model, fitting treatment as the main effect. The results of these analyses are presented in terms of geometric least-square means (LSMs) for each treatment, the treatment effect (ratio of each s.c. treatment geometric LSM/i.v. treatment geometric LSM) and the corresponding 90% confidence intervals.
Pharmacokinetic parameters were summarized by dose group using descriptive statistics, including n, mean and standard deviation (SD). For tmax only n, min, median, and max were reported. All CAT-354 concentration data were transformed from ng ml−1 to µg ml−1 for pharmacokinetic analyses and presentation. Final results were rounded and recorded to three significant figures for all pharmacokinetic parameters except tmax.
Results
Demographics and baseline characteristics
A total of 30 subjects were enrolled in the study between April and June 2008. All 30 subjects received their planned doses of study medication. One subject in the CAT-354 300 mg s.c. group did not complete the study because he was lost to follow-up, but his results were included in the pharmacokinetic and safety analyses.
All subjects were male and were predominantly white (77%). The mean age of the subjects was 32.5 years, the mean weight was 83.7 kg, the mean height was 180.0 cm and the mean BMI was 25.8 kg m−2. These characteristics were comparable across treatment groups and are summarized in Table 1.
Table 1.
Demographic characteristics of subjects at study entry
| Treatment group | |||
|---|---|---|---|
| CAT-354 150 mg i.v. | CAT-354 150 mg s.c. | CAT-354 300 mg s.c. | |
| Number of subjects | 10 | 10 | 10 |
| Age (years) | 30.4 ± 8.34 | 39.3 ± 8.55 | 27.8 ± 10.4 |
| Race | |||
| American Indian or Alaska Native | 0% | 0% | 10% |
| Asian | 0% | 10% | 0% |
| Black or African American | 10% | 20% | 10% |
| Other: Arabic | 10% | 0% | 0% |
| White | 80% | 70% | 80% |
| Ethnicity | |||
| Hispanic or Latino | 10% | 0% | 10% |
| Not Hispanic or Latino | 90% | 100% | 90% |
| Height (cm) | 179 ± 7.8 | 179 ± 6.0 | 182 ± 5.9 |
| Weight (kg) | 78.7 ± 14.7 | 87.3 ± 8.1 | 85.3 ± 12.2 |
| BMI (kg m−2) | 24.6 ± 3.47 | 27.1 ± 1.86 | 25.6 ± 2.99 |
Averages are presented as mean ± SD.
Pharmacokinetics and bioavailability results
Pharmacokinetic parameters for CAT-354 are listed in Table 2. The mean serum concentration–time profiles of CAT-354 over the 56-day study period (with detailed focus on period from infusion/injection through day 7) are illustrated in Figure 2. The highest sustained concentration was achieved with the 300 mg s.c. dose (see Figure 2).
Table 2.
Noncompartmental average pharmacokinetic parameters: CAT-354 administered s.c. or i.v.
| Treatment group | |||
|---|---|---|---|
| 150 mg i.v. | 150 mg s.c. | 300 mg s.c. | |
| Number of subjects | 10 | 10 | 10 |
| Cmax (µg ml−1) | 58.3 ± 14.4 | 17.1 ± 5.91 | 36.6 ± 13.1 |
| Cmax/D (µg ml−1 mg−1) | 0.389 ± 0.096 | 0.114 ± 0.039 | 0.122 ± 0.044 |
| tmax median (min, max) (days) | 0.063 (0.042, 1.02) | 5 (3, 9) | 5 (3, 9) |
| AUC(0,56 days) (µg ml−1 day) | 765 ± 220 | 467 ± 122 | 881 ± 287 |
| AUC(0,∞) (µg ml−1 day) | 903 ± 291 | 548 ± 143 | 1080 ± 315 |
| AUC extrapolated (%) | 14.3 ± 4.45 | 14.7 ± 4.98 | 18.2 ± 10.6 |
| AUC(0,∞)/D (µg ml−1 day mg−1) | 6.02 ± 1.94 | 3.66 ± 0.953 | 3.59 ± 1.05 |
| CL or CL/F (ml day−1) | 188 ± 84.0 | 292 ± 82.3 | 307 ± 109 |
| CL or CL/F (ml day−1 kg−1) | 2.40 ± 0.984 | 3.34 ± 0.828 | 3.60 ± 1.07 |
| VSS (ml) | 4960 ± 1440 | ND | ND |
| VSS (ml kg−1) | 63.6 ± 16.6 | ND | ND |
| t1/2 (days) | 21.4 ± 2.46 | 19.2 ± 3.1 | 19.4 ± 3.59 |
Averages are presented as mean ± SD except for tmax, which is presented as median (min, max); ND, not determined; Cmax, maximum observed concentration after infusion/injection; tmax, time at which Cmax is observed after infusion/injection; AUC(0,56 days), area under the serum concentration–time curve from time 0 to study day 56 (last observation); AUC(0,∞), area under the serum concentration–time curve from time 0 to infinity; CL or CL/F, systemic clearance; Vss, volume of distribution at steady state; t1/2, terminal phase elimination half-life.
