Abstract
The objective of the study was to describe the presentation of vaginal mesh erosions following Mersilene® suburethral slings for urinary incontinence. We performed a retrospective review of all Mersilene® suburethral slings placed at a tertiary referral center from 1996 to 2007. A total of 772 women underwent placement of a Mersilene® suburethral sling. We identified 62 women that underwent surgical revision due to mesh erosion for an overall erosion rate of 8%. The most common presenting symptom was vaginal discharge reported in 37% of women. Other symptoms included vaginal bleeding in 31%, pain or dyspareunia in 13%, and voiding dysfunction in 21% of women. Seven women were found to have the mesh in the bladder on cystoscopy. Cellulitis complicated 8.3% of erosions. Patients with erosions of Mersilene® mesh slings commonly complain of vaginal bleeding and discharge and may present up to 20 years after the surgery.
Keywords: Mersilene® sling, Erosions, Stress incontinence
Introduction
Suburethral sling procedures have been performed in women suffering from severe stress incontinence since Von Giordano first described the procedure in 1904 using gracilis muscle [1]. Goebell, Frangenheim, and Stoeckel modified the procedure in the early 1910s by using autologous grafts in combination with plication of the bladder neck [2]. Aldridge described using a strip of rectus fascia beneath the urethra in 1942. Later, Ridley modified the technique using fascia lata [3]. Each of these techniques required tissue harvesting, which added morbidity and time to the surgical procedure. In the early 1960s, graft materials were introduced, allowing pelvic surgeons to circumvent the morbidity associated with the harvesting process. Since multiple attempts have been made to find an ideal graft material, a synthetic graft should be permanent and consistent and avoid complications of erosion, infection, and material rejection.
The polyethylene teraphthalate ribbon (Mersilene® Ethicon, Sommerville, NJ, USA) for the suburethral sling procedure was first described in 1962 by Williams and TeLinde [4]. Unfortunately, the nonfenestrated ribbon was plagued by a high tissue rejection rate and several reports of urethral transection [5]. J Chassar Moir described the “gauze-hammock sling” in 1968 and used a woven mesh of Mersilene® instead of the ribbon [6]. The woven design of the Mersilene® mesh allows for it to be trimmed without unraveling or losing its bidirectional elastic properties; it also allows for better tissue in-growth and less host rejection compared to the ribbon. Postoperative complications of erosion and infection were less frequent with the woven mesh. The use of Mersilene® mesh and the gauze-hammock technique was further popularized by David Nichols in 1973 as the definitive treatment of severe recurrent stress urinary incontinence [7, 8].
The introduction of the minimally invasive midurethral slings using a monofilament polypropylene material for stress urinary incontinence in the mid-1990s has led many pelvic surgeons to adopt this procedure in lieu of the Mersilene® suburethral sling procedure. Of more recent is the use of synthetic materials for prolapse repairs. Regardless, pelvic surgeons and gynecologists may still encounter postoperative complications of any vaginal mesh placement, namely, and erosions, as has been seen in association with the Mersilene® suburethral sling. The primary aim of this study is to describe the presenting symptoms of Mersilene® mesh erosions seen after placement as a suburethral sling at a tertiary care center where the procedure has been routinely performed since 1972. Time to diagnosis, frequency of sling erosions, and need for reoperation are also described.
Materials and methods
We conducted a retrospective case series after approval from the Women and Infants’ Hospital Institutional Review Board. We identified women who underwent a Mersilene mesh suburethral sling and those who had surgical revision of a suburethral sling due to erosion, rejection, or infection as identified by ICD-9 procedure codes and CPT codes of “sling revision”, “sling erosion”, or “complications of a genitourinary device”. Charts of all women who underwent removal or revision of a Mersilene® suburethral sling at Women and Infants’ Hospital of Rhode Island between January 1996 and May 2007 were reviewed. We recorded demographic data, any preoperative conservative treatments, indications for surgery, presenting symptoms, and the time interval between sling placement and sling removal. Descriptive statistics and statistical analyses were performed with Stata SE Version 9.2 (StataCorp. College Station, TX, USA).
