Abstract
Central to theories of electron transfer (ET) is the idea that nuclear motion generates a transition state that enables electron flow to proceed, but nuclear motion also induces fluctuations in the donor-acceptor (DA) electronic coupling that is the rate-limiting parameter for nonadiabatic ET. The interplay between the DA energy gap and DA coupling fluctuations is particularly noteworthy in biological ET, where flexible protein and mobile water bridges take center stage. Here, we discuss the critical timescales at play for ET reactions in fluctuating media, highlighting issues of the Condon approximation, average medium versus fluctuation-controlled electron tunneling, gated and solvent relaxation controlled electron transfer, and the influence of inelastic tunneling on electronic coupling pathway interferences. Taken together, one may use this framework to establish principles to describe how macromolecular structure and structural fluctuations influence ET reactions. This framework deepens our understanding of ET chemistry in fluctuating media. Moreover, it provides a unifying perspective for biophysical charge-transfer processes and helps to frame new questions associated with energy harvesting and transduction in fluctuating media.
Keywords: coupling fluctuations, tunneling pathways, inelastic tunneling, pathway coherence, solvent dynamical effects, water-mediated tunneling
1. INTRODUCTION
Is the reactivity of biomolecules dictated by their average molecular structure, by routine thermal excursions from the average, or by rare large-scale conformational changes? Addressing these questions is central to establishing a description of bioenergetics at the molecular scale. Here, we address these and closely related issues in the framework of biological and bioinspired electron transfer (ET). We review how a new generation of theory and experiments is unveiling the role played by protein and solution structure, fluctuations, and relaxation in ET kinetics and mechanisms.
Early formulations of nonadiabatic ET reactions were based on Fermi’s golden rule and emphasized the probability of encountering configurations with quasi-degenerate donor and acceptor states (through the Franck-Condon factor), and the propensity for electron tunneling in these configurations (through the tunneling matrix element). Recent studies have focused on how structural fluctuations [those generating donor-acceptor (DA) degeneracy and others] may influence the transfer rate, revealing a richness of mechanistic behavior.
The familiar Fermi’s golden-rule ET rate constant is , where TDA is the DA electronic coupling interaction and (F.C.) is the Franck-Condon factor (1–7). The description of ET in terms of a single rate constant suggests that the ET kinetics is single exponential and it is appropriate if 1/kET is slow compared with timescales of fluctuations that may modulate the reaction rate. Such fluctuations could include conformational interconversions and solvent relaxation. These molecular fluctuations may affect both the DA energy gap and the DA tunneling matrix element, and the fluctuations may lead to regimes that require modification of the golden-rule rate constant, even in the limit of single exponential kinetics.
2. COUPLING FLUCTUATION EFFECTS IN ELECTRON TRANSFER
In the following, we discuss generalizations of the nonadiabatic ET rate expression when fluctuations of the molecular structure and of the solvent modulate TDA with a fluctuation timescale that is fast compared with 1/kET. When the coupling fluctuations are slow on the timescale of nuclear motion through the crossing region of the donor and acceptor potential surfaces (validating the Condon approximation), in the nonadiabatic rate expression should be replaced by its thermal average . If the coupling fluctuations are fast on the timescale of nuclear motion through the crossing region, the tunneling electron may exchange energy with the nuclear degrees of freedom of the bridge, enabling inelastic tunneling. This effect has a well-understood influence on the Franck-Condon factor and can also change TDA significantly, as inelastic markers left on tunneling pathways change the overall amplitude for tunneling (vide infra).
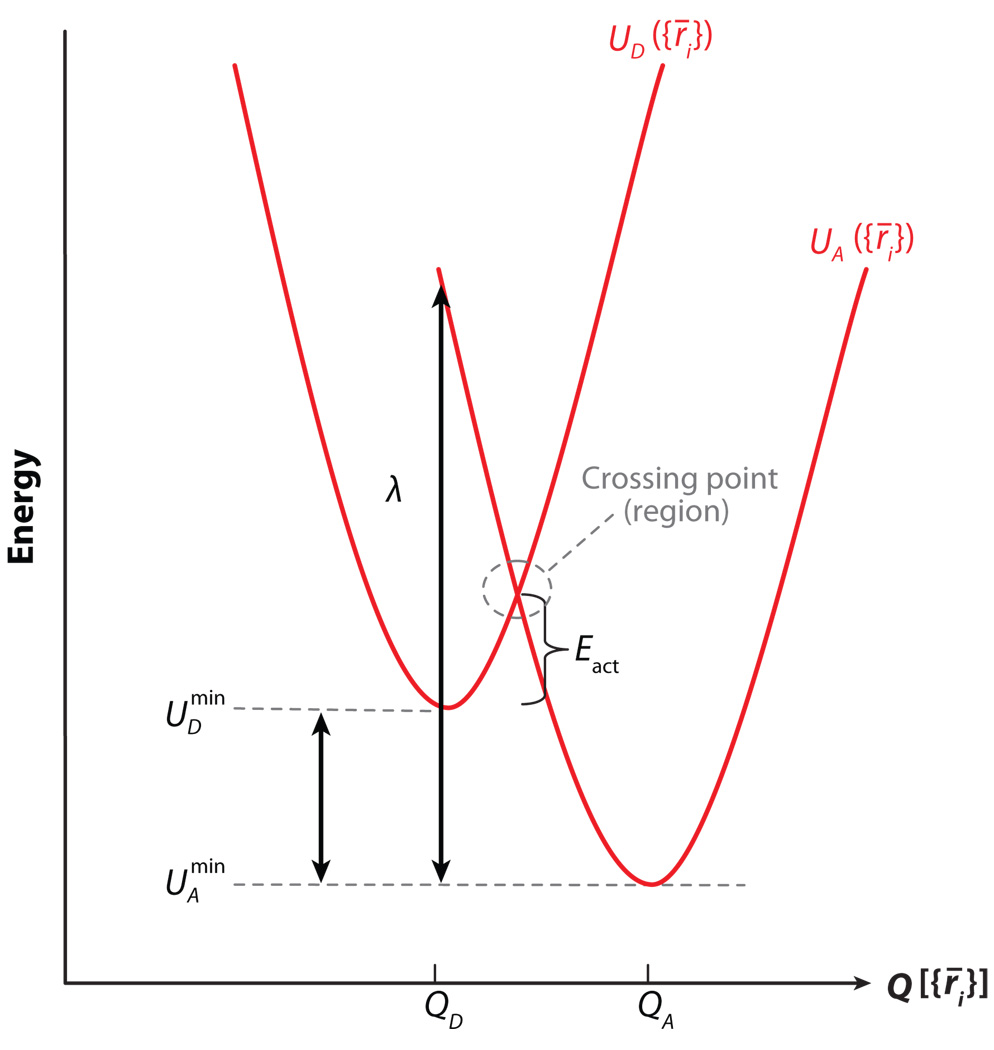
We now present a framework for exploring how bridge fluctuations influence ET kinetics. We use a time-dependent version of Fermi’s golden rule with a semiclassical approximation to explore the relevant timescales, both in general and for some specific systems. It is helpful to express the nonadiabatic ET rate constant as the Fourier transform of time-dependent correlation functions (see 8, 9). We consider an ET system described by the Hamiltonian Ĥ = ĤD + ĤA + ĤB + V̂, where ĤD and ĤA are electronic-vibrational (vibronic) Hamiltonians for the donor (D) and acceptor (A) diabatic potential surfaces (Figure 1), ĤB is the vibronic bridge Hamiltonian, and V̂ couples donor (acceptor) and bridge electronic states. In particular, , where ĤD(vi) and ĤA(vi) are the vibrational Hamiltonians for the UD and UA harmonic diabatic surfaces (Figure 1), and ĤB = ĤB(el) + ĤB(vi) + ĤB(e−v), where ĤB(el) and ĤB(vi) are the electronic and vibrational bridge Hamiltonians, respectively. ĤB(e−v) describes the bridge electronic-vibrational interactions. In this model, the nonadiabatic ET rate constant is
| (1) |
where is the frequency corresponding to the energy gap (Figure 1), CFC(t) is the time-dependent Franck-Condon factor,
| (2) |
and
| (3) |
is the correlation function of the vibronic DA electronic coupling (tunneling matrix element). 〈…〉D and 〈…〉B denote thermal averages over the ĤD(vi) and ĤB(vi) vibrational eigenstates, respectively. The vibronic tunneling matrix element to second order in V̂ is
| (4) |
where Etun is the total electronic-vibrational energy of the system when the electron is in the initial donor electronic state (10–12).
Figure 1.
Donor and acceptor diabatic potential energy curves as a function of the reaction coordinate Q, which is assumed to be a single normal mode ({r⃑i} denote atom coordinates). The diagram shows the crossing region, activation energy, energy gap , and reorganization energy λ = k(QD − QA)2/2, associated with the donor and acceptor potentials (k is the curvature of the surfaces).
In the high-temperature (classical) limit for the donor and acceptor vibrational degrees of freedom,
| (5) |
where λ is the reorganization energy (Figure 1), and is often called the Franck-Condon time (5, 7). The Franck-Condon time can be interpreted as the characteristic time for the system to move out of the DA diabatic surface crossing region (Figure 1) due to thermal fluctuations of the DA energy gap, i.e.,
In the classical limit for the bridge vibrational degrees of freedom, the electronic coupling depends parametrically on the atom trajectories, and the correlation function is
| (6) |
where 〈.…〉 denotes a classical thermal average. In practice, TDA(t) is computed along classical molecular dynamics (MD) trajectories, i.e., TDA(t) = TDA[{r⃑1(t), .… r⃑N(t)}], where r⃑i(t) is the position of atom i, and 〈‥〉 is the time (trajectory) average. A perturbative expression for TDA(t) in a nonorthogonal donor, acceptor, and bridge (iB, jB) basis is
| (7) |
where is the matrix element of the electronic bridge Green’s function; Sij and Vij are donor- (acceptor-) to-bridge overlap and electronic Hamiltonian matrix elements, respectively; and S̃B(el) and H̃B(el) are bridge overlap and electronic Hamiltonian matrices (13). is the tunneling energy of the electron. All quantities on the right-hand side of Equation 7, including the tunneling energy, should be evaluated at time t (14, 15).
TDA(t) is routinely computed using a variety of approaches, including Green’s function and energy-splitting analysis (11), generalized Mulliken-Hush analysis (16), and tunneling current methods (17). The timescale of TDA(t) fluctuations is characterized by the coherence time (τcoh) (8, 18, 19). τcoh is the decay time of the CTDA(t) correlation function; it measures how rapidly fluctuations of the electronic coupling randomize TDA(t) values, e.g.,
| (8) |
where . For small times compared with τcoh, CTDA(t) approaches the value ; for long times compared with τcoh, CTDA(t) approaches 〈TDA〉2. The strength of TDA(t) fluctuations is characterized by
| (9) |
which is known as the coherence parameter (20). When coupling fluctuations are large compared with the average coupling , the coherence parameter is small, Rcoh ≪ 1, and for small fluctuations , Rcoh ≈ 1.
The parameters Rcoh, τcoh, and τFC are useful to describe distinct nonadiabatic ET rate regimes (see 8, 18, 19, 21). We focus next on single exponential ET kinetics. In this regime, we have τFC, τcoh ≪ 1/kET [if any of the timescales is longer than the ET time, the kinetics may be multi-exponential or gated (22, 23)].
3. THE SLOW COUPLING–FLUCTUATION REGIME (τcoh > τFC)
In this regime, TDA(t) does not fluctuate while the donor and acceptor electronic states are in resonance. As a result, each DA energy crossing event is subject to an effectively constant coupling, equal to the value of TDA(t) at the time of the crossing (i.e., the Franck-Condon approximation is valid). In Equation 1, we can thus replace CTDA(t) by , and the rate is proportional to the product of and the Franck-Condon weighted density of states, [assuming the classical high-temperature expression for CFC(t) in Equation 5]. Here, one finds two limits: the weak TDA fluctuation regime (Rcoh ≈ 1) and the strong TDA fluctuation regime (Rcoh ≪ 1).
