Abstract
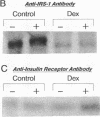
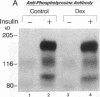
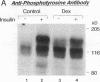
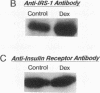
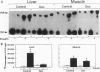
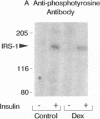
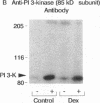
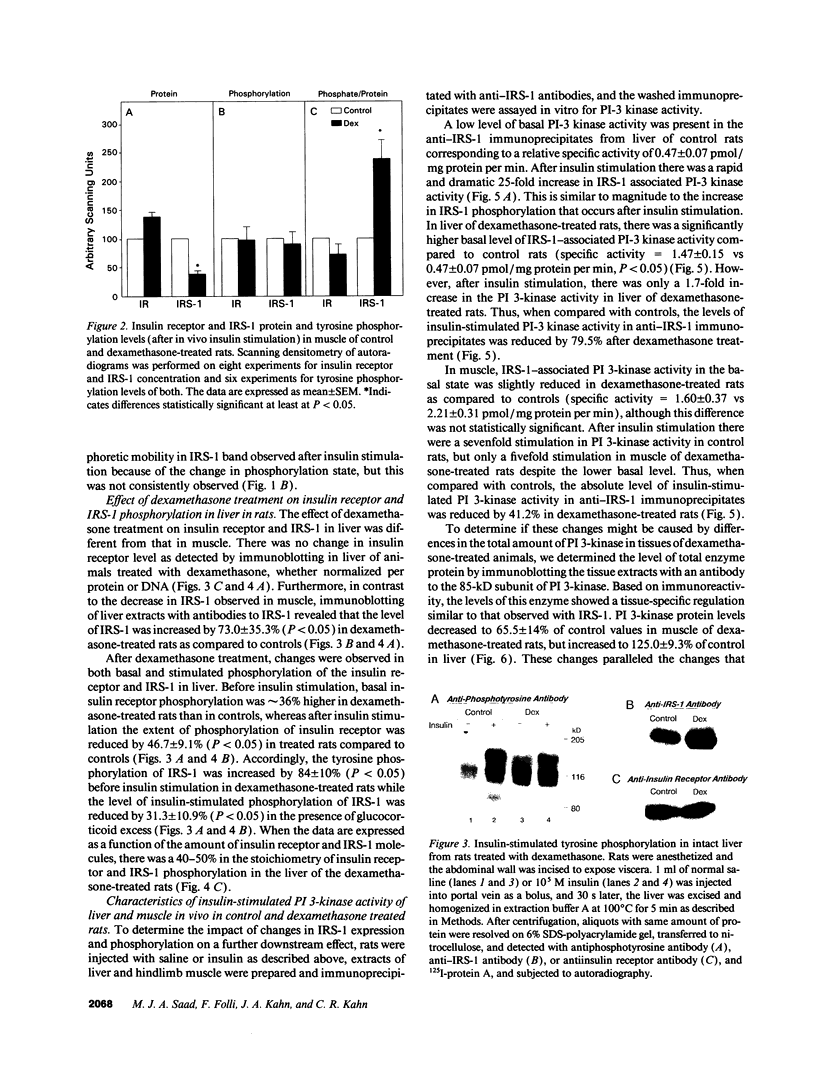
Insulin rapidly stimulates tyrosine kinase activity of its receptor resulting in phosphorylation of its cytosolic substrate, insulin receptor substrate-1 (IRS-1), which in turn associates with phosphatidylinositol 3-kinase (PI 3-kinase), thus activating the enzyme. Glucocorticoid treatment is known to produce insulin resistance, but the exact molecular mechanism is unknown. In the present study we have examined the levels and phosphorylation state of the insulin receptor and IRS-1, as well as the association/activation between IRS-1 and PI 3-kinase in the liver and muscle of rats treated with dexamethasone. After dexamethasone treatment (1 mg/kg per d for 5 d), there was no change in insulin receptor concentration in liver of rats as determined by immunoblotting with antibody to the COOH-terminus of the receptor. However, insulin stimulation of receptor autophosphorylation determined by immunoblotting with antiphosphotyrosine antibody was reduced by 46.7 +/- 9.1%. IRS-1 and PI 3-kinase protein levels increased in liver of dexamethasone-treated animals by 73 and 25%, respectively (P < 0.05). By contrast, IRS-1 phosphorylation was decreased by 31.3 +/- 10.9% (P < 0.05), and insulin stimulated PI 3-kinase activity in anti-IRS-1 immunoprecipitates was decreased by 79.5 +/- 11.2% (P < 0.02). In muscle, the changes were less dramatic, and often in opposite direction of those observed in liver. Thus, there was no significant change in insulin receptor level or phosphorylation after dexamethasone treatment. IRS-1 and PI 3-kinase levels were decreased to 38.6 and 65.6%, respectively (P < 0.01 and P < 0.05). IRS-1 phosphorylation showed no significant change in muscle, but insulin-stimulated IRS-1 associated PI 3-kinase was decreased by 41%. Thus, dexamethasone has differential effects on the proteins involved in the early steps in insulin action in liver and muscle. In both tissues, dexamethasone treatment results in a reduction in insulin-stimulated IRS-1-associated P I3-kinase, which may play a role in the pathogenesis of insulin resistance at the cellular level in these animals.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amatruda J. M., Livingston J. N., Lockwood D. H. Cellular mechanisms in selected states of insulin resistance: human obesity, glucocorticoid excess, and chronic renal failure. Diabetes Metab Rev. 1985;1(3):293–317. doi: 10.1002/dmr.5610010304. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Myers M. G., Jr, Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992 Sep;11(9):3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J. M., Schroeder G. G., Kahn C. R., Myers M. G., Jr, Wilden P. A., Cahill D. A., White M. F. Insulin stimulation of phosphatidylinositol 3-kinase activity maps to insulin receptor regions required for endogenous substrate phosphorylation. J Biol Chem. 1992 Jan 15;267(2):1367–1374. [PubMed] [Google Scholar]