Figure 2.
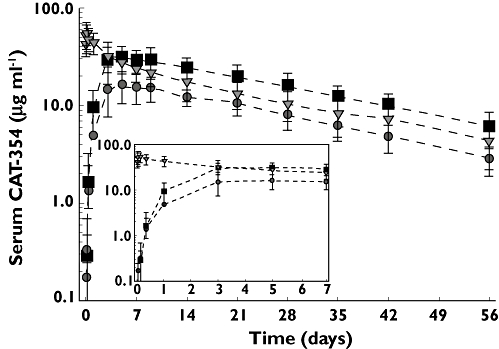
Serum concentration vs. time profile of single doses of CAT-354 at doses of 150 mg s.c. ( ), 300 mg s.c. (
), 300 mg s.c. ( ), and 150 mg i.v. (
), and 150 mg i.v. ( ) administered by s.c. injection or i.v., infusion
) administered by s.c. injection or i.v., infusion
Following i.v. dosing, CAT-354 exhibited biphasic kinetics (see Figure 2). The mean steady-state volume of distribution was 4960 ± 1440 ml (63.6 ± 16.6 ml kg−1), which is about 50% greater than plasma volume. The mean systemic clearance was 188 ± 84.0 ml day−1 (2.40 ± 0.984 ml kg−1). The mean elimination half-life was 21.4 ± 2.46 days.
Following s.c. administration, the pharmacokinetics of CAT-354 were linear over the dose range tested. AUC(0,∞) and Cmax increased approximately twofold with an increase in dose from 150 mg to 300 mg. The percent coefficients of variation (CV%) for AUC(0,∞) and Cmax were 35% and 26%, respectively, for the 150 mg s.c. dose and 36% and 29%, respectively, for the 300 mg s.c. dose. By comparison, the CV% after the 150 mg i.v. dose were 25% and 32%, respectively, for AUC(0,∞) and Cmax. Absorption of CAT-354 was slow, with a median tmax of 5 days (range, 3–9 days) observed in both s.c. dose groups.
The mean elimination half-life of CAT-354 was 19.2 ± 3.1 days and 19.4 ± 3.59 days following the 150 mg and 300 mg s.c. doses, respectively, which was similar to the half-life after i.v. dosing. The absolute bioavailability of the s.c. doses, as assessed by the geometric LSM ratios of AUC(0,∞)/D, is presented in Table 3. The bioavailability of CAT-354 was 62% and 60% following the 150 mg and 300 mg s.c. doses, respectively.
Table 3.