In the limit of weak TDA fluctuations, bridge fluctuations do not change TDA significantly. Hence, , and the rate is
| (10) |
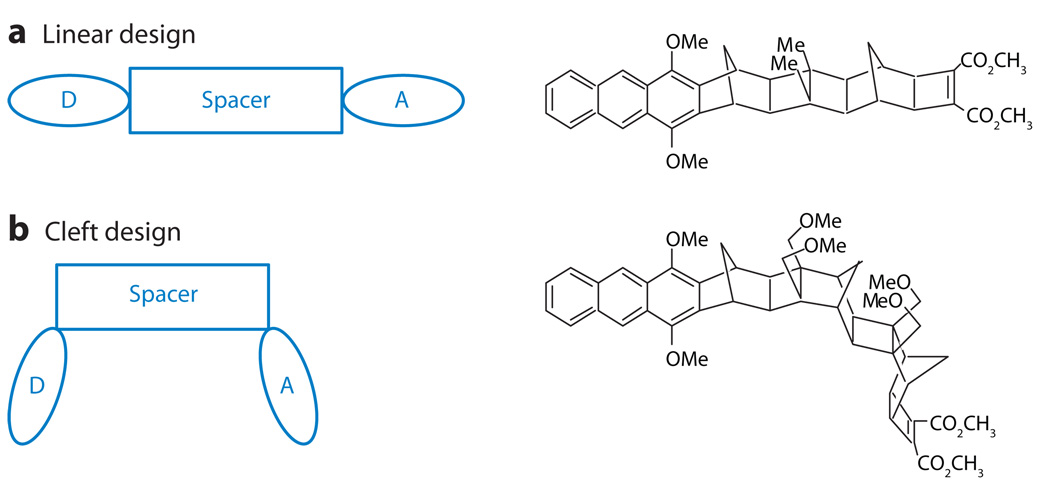
This limit applies to a broad range of ET model systems with the donor and acceptor linked by rigid bridges. Paddon-Row and coworkers (24, 25), Miller and coworkers (26), Newton and coworkers (27), and others (6) have investigated ET kinetics in chemical systems with rigid organic bridges (Figure 2). Through the use of beautifully designed molecular structures and many different kinetic probes, investigators have shown that nonadiabatic ET in such systems proceeds via bridge-mediated electron or hole tunneling. Furthermore, the tunneling propensity is described by TDA values that correspond to equilibrium bridge structures (see 4, 27, 28 for reviews of these studies). In these systems, thermal fluctuations of the bridge structure do not cause significant changes in TDA (i.e., ).
Figure 2.
(a) A schematic diagram and example molecular structure for a linear line-of-sight arrangement of donor-bridge-acceptor units in a supermolecule. (b) A schematic diagram and example molecular structure for a cleft geometry in which the line of sight between the acceptor and donor is through a gap rather than through a saturated bridge unit.
In the case of near-zero TDA values for the equilibrium bridge structure, TDA fluctuations can induce large changes in TDA compared with the average, i.e., . In this regime, bridge structural fluctuations enhance the coupling and determine the observed ET rate constant,
| (11) |
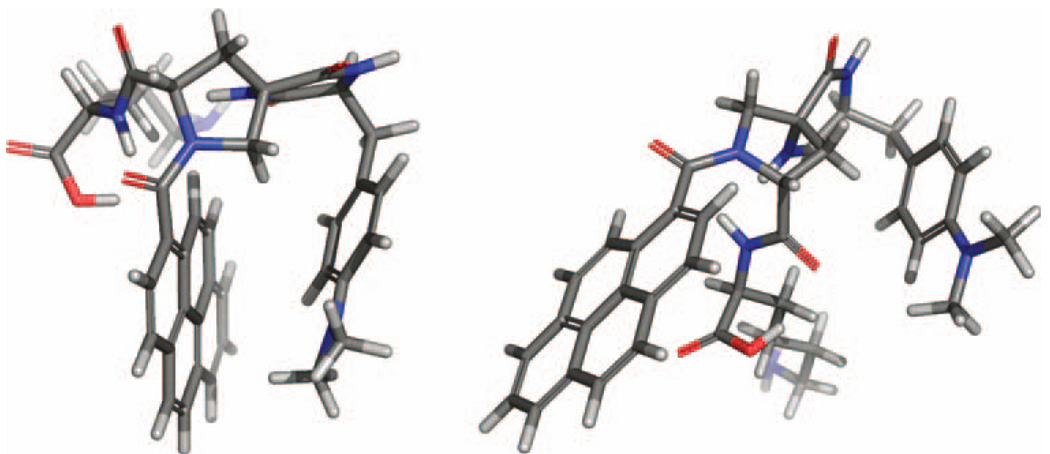
Here, one expects that the ET rate can increase with increasing temperature because the system accesses bridge conformations with larger couplings (9, 23). Whereas the regime of large TDA fluctuations has wide applicability in long-distance biological ET, the studies of Zimmt and Waldeck show that the fluctuation-dominated regime can also be relevant to small chemical ET systems (vide infra). Using synthetic donor-bridge-acceptor structures where a cleft (i.e., a C-clamp) forms between donor and acceptor, they (30, 31) showed that ET occurs by electron (hole) tunneling through a solvent in the cleft (Figure 2). Their studies reveal that the electronic coupling could not be described using a static perspective but must be described by a temperature-dependent dynamical perspective (32). By design, these C-clamp structures have a zero average electronic coupling because of symmetry, so configurational fluctuations (donor-solvent-acceptor) are essential for the enhancement of the DA coupling; such systems provide an important testing ground for understanding the fluctuation-controlled transfer regime (33). A recent extension of this idea to water-soluble cleft molecules (see Figure 3) promises to provide a way to investigate electron tunneling through water molecules.
Figure 3.
Molecular structures showing C-clamp molecules with a pyrene acceptor and a dimethylaniline donor. The D-SSS-A supermolecule has a well-defined cleft, whereas the D-SRR-A supermolecule does not.
Expanding CTDA(t) to second order in time, it is possible to write the ET rate in powers of (τFC/τcoh): CTDA(t) = CTDA(0) + ĊTDA(0)t + C̈TDA(0)t2/2. With , one finds
| (12) |
where δk is the non-Condon correction to the rate equation of the slow fluctuation regime (Equation 11), to lowest order (second order) in (τFC/τcoh) (8). Here, δk depends on Rcoh and [in addition to (τFC/τcoh)2], and it can be computed using MD simulations.
Numerous studies of ET reactions have examined the fluctuation dependence of the DA coupling (14, 15, 21, 28, 33–40), with the aim of identifying bridge conformations that enhance this coupling. Three studies (18, 19, 21) characterize known ET reactions in terms of Rcoh, τcoh, and δk (using MD simulations and semiempirical electronic structure analysis) to understand how these parameters relate to bridge structure and dynamics. Comparison of the results (18, 19, 21) is instructive because Reference 18 explores short-range solvent-mediated ET in the C-clamped molecules above, Reference 19 explores long-distance ET in the protein azurin, and Reference 21 studies ET in the photosynthetic reaction BPh− → QA in the bacterial photosynthetic reaction center. In these three cases, the computed non-Condon correction values are similar (δk ≤ 1%), which means that the ET rates for all these systems are well described using Equation 11. The coherence parameters vary from Rcoh ≤ 0.1 for References 18 and 19 (strong TDA fluctuations) to 0.4 for Reference 21 (moderate TDA fluctuations). Surprisingly, the τcoh values are similar for all ET reactions in these studies (τcoh is a few tens to 100 fs). In all cases, the tunneling matrix element fluctuations are slow (τcoh > τFC), and this observation may be understood using simple arguments. For ET reactions with λ ≈ 1 eV, the room-temperature Franck-Condon time is expected to be very short, [for azurin τFC ≈ 3 – 4 fs, as computed using MD simulations (41), and for the C-clamp molecules (Figure 2), a range of τFC values that depend on the solvent are possible and have magnitudes of a few femtoseconds (18)]. Constrained MD simulations for azurin (19) show that the fastest bridge motions that determine τcoh are largely valence angle vibrations with periods of a few tens of femtoseconds, much longer than the typical τFC times discussed above. Such vibrations have similar periods for a wide variety of chemical bridges, suggesting that the slow fluctuation regime is general for ET tunneling reactions at room temperature in polar media, with ~1-eV reorganization energies, normal (unstrained) chemical bridges, and Gibbs free energy gaps that do not place the ET reaction in the highly activated regime. Deviations from the regime of slow matrix element fluctuations could arise for systems with longer Franck-Condon times (see 42, 43 for deviations from the formula ) and are more likely for low-bridge-gap tunneling systems, where the tunneling matrix element is sensitive to bridge deformations (11, 19, 44).
3.1. The Characteristic Length rcrit
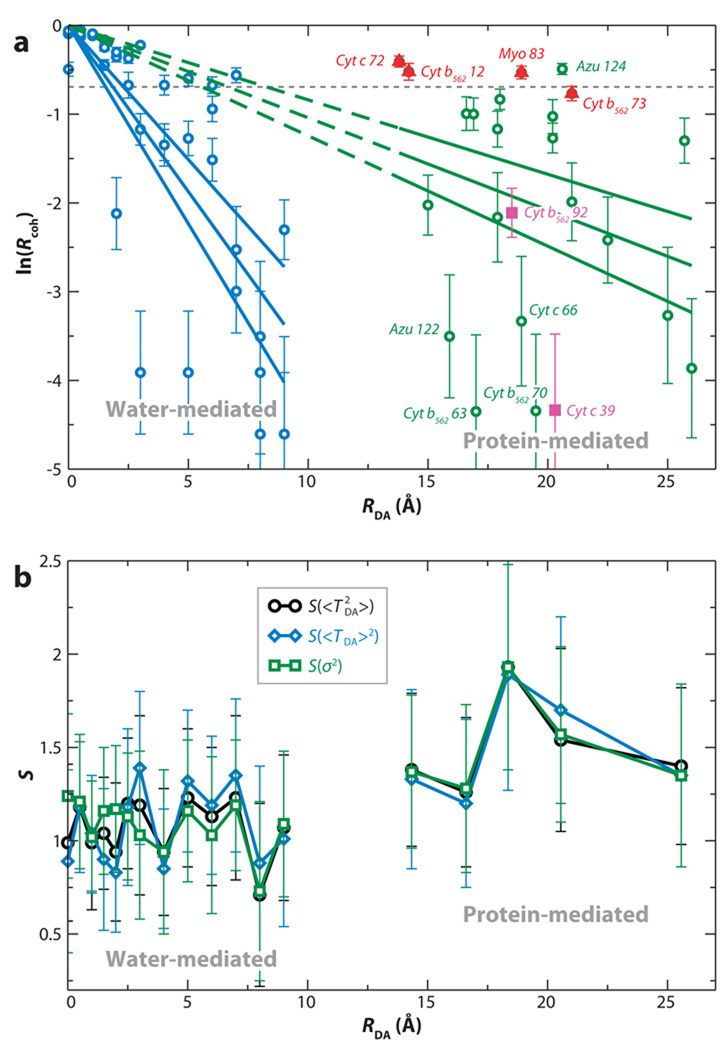
It is interesting to examine why short-distance (RDA ~ 7 Å) solvent-mediated ET in the C-clamp molecules (18) shows equally strong tunneling matrix element fluctuations as are found in long-distance (RDA ~ 17–24 Å) protein-mediated ET in azurin (19) (Rcoh ≤ 0.1 in both cases). Recent studies (45) suggest that there is a critical (medium-dependent) distance beyond which DA coupling fluctuations dominate the electron tunneling. Indeed, Reference 45 poses a statistical question regarding coupling: Is there a distance rcrit that characterizes, on average, a transition between 〈TDA〉2 and dominated kinetics? An answer to this question emerges from an electronic coupling analysis for MD sampled geometries of Ru-modified heme and blue-copper proteins (46), as well as for water-mediated self-exchange between cytochrome b5 molecules. (47) For the protein-mediated tunneling systems, the transition between the 〈TDA〉2-dominated regime and the -dominated regime occurs at distances of ~6–7 Å, about the size of an amino acid residue (see Figure 4a). For the water-mediated self-exchange reaction, the transition to a fluctuation-dominated coupling regime occurs at distances about the size of a water molecule, ~2–3 Å (Figure 4a). Importantly, these critical distances for protein and water tunneling media coincide with the computed mechanical (κij) and electron-tunneling (χij) spatial correlation functions (45):
| (13) |
Figure 4.