Mersilene® mesh suburethral slings are performed at our institution in the technique described by Nichols [7, 8]. The retropubic space is dissected from two separate 3-cm suprapubic incisions lateral to the midline after the rectus fascia has been incised. Vaginal dissection of the anterior vaginal wall is carried out to the superior pubic rami bilaterally, and the pubocervical fascia is plicated with interrupted absorbable suture at the operating surgeon’s discretion. A Raz-Pereya needle is then passed through abdominal incisions, traversing the retropubic space and exiting the periurethral space into the predissected space underneath the superior pubic rami onto the operator’s vaginal finger. The Mersilene® mesh is then affixed to the needle on either side, pulled through, and anchored to rectus fascia through the abdominal incisions. Consistent with the traditional description of suburethral slings, the belly of the mesh rests at the bladder neck and not midurethral. The vaginal wall is closed with vicryl suture.
Results
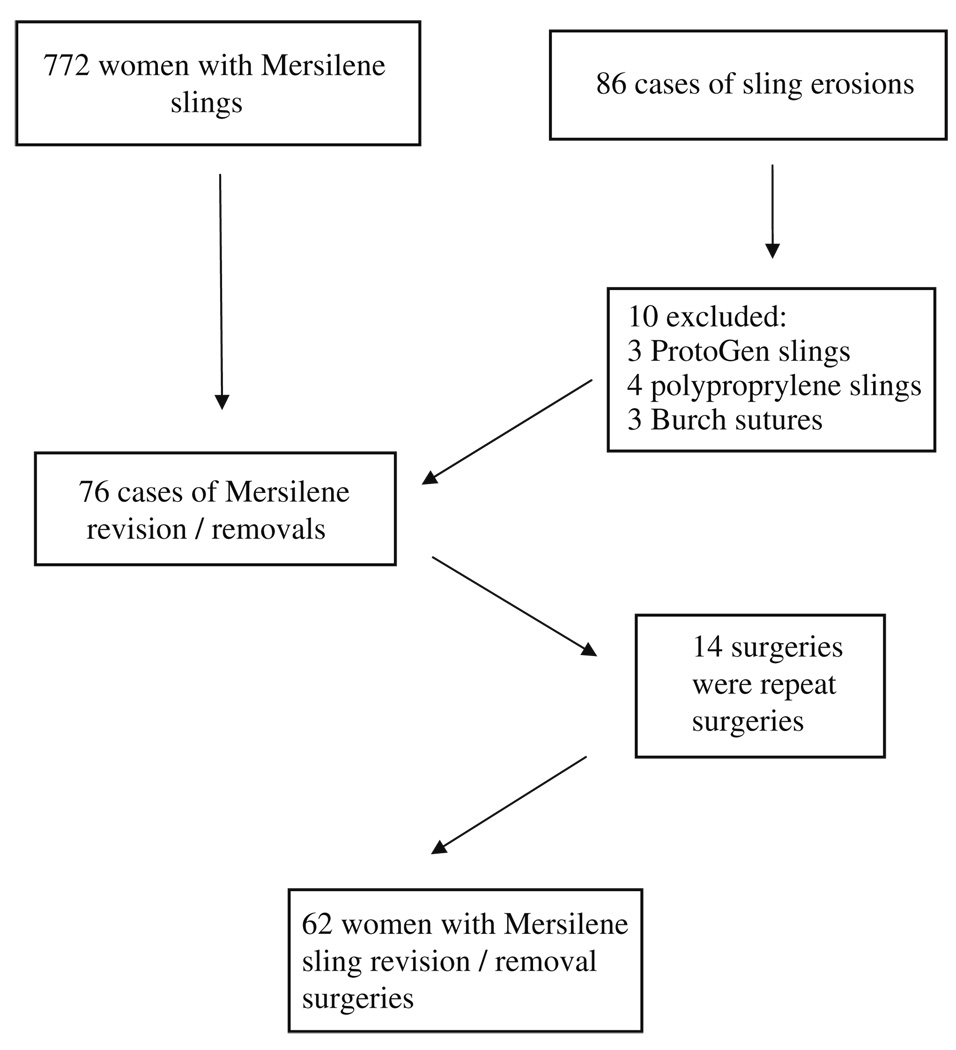
We identified 772 suburethral sling procedures utilizing Mersilene® mesh between January 1996 and May 2007. We identified 86 cases of sling revisions performed at the institution during that time period. Ten cases were excluded because they were revisions/removals of erosions from other synthetic materials: the ProtoGen sling in three cases, polyproprelene mesh in four cases, and permanent sutures following Burch urethropexy in three cases. Seventy-six cases were identified during the study period for complications from Mersilene® mesh. Fourteen women had undergone more than one surgical revision of the same mesh erosion (Fig. 1). Thus, a total of 62 separately identifiable women underwent surgical revision or excision of a Mersilene® sling, giving an estimated overall erosion rate of 8% (62 of 776).
