Abstract
Most small unmyelinated neurons in adult rat dorsal ganglia (DRG) express one or more of the co-receptors targeted by glial cell line-derived neurotrophic factor (GDNF), neurturin and artemin (GFRα1, GFRα2 and GFRα3 respectively). The function of these GDNF family ligands (GFLs) is not fully elucidated but recent evidence suggests GFLs could function in sensory neuron regeneration after nerve injury and peripheral nociceptor sensitisation. In this study, we used immunohistochemistry to determine if the DRG neurons targeted by each GFL change after sciatic nerve injury. We compared complete sciatic nerve transection and the chronic constriction model and found the pattern of changes incurred by each injury was broadly similar. In lumbar spinal cord, there was a widespread increase in neuronal GFRα1 immunoreactivity (IR) in the L1-6 dorsal horn. GFRα3-IR also increased but in a more restricted area. In contrast, GFRα2-IR decreased in patches of superficial dorsal horn and this loss was more extensive after transection injury. No change in calcitonin gene-related peptide-IR was detected after either injury. Analysis of double-immunolabelled L5 DRG sections suggested the main effect of injury on GFRα1- and GFRα3-IR was to increase expression in both myelinated and unmyelinated neurons. In contrast, no change in basal expression of GFRα2-IR was detected in DRG by analysis of fluorescence intensity and there was a small but significant reduction in GFRα2-IR neurons. Our results suggest the DRG neuronal populations targeted by GDNF, neurturin or artemin, and the effect of exogenous GFLs could change significantly after a peripheral nerve injury.
Keywords: nociceptor, pain, sprouting, regeneration, lumbosacral, spinal cord
Introduction
Injured adult primary sensory neurons undergo many chemical and structural changes, some of which are directly linked to regenerative growth (Navarro et al., 2007), while others may be maladaptive in the longer term and can lead to neuropathic pain (Costigan et al., 2009). Understanding the nature of these changes is therefore important for developing ways to promote regeneration while preventing the development of pain or, conversely, for developing new analgesics without adverse outcomes on neuronal structure.
Different classes of dorsal root ganglia (DRG) sensory neurons can be distinguished according to physiological properties, degree of myelination, neurotransmitter content and sensitivity to particular neurotrophic factors (Guo et al., 1999; Lawson, 2002; Ernsberger, 2008). In this study we have focused on C-type unmyelinated DRG neurons— which are predominantly nociceptors and many of which express the transient receptor potential vanilloid 1 (TRPV1) (Woolf and Ma, 2007). These neurons can be divided into two groups, “peptidergic” and “non-peptidergic”, based on whether or not they express neuropeptides such as substance P and calcitonin gene-related peptide (CGRP) (Averill et al., 1995; Molliver et al., 1995; Guo et al., 1999). Many non-peptidergic C-type neurons bind the lectin, bandeira simplicifolia I-isolectin B4 (IB4) (Silverman and Kruger, 1990; Kitchener et al., 1993) and in rat, a majority of IB4-binding neurons express TRPV1 (Guo et al., 1999; Michael and Priestley, 1999).
In adult rodents, peptidergic and non-peptidergic C-type DRG neurons differ in their sensitivity to neurotrophic factors. Neurotrophic factors are important pro-regenerative molecules (Sofroniew et al., 2001) but also sensitise nociceptors (Pezet and McMahon, 2006; Bespalov and Saarma, 2007). Peptidergic DRG neurons express TrkA and p75 neurotrophin receptors that are targeted by nerve growth factor (NGF) (Averill et al., 1995; Pezet and McMahon, 2006). A second group of neurotrophic factors that target C-type DRG neurons are members of the glial cell line-derived neurotrophic factor (GDNF) family (Sah et al., 2005; Malin et al., 2006). These comprise GDNF, neurturin and artemin, which signal using the tyrosine kinase RET, and specific co-receptors (respectively, GFRα1, GFRα2 and GFRα3) (Airaksinen and Saarma, 2002). Early reports suggested this family of ligands exclusively targets non-peptidergic neurons, but this has proven incorrect as the co-receptor for artemin, GFRα3, is primarily expressed by nociceptive peptidergic DRG neurons (Orozco et al., 2001). As GFRα3-immunoreactivity is expressed in approximately fifty percent of peptidergic neurons (Bennett et al., 2006; Kalous et al., 2009), it appears NGF-sensitive neurons comprise two classes, distinguished by their potential responsiveness to artemin. In addition, GDNF and neurturin target separate populations of non-peptidergic neurons. In summary, C-type DRG neurons can be divided into four categories: peptidergic/artemin-insensitive, peptidergic/artemin-sensitive, non-peptidergic/GDNF-sensitive, and non-peptidergic/neurturin-sensitive.