(a) Dependence of ln(Rcoh) on the donor-acceptor (DA) distance RDA. The vertical lines are error bars, and the horizontal line denotes the value of Rcoh where . (b) The scatter function (Equation 14), or , is plotted for different RDA distances. An SRDA of the scale of unity indicates that the specific protein structure largely determines the observable rate, whereas SRDA ≪ 1 means that an effective tunneling barrier could be used to describe the tunneling medium (45).
Here, r⃑i represent bridging atom positions, and Gij is the bridge electronic Green’s function between two-center bond orbitals i and j (Equation 7). κij describes the loss of correlation among atomic motions that arises from thermal fluctuations of the bridge structure, and χij describes the loss of correlation among electron-tunneling propagations through the bridge that involves different tunneling distances. The loss of tunneling correlation is also a function of the bridge structural fluctuations. For both water and protein media, the κij and χij decay (correlation) lengths coincide with rcrit for which, on average, . Thus, the onset of a fluctuation-dominated regime is related to the loss of mechanical and tunneling-propagation correlation in the bridge. For water-mediated tunneling, mechanical and tunneling correlation is lost, on average, for distances greater than the size of a water molecule, whereas for proteins, this distance is larger on average (45). The water results suggest that, for solvent-mediated ET in general, the fluctuation-dominated regime will occur at distances greater than the size of the solvent molecules. This analysis explains the results that find ET in C-clamp molecules (RDA ~ 7 Å) and in azurin (RDA ~ 17–24 Å) to be fluctuation dominated (18, 19). Even though the tunneling distances are different in the two systems, they are each larger than the critical distance for the respective tunneling media. It would be interesting to repeat the above analysis for the BPh− → QA ET reaction, where Rcoh = 0.4 (21).
In Gray & Winkler’s (46) tunneling-limited Ru-modified protein ET experiments, all derivatives with anomalously slow rates for their DA distance (in the heme proteins myoglobin, cytochrome c, and cytochrome b562) are in the weak fluctuation regime and have only a few coupling pathways, which are axial to the heme donor. Protein derivatives with fast and average rates (for their distance) fall in the strong fluctuation regime and have tunneling matrix elements that are determined by interactions among many alternative pathway structures, rather than by a few dominant paths (48). The above observations suggest a useful hierarchy for categorizing protein structural effects on ET. At a first coarse level, rates drop rapidly as a function of distance because of the underlying tunneling mechanism. At a second level, rates through β-strands are more rapid for their distance than for α-helices at the same distance as a consequence of tunneling pathway length effects (49). At a third level, edge-coupled hemes and chlorophylls tend to sample many interfering pathways and will have fluctuation-dominated and multipathway-mediated tunneling. As we discuss below, fluctuation-dominated coupling need not wash out the influence of the protein’s fold on the ET rate; rather, it seems likely that it may remove the possibility of a single weak link causing dramatic slowing (for a given distance) (50).
3.2. Fluctuations and Structure
A natural question that arises from an analysis of how fluctuations impact ET kinetics is whether fluctuations wash out the influence of medium structure on the ET rates. This question has been addressed within the database of protein-mediated ET (in the case of Ru proteins) and water-mediated ET (for cytochrome b5 self-exchange) (45). If tunneling medium fluctuations wash out structural features, the tunneling medium could be described by an effective structureless tunneling barrier. To address this key issue, which has deep connections to the role of evolution in determining tunneling pathway structure (48, 51–54), we computed the metric (45)
| (14) |
where the variance (var) and the average (avg) are computed for all distinct ET species with the same transfer distance. A value of SRDA ≪ 1 at a given RDA means that an average barrier model that reproduces gives, to a good approximation, for all ET species at RDA. A value of SRDA ≥ 1 means that the for any single ET species is not representative of the for the other ET species at that distance (Figure 5).
Figure 5.
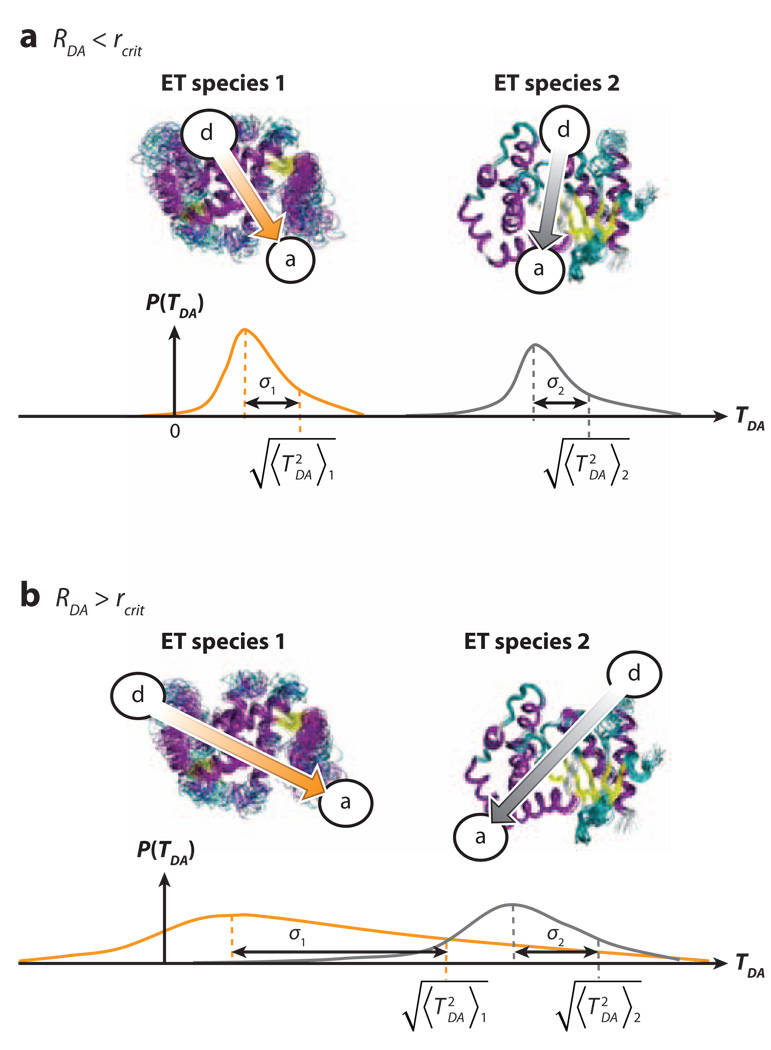
Schematic diagram explaining how donor-acceptor (DA) coupling fluctuations could wash out DA coupling structural differences with increasing DA distance. The diagram shows possible TDA probability densities for two pairs of different electron-transfer (ET) species, each pair having the same average values of RDA. σ1 and σ2 represent the root-mean-squared coupling fluctuations σTDA of each species in the pair. (a) For RDA < rcrit, coupling fluctuations are small and do not wash out structural differences in , i.e., . (b) For RDA > rcrit, the increase in coupling fluctuations could wash out structural differences in , i.e., SRDA>rcrit ≪ 1, leading to an average barrier limit. Surprisingly, our simulations do not observe this second regime (b) even though coupling fluctuations are large (45).
We find that SRDA is of order unity for all transfer distances (Figure 4b), indicating that the influence of structural differences between proteins on the mean-squared ET couplings is large. Even at large RDA, where dominates, the diversity of values at any distance is not washed out by structural fluctuations. Thus, the protein structure defines the tunneling rates, even when fluctuations dominate the ensemble of coupling values. This seems to be the case even for water-mediated interprotein ET (45).
The fluctuation analysis described here has been used mostly to study small-molecule and protein ET, although there is a growing body of related work on nucleic acids (see 55, and references therein). It will be interesting to use the parameters defined above to characterize fluctuations and structural disorder in DNA ET reactions. The DNA ET mechanism may vary from tunneling to thermally activated hopping, so that a simple tunneling element analysis may not describe the kinetics (44, 56, 57). Moreover, the folded structure of DNA does not show the wide variability found in proteins, so it may be interesting to study the dependency of Rcoh and SRDA on sequence, rather than on distance. The coherence parameter approach was recently applied to DNA charge transfer (58, 59).
MOLECULAR C-CLAMPS: INTERPOSING A WATER MOLECULE THAT MEDIATES TUNNELING BETWEEN DONOR AND ACCEPTOR
Modern synthetic tools allow the synthesis of tailored cleft or C-clamp structures, in which the dimensions of the C-clamp define the tunneling medium. The structure D-SSS-A (see Figure 3) has the dimensions and shape that will permit one water molecule to bridge between the donor and acceptor, but one DMSO molecule is too large to fit. The observed ET rate for D-SSS-A in water is much larger than that in DMSO. For the structure D-SRR-A, in which the dominant tunneling paths are through bonds, the ET rates are about the same in the two solvents (29).
WHAT ARE THE CHARACTERISTICS OF PROTEINS WITH LARGE VERSUS SMALL FLUCTUATIONS IN THE DONOR-ACCEPTOR TUNNELING INTERACTION, TDA?
Proteins are dynamical objects: Their geometries fluctuate so the interactions that they mediate between redox cofactors that bind to them also fluctuate. Figure 5 illustrates the small versus large DA fluctuation regimes. In the small fluctuation regime, the width of the TDA distributions for pairs of cofactors bound at different sites with the same DA separation distances (RDA) is narrow compared to the differences in the values. When fluctuations are large, the fluctuations may cause the differences in the mean-squared values to become quite similar.
4. THE FAST COUPLING–FLUCTUATION REGIME (τcoh ≤ τFC)
In this regime, TDA(t) fluctuates while the donor and acceptor are resonant, so the Franck-Condon approximation may not be valid. In this limit, the rate equation is more complex than suggested by Equation 11 because there is no clear separation between the CFC(t) and CTDA(t) timescales in Equation 1. Theoretical studies of how matrix element fluctuations influence ET rates demonstrate the existence of various kinetic regimes (8, 23, 60–68). In the fast fluctuation limit (τcoh < τFC) and for activationless (or nearly activationless) ET, the rate decreases with decreasing τcoh because fluctuations take the system out of the activationless regime. For large activation free energies, the fluctuations can enhance the rate. In general, this rate enhancement is more pronounced in the inverted region because of inelastic transitions, and the overall effect is to reduce the drop in rate with increasing energy gap (and to change the rate’s temperature dependence).
Inelastic tunneling introduces the exchange of energy between the tunneling electron and the bridge vibrations. If bridge vibrations are described quantum mechanically, the nonadiabatic rate expression is (8, 12, 65) kET = ∑i Pνi ∑f kET(νi → νf), where
| (15) |
assuming thermal equilibration of the vibrational degrees of freedom prior to ET. kET is a thermally weighted sum of individual rates kET(νi → νf), each associated with a νi-to-νf transition induced by the exchange of energy with the tunneling electron (νi and νf are initial and final bridge vibrational states, respectively). The statistical weights, Pνi, are Boltzmann populations of initial νi states. kET(νi → νf) in Equation 15 contains a vibronic tunneling matrix element 〈D; νi|T̂|A; νf〉 (T̂ is given in Equation 4) and a thermally weighted Franck-Condon factor ρFC(νi → νf). If the vibrational degrees of freedom associated with the donor and acceptor diabatic surfaces (Figure 1) are treated classically,
| (16) |
where ενi and ενf are the energies of bridge vibrational states νi and νf, and λ is the reorganization energy of the donor and acceptor diabatic surfaces (Figure 1). Equation 16 shows that the energy gap for elastic tunneling (ενi = ενf) is , regardless of the initial (final) bridge vibrational state. For inelastic tunneling (ενi ≠ ενf), the energy gap is shifted by an amount equal to the change in the bridge vibrational energy, given by , and it can have a strong effect on the ET rate. For an elastic ET reaction strongly in the inverted regime, the elastic ET rate is suppressed by the large activation energy; e.g., if . If a bridge vibration with frequency ℏω ≈ λ can interact with the tunneling electron, however, then inelastic tunneling is possible because the electron can deposit energy equal to λ in any state νi of the oscillator, exciting it to state νf with ενi − ενf = −ℏω = −λ. The corresponding rate kET(νi → νf) is activationless because its energy gap is reduced by λ, i.e., . Therefore, inelastic tunneling rates in the inverted region are enhanced, and the overall rate does not drop by the amount predicted for pure elastic tunneling. The effect is stronger for large bridges where the bridge vibrational spectrum is very dense (65). Enhanced ET rates in the inverted regime have been reported for small-molecule ET (69) and for biological ET (70), although it is not clear that the enhancement arises from inelastic tunneling.