Fig. 1.
Flowchart of identified cases of Mersilene® erosions
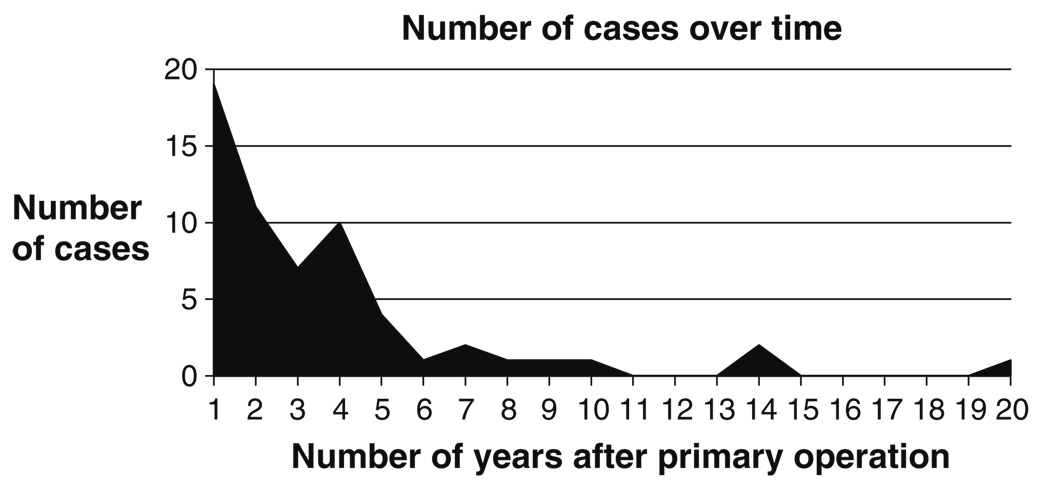
Of the 62 women undergoing sling revision between 1996 and 2007, eight women had their initial sling surgery placement prior to 1996. Sixty percent (37 of 62) of women with identified erosions underwent sling revision/removal within 3 years of their initial surgery (Fig. 2). Overall, the median time to reoperation was 2.0 years (range 9 days–20 years).
Fig. 2.
Number of mesh erosions per year after primary operation
The mean age of the 62 women was 58 years (range 37–88). The medical comorbidities of group were adult-onset diabetes (not requiring insulin), which was present in 3% of women (two of 62); chronic lung disease was noted in 23% (14 of 62); and hypertension was present in 35% (22 of 62). Eighteen percent (11 of 62) of women were smokers, and an additional 11% (seven of 62) had a history of tobacco use. The initial presenting signs and symptoms of sling erosion are listed in Table 1. The most common complaints were vaginal discharge (37%) and vaginal bleeding (31%). Pelvic pain/dyspareunia was reported by 13% (eight of 62) of women, and all these women presented within 3 years of the initial sling procedure. Twenty-one percent (13 of 62) of women complained of irritative voiding symptoms (e.g., urinary frequency, dysuria) and/or recurrent urinary tract infections as their presenting symptom. Cystoscopy identified six women (10%) with segments of Mersilene® mesh within the bladder. All of these women complained of irritative voiding symptoms prior to cystoscopy. Eleven percent (seven of 62) of erosions were complicated by concurrent cellulitis and 19% (12 of 62) had a concurrent abscess. Only a minority of the sling erosions 8% (five of 62) had no recorded complaints and had their erosion diagnosed on physical exam or at the time of another surgery.
Table 1.
Symptoms noted by the 62 women with Mersilene® mesh sling erosion
| Presenting symptoms | Samples | Percentage |
|---|---|---|
| Vaginal discharge | 23 | 37 |
| Vaginal bleeding | 19 | 31 |
| Irritative voiding symptoms | 13 | 21 |
| Pain/dyspareunia | 8 | 13 |
| Sling found in bladder | 6 | 10 |
| No presenting symptoms | 5 | 8 |
| Vaginal granulation tissue | 5 | 8 |
| Fevers | 3 | 5 |
Prior to undergoing complete surgical excision, some women had attempts at treatment of their Mersilene® mesh erosion with conservative strategies. Seven women underwent trimming of the exposed mesh in the office prior to their surgical procedures. Six women were treated with vaginal estrogen cream in an effort to promote healing.