A recent study of GFR-immunoreactive (IR) nerve fibers in the spinal cord has revealed an unexpected degree of complexity in the central targets of these four populations (Forrest and Keast, 2008). In L6 and S1 spinal cord segments of adult female rats, each GFR subtype showed a distinctive pattern of termination within the superficial laminae, providing strong evidence for selective activation of different spinal circuits. In rats and mice, L6 and S1 spinal levels are targeted by the inputs from the pelvic viscera and this same study showed that inflammation of the lower urinary tract causes an increase in intensity of GFRα1-immunofluorescence (but not GFRα2 or GFRα3) within the superficial laminae. This raises the further possibility that changes in GFR signalling may be causally linked to changes in bladder afferent or nociceptor behaviour.
The aim of the current study was to investigate the impact of peripheral injury on the central projections of DRG neurons expressing GFRs, many of which are non-peptidergic. A change in GFR expression or distribution of GFR-expressing nerve fibers could have significant impact on return of neuronal function or development of nociceptor sensitisation after injury. To address this, we have analysed the distribution of central terminations from three classes of GFR-immunoreactive neurons after unilateral sciatic nerve injury. Complete transection of this nerve causes profound changes in GFR mRNA levels within lumbar dorsal root ganglia (Bennett et al., 2000), but the impact of injury on their protein levels and central terminals is unknown. We have compared two well-known injury models, complete transection of the sciatic nerve and an incomplete injury model, chronic constriction injury (CCI), which is commonly used as a model of neuropathic pain (Bennett and Xie, 1988). A significant difference in the outcomes of these two types of injury could reveal changes specific to and potentially causally related to the development of pain. DRG were also examined to determine the likely contributions of phenotypic change or altered expression levels of GFRs.
Materials and Methods
Surgical procedures
All procedures were approved by the University of Sydney and Royal North Shore Hospital Animal Ethics Committees, as required by the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (National Health and Medical Research Council of Australia). Twenty-five adult male Sprague-Dawley rats (6–8 weeks old) were used for these experiments— 16 for sciatic nerve transection, 6 for sciatic chronic constriction injury, and 3 naive controls. Animals were purchased from the Animal Resources Centre (Murdoch, WA, Australia), were housed under a 12 h light-dark cycle and had free access to food and water. In all experiments, the side contralateral to injury served as the internal control. The immunohistochemical properties of the lumbar spinal cord in the naïve controls did not appear to differ from the contralateral side of operated animals so has not been described separately.
All surgical procedures were carried out under isoflurane anaesthesia (5% for induction and 2–3% for maintenance in O2). One group of rats underwent chronic constriction injury (CCI) of the left sciatic nerve as described previously (Bennett and Xie, 1988). Briefly, the left sciatic nerve was exposed at the mid-thigh level and freed from surrounding connective tissue. Five loose ligatures were made around the nerve, proximal to the sciatic trifurcation, using 6-0 chromic gut. The muscle (4-0) and then the skin (3-0) were closed with silk sutures. Another group of rats underwent sciatic nerve transection. Briefly, the left sciatic nerve was exposed at the mid-thigh level and a single ligature was made proximal to the trifurcation point using 6-0 chromic gut. The sciatic nerve was then cut just distal to the ligature using fine scissors. The muscle and then the skin were closed with silk sutures.
Behavioral testing
Animals that were to undergo CCI were allowed to acclimatise to their holding cages for 2–3 days before any procedures were carried out. Rats were then habituated to testing boxes for three days before baseline testing was performed with von Frey filaments (see below). The withdrawal response over three days of baseline testing was averaged for each rat to obtain a single value. On the next day, animals underwent sciatic nerve CCI (see above). On days 4 and 7 following CCI surgery, rats underwent behavioral testing. Only rats that developed mechanical allodynia by day 7 were used in this study (i.e. paw withdrawal thresholds <12 g, see below). On day 7 after injury, all animals were heavily anesthetised with sodium pentobarbitone (80 mg/kg i.p.) and perfused intra-cardially with freshly made 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4), prior to removal of spinal cord and DRGs. Spinal cords and DRGs were post-fixed overnight and then washed in 0.1M phosphate-buffered saline (PBS) and stored in PBS containing 0.1% sodium azide until sectioning.