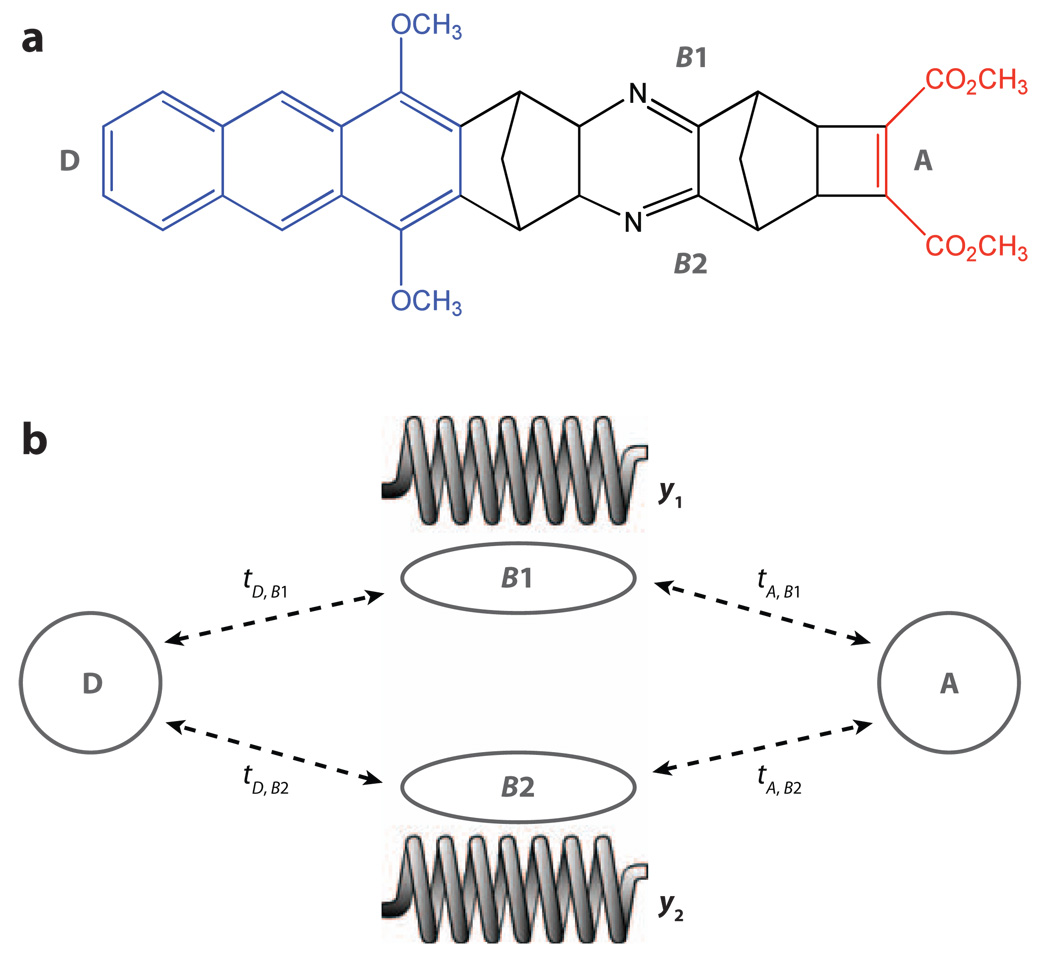
We now explore how tunneling interactions, 〈D; νi|T̂|A; νf〉 in Equation 4, change when the tunneling is inelastic. We consider a bridge with two parallel electronic pathways (bridge orbitals B1 and B2 both coupled to the D and A states; see Figure 6), where each bridge orbital energy is modulated by a pathway-specific bridge vibrational mode (y1 for B1 and y2 for B2). Assuming linear electronic-vibrational coupling, we have, in Equation 4, ĤB = ĤB(el) + ĤB(vi) + ĤB(e−v), where
| (17) |
| (18) |
and
| (19) |
Figure 6.
(a) A donor-bridge-acceptor supermolecule that might be used to realize a molecular double-slit experiment. (b) The vibrations of the bridge could act to measure which pathway the electron follows as it tunnels from the donor (D) to the acceptor (A), if some of the atoms in one pathway are isotopically substituted in order to create pathway-localized vibrations.
The tunneling process |D; νi〉 → |A; νf〉, where |νi〉 = |n(y1)〉|m(y2)〉 and |νf〉 = |n′(y1)〉|m′(y2)〉, is now described by the vibronic tunneling matrix element 〈D; n(y1)m(y2)|T̂|A; n′(y1)m′(y2)〉. Using Equation 4, one can show that the exchange of energy between the electron and the vibrations identifies the electronic path traversed by the electron. The reason is that, in this model, the vibrations are pathway specific (12, 71). Any elastic tunneling matrix element is the sum over the parallel pathway contributions (here, B1 and B2), which interfere coherently, e.g., 〈D; 0(y1), 0(y2)|T̂|A; 0(y1), 0(y1)〉 = Path(B1) + Path(B2). An inelastic matrix element, such as 〈D; 1(y1), 0(y2)|T̂|A; 0(y1), 0(y2)〉, comprises only Path(B1) (12, 71). Therefore, inelastic tunneling can erase the interference among coupling pathways; it is possible that such effects are relevant to protein ET (17). Recent studies have shown that, in a system in which elastic tunneling pathways interfere destructively and ET is symmetry forbidden, the enhancement of inelastic tunneling induced by exciting bridge vibrational modes with infrared irradiation can switch on ET (72). The field of inelastic tunneling ET reactions is closely related to inelastic tunneling spectroscopy (e.g., see 73, 74; reviewed in 75, 76).
A UNIMOLECULAR WHICH-WAY INTERFEROMETER
Leaving spatially localized vibrational energy behind on (or picking it up from) a tunneling pathway records the route that the electron traversed. Knowing this route with certainty removes all coupling pathways that do not include that specific coupling step (i.e., the group in which the vibrational energy was deposited) from the pathway sum (12). Figure 6 illustrates the concept of a molecular which-way interferometer. The key element is that spatially localized and distinguishable normal modes couple to bridge sites. Experiments to test these ideas are employing infrared excitation of bridge vibrational modes in a three-color experiment (77).
5. SOLVENT CONTROL OF ELECTRON TRANSFER
In the above sections, we explore nonadiabatic ET, in which the rate is limited by electron tunneling and tunneling fluctuations. It is well-known that for adiabatic ET, the rate-limiting step may be solvent relaxation, and the solvent fluctuation timescale becomes particularly important (1–7). The importance of solvent dynamics in the intermediate to nonadiabatic limits has not been well appreciated, however. Recent studies of the transition from solvent polarization control to tunneling control of ET, using the U-shaped molecules shown in Figure 7, are elucidating the relevant timescale considerations for such ET processes.
Figure 7.
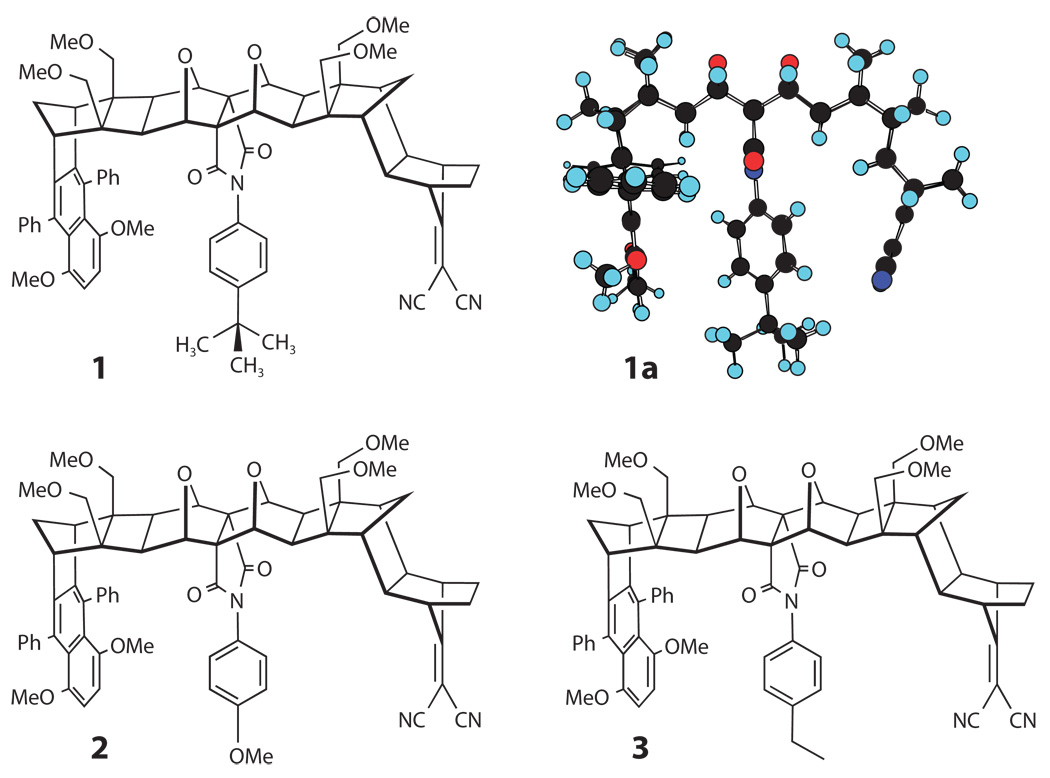
Molecular structures for the three donor-bridge-acceptor supermolecules, which have different pendant groups in the line of sight between the naphthalenic donor and the dicyanoethylene acceptor units, that were studied by Paddon-Row, Waldeck, and coworkers.
For the U-shaped molecules shown in Figure 7, TDA is smaller than kBT, but not that much smaller, and it is possible to observe a change in ET mechanism by changing the solvent friction. Three different limiting regimes, or mechanisms, are observed in ET reactions: nonadiabatic ET, adiabatic ET, and solvent-controlled ET. Criteria for transitions between the adiabatic and nonadiabatic regime are related to the nature of the nuclear motion through the crossing (e.g., ballistic or overdamped) and can be formulated in terms of the Landau-Zener curve-crossing problem (1–7). In the nonadiabatic case (discussed in the previous sections), the electronic coupling is very weak in the sense that the system has to move through the curve-crossing region many times before the electronic state changes from the donor to the acceptor (see Figure 1). In the discussion that follows, we use Equation 11 for the nonadiabatic rate constant and denote the nonadiabatic rate kNA rather than kET. Furthermore, we assume that in addition to the classical mode that modulates the DA energy gap (UD and UA in Figure 1), there is a high-frequency quantum mode that also modulates the gap (with reorganization energy λ̃ and frequency ω̃ = 2πν̃). In this case the Franck-Condon factor has both classical mode and quantum mode contributions, and the nonadiabatic rate constant becomes
| (20) |
where λ is the reorganization energy of the classical mode of Figure 1 , and S = λ̃/hν̃ is the Huang-Rhys factor associated with the reorganization energy and frequency of the quantum mode (1–7). Equation 20 assumes that the initial vibrational state of the quantum mode is the ground state (i.e., hν̃ ≫ kBT). The Franck-Condon factor in Equation 20 is similar to ρFC(νi → νf) of Equation 16. Both Franck-Condon factors describe the exchange of energy between the electron and quantum vibrations of the system. In Equation 16 the vibrations modulate the DA coupling, whereas in Equation 20, they modulate the DA energy gap (8, 65).