- Block N. E., Buse M. G. Effects of hypercortisolemia and diabetes on skeletal muscle insulin receptor function in vitro and in vivo. Am J Physiol. 1989 Jan;256(1 Pt 1):E39–E48. doi: 10.1152/ajpendo.1989.256.1.E39. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caro J. F., Amatruda J. M. Glucocorticoid-induced insulin resistance: the importance of postbinding events in the regulation of insulin binding, action, and degradation in freshly isolated and primary cultures of rat hepatocytes. J Clin Invest. 1982 Apr;69(4):866–875. doi: 10.1172/JCI110526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pirro R., Green A., Kao M. Y., Olefsky J. M. Effects of prednisolone and dexamethasone in vivo and in vitro: studies of insulin binding, deoxyglucose uptake and glucose oxidation in rat adipocytes. Diabetologia. 1981 Aug;21(2):149–153. doi: 10.1007/BF00251283. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endemann G., Yonezawa K., Roth R. A. Phosphatidylinositol kinase or an associated protein is a substrate for the insulin receptor tyrosine kinase. J Biol Chem. 1990 Jan 5;265(1):396–400. [PubMed] [Google Scholar]
- Fantus I. G., Saviolakis G. A., Hedo J. A., Gorden P. Mechanism of glucocorticoid-induced increase in insulin receptors of cultured human lymphocytes. J Biol Chem. 1982 Jul 25;257(14):8277–8283. [PubMed] [Google Scholar]
- Ferrannini E., Bjorkman O., Reichard G. A., Jr, Pilo A., Olsson M., Wahren J., DeFronzo R. A. The disposal of an oral glucose load in healthy subjects. A quantitative study. Diabetes. 1985 Jun;34(6):580–588. doi: 10.2337/diab.34.6.580. [DOI] [PubMed] [Google Scholar]
- Folli F., Saad M. J., Backer J. M., Kahn C. R. Insulin stimulation of phosphatidylinositol 3-kinase activity and association with insulin receptor substrate 1 in liver and muscle of the intact rat. J Biol Chem. 1992 Nov 5;267(31):22171–22177. [PubMed] [Google Scholar]
- Folli F., Saad M. J., Backer J. M., Kahn C. R. Regulation of phosphatidylinositol 3-kinase activity in liver and muscle of animal models of insulin-resistant and insulin-deficient diabetes mellitus. J Clin Invest. 1993 Oct;92(4):1787–1794. doi: 10.1172/JCI116768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn J. M., Tilghman S. M., Hanson R. W., Reshef L., Ballard F. J. Effects of cyclic adenosine monophosphate, dexamethasone and insulin on phosphoenolpyruvate carboxykinase synthesis in Reuber H-35 hepatoma cells. Biochemistry. 1975 Jun 3;14(11):2350–2357. doi: 10.1021/bi00682a012. [DOI] [PubMed] [Google Scholar]
- Haber R. S., Weinstein S. P. Role of glucose transporters in glucocorticoid-induced insulin resistance. GLUT4 isoform in rat skeletal muscle is not decreased by dexamethasone. Diabetes. 1992 Jun;41(6):728–735. doi: 10.2337/diab.41.6.728. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Goldfine I. D., Neville D. M., Jr, De Meyts P. Alterations in insulin binding induced by changes in vivo in the levels of glucocorticoids and growth hormone. Endocrinology. 1978 Oct;103(4):1054–1066. doi: 10.1210/endo-103-4-1054. [DOI] [PubMed] [Google Scholar]
- Kapeller R., Chen K. S., Yoakim M., Schaffhausen B. S., Backer J., White M. F., Cantley L. C., Ruderman N. B. Mutations in the juxtamembrane region of the insulin receptor impair activation of phosphatidylinositol 3-kinase by insulin. Mol Endocrinol. 1991 Jun;5(6):769–777. doi: 10.1210/mend-5-6-769. [DOI] [PubMed] [Google Scholar]
- Karasik A., Kahn C. R. Dexamethasone-induced changes in phosphorylation of the insulin and epidermal growth factor receptors and their substrates in intact rat hepatocytes. Endocrinology. 1988 Nov;123(5):2214–2222. doi: 10.1210/endo-123-5-2214. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Kelly K. L., Ruderman N. B., Chen K. S. Phosphatidylinositol-3-kinase in isolated rat adipocytes. Activation by insulin and subcellular distribution. J Biol Chem. 1992 Feb 15;267(5):3423–3428. [PubMed] [Google Scholar]
- Knutson V. P. The acute and chronic effects of glucocorticoids on insulin receptor and insulin responsiveness. Transient fluctuations in intracellular receptor level parallel transient fluctuations in responsiveness. J Biol Chem. 1986 Aug 5;261(22):10306–10312. [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maegawa H., Olefsky J. M., Thies S., Boyd D., Ullrich A., McClain D. A. Insulin receptors with defective tyrosine kinase inhibit normal receptor function at the level of substrate phosphorylation. J Biol Chem. 1988 Sep 5;263(25):12629–12637. [PubMed] [Google Scholar]