To assess mechanical allodynia, the mechanical paw withdrawal threshold (PWT) was measured using a series of von Frey filaments (range 2–15 g). Rats were placed in elevated perspex boxes (28 cm × 15 cm × 18 cm) with wire mesh bases in which they had been habituated. Rats were tested on day 4 and day 7 after surgery and given 20 – 30 min to acclimatise to the testing environment. PWT was measured using the up-down method (Chaplan et al., 1994) beginning with the 2 g filament; each von Frey filament was pressed perpendicularly against the plantar surface of the hindpaw and held in place for 1–2 s, five times at random locations of the hindpaw. A positive withdrawal response was noted if the paw was sharply removed in response to the application the filament. If a rat showed a positive withdrawal response, the next lighter filament was used. Once there was a withdrawal to a filament, only four more filaments were tested. If no response was observed, the next heaviest filament was used, stopping at 15 g. The mechanical PWT was calculated using the up-down paradigm (Chaplan et al., 1994). If animals withdrew from all filaments, the PWT was assigned 0.2 g. If animals did not withdraw to any of the filaments, the PWT was assigned 15 g.
Antibody characterization
Affinity purified antisera against each GFR raised in goat were purchased from R&D Systems (Minneapolis, MN; GFRα1, Cat. No. AF560, Batch No, BQEO24081; GFRα2, Cat. No. AF429, Batch No. BTM02; GFRα3, Cat. No. AF2645, Batch No. VFU01). The specificities of the all three antibodies have been demonstrated using Western blotting, showing a single band (manufacturer’s technical information). Western blotting of GFRs can be problematical because of multiple bands, due to variable dimerization, glycosylation states and ligand binding (Jing et al., 1996). Furthermore, GFRα1 and GFRα2 have very similar molecular weights, so Western blotting alone is not ideal for identifying non-specificity. In the present study, the completely distinctive patterns of staining within the spinal cord and DRG using these antibodies demonstrated that they do not show significant cross-reactivity with an inappropriate GFR.
GFRα1 antiserum was raised against recombinant rat GFRα1 extracellular domain (see Accession Number U59486). The immunogen consisted of amino acid residues Ala19-Leu445 of the rat GFRα1 extracellular domain fused to Fc with a linker sequence CSIEGRMD; the Fc was then cleaved, leaving Ala19-Leu445-CSIEGR. This antibody is specific for GFRα1: it blocks binding of GDNF to immobilized GFRα1/Fc chimera in a functional enzyme-linked immunosorbent assay (ELISA); in ELISA or western blotting this antibody shows no cross-reactivity with RET or GFRα3 and 10% cross-reactivity with GFRα2 (manufacturer’s technical information) but in our study showed staining patterns distinct from GFRα2. The same antibody showed a band of ~55 kDa in extracts of cultured sympathetic ganglion neurons (Pierchala et al., 2006). It produced a similar pattern of immunoreactivity in the lumbar dorsal horn as shown previously in more rostral segments (Widenfalk et al., 2001); in this previous study, using the same antibody as our study, the GFRα1 immunostaining in rat dorsal root ganglion and kidney closely matched the pattern of GFRα1 mRNA as determined by in situ hybridisation. In addition, following spinal cord injury, there was a parallel increase in GFRα1 immunoreactivity and GFRα1 mRNA levels in spinal cord. GFRα1-IR distribution in DRG of our current study closely matched the results of previous in situ hybridisation studies on rat DRG (Kashiba et al., 2003), which showed neurons of similar frequency, distribution and size as we observed using immunofluorescence. In rat striatum, immunostaining with this antibody closely matches results from in situ hybridisation (Cho et al., 2004). Furthermore, this antibody immunostains many DRG neurons in wild type but not GFRα1 knockout mice (Rakowicz et al., 2002).