In the adiabatic case of large coupling, the reaction proceeds by nuclear motion through the transition state on a single electronic potential energy surface (the lower adiabatic energy surface arising from the coupling TDA between UD and UA in Figure 1). The effect of TDA on the rate constant is manifest only through its role in determining the adiabatic surface energy barrier, which is less than Eact of Figure 1. In the solvent-controlled limit, the electronic coupling may still be small; however, the rate constant is affected by frictional coupling. In this case, the characteristic time spent in the curve-crossing region is long enough that the electronic state changes from D to A for nearly every approach to the DA crossing, even though the coupling is weak. Hence the reaction appears adiabatic in the sense that the rate is limited by nuclear dynamics rather than by the electron-tunneling probability.
Zusman (78, 79) generalized the rate constant expression for ET, kET, to describe a transition between the normal nonadiabatic limit kNA and a solvent-controlled limit kSC, namely
| (21) |
which shows that the measured ET rate kET can be limited by either the electronic motion (where kNA is small) or the nuclear motion (when kSC is small). The slower process is rate controlling. In the classical limit he found
| (22) |
in which the ET rate is proportional to the solvation rate, 1/τs, where τs is the solvation time. It is the characteristic time that it takes for the solvent to respond to a change in the charge distribution of a solute and has been quantified in a number of different ways (vide infra). In Equation 22, ΔG≠ = (ΔG + λ)2/4λ (in the notation of Figure 1, ). Because the solvation time τs increases dramatically with decreasing temperature, especially in viscous solvents, the solvation time becomes more important as the temperature is lowered. Zusman used the inequality
| (23) |
to assess when the solvent friction should be important for the ET rate. In the context of our previous discussion of fluctuating DA couplings, |TDA| in Equation 23 should be interpreted as . For small driving forces, |ΔG| ≪ λ, this criterion is . Hence, the solvent-controlled regime may be interpreted to arise from a solvent-driven change of adiabaticity, characterized by an adiabaticity parameter g, where (80)
| (24) |
When g ≫ 1, the reaction is solvent controlled, and when g ≪ 1, it is nonadiabatic.
In addition to Zusman’s model, there have been several theoretical approaches to the problem of solvent-controlled ET and to the transition from nonadiabatic to adiabatic ET [e.g., for reviews, see Calef & Wolynes (81), Morillo & Cukier (82) Hynes (83), Sumi and Marcus (84), Onuchic and coworkers (86), Sparpaglione & Mukamel (87, 88), Rips & Jortner (89), and Jortner & Bixon (4)]. Sumi & Marcus (84) considered the combined effects of intramolecular vibrations and diffusive solvent orientational motions on ET. They described the reaction as proceeding along a two-dimensional effective potential energy surface, V(q, X). The coordinate X corresponds to the solvent polarization (the polarization response of the solvent to changes of the charge distribution), and q is an intramolecular vibrational coordinate, which includes the fast nuclear motions typical of an ET reaction in the nonadiabatic or adiabatic limit. To find the reaction rate, they solved the Fokker-Planck equation for diffusive motion along X and treated the motion along q through a rate constant k(X) that depends on the fast motions in the normal way (e.g., Equation 1) and depends parametrically on X (84, 85).
It was found that the electron transfer rate of the U-shaped molecules in Figure 7 in fast solvents (90, 91) could be modeled by using Equation 21 with an internal (quantum mode) reorganization energy λ̃ of 0.65 eV and an effective quantum mode frequency of 1600 cm−1. Comparison with solvation models indicated that the solvent (classical mode) reorganization energy λ was between 1.2 eV and 1.4 eV for molecules 1 and 2 in Figure 7 in NMA (N-methylacetamide) and NMP (N-methylpropionamide) (vide infra). The ratio λ̃/λ is thus approximately 0.5, which places these reactions in the narrow reaction window limit of Sumi & Marcus (84). This limit is also one in which Zusman’s predictions (Equations 21 and 22) should apply. Sumi & Marcus pointed out the nonexponential character in the narrow reaction window limit; however, Zusman’s treatment does not address this feature.
By studying chemical systems in different solvents with a range of solvent relaxation times, Waldeck and coworkers (29, 85, 92, 93) explored the limit in which the ET time τET = 1/kET is controlled by τs, which is commonly modeled by a characteristic dielectric relaxation (Debye) time (79) or the characteristic time measured in a dynamic Stokes shift experiment (94). They reported a transition from the nonadiabatic ET regime to the solvent-controlled limit and showed how it depends on the solvent relaxation time and the electronic coupling strength. The molecules shown in Figure 7 (molecules 1, 2, and 3) have empirically determined electronic couplings in the range of tens to hundreds of wave numbers (depending on the pendant group that is placed in the cleft). In solvents with rapid polarization fluctuations, as measured by their dynamic Stokes shift relaxation times, the ET followed a first-order (i.e., exponential) rate and proceeded in the nonadiabatic limit. However, in slow solvents (e.g., NMP), the rate displayed a nonexponential behavior that was controlled by the solvent relaxation rate.
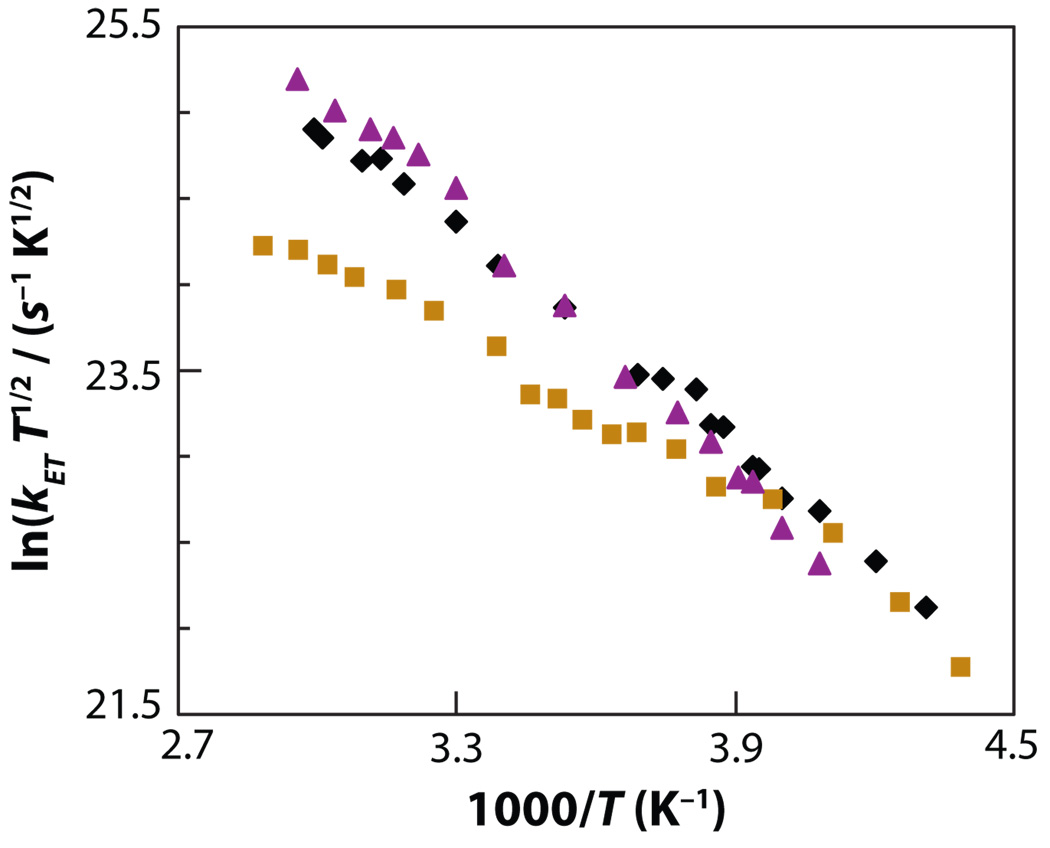
Because the rate was nonexponential, Waldeck and coworkers (93, 95) defined a rate constant kET from the inverse of the correlation time for the decay of the reactant state (i.e., the time integral of the temporal profile of the initially excited state), so it was possible to plot the ln(kET T1/2) versus the inverse of the temperature T (see Figure 8). The rate constants for all three compounds were similar at low temperatures and deviate from one another at higher temperatures. The deviation occurred first for compound 1 (at 260 K to 270 K; NMP solvation time of approximately 240 ps) and then for compounds 2 and 3 at higher temperature (above 310 K; NMP solvation time of approximately 55 ps). Thus, the trend in characteristic times for the different solutes correlated with the change in electronic coupling |TDA| (see Table 1) that was determined for these three molecules in aromatic solvents and acetonitrile, which have a rapid solvation response (τs is small).
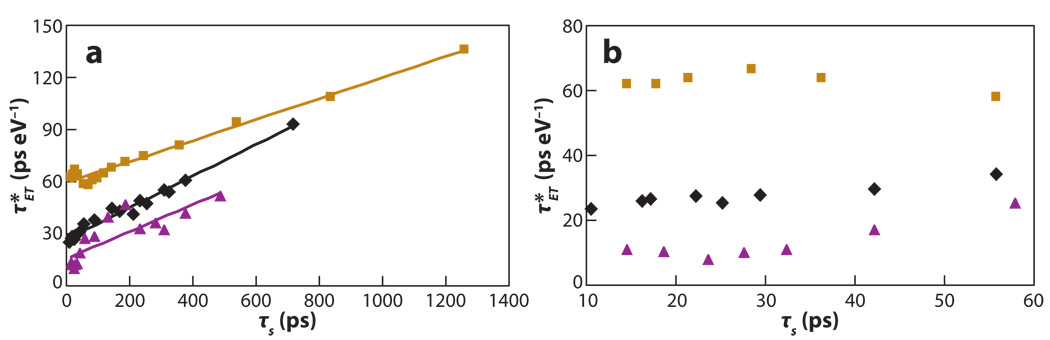
Figure 8.
Experimental electron transfer rate constants for compounds 1 (gold squares), 2 (purple triangles), and 3 (black diamonds) in NMP plotted versus the inverse of the temperature. Figure adapted with permission from Reference 93.
Table 1.
Fitting parameters for compound 1, 2, and 3 in N-methylpropionamide (NMP) at 295Ka
| Compound | |TDA| (cm−1) | λ (eV) | ΔG (eV) |
|---|---|---|---|
| 1 | 90 | 1.24 | −0.35 |
| 2 | 273 | 1.59 | −0.57 |
| 3 | 147 | 1.50 | −0.52 |
See Reference 29.
The data in Figure 8 show that the ET rate of compound 2 is higher than that of compound 3 in NMP, and compound 1 has the lowest ET rate. This trend is reflected in the electronic coupling values extracted from a fit of the rate data in solvents with a fast solvation response by the semiclassical equation (Equation 20; see Table 1). The electronic coupling magnitude of compound 2 with a methoxy substituted pendant group is the largest among the molecules, and this large value can be rationalized in terms of that group’s electron affinity and ionization potential. The reorganization energy and Gibbs free energy parameters in Table 1 vary somewhat among the compounds, but the activation barrier for the reaction ΔG≠ = (ΔG + λ)2/4λ is similar for the three structures at 295 K (from 0.160 eV to 0.164 eV). The similarity of the activation barriers (and energetic parameters) is consistent with the similar size, shape, and chemical structure of the molecules. This similarity is found even though the rate data appear to deviate substantially from one another as the temperature changes.
The self-consistency of this analysis can be assessed by considering the dependency of the rate constant on the solvation time. The different kinetic models predict that the ET rate constant is inversely proportional to the solvation time in the solvent-controlled regime, but that it becomes independent of solvent friction when the solvation time is short.
Figure 9 plots a reduced ET time (Equation 25), defined in terms of Zusman’s timescale (78, 96, 97) as
| (25) |
versus the solvation time of NMP. For all of these molecules, a linear correlation exists between and the solvation time at low temperature, and the ET times become independent of the solvation time at high temperatures. The Sparpaglione-Mukamel treatment (87, 88) is similar. Both models fail to predict the observed slopes quantitatively, although they otherwise provide an accurate description of the data. As discussed above, when g ≫ 1 the reaction is solvent controlled, and when g ≪ 1 it is nonadiabatic. This analysis predicts that the solvent-controlled regime is reached when τS ≫ 24 ps for compound 1, τS ≫ 2 ps for compound 2, and τS ≫ 6 ps for compound 3 in NMP. The experimental results (Figure 7) indicate that compounds 2 and 3 are in the solvent-controlled limit (coalescence of rates) when τS is near 56 ps, and for compound 1 the solvent-controlled limit is reached at around 240 ps. Thus, the trend in the rate data can be understood via the Zusman model.
