INTRODUCTION
Lumbar discectomy is the most common surgical procedure performed in the US for patients with back pain and leg symptoms due to intervertebral disc herniation (IDH) 1, Disc herniation most frequently occurs among persons between 33 and 55 years of age; however, rates of spine surgery in the Medicare population--those 65 and older--rose dramatically in the United States over the period 1992 through 2003, with total Medicare spending on lumbar discectomy/laminectomy estimated at $306 million.2. While recent clinical evidence shows a health benefit for those undergoing surgery,3,4 the cost-effectiveness of operative intervention compared with non-operative care remains poorly characterized.
One study reported moderate cost-effectiveness for surgical treatment of IDH,5 but had several important limitations. First, cost and health outcome data came from different populations. Second, transitions between health states were not observed with prospectively collected data appropriate for estimating impact on quality-adjusted life years (QALYs), but were modeled using decision analysis. Third, the effect of surgery on worker productivity (i.e., indirect costs) was not addressed. The results from the Maine Lumbar Spine Study suggest that indirect costs are important to evaluate because, although spine surgery was associated with pain reduction, it was not associated with increased labor force participation.6 A recent Swedish study suggested that surgery for IDH may be cost-saving when lost productivity due to permanent disability is considered.7
To comprehensively address the economic value of surgery for treatment of IDH, the multicenter Spine Patient Outcomes Research Trial (SPORT), included patients with confirmed diagnosis of IDH and tracked the impact of treatment on QALYs using a validated instrument (EQ-5D), resource utilization, and indirect costs .8 Reports of the SPORT primary outcomes among 1,244 patients with IDH suggest that both surgically and non-operatively treated patients improve over time 3,4 In this paper, we report on the cost-effectiveness of surgery for IDH using SPORT two-year cost and outcomes data.
MATERIALS AND METHODS
This cost-effectiveness analysis is based on analysis of the pooled SPORT randomized and observational cohorts. Further details of SPORT’s design, conduct and analytic methods are provided elsewhere.3,4,8 In brief, the patient population included men and women age 18 and older diagnosed with IDH by their treating physicians and confirmed as surgical candidates by a participating surgeon. All participants were required to have symptoms for at least six weeks. The surgical intervention was a standard open laminotomy/laminectomy with removal of the herniation and examination of the involved nerve root. As is usual practice, surgeons only performed other procedures when it was deemed necessary for the treatment of an individual patient. Non-operative treatments were usual care chosen individually by patients and physicians. A standardized protocol was approved by the human subjects committees at each participating institution and SPORT was monitored by an independent Data Safety and Monitoring Board.
To evaluate treatment effectiveness using quality-adjusted life years (QALYs), health state values were obtained using EQ-5D with U.S. scoring 9,10 at baseline, 6 weeks, 3, 6, 12 and 24 months. Treatment costs were estimated from patient-reported lumbar-spine-related medical resource utilization. Indirect costs were based on patient-reported time away from work and/or other usual activities as collected at 6 weeks, 3, 6, 12 and 24 months. The recall period for the self-reports of spine-related resource utilization and time away from work/usual activities was 6 weeks for the 6 week and 3 month visits. For all other visits, a one-month recall period was used. Participants were provided a health care diary to assist in tracking resource utilization and missed work/ housekeeping days.
Treatment Effectiveness
QALYs, which account for both quality and length of life, are recommended for assessing the value of interventions in health and medicine.11 Time-weighted sums of EQ-5D values, adjusted to the overall mean baseline health state value, provided an estimate of QALYs for each treatment group.
Resource Utilization
Patient-reported resource utilization data were collected at each follow-up time regarding spine-related outpatient visits (surgeons, chiropractors, other physicians, physical therapists, acupuncturists, or other health care providers); diagnostic tests (X-ray, CAT scan, MRI, and EMG); injections; devices (braces, canes, walkers, shoe inserts, etc.); emergency-room visits; and rehabilitation or nursing home days. In a nurse-administered survey, participants were asked in detail about their use of specific medications, including non-steroidal anti-inflammatories (NSAIDS) and Cox-2 inhibitors, oral steroids, narcotics, muscle relaxants, and antidepressants.
To estimate direct medical cost at each time point, self-reported instances of medical resource use were multipled by unit costs for each cost component as shown in Table 1. Unit costs for office visits, hospitalizations, diagnostic tests and procedures were based on 2004 Medicare national allowable payment amounts. Medication prices were based on 2004 Redbook prices. All costs were adjusted for inflation and are expressed in 2004 U. S. dollars with a 3% annualized discount rate used in the analysis of both costs and QALYs.
Table 1.
Mean surgery cost and associated unit costs by cost component.
| Mean and Unit Costs |
|
|---|---|
| Cost Components | |
| Direct Cost | |
| Health Care Visits | |
| Surgeon | $37.88 |
| Physician | $41.07 |
| Chiropractor | $21.11 |
| Physical Therapy | $45.68 |
| Accupuncture | $65.05 |
| Other | $38.89 |
| Diagnostic Tests | |
| MRI without contrast | $566.54 |
| MRI with contrast (after surgery) | $622.93 |
| X-ray | $66.10 |
| CAT scan | $292.78 |
| EMG | $103.64 |
| Other Health Care Services | |
| Injections | $122.84 |
| ER Visits | $87.10 |
| Rehab or Nursing Home | $255.27 |
| Paid caregiver | $29.87/hr |
| Medications ** | |
| NSAIDS/ Cox-2 Inhibitors | $4.26 |
| Oral Steroids | $10.18 |
| Narcotics | $5.24 |
| Muscle Relaxants | $5.08 |
| Antidepressants | $2.59 |
| Other | $5.10 |
| Over-the-counter | $0.73 |
| Alternative medications | $0.57 |
| Surgery | |
| Primary Surgery Mean Cost* | $5,660/$13,235 |
| Discectomy (DRG 500) | $5,470/$12,750 |
| Discectomy with complications (DRG 499) | $7,780/$19,060 |
| Discectomy with fusion (DRG 498) | $16,634/$16,630 |
| Discectomy and fusion with complications (DRG 497) | $22,841/$54,100 |
| Repeat surgery mean cost† | $8,975/$23,341 |
| Indirect Cost Components | |
| Lost Productivity‡ | |
| Missed work | 28.42/hr |
| Unpaid caregiver time | $16.29/hr |
| Missed housekeeping | $15.00/hr |
Includes surgeon costs, anesthesiology costs and hospitalization costs, which were estimated for both the Medicare and general adult populations.
