Introduction
Fixed drug eruption (FDE) is characterized by single or multiple skin lesions which occur at the same site each time the drug is administered [1]. Lesions are usually round or oval and well defined, however their number and severity may increase after each exposure. Swelling and redness of skin are typically seen within 30 min to 8 h after exposure. Lesions are more commonly seen on extremities, genitals and perianal areas but they may appear on any location. Persistent hyperpigmentation at the site of the lesion is normally seen after healing. Accompanying systemic symptoms are mild with FDE. Cross-sensitivity may occur with structurally similar drugs. The offending drug is thought to function as a hapten that preferentially binds to basal keratinocytes, thereby releasing lymphokines and antibodies thus damaging the basal cell layer [2].
Nitroimidazoles are low molecular weight antimicrobial compounds with excellent activity against anaerobic micro-organisms and protozoa. They are the first line drugs for hepatic and intestinal amoebiasis. All the nitroimidazoles: metronidazole, tinidazole, ornidazole, secnidazole and satranidazole, have a similar nitroimidazole ring but different side chains. Although all these molecules have been used for a long time without many side-effects, only metronidazole and tinidazole have been reported to cause FDEs with cross sensitivity to each other [3, 4] and are included in the list of drugs causing FDE. We report a case of FDE caused by ornidazole which showed cross-sensitivity to secnidazole but not to metronidazole, tinidazole or satranidazole.
Case report
This is the case of a 25 year old male patient. He presented in Dermatology OPD with an itchy erythymatous macule on the antero-lateral aspect of his right thigh. On history, he revealed self medication with ornidazole 500 mg twice daily for amoebiasis since 3 days. On examination, a round solitary, ill defined, dusky red macule, 5–6 cm in size was seen. The lesion faded at the periphery where it merged with normal skin. Temporal relation with ornidazole, without any other relevant history led to the clinical diagnosis of FDE due to ornidazole.
The drug was withdrawn and a punch biopsy was taken from the lesion. The histology showed interface dematitis with vacuolar changes, basal cell degeneration, spongiosis and dermal oedema. Pigmentary incontinence within the papillary dermis and perivascular and scattered lymphoplasmacytoid infiltrate containing many neutrophils and few eosinophils was also present. These findings confirmed the clinical diagnosis of FDE.
The lesion healed completely with the use of flucinolone acetonide 0.025% cream applied twice daily for 1 week. A slight post-inflammatory hyperpigmentation remained. After obtaining approval from Institutional Review Board, Government Medical College, Bhavnagar, Gujarat (India), the written informed consent of the patient was taken to test for cross-sensitivity among various nitroimidazole derivatives.
The patient was subjected to oral provocation tests with all the available nitroimidazole derivatives i.e. metronidazole, tinidazole, secnidazole, satranidazole and ornidazole, to find a safer alternative for future use. The provocation test was started with one-fourth of a single therapeutic dose for that drug. If no reactivation of the lesion (itching/burning, erythema, erosion) was seen in 24 h, the dose was doubled until the therapeutic dose was reached. In case of reactivation, the provocation test with another drug was done after 2 weeks of complete resolution of the eruption with topical steroids. The patient showed reactivation of the lesion with secnidazole 250 mg orally within 6 h but not with full oral therapeutic doses of metronidazole, tinidazole and satranidazole.
Knowing the temporal relation of the lesion with ornidazole, re-challenge with this drug was done last. Provocation with 125 mg ornidazole not only reactivated the lesion at the previous site but also led to the development of two new erythymatous lesions on the left calf and lower lip. This confirmed that the patient had FDE to ornidazole (causality analysis shows ‘definite’ relationship with score of 10 on Naranjo algorithm [5]) with cross-sensitivity to secnidazole but not to metronidazole, tinidazole and satranidazole.