Absolute bioavailability of CAT-354
| Geometric LSM | Ratio of LSM % (90% CI) | ||||
|---|---|---|---|---|---|
| Parameter | 150 mg i.v. | 150 mg s.c. | 300 mg s.c. | 150 mg s.c. : i.v. | 300 mg s.c. : i.v. |
| Cmax/D (µg ml−1 mg−1) | 0.379 | 0.109 | 0.115 | 28.7 (22.5, 36.6) | 30.3 (23.7, 38.6) |
| AUC(0,∞)/D (µg ml−1 day mg−1) | 5.70 | 3.54 | 3.43 | Absolute bioavailability*(90% CI) | |
| 62.1 (48.5, 79.6) | 60.1 (46.9, 77.1) | ||||
Assessed from geometric LSM ratios of AUC(0,∞)/D. LSM, least-square means; Cmax/D, dose-normalized maximum concentration after each infusion/injection; AUC(0.∞)/D, dose-normalized area under the serum concentration–time curve from time 0 to infinity.
Compartmental modelling results
The model-estimated parameters are summarized in Table 4. Figure 3 shows these plots for the 150 mg s.c. (Panel A), 300 mg s.c. (Panel B), and 150 mg i.v. (Panel C) groups. The model predicted mean (50%, solid line) and the 90% prediction interval (5% and 95%, dashed lines) were plotted together with observed data to allow visual predictive checks of the model performance.
Table 4.
Two-compartment model-estimated parameters for CAT-354 pharmacokinetics
| Parameter | Final estimates | SEE (%) |
|---|---|---|
| CL (ml day−1) | 186 | 13.5 |
| Vcentral (ml) | 3060 | 8.17 |
| Q (ml day−1) | 294 | 37.4 |
| Vperipheral (ml) | 1770 | 24.9 |
| F (%) | 66.2 | 12.4 |
| tlag (days) | 0.245 | 35.3 |
| ka1 (day−1) | 0.110 | 221 |
| ka2 (day−1) | 0.270 | 35.3 |
| Fraction Abs2 (%) | 0.847 | 69.4 |
CL, systemic clearance; Vcentral, volume of central compartment; Q, distributional clearance between central and peripheral compartments; Vperipheral, volume of peripheral compartment; F, absolute bioavailability; tlag, absorption lag time; ka1, first-order absorption rate constant; ka2, delayed first-order absorption rate constant; Fraction Abs2, fraction of CAT-354 absorbed by the delayed absorption process; SEE, standard error of the estimate.
Figure 3.
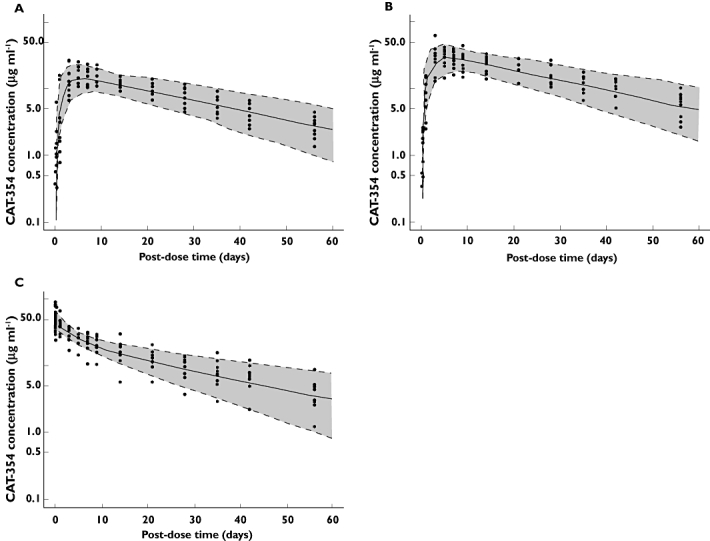
(A) Observed and model-predicted profiles of CAT-354 following 150 mg s.c. administration. Solid line: mean prediction. Dashed lines: 5th and 95th quantiles. Shaded area: 90% prediction interval. (B) Observed and model-predicted profiles of CAT-354 following 300 mg s.c. administration. Solid line: mean prediction. Dashed lines: 5th and 95th quantiles. Shaded area: 90% prediction interval. (C) Observed and model-predicted profiles of CAT-354 following 150 mg i.v. administration. Solid line: mean prediction. Dashed lines: 5th and 95th quantiles. Shaded area: 90% prediction interval
The absorption kinetics of CAT-354 were described by parallel mechanisms: (i) a first-order absorption process and (ii) a delayed first-order absorption process. The delayed absorption process accounted for approximately 85% of the absorbed dose. The central volume of distribution was approximately equal to plasma volume. The steady-state volume of distribution was 4830 ml, consistent with the Vss determined by noncompartmental analysis. Clearance and bioavailability determined by compartmental analysis also were similar to the values determined by noncompartmental methods.