Figure 9.
Plot of (Equation 25) versus τS for compound 1 (gold squares), compound 2 (purple triangles), and compound 3 (black diamonds) in NMP. (a) The plot over the whole range of data. (b) Expansion of the plot in the high-temperature region 0 ≤ τS ≤ 60 ps (60 ps corresponds to the room temperature) for compounds 1, 2, and 3. Figure adapted with permission from Reference 95.
6. GATED ELECTRON TRANSFER VERSUS DYNAMICAL CONTROL
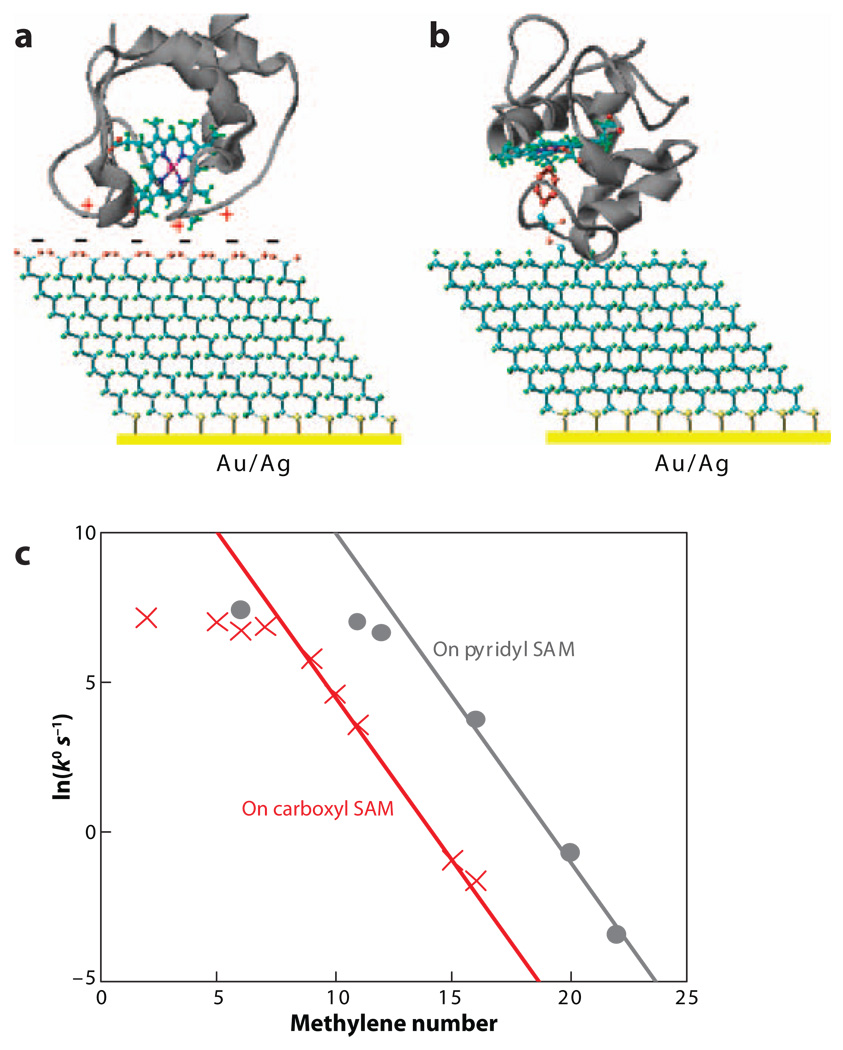
If any of the timescales discussed above is longer than the ET time, the ET kinetics may be multi-exponential or gated. Figure 10 shows rate constants for cytochrome c immobilized in two different ways in monolayer films (electrostatic adsorption and ligation of the iron heme) (98–100). Although the thickness dependencies of the rates are shifted from one another, determined by the immobilization, both tethering schemes show an exponential distance dependence for thick films and a weaker distance dependence for thin films. A number of studies (101–107) have found a change in the ET reaction mechanism with distance from an electrode for immobilized proteins. For cytochrome c immobilized by heme ligation, the tunneling regime is reached for long tethers (n ≥ 12) and is controlled by medium relaxation for short tethers (98, 108). The soft distance dependence of the rate and its sensitivity to the medium friction can be rationalized in different ways. For a gated mechanism, the conformation changes are slow with respect to the ET time and limit the rate for thin films; i.e., the system fluctuates to a geometry of large DA coupling. Alternatively, the experimental data can be explained by a change in the ET mechanism from nonadiabatic at long distances to solvent controlled at shorter distances.
Figure 10.
Protein immobilized on self-assembled monolayer (SAM) surfaces: (a) Cytochrome c is absorbed electrostatically to a SAM of carboxylic acid terminated thiols, and (b) cytochrome c is tethered to a SAM by a pyridyl group that replaces Met 80 as an axial ligand. (c) Plots of ln(k°) versus the number of methylene groups in alkyl SAMs on Au.
Yue and coworkers (99) distinguished tunneling control from relaxation control mechanisms by measuring the dependence of the ET rate on the overpotential. For electrostatic assemblies on silver, they found no dependence on the overpotential, indicating that ET is gated. For ligated-protein assemblies on gold electrodes, they found an overpotential dependence, suggesting that ET proceeds in an effective adiabatic/medium-controlled limit. Yue and coworkers (99) also performed a temperature-dependent study of protein ET and found that the activation barrier has contributions from both the ET reorganization energy and the medium friction’s apparent activation. These findings suggest that the so-called unusual distance dependence of the heterogeneous ET rate, reported for different proteins immobilized on metals, may be explained by the same underlying principles.
7. CONCLUSIONS AND PROSPECTS
Molecular fluctuations in ET systems not only enable access to the activated complex, but also cause DA electronic interactions to fluctuate. The magnitudes and timescales of these fluctuations can determine the reaction mechanism. The interplay among electronic coupling and nuclear fluctuations is particularly important in biological electron transfer because protein and water bridges dominate the electron-tunneling pathways. Simulations indicate that the Condon approximation appears to be valid for most of the proteins studied so far. For long-range ET, the rates tend to be controlled by fluctuations of the coupling away from its mean value. Indeed, because of destructive interferences among multiple paths, the mean coupling value tends to be quite small. Small-molecule studies show that solvent relaxation can change the adiabaticity of ET and, for highly sluggish solvents, limit the ET rate even for intermediate (approximately 100 cm−1) couplings. Such effects are expected to be important for proteins in which a wide distribution of relaxation processes and timescales are present (see 109). Electrochemical studies of immobilized proteins are being used to reveal how the ET rate changes from the tunneling limit to relaxation control. Electron tunneling, gated ET, and solvent relaxation controlled ET are now well documented in both small-molecule and protein systems alike.
It is clear that inelastic tunneling, in addition to modifying the free energy dependence of ET kinetics, could have substantial effects on coupling pathway interferences. The quantitative theoretical framework that has emerged from these recent studies suggests that one may now be able to formulate general principles linking the structure, fluctuations, and function with ET kinetics. This framework also appears likely to frame new questions in molecular biophysical chemistry. For example, single-molecule studies of ET suggest the possibility of directly probing the role of fluctuations in ET reactions experimentally. Xie and colleagues (110) have reported measurements of ET in a flavoprotein, and Barbara and coworkers (111) have reported single-molecule measurements of ET at electrodes. Studies of this kind in systems with structural flexibility offer the opportunity to explore, in great detail, the role of fluctuations in bioenergetics. Indeed, Rubtsov and coworkers (77) just reported an ultrafast multipulse experiment that uses infrared excitation to perturb electron DA interactions during the course of an ET reaction.
FUTURE ISSUES
Experimental probes of pathway coherence, including inelastic labeling of paths, should be developed.
Experiments should be designed that are directly sensitive to coupling fluctuations.
Electron tunneling across organized water structures needs to be investigated.
Experiments should be designed that access multiple ET regimes in single systems: few-path tunneling, multipath tunneling, fluctuation-controlled tunneling, protein-gated tunneling, solvent relaxation limited transport, and carrier injection limited transport.
ACKNOWLEDGMENTS
S.S.S. thanks the University of Cyprus, D.H.W. thanks the NSF (CHE-0718755), and D.N.B. thanks the NSF (CHE-0718043) and the NIH (GM-48043) for support of these research activities.