Discussion
Mesh erosions are thought to be caused by some degree of bacterial colonization/infection within the mesh [9]. The higher erosion rates of erosions found in this study are consistent with the properties of the Mersilene® mesh. Mersilene® is a permanent macroporous mesh with multifilament microporous components. The pore size of Mersilene® mesh is smaller than the typical diameter of a macrophage (10 µm) but larger than the diameter of bacterial cells (typically less than 1 µm). For this reason, synthetic materials with woven microporous multifilament structures have been criticized as potential bacterial havens, allowing bacteria to colonize where macrophages cannot reach. Indeed, previous studies comparing different types of mesh used in incontinence surgery have described higher rates of erosion with microporous materials (3.1% to 19.5%) than macroporous monofilament meshes such as polypropylene (0.9%) [10, 11]. Furthermore, we found unexpectedly high rates of concurrent cellulitis and abscess formation with Mersilene® mesh in our study.
Only a few studies have documented the erosion rate attributable to Mersilene® mesh alone. In abdominal sacrocolpopexies, Mersilene® erosions have been reported in the range of 2.5% to 4% [12, 13]. Using it in an antiincontinence procedure, Young et al. reported a 1.8% erosion rate 1 year following the procedure [14]. Our study yielded similar results at 1 year with 2.5% of women presenting with a mesh erosion. Although we found that the median time to reoperation was 2 years, our study did include 12 women who had undergone Mersilene® sling placement 4–10 years prior to reoperation and one woman who had undergone sling surgery 20 years prior. Our longer study time span demonstrates that erosions from permanent materials can be quite delayed and that diligence is needed in the follow-up care for women after any surgery placing permanent material, as complications from the mesh can present so much later than the index surgery.
Previous reports have suggested that conservative management of sling erosions is successful in the early postoperative period [15]. Our study did not investigate the long-term success of conservative management of sling erosions, but rather investigated those which were managed in the operating room. Our study did include 14 women who had undergone repeat procedures, which suggests that surgical revision, i.e., not removal, is likely to be of benefit only when the sling is not colonized with vaginal or anaerobic flora. We theorize that conservative management of sling erosion found more than 6 months postoperatively may ultimately lead to chronic infection, erosion, or sinus tract formation from the remaining colonized mesh. This emphasizes the importance of seeing women early and frequently in the postoperative period following any mesh placement to detect erosion.
Our study is limited by the retrospective design. We assumed that all erosions of Mersilene® mesh presented back to our institution where the revision/removal surgery was performed. The state of Rhode Island is home to 11 hospitals providing gynecology services; Women and Infants’ Hospital performs 61.2% of all inpatient gynecological procedures [16]. Therefore, it is possible that some patients with Mersilene® sling complications/erosions sought care at a different hospital. This would underestimate our reported erosion rate of 8%.
Surgical data from the index case of the initial mesh placement and from those women with Mersilene® mesh whom did not present with erosions were not collected. Therefore, risk factors for mesh erosion cannot be inferred from our study. For example, the prevalence of tobacco use in the adult population of our state at the time of the study (19%) was similar to the rate reported by women with mesh erosions (18%), but tobacco use among the women without mesh erosion is not known [17]. In addition, women at a tertiary care center may be more likely to undergo multiple surgical procedures at the time of initial mesh placement, which may increase a woman’s overall risk of mesh erosion.
This study however is strengthened by its review of a large number of Mersilene® mesh sling procedures over a 10-year period at an institution that performs a high number of gynecological surgeries and that many residents of the state of Rhode Island remain in the state and do have their gynecologic surgery at our institution, thus providing longer term follow-up.
The most common presenting symptoms of sling erosion were vaginal discharge and vaginal bleeding. Symptoms of vaginal discharge and bleeding should be presumed to be caused by mesh erosion in women with a prior history of any mesh placement until proven otherwise. Mesh erosion or exposure should be considered as a possible cause of chronic persistent vaginal discharge. Pelvic examinations must be very meticulous to detect erosions within the vaginal ruggae and sulci. Cystoscopy should be considered for women with irritative voiding symptoms and a history of mesh placement as these symptoms were present in women noted to have mesh within the bladder on cystoscopy.