GFRα2 antiserum was raised against recombinant mouse GFRα2 extracellular domain (Accession Number NM008115). The immunogen consisted of amino acid residues Thr160-Ser441 of the GFRα2 extracellular domain fused to Fc with a linker sequence DIEGRMD; the Fc was then cleaved, leaving Thr160-Ser441-CSIEGR. As well as the control western blotting studies described above, this antibody produces labeling in pelvic ganglia and brain of wild type but not GFRα2 knockout mice (Voikar et al., 2004; Wanigasekara et al., 2004). This antiserum shows specific binding and functional reactivity in ELISA and western blotting assays, where it shows a single band; it also shows no cross-reactivity with GFRα3 (manufacturer’s technical information) and a similar pattern of staining in dorsal horn of rat spinal cord as another antibody (goat anti-GFRα2, Cat. No. GT15005, Lot 40058, Neuromics, Edina, MN). The spinal cord immunostaining in the current study was carried out with the antibody provided by R&D Systems described above but DRG analyses were conducted with the Neuromics antibody that also showed indistinguishable results in pilot studies on naïve DRG. The Neuromics antibody was affinity purified and raised against the extracellular domain of recombinant mouse GFRα2; it shows specificity for GFRα2 in Western blotting, direct ELISA and immunohistochemistry (manufacturer’s information).
GFRα3 antiserum was raised against purified extracellular domain of the recombinant mouse GFRα3 protein (amino acid sequence 17–386; see Accession number O35118) linked to an Fc sequence as described above. The antibody was affinity purified, using methods identical to those described above for GFRα1 and GFRα2 antisera. According to the manufacturer’s technical information, in direct ELISA this antibody shows less than 2% cross-reactivity with GFRα2 and GFRα4 and in Western blot reacts with recombinant GFRα3, showing a 70 kDa band under reducing conditions (for the Fc chimera protein, comprising extracellular domain and Fc), and a 43 kDa band (for the extracellular domain alone). In our hands, this antibody shows identical patterns of localisation in DRG as described previously using the same antibody (Malin et al., 2006) and the same as described previously using another antibody, raised against residues 108-120 of murine GFRα3 and shown to be specific to GFRα3 using Western blotting and ELISA (Orozco et al., 2001). The pattern of staining in our study mimics the results of in situ hybridisation for GFRα3 mRNA in rat DRG (Bennett et al., 2000; Bennett et al., 2006). Using this antibody, we have also found that ~50% of the GFRα3 neurons in adult rat sacral DRG express CGRP (Kalous et al., 2009) comparable to the coexpression shown with substance P described previously, using in situ hybridisation for GFRα3 mRNA (Bennett et al., 2006).
Rabbit anti-calcitonin gene-related peptide (CGRP) polyclonal antibody (Chemicon/Millipore, North Ryde, NSW, Australia; Cat No. AB5920, Batch No. LV1496277) was raised against synthetic rat CGRP. This antibody reacts with rat CGRP but does not cross-react (<0.01%) with calcitonin, somatostatin or amylin 8–37; specific reactivity is eliminated by pre-incubation of the antiserum with 10 mM rat CGRP (manufacturer’s technical information). This antibody produces a similar pattern of immunoreactivity in the rat primary afferent neurons as numerous previous publications, e.g. Chao et al., 2008).
Mouse anti-neuronal nuclei (NeuN) monoclonal antibody (Chemicon; Cat No. MAB377, Batch No. 22060766) was raised against purified nuclei from mouse brain and recognises most neuronal cell types in the nervous system of numerous vertebrates (see manufacturer’s technical information sheet for list of species). In Western blots, this antibody recognises 2–3 bands in the 46–48 kDa range and another band at 66 kDa (manufacturer’s technical information). This antibody produced a similar pattern of neuronal staining as previously described (Mullen et al., 1992; Yang et al., 2008)
Mouse anti-neurofilament 200 monoclonal antibody (NF200, Sigma, Castle Hill, NSW, Australia; Cat No. N0142, Batch No. 17154802) is derived from a hybridoma produced by the fusion of mouse myeloma cells and splenocytes from an immunised mouse. The carboxyterminal tail segment of enzymatically dephosphorylated pig neurofilament H-subunit was used as the immunogen (manufacturer’s technical information). Anti-NF200 specifically localises the neurofilaments of molecular weight 200 kDa in rat spinal cord using an immunoblotting technique and does not cross-react with other intermediate filament proteins (manufacturer’s technical information). This antibody produced a similar pattern of staining in the DRG and spinal cord as reported previously (Schumacher et al., 1999; Chao et al., 2008).