Discussion
The case we present here is just the second case report of FDE with ornidazole [6], but our case is unique in its cross-sensitivity pattern. Our patient showed sensitivity to ornidazole and secnidazole but not to metronidazole, tinidazole and satranidazole; even though all the five drugs belong to the same pharmacological group and have a similar structure with nitro-imidazole rings. The difference in sensitivity can be attributed to the different side chains in their structure (Figure 1): metronidazole has ethanol, tinidazole has ethylsulphonyl ethyl, ornidazole has chloro propanol, secnidazole has a propanol group and satranidazole has methlysulphonyl imidazoline. Ornidazole and secnidazole have very closely resembling propanol side chains attached to the imidazole ring. This side chain may be functioning as an antigen for provoking the drug reaction.
Figure 1.
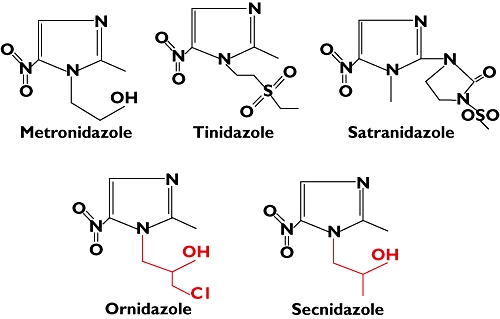
Structure of nitro-imidazole compounds having different side chains
It is generally believed that if an individual develops FDE to a particular drug, he should preferably avoid structurally similar drugs from the same pharmacologic group, as they may also result in FDE. The present patient had sensitivity to ornidazole and secnidazole but not to other drugs of the same group. Similar cross-sensitivity patterns with tetracyclines, azole antifungals and other nitroimidazoles suggest that cross-sensitivity of drugs within a pharmacologic group is not necessarily an all or none phenomenon [3, 4, 7]. Testing for sensitivity with structurally related drugs may help us in selecting alternative drugs from the same group. Although a graded oral provocation test is the most prudent way to establish sensitivity of drugs in the case of non-life threatening FDE [8], it should be done with the utmost care as it might lead to eruption of new lesions, as happened with the last rechallenge in our case. These new lesions may appear on unwanted sites and may result in cosmetic disfigurement.
To conclude, ornidazole and secnidazole should be added in the list of drugs causing FDEs and provocation tests to find safer, non-reacting drugs of the same group should be done with the utmost care and only when necessary.
Competing interests
There are no competing interests to declare.
REFERENCES
- 1.Korkij W, Soltani K. Fixed drug eruptions – a brief review. Arch Dermatol. 1984;120:520–4. [PubMed] [Google Scholar]
- 2.Weedon D. Skin Pathology. 2nd. London: Churchill Livingstone; 2002. The lichenoid reaction pattern (‘interface dermatitis’) pp. 42–3. [Google Scholar]
- 3.Kanwar AJ, Sharma R, Rajgopalan M, Kaur S. Fixed drug eruption due to tinidazole with cross sensitivity to metronidazole. Dermatologica. 1990;180:277. doi: 10.1159/000248048. [DOI] [PubMed] [Google Scholar]
- 4.Thami GP, Kanwar AJ. Fixed drug eruption due to metronidazole and tinidazole without cross-sensitivity to secnidazole. Dermatology. 1998;196:368. [PubMed] [Google Scholar]
- 5.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruis I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Jain VK, Aggarwal K, Gupta S, Mahendra A. Fixed drug eruption caused by ornidazole. Contact Dermatitis. 2005;53:300–1. doi: 10.1111/j.0105-1873.2005.0654b.x. [DOI] [PubMed] [Google Scholar]
- 7.Gupta R, Thami GP. Fixed drug reaction by itraconazole reactivity and cross reactivity. J Am Acad Dermatol. 2008;58:521–2. doi: 10.1016/j.jaad.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Pierre-Dominique G, Emile G. Fixed drug eruption due to fluconazole: a third case. J Am Acad Dermatol. 2002;46:467. doi: 10.1067/mjd.2002.118356. [DOI] [PubMed] [Google Scholar]