Repeat surgery mean cost comprised of DRGs 415, 443, 498, 499, 500 and 538.
Cost per day;
Cost per hour
Surgery costs depended on the procedure performed and whether or not intraoperative complications occurred. These factors determined the diagnosis related group (DRG), with associated costs assigned in two ways. First, a cost approximating the value paid by non-Medicare insurers was estimated at 70% of the mean amount billed to Medicare in 2004. Second, the observed 2004 Medicare mean total DRG price was used to reflect hospital-related surgery costs for the age 65 and older population. Surgeon costs were based on 2004 Medicare allowable amounts using the resource-based relative value scale (RBRVS)12, and anesthesiology costs were estimated using operative time with a fixed amount of time added for post-acute care according to whether or not intraoperative complications occurred. For hospitalizations that were not associated with a spine surgery, costs were based on the DRG and priced using mean observed Medicare prices in 2004 for each admission.
Indirect Costs
At each follow-up, productivity losses due to spine-related problem (i.e., missed work days for those employed outside of the home and missed homemaking days for those who reported housekeeping as their primary activity) were recorded. Use of unpaid caregivers (including spousal care giving) was also obtained. Costs were estimated using the standard human capital approach13 by multiplying the change in hours worked by the gross-of-tax wage rate based on self-reported wages at study entry. Costs for missed days of housekeeping and unpaid caregivers were valued based on average wages plus non-health benefits for individuals ages 35 and older.14–16
Incremental Cost-Effectiveness Ratio
The primary endpoint for the cost-effectiveness analysis is the incremental cost-effectiveness ratio (ICER). To estimate the ICER, average total costs and average QALYs from baseline to two-years are estimated for both treatment groups. The ICER is defined as the difference in mean total costs between surgical and non-operative groups, divided by the difference in mean QALYs as follows:
Statistical Analysis
As previously reported, both the randomized and observational cohort experienced high rates of non-adherence to assigned treatment groups.3,4 Among those with any follow-up data available in the randomized cohort, non-adherence was 16% (114/735) in those initially assigned to surgery and 34% (155/456) in those initially assigned to non-operative care.
To complete the cost-effectiveness analysis, the two cohorts were combined and analyzed according to treatment received using robust regression models for longitudinal data via generalized estimating equations.17,18 Separate models were fit for EQ-5D and for 30-day cost rates as measured at 6 weeks, 3, 6, 12 and 24 months after surgery or the beginning of non-operative therapy. The 30-day cost rates were based on reported utilization rates at each time period taking into account the recall period used in the questionnaire and were used to generate mean costs for the intervals 0 to 6 weeks, 6 weeks to 3 months, 3 to 6 months, 6 to 12-months and 12 to 24 months. Mean costs over each interval were summed to provide estimates of total mean costs for each treatment group. If a patient was missing a visit, all other available visits for that patient were included in the analysis. The treatment indicator (i.e. surgery versus non-operative) was a time-dependent covariate, allowing for variable surgery time. This procedure would have the effect of incorporating the non-operative experience of patients who postponed surgery beyond three months from enrollment.
The longitudinal regression models, done using PROC GENMOD (SAS version 9.1 Windows XP Pro, Cary, NC), were specified with a compound symmetry assumption for the working covariance matrix to account for correlations among repeated measurements for individuals, including observations before and after surgery.
Following surgery, outcomes were assigned to the surgical group with follow-up times measured from the surgery date. Due to the allowable windows for scheduled visits and the procedure for crossover, the actual time of outcome assessment varied (e.g. a six week follow-up might occur at five weeks or seven weeks). To adjust for this variation, individual visit times were included as adjusting variables in the longitudinal regression. To adjust for potential confounding, baseline variables associated with missing data or treatment received were included as covariates (age, sex race, marital status, work status, compensation, body mass index, smoking status, joint problems, migraines, any neurological deficit, herniation (type, level, location), baseline evaluation score, baseline sciatica bothersomeness, baseline satisfaction with symptoms, self-rated health trend, center and insurance).3,4 This procedure had the effect of controlling for systematic factors that may be associated with incomplete data collection.
Based on the adjusted mean difference in EQ-5D from the longitudinal regression model at 6 weeks, 3, 6, 12 and 24 months, an area under the curve/time-weighted average was formed to estimate the difference in QALYs between the surgical and non-operative treatments, adjusted to a common baseline value. For costs, the adjusted mean 30-day difference in rates at 6 weeks, 3, 6, 12 and 24 months was used to form a mean difference in total costs over 2 years by multiplying each adjusted rate by the corresponding time interval between visits and summing to obtain total costs in each treatment group. To estimate an ICER confidence interval, a bootstrap method was applied using 1000 samples taken with replacement from the original sample with the individual as the unit of observation.