The recent shift in the development of synthetic materials has favored a macroporous monofilament structure that allows tissue in-growth while also allowing macrophages to traverse its framework in search of residing bacteria. Thus, the use of Mersilene® mesh has fallen out of favor. However, providers need to maintain a high index of suspicion for mesh erosion in this group of women even many years beyond from their initial surgery, as we have shown that complications from Mersilene® mesh may present as far as 20 years after initial placement.
Footnotes
Presented at the 34th Annual SGS Scientific Meeting April 14th to 16th, 2008 in Savannah, GA, USA.
Conflicts of interest None.
References
- 1.Hohenfellner R, Petrie E. Sling procedures. In: Stanton SL, Tanagho E, editors. Surgery of Female Incontinence. 2nd edn. Berlin: Springer; 1986. pp. 105–113. [Google Scholar]
- 2.Tizzano A. Historical milestones in female pelvic surgery, gynecology, and female urology. In: Walters MD, Karram MM, editors. Urogynecology and Reconstructive Pelvic Surgery. ed 3. Mosby; 2007. pp. 4–12. [Google Scholar]
- 3.Ridley JH. Surgical treatment of stress urinary incontinence in women. J Med Assoc Georgia. 1955;44:135. [PubMed] [Google Scholar]
- 4.Williams TJ, TeLinde RW. The sling operation for urinary incontinence using mersilene ribbon. Obstet Gynecol. 1962;19(2):241–245. [PubMed] [Google Scholar]
- 5.Young SB, Rosenblatt PL, Pingeton DM, Howard AE, Baker SP. The Mersilene mesh suburethral sling: a clinical and urodynamic evaluation. Am J Obstet Gynecol. 1995;173(6):1719–1726. doi: 10.1016/0002-9378(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 6.Moir JC. The Gauze-Hammock operation. Br J Obstet Gynaecol. 1968;75(1):1–9. doi: 10.1111/j.1471-0528.1968.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 7.Nichols DH, Randall CL. Choice of operation for urinary stress incontinence. In: Nichols DH, Randall CL, editors. Vaginal surgery. 4th edn. Baltimore: Williams & Wilkins; 1996. pp. 384–421. [Google Scholar]
- 8.Nichols DH. The Mersilene mesh gauze-hammock for severe urinary stress incontinence. Obstet Gynecol. 1973;41(1):88–93. [PubMed] [Google Scholar]
- 9.Persson J, Iosif C, Wolner-Hanssen P. Risk factors for rejection of synthetic suburethral slings for stress urinary incontinence: a case–control study. Obstet Gynecol. 2002;99(4):629–634. doi: 10.1016/s0029-7844(02)01659-9. [DOI] [PubMed] [Google Scholar]
- 10.Karram MM, Segal JL, Vassallo BJ, Kleeman SD. Complications and untoward effects of the tension-free vaginal tape procedure. Obstet Gynecol. 2003;101(5):929–932. doi: 10.1016/s0029-7844(03)00122-4. [DOI] [PubMed] [Google Scholar]
- 11.Iglesia CB, Fenner DE, Brubaker L. The use of mesh in gynecologic surgery. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8(2):105–155. doi: 10.1007/BF02764826. [DOI] [PubMed] [Google Scholar]
- 12.Visco AG, Weidner AC, Barber MD, Myers ER, Cundiff GW, Bump RC, Addison WA. Vaginal mesh erosion after abdominal sacral colpopexy. Obstet Gynecol Surv. 2001;56(7):410–411. doi: 10.1067/mob.2001.109654. [DOI] [PubMed] [Google Scholar]
- 13.Timmons MC, Addison WA. Mesh erosion after abdominal sacrocolpopexy. J Pelvic Surg. 1997;3:75–80. [Google Scholar]
- 14.Young SB, Howard AD, Baker SP. Mersilene mesh sling: short- and long-term clinical and urodynamic outcomes. Am J Obstet Gynecol. 2001;185(1):32–40. doi: 10.1067/mob.2001.116370. [DOI] [PubMed] [Google Scholar]
- 15.Myers DL, LaSala CA. Conservative surgical management of Mersilene mesh suburethral sling erosion. Am J Obstet Gynecol. 1998;179(6):1424–1429. doi: 10.1016/s0002-9378(98)70005-5. [DOI] [PubMed] [Google Scholar]
- 16.Care New England Health System Annual Report. Fiscal Year. 2006
Further Reading
- 17.Website: Rhode Island Department of Health. www.health.ri.gov/chic/statistics/BRFSS2004Summary_smk.pdf.