Glossary
- ET
electron transfer
- TDA
tunneling matrix element
- σTDA
variance of the tunneling matrix element
- Rcoh
coherence parameter
- NMP
N-methylpropionamide
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Marcus RA, Sutin N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta. 1985;811:265–322. [Google Scholar]
- 2.Jortner J, Ratner MA. Molecular Electronics. Oxford: Blackwell Science; 1997. [Google Scholar]
- 3.Kuznetsov AM, Ulstrup J. Electron Transfer in Chemistry and Biology. Chichester, UK: Wiley; 1999. [Google Scholar]
- 4.Jortner J, Bixon M, editors. Electron Transfer: From Isolated Molecules to Biomolecules. Adv. Chem. Phys. Ser. New York: Wiley Intersci.; 1999. pp. 106–107. [Google Scholar]
- 5.May V, Kuhn O. Charge and Energy Transfer Dynamics in Molecular Systems. Berlin: Wiley-VCH; 2000. [Google Scholar]
- 6.Balzani V, Piotrowiak P, Rodgers MAJ, Mattay J, Astruc D, et al. Electron Transfer in Chemistry. Vols. I–V. Weinheim: Wiley-VCH; 2001. [Google Scholar]
- 7.Nitzan A. Chemical Dynamics in Condensed Phases. Oxford: Oxford Univ. Press; 2006. [Google Scholar]
- 8.Troisi A, Nitzan A, Ratner MA. A rate constant expression for charge transfer through fluctuating bridges. J. Chem. Phys. 2003;119:5782–5788. [Google Scholar]
- 9.Skourtis SS, Lin J, Beratan DN. The effects of bridge motion on electron transfer reactions mediated by tunneling. In: Starikov EB, Lewis JP, Tanaka S, editors. Modern Methods for Theoretical Physical Chemistry of Biopolymers. Boston: Elsevier; 2006. pp. 357–382. [Google Scholar]
- 10.Skourtis SS, Beratan DN, Onuchic JN. The two-state reduction for electron and hole transfer in bridge-mediated electron-transfer reactions. Chem. Phys. 1993;176:501–520. [Google Scholar]
- 11.Skourtis SS, Beratan DN. Theories of structure-function relationships for bridge-mediated electron transfer reactions. Adv. Chem. Phys. 1999;106:377–452. [Google Scholar]
- 12.Skourtis SS, Waldeck DH, Beratan DN. Inelastic electron tunneling erases coupling-pathway interferences. J. Phys. Chem. B. 2004;108:15511–15518. [Google Scholar]
- 13.Teklos A, Skourtis SS. Comparative studies of perturbative methods for computing electron transfer tunneling matrix elements for a nonorthogonal basis set. J. Chem. Phys. 2006;125:244103–244109. doi: 10.1063/1.2403859. [DOI] [PubMed] [Google Scholar]
- 14.Xie Q, Archontis G, Skourtis SS. Protein electron transfer: a numerical study of tunneling through fluctuating bridges. Chem. Phys. Lett. 1999;312:237–246. [Google Scholar]
- 15.Skourtis SS, Archontis G, Xie Q. Electron transfer through fluctuating bridges: on the validity of the superexchange mechanism and time-dependent tunneling matrix elements. J. Chem. Phys. 2001;115:9444–9462. [Google Scholar]
- 16.Cave RJ, Newton MD. Generalization of the Mulliken-Hush treatment for the calculation of electron transfer matrix elements. Chem. Phys. Lett. 1996;249:15–19. [Google Scholar]
- 17.Stuchebrukhov AA. Toward ab initio theory of long-distance electron tunneling in proteins: tunneling currents approach. Adv. Chem. Phys. 2001;118:1–44. [Google Scholar]
- 18.Troisi A, Ratner MA, Zimmt MB. Dynamic nature of the intramolecular electronic coupling mediated by a solvent molecule: a computational study. J. Am. Chem. Soc. 2004;126:2215–2224. doi: 10.1021/ja038905a. [DOI] [PubMed] [Google Scholar]
- 19.Skourtis SS, Balabin IA, Kawatsu T, Beratan DN. Protein dynamics and electron transfer: electronic decoherence and non-Condon effects. Proc. Natl. Acad. Sci. USA. 2005;102:3552–3557. doi: 10.1073/pnas.0409047102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balabin IA, Onuchic JN. Dynamically controlled protein tunneling paths in photosynthetic reaction centers. Science. 2000;290:114–117. doi: 10.1126/science.290.5489.114. [DOI] [PubMed] [Google Scholar]
- 21.Nishioka H, Kimura A, Yamato T, Kawatsu T, Kakitani T. Interference, fluctuation, and alternation of electron tunneling in protein media. 2. Non-Condon theory for the energy gap dependence of electron transfer rate. J. Phys. Chem. B. 2005;109:15621–15635. doi: 10.1021/jp051606i. [DOI] [PubMed] [Google Scholar]
- 22.Bixon M, Jortner J. Effects of configurational fluctuation on electronic coupling for charge transfer dynamics. Russ. J. Electrochem. 2003;39:3–8. [Google Scholar]
- 23.Berlin YA, Grozema FC, Siebbeles LDA, Ratner MA. Charge transfer in donor-bridge-acceptor systems: static disorder, dynamic fluctuations, and complex kinetics. J. Phys. Chem. C. 2008;112:10988–11000. [Google Scholar]
- 24.Jordan KD, Paddon-Row MN. Analysis of the interactions responsible for long-range through-bond-mediated electronic coupling between remote chromophores attached to rigid polynorbornyl bridges. Chem. Revs. 1992;92:395–410. [Google Scholar]
- 25.Jordan KD, Paddon-Row MN. Long-range interactions in a series of rigid nonconjugated dienes. 1. Distance dependence of the π+, π− and π* splittings determined by ab initio calculations. J. Phys. Chem. 1992;96:1188–1196. [Google Scholar]
- 26.Curtiss LA, Naleway CA, Miller JR. Superexchange pathway calculation of long-distance electronic coupling in H2C(CH2)M-2CH2 chains. Chem. Phys. 1993;176:387–405. [Google Scholar]
- 27.Liang CX, Newton MD. Ab initio studies of electron transfer: pathway analysis of effective transfer integrals. J. Phys. Chem. 1992;96:2855–2866. [Google Scholar]
- 28.Newton MD. Modeling donor/acceptor interactions: combined roles of theory and computation. Int. J. Quantum Chem. 2000;77:255–263. [Google Scholar]
- 29.Chakrabarti S, Liu M, Waldeck DH, Oliver AM, Paddon-Row MN. Solvent dynamical effects on electron transfer in U-shaped donor-bridge-acceptor molecules. J. Phys. Chem. A. 2009;113:1040–1048. doi: 10.1021/jp807412c. [DOI] [PubMed] [Google Scholar]
- 30.Kumar K, Lin Z, Waldeck DH, Zimmt MB. Electronic coupling in C-clamp-shaped molecules: solvent-mediated superexchange pathways. J. Am. Chem. Soc. 1996;118:243–244. [Google Scholar]
- 31.Read I, Napper A, Kaplan R, Zimmt MB, Waldeck DH. Solvent-mediated electronic coupling: the role of solvent placement. J. Am. Chem. Soc. 1999;121:10976–10986. [Google Scholar]
- 32.Napper AM, Read I, Waldeck DH, Kaplan RW, Zimmt MB. Electron transfer reactions of C-shaped molecules in alkylated aromatic solvents: evidence that the effective electronic coupling magnitude is temperature-dependent. J. Phys. Chem. A. 2002;106:4784–4793. [Google Scholar]
- 33.Zimmt MB, Waldeck DH. Exposing solvent’s roles in electron transfer reactions: tunneling pathway and solvation. J. Phys. Chem. A. 2003;107:3580–3597. [Google Scholar]
- 34.Wolfgang J, Risser SM, Priyadarshy S, Beratan DN. Secondary structure conformations and long range electronic interactions in oligopeptides. J. Phys. Chem. B. 1997;101:2986–2991. [Google Scholar]
- 35.Daizadeh I, Medvedev ES, Stuchebrukhov AA. Effect of protein dynamics on biological electron transfer. Proc. Natl. Acad. Sci. USA. 1997;94:3703–3708. doi: 10.1073/pnas.94.8.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ungar LW, Newton MD, Voth GA. Classical and quantum simulation of electron transfer through a polypeptide. J. Phys. Chem. B. 1999;103:7367–7382. [Google Scholar]
- 37.Miller NE, Wander MC, Cave RJ. A theoretical study of the electronic coupling element for electron transfer in water. J. Phys. Chem. A. 1999;103:1084–1093. [Google Scholar]
- 38.Castner EW, Kennedy D, Cave RJ. Solvent as electron donor: Donor/acceptor electronic coupling is a dynamical variable. J. Phys. Chem. A. 2000;104:2869–2885. [Google Scholar]
- 39.Troisi A, Orlandi G. Hole migration in DNA: a theoretical analysis of the role of structural fluctuations. J. Phys. Chem. B. 2002;106:2093–2101. [Google Scholar]
- 40.Kawatsu T, Kakitani T, Yamato T. Destructive interference in the electron tunneling through protein media. J. Phys. Chem. B. 2002;106:11356–11366. [Google Scholar]
- 41.Lockwood DM, Cheng YK, Rossky PJ. Electronic decoherence for electron transfer in blue copper proteins. Chem. Phys. Lett. 2001;345:159–165. [Google Scholar]
- 42.Warshel A, Chu ZT, Parson WW. Dispersed polaron simulations of electron transfer in photosynthetic reaction centers. Science. 1989;246:112–116. doi: 10.1126/science.2675313. [DOI] [PubMed] [Google Scholar]
- 43.Warshel A, Parson WW. Dynamics of biochemical and biophysical reactions: insight from computer simulations. Q. Rev. Biophys. 2001;34:563–679. doi: 10.1017/s0033583501003730. [DOI] [PubMed] [Google Scholar]
- 44.Teklos A, Skourtis SS. Electron transfer through time dependent bridges: differences between Franck-Condon and Born-Oppenheimer breakdown. Chem. Phys. 2005;319:52–68. [Google Scholar]
- 45.Balabin IA, Beratan DN, Skourtis SS. Persistence of structure over fluctuations in biological electron-transfer reactions. Phys. Rev. Lett. 2008;101:158102. doi: 10.1103/PhysRevLett.101.158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray HB, Winkler JR. Electron tunneling through proteins. Q. Rev. Biophys. 2003;36:341–372. doi: 10.1017/s0033583503003913. [DOI] [PubMed] [Google Scholar]
- 47.Lin JP, Balabin IA, Beratan DN. The nature of aqueous tunneling pathways between electron-transfer proteins. Science. 2005;310:1311–1313. doi: 10.1126/science.1118316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prytkova TR, Kurnikov IV, Beratan DN. Coupling coherence distinguishes structure sensitivity in protein electron transfer. Science. 2007;315:622–625. doi: 10.1126/science.1134862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beratan DN, Betts JN, Onuchic JN. Protein electron-transfer rates set by the bridging secondary and tertiary structure. Science. 1991;252:1285–1288. doi: 10.1126/science.1656523. [DOI] [PubMed] [Google Scholar]
- 50.Beratan DN, Skourtis SS, Balabin IA, Balaeff A, Keinan S, et al. Steering electrons on moving pathways. Accounts Chem. Res. 2009;42:1669–1678. doi: 10.1021/ar900123t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edwards PP, Gray HB, Lodge MTJ, Williams RJP. Electron transfer and electronic conduction through an intervening medium. Angew. Chem. Int. Ed. Engl. 2008;47:6758–6765. doi: 10.1002/anie.200703177. [DOI] [PubMed] [Google Scholar]
- 52.Onuchic JN, Kobayashi C, Miyashita O, Jennings P, Baldridge KK. Exploring biomolecular machines: energy landscape control of biological reactions. Philos. Trans. R. Soc. Lond. Ser. B. 2006;361:1439–1443. doi: 10.1098/rstb.2006.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moser CC, Page CC, Dutton PL. Darwin at the molecular scale: selection and variance in electron tunnelling proteins including cytochrome c oxidase. Philos. Trans. R. Soc. Lond. Ser. B. 2006;361:1295–1305. doi: 10.1098/rstb.2006.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones ML, Kurnikov IV, Beratan DN. The nature of tunneling pathway and average packing density models for protein-mediated electron transfer. J. Phys. Chem. A. 2002;106:2002–2006. [Google Scholar]
- 55.Schuster GB, editor. Topics in Current Chemistry. Vols. 236–237. Berlin: Springer; 2004. [Google Scholar]
- 56.Skourtis SS. Electron transfer through time-dependent bridges: tunneling by virtual transitions that break the Born-Oppenheimer approximation. Chem. Phys. Lett. 2003;372:224–231. [Google Scholar]
- 57.Hatcher E, Balaeff A, Keinan S, Venkatramani R, Beratan DN. PNA versus DNA: effects of structural fluctuations on electronic structure and hole-transport mechanisms. J. Am. Chem. Soc. 2008;130:11752–11761. doi: 10.1021/ja802541e. [DOI] [PubMed] [Google Scholar]
- 58.Gutierrez R, Caetano RA, Woiczikowski BP, Kubar T, Elstner M, Cuniberti G. Charge transport through biomolecular wires in a solvent: bridging molecular dynamics and model Hamiltonian approaches. Phys. Rev. Lett. 2009;102:208102. doi: 10.1103/PhysRevLett.102.208102. [DOI] [PubMed] [Google Scholar]