Sensitivity analyses were undertaken to assess the impact of assumptions and/or analytic approach on cost-effectiveness results. Several analyses considered the impact of limiting the costs included in the analysis to direct medical costs or direct medical costs plus costs of work loss for those employed in the workforce.
RESULTS
The cost-effectiveness analysis utilized data from 1,191 participants, including 775 who underwent surgery and 416 who were treated non-operatively for the entire follow-up period based on data collected through December 5, 2006. Differences in baseline characteristics by treatment received are shown in Table 2. Surgical patients were younger, less likely to be working fulltime and more likely to be receiving or have applied for disability or social security compensation and more likely to have L5-S1 herniation. At baseline, patients who went on to surgery had significantly worse bodily pain, physical function, mental health and Oswestry Disability Index and EQ-5D scores than non-operative patients.
Table 2.
Patient baseline demographic characteristics and health status
| Surgery (n=775) |
Non-Operative (n=416) |
p-value | |
|---|---|---|---|
| Mean Age (SD) | 40.7 (10.8) | 43.8 (12.1) | <0.001 |
| Female | 338 (44%) | 169 (41%) | 0.35 |
| Ethnicity - Non Hispanic | 740 (95%) | 397 (95%) | 0.92 |
| Race - White | 683 (88%) | 350 (84%) | 0.06 |
| Education - At least some college | 562 (73%) | 321 (77%) | 0.09 |
| Income - Under $50,000 | 363 (47%) | 172 (41%) | 0.08 |
| Marital Status - Married | 541 (70%) | 293 (70%) | 0.87 |
| Work Status | 0.004 | ||
| Full Time or Part Time | 444 (57%) | 277 (67%) | |
| Disabled | 120 (15%) | 38 ( 9%) | |
| Homemaker | 55 ( 7%) | 24 ( 6%) | |
| Other | 155 (20%) | 77 (19%) | |
| Compensation* | 157 (20%) | 51 (12%) | <0.001 |
| Time since recent episode < 6 months | 601 (78%) | 328 (79%) | 0.66 |
| Herniation Level‡ | <0.001 | ||
| L2–L3 / L3–L4 | 41 ( 5%) | 47 (11%) | |
| L4–L5 | 300 (39%) | 156 (38%) | |
| L5-S1 | 434 (56%) | 212 (51%) | |
| Herniation Type | 0.13 | ||
| Protruding | 198 (26%) | 124 (30%) | |
| Extruded | 525 (68%) | 257 (62%) | |
| Sequestered | 52 ( 7%) | ( 8%) | |
| Posterolateral Herniation | 617 (80%) | 301 (72%) | 0.006 |
| EQ-5D | 0.49 (0.2) | 0.64 (0.2) | <0.001 |
| SF-36 Bodily Pain (BP)§ | 22.1 (16.1) | 32.9 (19.7) | <0.001 |
| SF-36 Physical Function (PF)§ | 32 (23.2) | 48.3 (26.3) | <0.001 |
| SF-36 Mental Component Summary (MCS)§ |
44.6 (11.4) | 46.3 (11.8) | 0.02 |
| Oswestry Disability Index (ODI)‖ | 55.2 (19.4) | 38.9 (20.5) | <0.001 |
Receiving workers compensation, social security compensation, or other compensation, or application(s) pending
The diagnoses for approximately 97% of patients were evaluated with MRI and 3% with CT.
Higher SF-36 scores indicate less severe symptoms.
Lower ODI scores indicate less severe symptoms.
Mean health state values improved over time for both surgical and non-operative patients as shown in Figure 1. Total mean discounted QALYs were 1.64 (95%CI: 1.62, 1.67) for surgical patients and 1.44 (95%CI: 1.41, 1.47) for non-surgical patients, a difference of 0.21 (95%CI: 0.16, 0.25).
Figure 1.
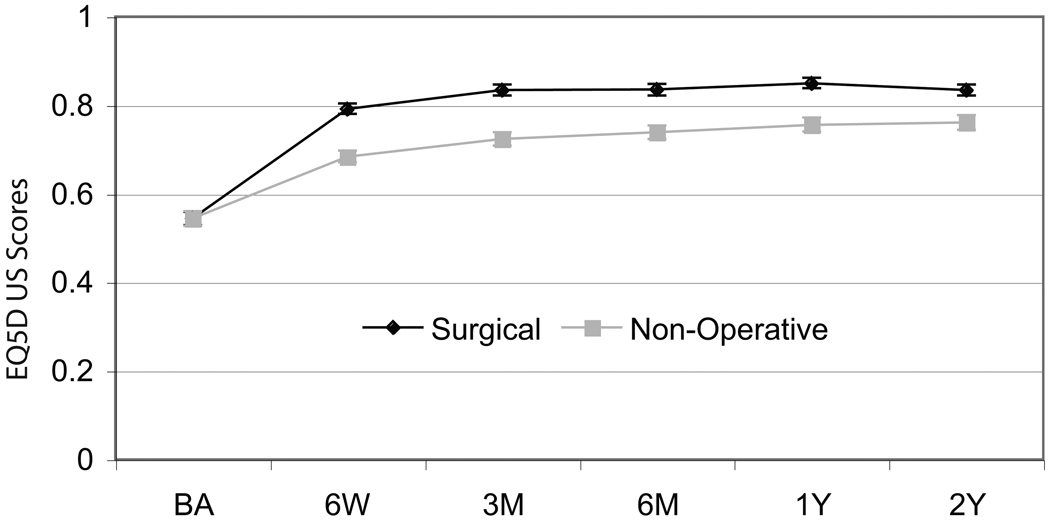
Mean health state values and 95% confidence intervals over time by treatment received.