Safety assessment results
No AEs were considered by the investigator to be probably or definitely related to CAT-354. Four AEs were assessed as possibly related to CAT-354: three AEs in the 150 mg i.v. treatment group (headache, dizziness and feeling hot), and one AE in the 150 mg s.c. treatment group (feeling cold). Six additional AEs were assessed as remotely related: one AE in the 150 mg i.v. treatment group (headache) and five AEs in the 150 mg s.c. treatment group (three AEs of headache, and one AE each of dizziness and sinus headache). The remaining 30 AEs were assessed as unrelated to CAT-354.
Fourteen subjects reported a total of 40 treatment-emergent AEs. Thus, approximately half of all subjects in all treatment groups experienced at least one AE. The number of subjects experiencing AEs was comparable across treatment groups. There were more AEs reported in the 150 mg i.v. treatment group (17) compared with either of the s.c. treatment groups (150 mg s.c.: 13; 300 mg s.c.: 10). The incidence of all AEs reported by one or more subjects after administration of CAT-354 is summarized in Table 5.
Table 5.
Summary of all reported adverse events (AEs) by relationship to CAT-354
| Relationship to CAT-354* | |||||||
|---|---|---|---|---|---|---|---|
| Subjects with AEs | Number of AEs | None | Remote | Possible | Probable | Definite | |
| 150 mg i.v. (n= 10) | |||||||
| All AEs | 5 | 17 | 13 | 1 | 3 | 0 | 0 |
| Headache | 4 | 4 | 2 | 1 | 1 | 0 | 0 |
| Back pain | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Cough | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Dizziness | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| Eye pruritus | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Feeling hot | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| Joint swelling | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Nasal congestion | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Pain in extremity | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Pharyngolaryngeal pain | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Post-traumatic pain | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Rhinorrhoea | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Sinus congestion | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Sneezing | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| 150 mg s.c. (n= 10) | |||||||
| All AEs | 5 | 13 | 7 | 5 | 1 | 0 | 0 |
| Headache | 4 | 6 | 3 | 3 | 0 | 0 | 0 |
| Paranasal sinus hypersecretion | 2 | 2 | 2 | 0 | 0 | 0 | 0 |
| Abdominal pain | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Dizziness | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| Feeling cold | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| Sinus congestion | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Sinus headache | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| 300 mg s.c. (n= 10) | |||||||
| All AEs | 4 | 10 | 10 | 0 | 0 | 0 | 0 |
| Headache | 1 | 3 | 3 | 0 | 0 | 0 | 0 |
| Sunburn | 1 | 2 | 2 | 0 | 0 | 0 | 0 |
| Breast mass | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Breast tenderness | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Epistaxis | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Melanocytic naevus | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Procedural pain | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
All AEs were described using MedDRA (Medical Dictionary for Regulatory Activities) Version 11.0 and graded by severity (mild, moderate, severe) and attributional relationship to CAT-354.
Across all subjects, AEs from the MedDRA System Organ Class (SOC) ‘Nervous system disorders’ were reported by the largest number of subjects (9/30), and consisted of headache (most frequent), dizziness and sinus headache. More subjects in the 150 mg i.v. (4/10) and 150 mg s.c. (4/10) treatment groups experienced nervous system disorders compared with the 300 mg s.c. treatment group (1/10).
Subjects also reported AEs from MedDRA SOC ‘Respiratory, thoracic and mediastinal disorders’ (6/30), including paranasal sinus hypersecretion and sinus congestion. Respiratory, thoracic and mediastinal disorders were reported by more subjects in the 150 mg i.v. group (six events, three subjects), compared with the 150 s.c. group (three events, two subjects) and the 300 mg s.c. group (one event, one subject).