- O'Brien R. M., Granner D. K. PEPCK gene as model of inhibitory effects of insulin on gene transcription. Diabetes Care. 1990 Mar;13(3):327–339. doi: 10.2337/diacare.13.3.327. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1975 Dec;56(6):1499–1508. doi: 10.1172/JCI108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Johnson J., Liu F., Jen P., Reaven G. M. The effects of acute and chronic dexamethasone administration on insulin binding to isolated rat hepatocytes and adipocytes. Metabolism. 1975 Apr;24(4):517–527. doi: 10.1016/0026-0495(75)90076-1. [DOI] [PubMed] [Google Scholar]
- Pang D. T., Sharma B. R., Shafer J. A. Purification of the catalytically active phosphorylated form of insulin receptor kinase by affinity chromatography with O-phosphotyrosyl-binding antibodies. Arch Biochem Biophys. 1985 Oct;242(1):176–186. doi: 10.1016/0003-9861(85)90491-6. [DOI] [PubMed] [Google Scholar]
- Rannels S. R., Jefferson L. S. Effects of glucocorticoids on muscle protein turnover in perfused rat hemicorpus. Am J Physiol. 1980 Jun;238(6):E564–E572. doi: 10.1152/ajpendo.1980.238.6.E564. [DOI] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Rothenberg P. L., Lane W. S., Karasik A., Backer J., White M., Kahn C. R. Purification and partial sequence analysis of pp185, the major cellular substrate of the insulin receptor tyrosine kinase. J Biol Chem. 1991 May 5;266(13):8302–8311. [PubMed] [Google Scholar]
- Ruderman N. B., Kapeller R., White M. F., Cantley L. C. Activation of phosphatidylinositol 3-kinase by insulin. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1411–1415. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M. J., Araki E., Miralpeix M., Rothenberg P. L., White M. F., Kahn C. R. Regulation of insulin receptor substrate-1 in liver and muscle of animal models of insulin resistance. J Clin Invest. 1992 Nov;90(5):1839–1849. doi: 10.1172/JCI116060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhanick A. I., Krupp M. N., Amatruda J. M. Dexamethasone stimulates insulin receptor synthesis in cultured rat hepatocytes. J Biol Chem. 1983 Dec 10;258(23):14130–14135. [PubMed] [Google Scholar]
- Sun X. J., Miralpeix M., Myers M. G., Jr, Glasheen E. M., Backer J. M., Kahn C. R., White M. F. Expression and function of IRS-1 in insulin signal transmission. J Biol Chem. 1992 Nov 5;267(31):22662–22672. [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Tobe K., Koshio O., Tashiro-Hashimoto Y., Takaku F., Akanuma Y., Kasuga M. Immunological detection of phosphotyrosine-containing proteins in rat livers after insulin injection. Diabetes. 1990 May;39(5):528–533. doi: 10.2337/diab.39.5.528. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truglia J. A., Hayes G. R., Lockwood D. H. Intact adipocyte insulin-receptor phosphorylation and in vitro tyrosine kinase activity in animal models of insulin resistance. Diabetes. 1988 Feb;37(2):147–153. doi: 10.2337/diab.37.2.147. [DOI] [PubMed] [Google Scholar]
- White M. F., Maron R., Kahn C. R. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985 Nov 14;318(6042):183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- Wilden P. A., Backer J. M., Kahn C. R., Cahill D. A., Schroeder G. J., White M. F. The insulin receptor with phenylalanine replacing tyrosine-1146 provides evidence for separate signals regulating cellular metabolism and growth. Proc Natl Acad Sci U S A. 1990 May;87(9):3358–3362. doi: 10.1073/pnas.87.9.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]