Most surgeries (96%, 741/771) were classified under DRG 500 (back and neck procedures without complications) and were assigned mean surgery costs of $12,754 (95%CI: $12.740, 12,760). A total of 22/771 (3%) primary surgeries were classified as having complications (DRG 499) with mean surgery costs estimated at $19,063 (95%CI: $18.960, 19,160). Spinal fusion was uncommon among primary surgeries, but occurred in 8 patients. A total of 63 repeat surgeries occurred in 53 (6.8%) surgery patients, with a mean cost of $28,019 (95%CI: $19,950, 26,730). For each surgery type, use of Medicare pricing decreased surgery costs substantially with mean costs of $6,828 for DRG 500, $7,082 for DRG 499, and $9,757 for repeat surgery.
Total mean costs were $27,273 (95%CI: $26,009, $28,644) for surgically treated patients and $13,135 (95%CI: $11,244, $14,902) for non-operative patients (Table 3). Reported use of any health care visits did not differ between the treatment groups (90% surgery vs. 88% non-operative, p=0.16). Fewer than half of participants reported physical therapy visits (50% surgery vs, 44% non-operative, p=0.064); chiropractor visits were infrequent (13% surgery vs. 15% non-operative, p=0.49); and use of acupuncture was reported by only 5% of participants. Those treated surgically reported more diagnostic test use (53% of surgery vs. 34% of non-operative patients, p<0.001) and medication use (96% of surgery vs. 89% of non-operative patients, p<0.001) than non-operative patients, with use in both groups declining over time. Treatment groups differed significantly in use of oral steroids (8% surgery vs. 4% non-operative, p=0.011) and narcotics (77% surgery vs. 32% non-operative, p<0.001). Device use was similar in both groups and occurred among 40% of participants. Among surgery patients, the most frequently reported devices were brace, cane and orthopedic pillow, which were reported by 11%, 11% and 12%, respectively. Among non-operative patients orthopedic pillow (15%) and shoe inserts (15%) were among the most commonly reported devices.
Table 3.
| Treatment Received | |||
|---|---|---|---|
| Surgery (n=775) |
Non-operative (n=416) |
||
| Direct Costs | General Population | Medicare Population | |
| Surgery | $15,139 ($14,487, $15,792) $8,063 ($7,727, $8,400) | ||
| Health Care Visits | $1,500 ($1,357, $1,642) | $1444 ($1265, $1622) | |
| Diagnostic Tests | $826 ($639, $1,012) | $882 ($645, $1119) | |
| Medications | $1,592 ($1,405, $1,779) | $1877 ($1643, $2111) | |