Of note, no subject in any treatment group experienced a local reaction to infusion or injection of CAT-354. No deaths or SAEs occurred in any treatment group during the study, and no subject discontinued the study because of an AE.
Immunogenicity testing results
The immunogenicity of CAT-354 was evaluated by analysis of samples collected pre-dose and on day 56. On day 56, the maximum concentrations of CAT-354, which could potentially interfere with detection of ADA, measured in samples from individual volunteers were 8.9 µg ml−1, 4.5 µg ml−1 and 10 µg ml−1 in the 150 mg i.v., 150 mg s.c. and 300 mg s.c. treatment groups, respectively. The ADA assay could measure less than 500 ng ml−1 ADA in the presence of 10 µg ml−1 CAT-354; therefore, the ADA assay was sufficiently sensitive and drug tolerant to detect clinically relevant concentrations of ADA on day 56 [18]. All samples were negative for the presence of ADA. All individual pharmacokinetic profiles were consistent with the absence of an ADA response.
Discussion
Despite adequate treatment with inhaled corticosteroids, long-acting β2-adrenoceptor agonists, and other controller medications such as leukotriene antagonists and omalizumab, a significant medical need still remains for alternative treatments for patients with poorly controlled asthma. Alternative therapies for asthma targeting IL-13-expressing cells probably constitute a reasonable goal for advancement in the treatment of asthma.
The targeting of IL-13 in the management of pulmonary diseases has been recently reviewed by Blease, with a summary specific to CAT-354 [19]. Early therapeutic potential of CAT-354 for asthma was demonstrated by Blanchard et al. via a murine model in which CAT-354 significantly inhibited IL-13-induced AHR and airway eosinophilia [14]. Kaur et al. demonstrated that human lung mast cells (HLMCs) expressed IL-13, that IL-13 can up-regulate allergic functions of HLMCs and, specifically, that CAT-354 inhibited all of these IL-13-mediated effects in HLMCs [15].
Preclinical pharmacokinetics and interspecies scaling analyses by Vugmeyster et al. suggested that i.v. or s.c. administration of anti-IL-13 antibodies with safe and favourable pharmacokinetic profiles was feasible [20]. Recent data from a study of three dose levels of CAT-354 (1, 5 and 10 mg kg−1) presented by Bhowmick et al. demonstrated linear pharmacokinetics and an acceptable safety profile after multiple i.v. doses in 23 patients with asthma; most AEs observed were mild or moderate and not related to CAT-354 [16]. Overall, the CAT-354 pharmacokinetic parameters demonstrated in the current study were very similar to those of the single-dose pharmacokinetic behaviour of CAT-354 as reported by those authors, although its half-life in that study was reported as somewhat shorter (12–17 days) [16]. Thus, the required characterization of the safety and pharmacokinetic profile of CAT-354 during its development as an asthma therapeutic has been encouraging.
The current study is the first to compare s.c. injection with i.v. infusion of single doses of CAT-354 with regard to bioavailability, safety and pharmacokinetics in healthy subjects. The safety profile of CAT-354 was encouraging in this study, and the most commonly reported AEs (headache, sinus hypersecretion) were similar to those observed by Bhowmick et al. in patients with asthma (nasopharyngitis, headache [also reported by that study's placebo arm]) [16].
The most common AEs overall in the current study were reported with the lowest frequency in the 300 mg s.c. group; this was especially apparent with AEs coded under the MedDRA ‘Respiratory, thoracic and mediastinal disorders’ SOC, which occurred more frequently in the 150 mg i.v. group (six AEs compared with one AE in the 300 mg s.c. group).
Of particular note was the absence of injection site or infusion reactions observed in this study, together with the absence of immunogenicity demonstrated by CAT-354. Again, a monoclonal antibody administered subcutaneously should ideally produce as few injection-site reactions as possible.