- 59.Woiczikowski PB, Kubar T, Gutierrez R, Caetano RA, Cuniberti G, Elstner M. Combined density functional theory and Landauer approach for hole transfer in DNA along classical molecular dynamics trajectories. J. Chem. Phys. 2009;130:215104. doi: 10.1063/1.3146905. [DOI] [PubMed] [Google Scholar]
- 60.Onuchic JN, DaGama AAS. Influence of intersite modes on the exchange interaction in electron-transfer at large distances. Theor. Chim. Acta. 1986;69:89–100. [Google Scholar]
- 61.Mikkelsen KV, Ulstrup J, Zakaraya MG. Free-energy dependence of the electronic factor in biological long-range electron transfer. J. Am. Chem. Soc. 1989;111:1315–1319. [Google Scholar]
- 62.Goldstein RF, Franzen S, Bialek W. Nonperturbative approach to non-Condon effects: Must a nonadiabatic transition always occur at the potential surface crossing? J. Phys. Chem. 1993;97:11168–11174. [Google Scholar]
- 63.Tang J. Effects of a fluctuating electronic coupling on electron-transfer rate. J. Chem. Phys. 1993;98:6263–6266. [Google Scholar]
- 64.Goychuk IA, Petrov EG, May V. Bridge-assisted electron-transfer driven by dichotomically fluctuating tunneling coupling. J. Chem. Phys. 1995;103:4937–4944. [Google Scholar]
- 65.Medvedev ES, Stuchebrukhov AA. Inelastic tunneling in long-distance biological electron transfer reactions. J. Chem. Phys. 1997;107:3821–3831. [Google Scholar]
- 66.Liao JL, Voth GA. Numerical approaches for computing nonadiabatic electron transfer rate constants. J. Chem. Phys. 2002;116:9174–9187. [Google Scholar]
- 67.Milischuk A, Matyushov DV. Non-Condon theory of nonadiabatic electron transfer reactions in V-shaped donor-bridge-acceptor complexes. J. Chem. Phys. 2003;118:5596–5606. [Google Scholar]
- 68.Jang SJ, Newton MD. Theory of torsional non-Condon electron transfer: a generalized spin-boson Hamiltonian and its nonadiabatic limit solution. J. Chem. Phys. 2005;122:024501. doi: 10.1063/1.1828431. [DOI] [PubMed] [Google Scholar]
- 69.Rehm D, Weller A. Kinetics of fluorescence quenching by electron and H-atom transfer. Isr. J. Chem. 1970;8:259–271. [Google Scholar]
- 70.Mines GA, Bjerrum MJ, Hill MG, Casimiro DR, Chang IJ, et al. Rates of heme oxidation and reduction in Ru(His33)cytochrome c at very high driving forces. J. Am. Chem. Soc. 1996;118:1961–1965. [Google Scholar]
- 71.Skourtis SS, Beratan DN. A molecular double slit paradigm. AIP Conf. Proc. 2007;963:809–812. doi: 10.1063/1.2836174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao D, Skourtis SS, Rubtsov IV, Beratan DN. Turning charge transfer on and off in a molecular interferometer with vibronic pathways. Nano Lett. 2009;9:1818–1823. doi: 10.1021/nl8037695. [DOI] [PubMed] [Google Scholar]
- 73.Maddox JB, Harbola U, Liu N, Silien C, Ho W, et al. Simulation of single molecule inelastic electron tunneling signals in paraphenylene-vinylene oligomers and distyrylbenzene[2.2]paracyclophanes. J. Phys. Chem. A. 2006;110:6329–6338. doi: 10.1021/jp061590b. [DOI] [PubMed] [Google Scholar]
- 74.Troisi A, Beebe JM, Picraux LB, van Zee RD, Stewart DR, et al. Tracing electronic pathways in molecules by using inelastic tunneling spectroscopy. Proc. Natl. Acad. Sci. USA. 2007;104:14255–14259. doi: 10.1073/pnas.0704208104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galperin M, Ratner MA, Nitzan A. Molecular transport junctions: vibrational effects. J. Phys. Condens. Matter. 2007;19:103201. [Google Scholar]
- 76.Kohler S, Lehmann J, Hanggi P. Driven quantum transport on the nanoscale. Phys. Rep. 2005;406:379–443. [Google Scholar]
- 77.Lin Z, Lawrence CM, Xiao D, Kireev VV, Skourtis SS, et al. Modulating unimolecular charge transfer by exciting bridge vibrations. J. Am. Chem. Soc. 2009;131:18060–18062. doi: 10.1021/ja907041t. [DOI] [PubMed] [Google Scholar]
- 78.Zusman LD. Outer-sphere electron transfer in polar solvents. Chem. Phys. 1980;49:295–304. [Google Scholar]
- 79.Zusman LD. Dynamical solvent effects in electron-transfer reactions. Z. Phys. Chem. 1994;186:1–29. [Google Scholar]
- 80.Beratan DN, Onuchic JN. Adiabaticity and nonadiabaticity in bimolecular outer-sphere charge-transfer reactions. J. Chem. Phys. 1988;89:6195–6203. [Google Scholar]
- 81.Calef DF, Wolynes PG. Classical solvent dynamics and electron transfer. 1. Continuum theory. J. Phys. Chem. 1983;87:3387–3400. [Google Scholar]
- 82.Morillo M, Cukier RI. The transition from nonadiabatic to solvent controlled adiabatic electron transfer kinetics: the role of quantum and solvent dynamics. J. Chem. Phys. 1988;89:6736–6743. [Google Scholar]
- 83.Hynes JT. Outer-sphere electron-transfer reactions and frequency dependent friction. J. Phys. Chem. 1986;90:3701–3706. [Google Scholar]
- 84.Sumi H, Marcus RA. Dynamic effects in electron-transfer reactions. J. Chem. Phys. 1986;84:4894–4914. [Google Scholar]
- 85.Liu M, Waldeck DH, Oliver AM, Head NJ, Paddon-Row MN. Observation of dynamic solvent effect for electron tunneling in U-shaped molecules. J. Am. Chem. Soc. 2004;126:10778–10786. doi: 10.1021/ja049539d. [DOI] [PubMed] [Google Scholar]
- 86.Garg A, Onuchic JN, Ambegaokar J. Effect of friction on electron transfer in biomolecules. J. Chem. Phys. 1985;83:4491–4503. [Google Scholar]
- 87.Sparpaglione M, Mukamel S. Dielectric friction and the transition from adiabatic to nonadiabatic electron-transfer in condensed phases. 2. Application to non-Debye solvents. J. Chem. Phys. 1988;88:4300–4311. [Google Scholar]
- 88.Sparpaglione M, Mukamel S. Dielectric friction and the transition from adiabatic to nonadiabatic electron transfer in condensed phases. 1. Solvation dynamics in Liouville space. J. Chem. Phys. 1988;88:3263–3280. [Google Scholar]
- 89.Rips I, Jortner J. Dynamic solvent effects in outer sphere electron transfer. J. Chem. Phys. 1987;87:2090–2104. [Google Scholar]
- 90.Napper AM, Read I, Waldeck DH, Head NJ, Oliver AM, Paddon-Row MN. An unequivocal demonstration of the importance of nonbonded contacts in the electronic coupling between electron donor and acceptor units of donor-bridge-acceptor molecules. J. Am. Chem. Soc. 2000;122:5220–5221. [Google Scholar]
- 91.Napper AM, Head NJ, Oliver AM, Shephard MJ, Paddon-Row MN, et al. Use of U-shaped donor-bridge-acceptor molecules to study electron tunneling through nonbonded contacts. J. Am. Chem. Soc. 2002;124:10171–10181. doi: 10.1021/ja025683s. [DOI] [PubMed] [Google Scholar]
- 92.Chakrabarti S, Liu M, Waldeck DH, Oliver AM, Paddon-Row MN. Competing electron-transfer pathways in hydrocarbon frameworks: short-circuiting through-bond coupling by nonbonded contacts in rigid U-shaped norbornylogous systems containing a cavity-bound aromatic pendant group. J. Am. Chem. Soc. 2007;129:3247–3256. doi: 10.1021/ja067266b. [DOI] [PubMed] [Google Scholar]
- 93.Liu M, Ito N, Maroncelli M, Waldeck DH, Oliver AM, Paddon-Row MN. Solvent friction effect on intramolecular electron transfer. J. Am. Chem. Soc. 2005;127:17867–17876. doi: 10.1021/ja055596a. [DOI] [PubMed] [Google Scholar]
- 94.Maroncelli M. The dynamics of solvation in polar liquids. J. Mol. Liq. 1993;57:1–37. [Google Scholar]
- 95.Chakrabarti S, Liu M, Waldeck DH, Oliver AM, Paddon-Row MN. Solvent dynamical effects on electron transfer in U-shaped donor-bridge-acceptor molecules. J. Phys. Chem. A. 2009;113:1040–1048. doi: 10.1021/jp807412c. [DOI] [PubMed] [Google Scholar]
- 96.Zusman L. Dynamical solvent effect in electron-transfer reactions occurring in a mixture of two polar solvents. J. Chem. Phys. 1995;102:2580–2584. [Google Scholar]
- 97.Zusman LD. Solvent dynamic effects of polarization diffusion in the rate-constant of electron transfer. Electrochim. Acta. 1991;36:395–399. [Google Scholar]
- 98.Khoshtariya DE, Wei JJ, Liu HY, Yue HJ, Waldeck DH. Charge-transfer mechanism for cytochrome c adsorbed on nanometer thick films: distinguishing frictional control from conformational gating. J. Am. Chem. Soc. 2003;125:7704–7714. doi: 10.1021/ja034719t. [DOI] [PubMed] [Google Scholar]
- 99.Yue HJ, Khoshtariya D, Waldeck DH, Grochol J, Hildebrandt P, Murgida DH. On the electron transfer mechanism between cytochrome c and metal electrodes: evidence for dynamic control at short distances. J. Phys. Chem. B. 2006;110:19906–19913. doi: 10.1021/jp0620670. [DOI] [PubMed] [Google Scholar]
- 100.Yue HJ, Waldeck DH. Understanding interfacial electron transfer to monolayer protein assemblies. Curr. Opin. Solid State Mater. 2005;9:28–36. [Google Scholar]
- 101.Avila A, Gregory BW, Niki K, Cotton TM. An electrochemical approach to investigate gated electron transfer using a physiological model system: cytochrome c immobilized on carboxylic acid-terminated alkanethiol self-assembled monolayers on gold electrodes. J. Phys. Chem. B. 2000;104:2759–2766. [Google Scholar]
- 102.Niki K, Hardy WR, Hill MG, Li H, Sprinkle JR, et al. Coupling to lysine-13 promotes electron tunneling through carboxylate-terminated alkanethiol self-assembled monolayers to cytochrome c. J. Phys. Chem. B. 2003;107:9947–9949. [Google Scholar]
- 103.Davis KL, Drews BJ, Yue H, Waldeck DH, Knorr K, Clark RA. Electron-transfer kinetics of covalently attached cytochrome c/SAM/Au electrode assemblies. J. Phys. Chem. C. 2008;112:6571–6576. [Google Scholar]
- 104.Murgida DH, Hildebrandt P. Proton-coupled electron transfer of cytochrome c. J. Am. Chem. Soc. 2001;123:4062–4068. doi: 10.1021/ja004165j. [DOI] [PubMed] [Google Scholar]
- 105.Wackerbarth H, Hildebrandt P. Redox and conformational equilibria and dynamics of cytochrome c at high electric fields. Chem. Phys. Chem. 2003;4:714–724. doi: 10.1002/cphc.200200618. [DOI] [PubMed] [Google Scholar]
- 106.Dolidze TD, Khoshtariya DE, Waldeck DH, Macyk J, van Eldik R. Positive activation volume for a cytochrome c electrode process: evidence for a “protein friction” mechanism from high-pressure studies. J. Phys. Chem. B. 2003;107:7172–7179. [Google Scholar]
- 107.Fujita K, Nakamura N, Ohno H, Leigh BS, Niki K, et al. Mimicking protein-protein electron transfer: voltammetry of Pseudomonas aeruginosa azurin and the Thermus thermophilus Cu-A domain at ω-derivatized self-assembled-monolayer gold electrodes. J. Am. Chem. Soc. 2004;126:13954–13961. doi: 10.1021/ja047875o. [DOI] [PubMed] [Google Scholar]
- 108.Wei JJ, Liu HY, Khoshtariya DE, Yamamoto H, Dick A, Waldeck DH. Electron-transfer dynamics of cytochrome c: a change in the reaction mechanism with distance. Angew. Chem. Int. Ed. Engl. 2002;41:4700–4703. doi: 10.1002/anie.200290021. [DOI] [PubMed] [Google Scholar]
- 109.LeBard DN, Matyushov DV. Dynamical transition, hydrophobic interface, and the temperature dependence of electrostatic fluctuations in proteins. Phys. Rev. E. 2008;78:061901. doi: 10.1103/PhysRevE.78.061901. [DOI] [PubMed] [Google Scholar]
- 110.Yang H, Luo GB, Karnchanaphanurach P, Louie TM, Rech I, et al. Protein conformational dynamics probed by single-molecule electron transfer. Science. 2003;302:262–266. doi: 10.1126/science.1086911. [DOI] [PubMed] [Google Scholar]
- 111.Palacios RE, Fan FRF, Bard AJ, Barbara PF. Single-molecule spectroelectrochemistry (SMS-EC) J. Am. Chem. Soc. 2006;128:9028–9029. doi: 10.1021/ja062848e. [DOI] [PubMed] [Google Scholar]
RELATED RESOURCES
- Bendall DS, editor. Protein Electron Transfer. Oxford, UK: BIOS Sci.; 1996. [Google Scholar]
- Bertini I, Gray HB, Stiefel EI, Valentine JS. Bioinorganical Inorganic Chemistry: Structure and Reactivity. Sausalito, CA: Univ. Sci.; 2007. [Google Scholar]
- Gray HB, Halpern J, editors. Proc. Natl. Acad. Sci. USA. 1. Vol. 102. 2005. Long-range electron transfer special feature. [DOI] [PMC free article] [PubMed] [Google Scholar]