| Other Health Care Services | $859 ($513, $1206) | $1447 ($1012, $1881) | |
| Total Direct Costs¶ | $20,237 ($19,314, $21,160) $13,056 ($12,365, $13,746) | $5,804 ($4,639, $6,969) | |
| Indirect Costs¶ | |||
| Missed work | $5,218 ($4,449, $5,986) | $3,614 ($2,641, $4,587) | |
| Unpaid caregiver | $80 ($-78, $238) | $399 ($200, $598) | |
| Missed homemaking | $2,079 ($1,459, $2,699) | $3,321 ($2,547, $4,095) | |
| Total Indirect Costs¶ | $7,089 ($6,155, $8,022) | $7,399 ($6,221, $8,577) | |
| TOTAL COSTS¶ | $27,341 ($25,882, $28,799) $20,150 ($18,840, $21,460) | $13,135 ($11,244, $14,902) | |
Estimate based on data aggregated at the level of the individual in adjusted as-treated analyses.
As shown in Table 4, the proportion of participants reporting any missed work days was higher for those undergoing surgery than those treated non-operatively (58% surgery vs 36% non-operative, p<0.001). There were no differences between groups in missed homemaking days or unpaid caregiver use.
Table 4.
Missed work, missed homemaking, and days requiring unpaid caregivers by treatment. “Surgery” means surgery within 24 months of enrollment.
| Treatment Received | |||
|---|---|---|---|
| Surgery (n=775) |
Non- Operative (n=416) |
p-value | |
| Any missed work days (%) | 58% | 36% | <0.001 |
| Mean work days missed (SD) | 27.7 (41.2) | 15.7 (37.9) | <0.001 |
| Any missed homemaker days (%) | 21% | 17% | 0.21 |
| Mean homemaker days missed (SD) | 20.6 (67.3) | 22.7 (80.3) | 0.30 |
| Any unpaid caregiver days (%) | 13% | 11% | 0.28 |
| Mean unpaid caregiver days (SD) | 2.7 (19.5) | 3.8 (32.8) | 0.24 |
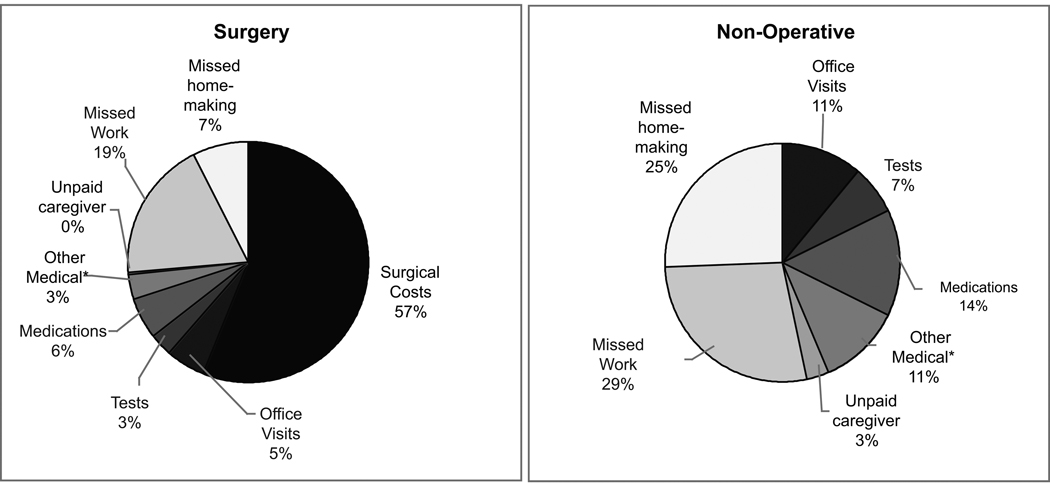
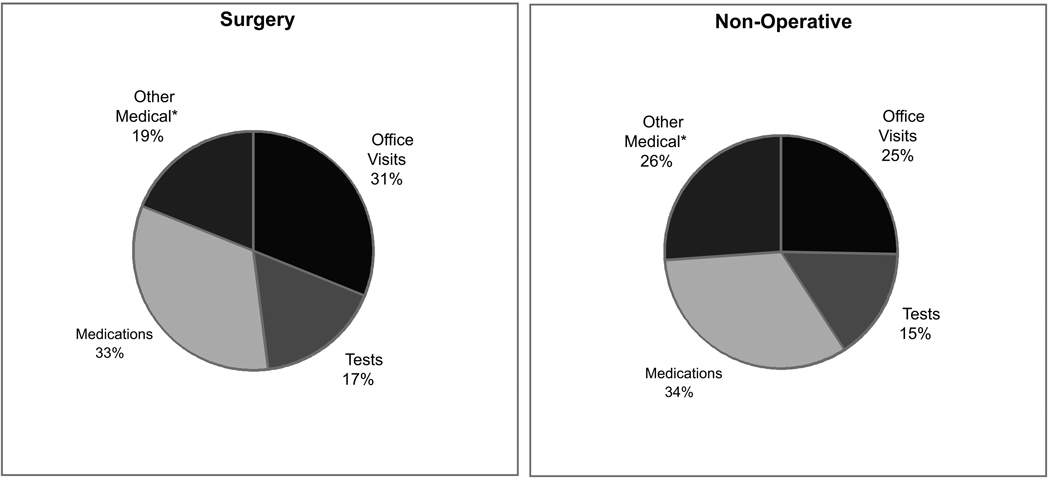
As depicted in Figure 2, over the two-year period, indirect costs accounted for a substantial proportion of total costs in both groups (26% of cost for surgical patients and 57% of costs for non-operative patients). The distribution of non-surgical direct medical costs was similar between groups.
Figure 2.
Cost distributions by treatment received over 24 months for A) total costs and B) direct medical non-surgical costs.
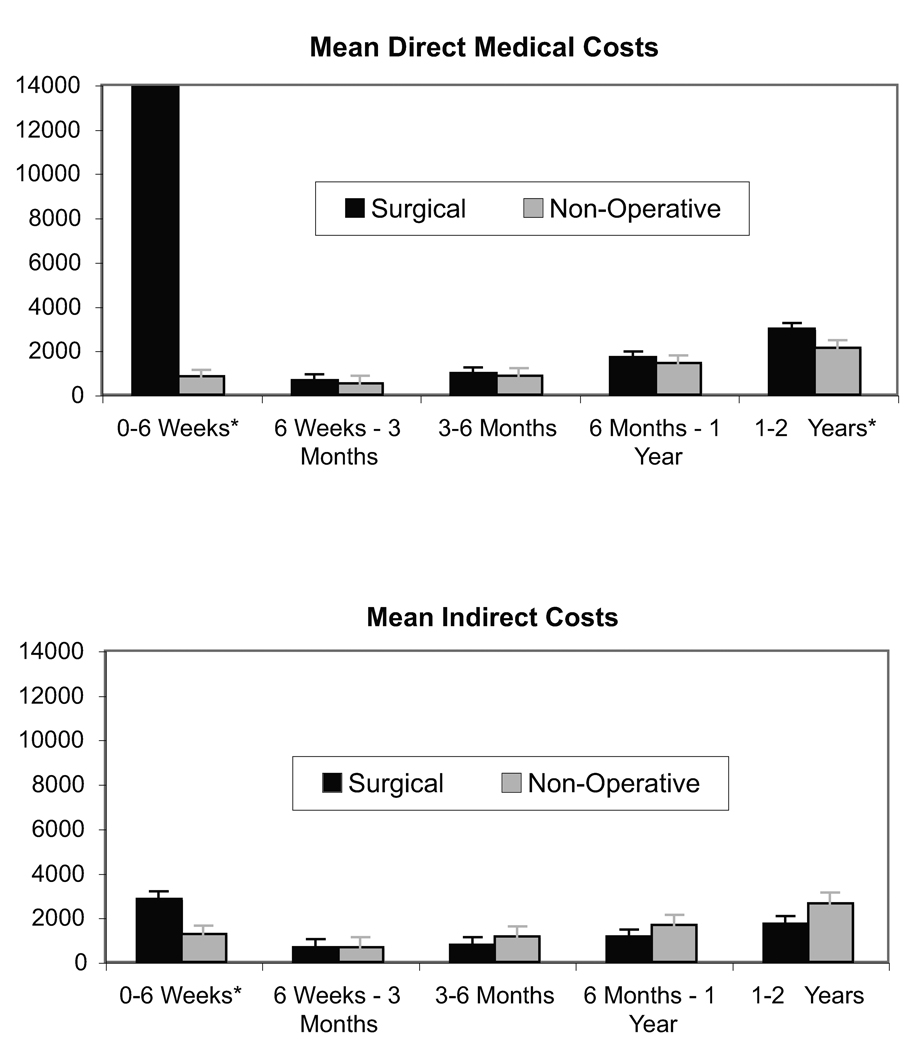
Direct medical costs and indirect costs for each time period are shown in Figure 3. Both types of cost were highest during the first six weeks among those undergoing surgery. Mean indirect costs for non-operative patients tended to be higher over time than for surgically treated patients.