CAT-354 demonstrated linear pharmacokinetics over the dose range tested, with a half-life that was essentially independent of dose and route of administration (ranging between 19.2 and 21.4 days across groups and routes) and an AUC that was dose-proportional within the s.c. route. The systemic clearance and elimination half-life after i.v. dosing were consistent with endogenous IgG, elimination of CAT-354 by the reticuloendothelial system and the absence of an antigen sink [21]. The apparent clearances and elimination half-lives observed were similar between the two s.c. dose groups.
Whilst the AUC associated with the 150 mg s.c. dose was slightly greater than half that achieved with the 150 mg i.v. dose, the 300 mg s.c. dose produced mean and median AUCs that were in fact higher than that achieved by the 150 mg i.v. dose. Since the 300 mg s.c. dose demonstrated a bioavailability of 60%, the simpler route of administration could provide therapeutic benefit equivalent to that afforded by a 150 mg i.v. infusion and thus an advantage for patients. A comparison of the interpatient pharmacokinetic variability following i.v. and s.c. dosing suggests that s.c. administration will not increase variability compared with the i.v. route.
A bisegmental absorption model has been used to describe the s.c. absorption of other therapeutic proteins; this model reflects direct absorption into the blood and absorption through the lymphatics [22–24]. Compartmental modelling was used in the current study to describe the absorption, distribution and elimination kinetics of CAT-354. After s.c. dosing, the absorption kinetics of CAT-354 were modelled by a two-phase absorption process. The bioavailability, clearance and Vss parameters derived from compartmental modelling were similar to parameters derived by noncompartmental analysis. The compartmental pharmacokinetics model can be used for future simulation of repeat-dose s.c. dosing regimens of CAT-354. CAT-354 exhibited a pharmacokinetic profile consistent with endogenous human IgG, and an absolute bioavailability of approximately 60% when given s.c. to healthy male subjects.
In conclusion, CAT-354 showed good bioavailability and a good tolerability profile in this study of healthy male subjects, with encouraging results shown with s.c. administration. The similar safety profile demonstrated by this study to that already demonstrated by CAT-354 in patients with asthma [16] might be expected to predict an acceptable safety profile in larger efficacy studies that would provide further confirmation of the bioavailability results demonstrated in the current study.
Acknowledgments
This study was funded by MedImmune, LLC.
The authors would like to thank Scott Saunders, DDS, MirrorMonitor Creativity (Royersford, PA, USA), and Miriam Gitler, PhD, MedImmune, LLC, for medical writing and editorial contributions to this manuscript, funded by MedImmune, LLC.
Competing interests
Rosamund Wilson, PhD, is a consultant to MedImmune, LLC, and has received compensation from MedImmune, LLC, for statistical consultancy.
REFERENCES
- 1.Bateman ED, Boshey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, Perdersen SE GOAL Investigators Group. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma controL study. Am J Respir Crit Care Med. 2004;170:836–44. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, Beeh KM, Ramos S, Canonica GW, Hedgecock S, Fox H, Blogg M, Surrey K. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–16. doi: 10.1111/j.1398-9995.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 3.Brightling CE, Symon FA, Birring SS, Bradding P, Pavord ID, Wardlaw AJ. TH2 cytokine expression in bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. J Allergy Clin Immunol. 2002;110:899–905. doi: 10.1067/mai.2002.129698. [DOI] [PubMed] [Google Scholar]
- 4.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 5.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–90. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 6.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 7.Berry MA, Parker D, Neale N, Woodman L, Morgan A, Monk P, Bradding P, Wardlaw AJ, Pavord ID, Brightling CE. Sputum and bronchial submucosal IL-13 expression in asthma and eosinophilic bronchitis. J Allergy Clin Immunol. 2004;114:1106–9. doi: 10.1016/j.jaci.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Saha SK, Berry MA, Parker D, Siddiqui S, Morgan A, May R, Monk P, Bradding P, Wardlaw AJ, Pavord ID, Brightling CE. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol. 2008;121:685–91. doi: 10.1016/j.jaci.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brightling CE, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID, Bradding P. Interleukin-4 and -13 expression is co-localized to mast cells within the airway smooth muscle in asthma. Clin Exp Allergy. 2003;33:1711–6. doi: 10.1111/j.1365-2222.2003.01827.x. [DOI] [PubMed] [Google Scholar]
- 10.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, Foster PS, Rothenberg ME. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001;108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–88. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, Hogan SP, Koskinen A, McKenzie AN, Dent LA, Rothenberg ME, Matthaei KI, Young IG, Foster PS. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med. 2002;195:1433–44. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanchard C, Mishra A, Saito-Akei H, Monk P, Anderson I, Rothenberg ME. Inhibition of human interleukin-13-induced respiratory and oesophageal inflammation by anti-human-interleukin-13 antibody (CAT-354) Clin Exp Allergy. 2005;35:1096–103. doi: 10.1111/j.1365-2222.2005.02299.x. [DOI] [PubMed] [Google Scholar]
- 15.Kaur D, Hollins F, Woodman L, Yang W, Monk P, May R, Bradding P, Brightling CE. Mast cells express IL-13R alpha 1: IL-13 promotes human lung mast cell proliferation and Fc epsilon RI expression. Allergy. 2006;61:1047–53. doi: 10.1111/j.1398-9995.2006.01139.x. [DOI] [PubMed] [Google Scholar]
- 16.Bhowmick B, Singh D, Molfino NA, Cranmer H, Birrell C, Faggioni R. A double-blind placebo controlled study to assess the pharmacokinetics, safety and tolerability of multiple ascending intravenous doses of CAT-354, a recombinant human anti-IL13 antibody in subjects with moderate asthma. Abstract No. 3021]. European Respiratory Society Annual Congress 2008.
- 17.Klakamp SL, Lu H, Tabrizi M, Funelas C, Roskos LK, Coleman D. Application of analytical detection concepts to immunogenicity testing. Anal Chem. 2007;79:8176–84. doi: 10.1021/ac071364d. [DOI] [PubMed] [Google Scholar]
- 18.Mire-Sluis AR, Barrett YC, Devanarayan V, Koren E, Liu H, Maia M, Parish T, Scott G, Shankar G, Shores E, Swanson SJ, Taniguchi G, Wierda D, Zuckerman LA. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J Immunol Methods. 2004;289:1–16. doi: 10.1016/j.jim.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Blease K. Therapeutics targeting IL-13 for the treatment of pulmonary inflammation and airway remodeling. Curr Opin Investig Drugs. 2008;9:1180–4. [PubMed] [Google Scholar]
- 20.Vugmeyster Y, Szklut P, Tchistiakova L, Abraham W, Kasaian M, Xu X. Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of humanized monoclonal anti-IL-13 antibodies with different IL-13 neutralization mechanisms. Int Immunopharmacol. 2008;8:477–83. doi: 10.1016/j.intimp.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81–8. doi: 10.1016/S1359-6446(05)03638-X. [DOI] [PubMed] [Google Scholar]
- 22.McLennan DN, Porter CJ, Edwards GA, Brumm M, Martin SW, Charman SA. Pharmacokinetic model to describe the lymphatic absorption of r-metHu-leptin after subcutaneous injection to sheep. Pharm Res. 2003;20:1156–62. doi: 10.1023/a:1025036611949. [DOI] [PubMed] [Google Scholar]
- 23.McLennan DN, Porter CJ, Edwards GA, Heatherington AC, Martin SW, Charman SA. The absorption of darbepoetin alfa occurs predominantly via the lymphatics following subcutaneous administration to sheep. Pharm Res. 2006;23:2060–6. doi: 10.1007/s11095-006-9064-8. [DOI] [PubMed] [Google Scholar]
- 24.Wang B, Ludden TM, Cheung EN, Schwab GG, Roskos LK. Population pharmacokinetic-pharmacodynamic modeling of filgrastim (r-metHuG-CSF) in healthy volunteers. J Pharmacokinet Pharmacodyn. 2001;28:321–42. doi: 10.1023/a:1011534529622. [DOI] [PubMed] [Google Scholar]