Figure 3.
Mean costs by time period and treatment received. Asterisks show time period differences between treatment groups with p-value<0.05.
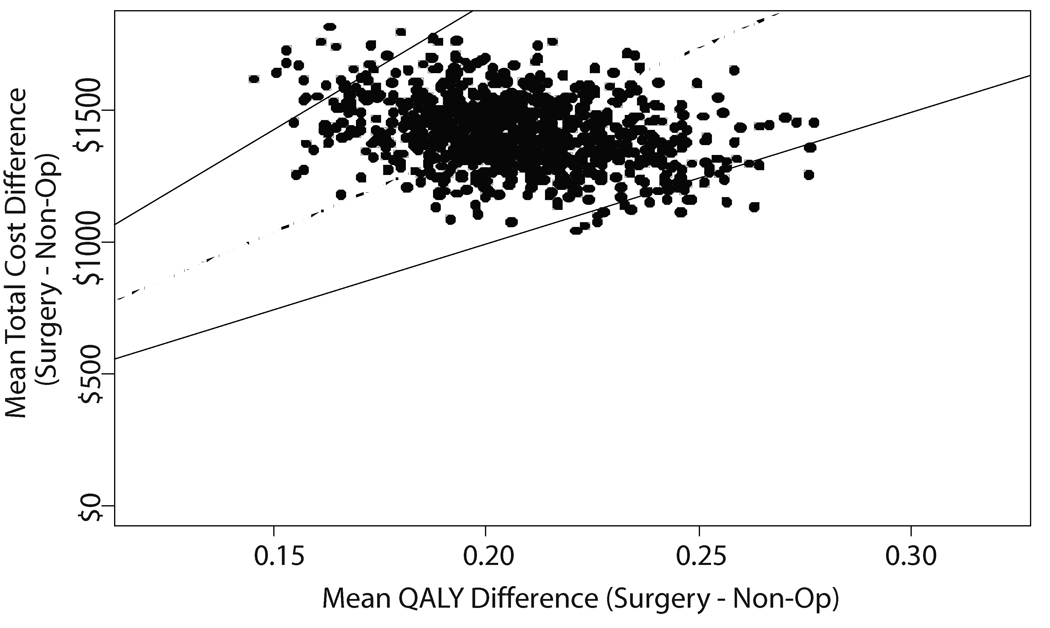
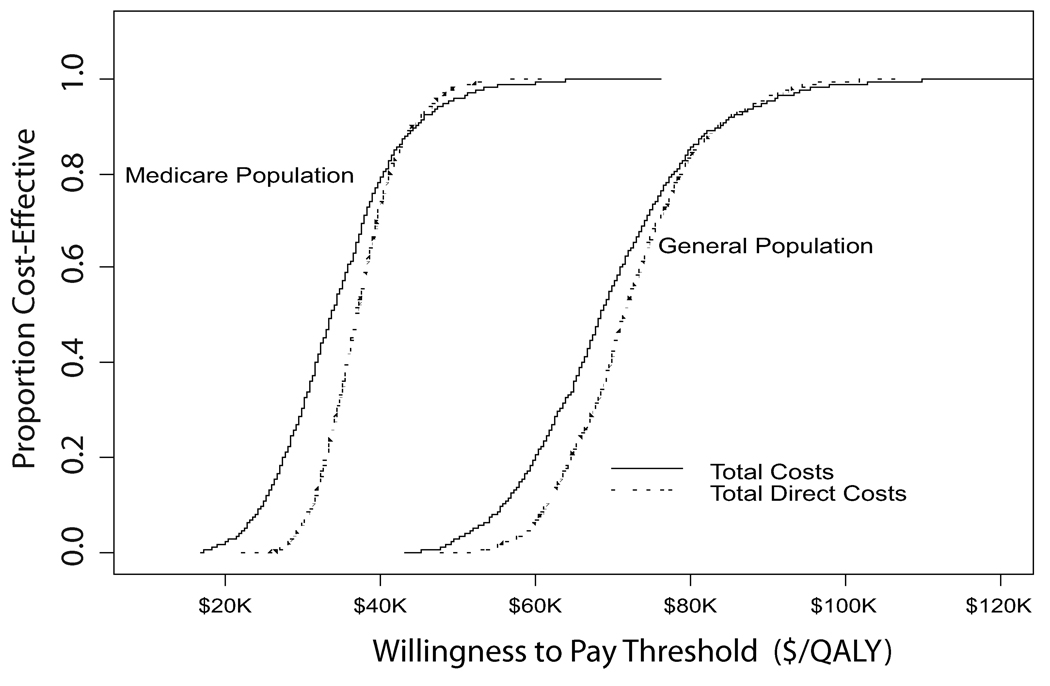
When all costs were considered, the cost per QALY gained for surgical treatment relative to non-operative care in the general population was $69,403 (95%CI: $49,523, $94,999) (Figure 4). For those age 65 and older for whom Medicare surgery costs are more appropriate, the cost per QALY gained decreased to $34,355 (95%CI: $20,419, $25,512). Limiting costs considered to direct medical costs alone or to direct medical costs together with lost work days had little impact on the value of surgery relative to non-operative care (Table 5). Likewise, excluding those who reported that they were receiving compensation or had it pending at baseline had little impact on the ICER, which was estimated at $33,176 (95%CI: $18,348, $54,157) under Medicare pricing.
Figure 4.
Incremental cost-effectiveness analysis results. A) Results from 1,000 bootstrap estimates of the difference in total costs and difference in QALYs with the mean incremental costeffectiveness ratio shown as a dashed line and the 95% confidence interval shown as solid lines for the General Population and B) Cost-effectiveness acceptability curves for surgery relative to nonoperative care when total costs or direct medical costs alone are considered by population group.
Table 5.
Mean cost per QALY gained for surgery relative to non-operative care for the general population and for the Medicare population (95% confidence intervals) when various costs are included.
| Costs included | General Population* | Medicare Population+ |
|---|---|---|
| Total costs | $69,403 ($49,523, $ 94,999) | $34,355 ($20,419, $52,512) |
| Direct medical costs | $72,181 ($56,473, $ 92,394) | $37,285 ($28,364, $48,993) |
| Direct medical costs & productivity costs++ | $77,300 ($60,009, $99,544) | $42,111 ($30,976, $56,284) |
Surgery hospitalization cost estimated at 70% of amount billed to Medicare in 2004
Surgery hospitalization cost estimate based on mean Medicare prices in 2004.
estimated as lost work days only for those working outside of the home
DISCUSSION
We utilized data collected on resource utilization, work loss and health-related quality of life to estimate cost per QALY gained for surgical treatment relative to non-operative care of herniated disc in a population with persistent back and leg pain. Although surgery was more costly than non-operative treatment, health outcomes over two years were better among those treated surgically. We estimated costs per QALY gained with surgery compared to non-operative treatment ranging from $34,355 to $69,403 depending on the cost of surgery. While the lower ratio compares very favorably with the economic value of commonly accepted medical and surgical interventions such as antihypertensive treatment of 60 year old males with diastolic blood pressure of 110mgHg, which is associated with an ICER of $56,500 in 2002 US dollars19 ($59, 500 in 2004 dollars20), cost-effectiveness for the general population is less favorable. Our estimated cost-effectiveness ratios for surgery relative to non-operative treatment of herniated disc were comparable to the $52,200 per QALY gained ($33,900 inflated from ’93 to ’04 dollars) estimate reported by Malter, et al.5 Although Malter’s report relied on costs and health outcomes derived from two different populations, which were integrated in a model-based cost-effectiveness analysis, it appears that our prospective follow-up of SPORT participants provides a qualitatively similar estimate of economic value.
One important difference between our analysis and that of Malter, et al5 was the time horizon. We report on the cost-effectiveness of surgery for disc herniation over a two-year period, whereas Malter’s report was for a 10-year horizon. To the extent that differences in health state values between those treated surgically and non-surgically are maintained beyond two years, the incremental value of surgery may be greater. Continuing follow-up of SPORT participants over time should allow for evaluation of economic value over a longer time horizon.
A strength of our economic evaluation was our ability to consider work-related productivity costs in the analysis. To understand the cost-effectiveness of surgical treatment from a societal perspective, it is important for such costs to be included. However, while we tracked self-reported work days lost due to spine-related problems, we acknowledge that more subtle productivity losses ( e.g., less efficient work due to pain) were not measured. Nonetheless, when productivity costs were omitted from the analysis, the value of surgery changed little. Likewise, exclusion of those who were receiving compensation for disability or had such applications pending had virtually no impact on the estimated economic value of surgical intervention. These findings contrast with results reported in a recent Swedish study, where surgery for IDH was estimated to save money.7 Although the Swedish study utilized a 2-year time horizon, all future earnings were counted as lost productivity among those who became disabled within the 2-year period studied. Exclusion of these future costs relative to mean QALY changes would result in a cost per QALY gained of approximately $75,000, which is fairly consistent with our cost-effectiveness estimates.
A further strength of our study was use of a validated EuroQol instrument to obtain societal health state values appropriate for use in economic analyses. Although EQ-5D has seen limited application in assessing low-back surgery, a recent Norwegian study that compared it with the Oswestry Disability Index reinforces its validity for use in cost-utility analyses of low-back surgery.21
The analysis has several limitations. First, we relied on patient self-reports of resource use and productivity losses to estimate total costs. Although more complete capture of resource use may have been possible through linkage with electronic billing records, such an approach may have resulted in biased cost ascertainment with near complete capture of some types of service (e.g., surgery) relative to non-operative care (e.g., chiropractor, physical therapy, etc).
Second, to minimize recall issues, we limited the recall window to six weeks at early visits and one-month at annual visits for non-hospital based care. These snapshots of utilization were used to infer resource use over a longer time period. To the extent that chronic problems are likely to incur ongoing costs, this approach may have identified these while missing some acute care costs. Large costs including hospitalizations and repeat surgeries were not limited by the recall period.
Third, we relied on Medicare payment schedules to estimate costs for most medical resource consumption. Although regulated payments may more accurately reflect the resources necessary to produce a service (i.e., actual cost), they do not reflect true costs. In addition, surgical technique was not controlled for in this study. At present there is little evidence to support a difference in cost or in clinical outcomes for open discectomy compared with percutaneous discectomy techniques.22 In both cases, our analyses highlight the substantial impact that surgery cost has on the estimated economic value of surgical intervention.
Finally, our evaluation is based on an observational rather than randomized measure of outcomes. Due to the high degree of crossover in both the observational and randomized cohorts, we present a pooled analysis that utilized longitudinal modeling to evaluate costs and outcomes of participants as they were treated. Although this involves complex statistical models, an advantage of this approach is that we utilized the experience of a large number of persons with IDH whose overall characteristics did not differ statistically between cohorts (e.g., observational vs. randomized cohorts).
Changing trends in spine surgery in the United States, combined with continued escalation in health care expenditures, highlights the importance of understanding the economic value of common surgical intervention. To date, relative to spinal fusion, the economic value of surgery for disc herniation has received relatively little attention 23. Our comprehensive analysis suggests that surgical treatment of herniated disc represents a reasonably cost-effective health care intervention when compared with other common health care interventions.
ACKNOWLEDGEMENTS
We gratefully acknowledge Catherine C. Lindsay, S.M. for extensive work in the development of the cost weights utilized in this analysis, and Tamara S. Morgan for creation of the patient diaries and for her assistance in preparing this manuscript.
The authors would like to acknowledge funding from the following sources: The National Institute of Arthritis and Musculoskeletal and Skin Diseases (U01-AR45444-01A1) and the Office of Research on Women's Health, the National Institutes of Health, and the National Institute of Occupational Safety and Health, the Centers for Disease Control and Prevention. The Multidisciplinary Clinical Research Center in Musculoskeletal Diseases is funded by NIAMS (P60-AR048094-01A1). Dr. Lurie is supported by a Research Career Award from NIAMS (1 K23 AR 048138-01).
These sources had no role in design or conduct of the study; collection, management, analysis, or interpretation of the data; nor in preparation, review, or approval of the manuscript. The principal investigator (Dr. Anna Tosteson) had full access to all the data in the study and takes responsibility for the accuracy and integrity of the data analysis.
Funding:
This research was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U01-AR45444 and P60-AR048094)
REFERENCES
- 1.JW PI. Dartmouth Atlas Working Group. Dartmouth Atlas of Musculoskeletal Health Care. Chicago, IL: American Hospital Association Press; 2000. [Google Scholar]
- 2.Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States' trends and regional variations in lumbar spine surgery: 1992–2003. Spine. 2006 Nov 1;31(23):2707–2714. doi: 10.1097/01.brs.0000248132.15231.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malter AD, Larson EB, Urban N, Deyo RA. Cost-effectiveness of lumbar discectomy for the treatment of herniated intervertebral disc. Spine. 1996 May 1;21(9):1048–1054. doi: 10.1097/00007632-199605010-00011. discussion 1055. [DOI] [PubMed] [Google Scholar]
- 4.Atlas SJ, Deyo RA, Keller RB, et al. The Maine Lumbar Spine Study, Part II. 1-year outcomes of surgical and nonsurgical management of sciatica. Spine. 1996 Aug 1;21(15):1777–1786. doi: 10.1097/00007632-199608010-00011. [DOI] [PubMed] [Google Scholar]
- 5.Hansson E, Hansson T. The cost-utility of lumbar disc herniation surgery. Eur Spine J. 2007;16:329–337. doi: 10.1007/s00586-006-0131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birkmeyer NJ, Weinstein JN, Tosteson AN, et al. Design of the Spine Patient outcomes Research Trial (SPORT) Spine. 2002 Jun 15;27(12):1361–1372. doi: 10.1097/00007632-200206150-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006 Nov 22;296(20):2451–2459. doi: 10.1001/jama.296.20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006 Nov 22;296(20):2441–2450. doi: 10.1001/jama.296.20.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold M, Siegel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 10.EuroQol--a new facility for the measurement of health-related quality of life. The EuroQol Group.[comment] Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 11.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005 Mar;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 12.RBRVS Fee Schedule: A Plain-English Guide. Rockville, MD: Decision Health; 2004. [Google Scholar]
- 13.Hodgson T, Meiners M. Cost-of-illness medthodology: A guide to current practices and procedures. Milbank Memorial Fund Quarterly. 1982;60(3):429–462. [PubMed] [Google Scholar]
- 14.Bureau USC. Income, Expenditures and Wealth. Statistical Abstract of the United States: 2004–2005. [ [Google Scholar]
- 15.Bureau of Labor Statistics. Employer Costs for Employee Compensation Summary. [Accessed September 7, 2005];2005 http://www.bls.gov/news.release/ecec.nr0.htm.
- 16.Bureau of Labor Statistics. Employment Cost Index. [Accessed September 7, 2006];2005 http://data.bls.gov/PDQ/servlet/SurveyOutputServlet?data_tool=latest_numbers&series_id=ECU10001A.
- 17.Diggle P, Haeagerty P, Liang K, Zeger S. The analysis of longitudinal data. 2nd ed. Oxford, England: Oxford University Press; 2002. [Google Scholar]
- 18.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. Philadelphia, PA: John Wiley & Sons; 2004. [Google Scholar]
- 19.CEA Registry. [Accessed April 19, 2007]; http://www.tufts-nemc.org/cearegistry/data/default.asp.
- 20.Bureau of Labor Statistics. Consumer Price Index, U.S. City Average--Medical Care. Washington, D.C: U.S. Department of Labor; 2006. [Google Scholar]
- 21.Solberg TK, Olsen JA, Ingebrigtsen T, Hofoss D, Nygaard OP. Health-related quality of life assessment by the EuroQol-5D can provide cost-utility data in the field of low-back surgery. Eur Spine J. 2005 Dec;14(10):1000–1007. doi: 10.1007/s00586-005-0898-2. [DOI] [PubMed] [Google Scholar]
- 22.Haines SJ, Jordan N, Boen JR, et al. Discectomy strategies for lumbar disc herniation: results of the LAPDOG trial. J Clin Neurosci. 2002 Jul;9(4):411–417. doi: 10.1054/jocn.2002.1120. [DOI] [PubMed] [Google Scholar]
- 23.Soegaard R, Christensen FB. Health economic evaluation in lumbar spinal fusion: a systematic literature review anno 2005. Eur Spine J. 2006 Aug;15(8):1165–1173. doi: 10.1007/s00586-005-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]