Immunohistochemistry
Single-labeling experiments
Prior to sectioning, a small cut was made in the ventral horn of the spinal cord on the side contralateral to injury. Segments (L1-L6) were then blocked and embedded in an albumin-gelatine matrix (Llewellyn-Smith et al., 2007), prior to cryoprotection overnight in phosphate-buffered saline (PBS) containing 30% sucrose. Three spinal segments from the same animal (L1-L3 and L4-L6) were blocked and processed together. Transverse cryostat sections (40μm) were processed free floating (Kalous et al., 2009), using antisera raised against GFRα1 (1:400), GFRα2 (1:1000), GFRα3 (1:300) and CGRP (1:2000). Sections were distributed sequentially into a one in four series and processed for immunohistochemistry using the glucose oxidase/nickel-enhanced diaminobenzidine (DAB) method. Sections were blocked for 30 min in 0.1 M phosphate buffer (PB; pH 7.4) containing 5% non-immune horse serum and then incubated for 48 h at room temperature on a shaker with primary antibody in PB containing 2% normal horse serum and 0.2% triton X-100. After washes in PB, sections were incubated overnight at room temperature on a shaker in biotin-conjugated donkey anti-sheep (Jackson ImmunoResearch Laboratories, West Grove, PA; Cat. No. 713-065-003, Batch No. 57583, 1:1000) or donkey anti-rabbit (Jackson, Cat. No. 711-065-152, Batch No. 73931, 1:1000). Sections were then incubated for 2 h in avidin-biotin complex, followed by 15 min in nickel-enhanced DAB solution (0.2% D-glucose, 0.04% ammonium chloride, 0.025% DAB/100 ml 0.1M acetate buffer pH 6.0, 2% nickel sulfate). Black reaction product was obtained by adding glucose oxidase (0.002%), and the reaction was stopped using large volume washes of acetate buffer. Sections were mounted onto 1% gelatinised slides, dried overnight, dehydrated with increasing concentrations of ethanol, followed by histolene, and then coverslipped with DPX water-free mounting media (Crown Scientific, Mulgrave, Vic, Australia).
Double-labeling experiments
Double-labelling immunofluorescence was performed on L5 DRG from a separate group of rats that had undergone sciatic nerve transection. DRGs and spinal cord tissue were cryoprotected overnight in PBS containing 30% sucrose and embedded in an inert mounting medium (OCT Tissue-Tek, Sakura, Torrance, CA) prior to sectioning.
For DRG studies, ganglia from each side were placed in the same cryomold, sectioned (14 μm), direct mounted on 1% gelatine-subbed slides and processed simultaneously. Sections were distributed sequentially between sets of five slides to avoid double-counting. Sections were blocked with PBS containing 10% horse serum and 0.1 triton X-100 for 1–2 h, followed by overnight incubation at room temperature in combinations of primary antibodies: GFRα1 with CGRP, GFRα1-3 with NeuN (mouse, 1:10000), GFRα1 with NF200 (mouse, 1:4000), GFRα3 with CGRP and GFRα3 with NF200. Antibodies were made up in hypertonic PBS (0.1M phosphate, 0.3M NaCl, pH 7.2). Appropriate secondary antibodies were applied for 2–3 h: donkey anti-goat Cy3 (Jackson, Cat. No. 705-165-147, Batch No. 79773 1:1000), donkey anti-rabbit (Molecular Probes/Invitrogen, Mulgrave, Vic, Australia; Cat. No. A21206, Batch No. 57542A, 1:500) and donkey anti-mouse (Molecular Probes, Cat. No. A-21202, Batch No. 84D1-1, 1:500), the sections were washed, and then the slides were coverslipped with 0.5 M bicarbonate-buffered glycerol (pH 8.6).
Fibre density and distribution measurements
Single-labelled sections were visualised using an Olympus BX51 microscope (Olympus Australia, Melbourne, Australia) and images captured with a Zeiss AxioCam HRc camera (Munchen-Hallbergmoos, Germany) and Axiovision 4.2 software. Image analysis was performed on 8-bit monochrome TIFF images (1300 × 1030 pixels) using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). For each antibody, spinal cord sections from the same rat were imaged using the same camera settings and exposure time, under a 10× objective. For quantitative studies of immunolabelling intensity, images were obtained from 5 randomly selected sections of L5 spinal cord (left and right sides). Optical density was assessed in areas of interest (AOI, 130 ×130 μm) within the dorsal horn, as shown in Figure 1, where boxes 1–3 were used for each antibody but box 4 only for GFRα1. For each antibody, the optical density value from each of the five sections was averaged to obtain a single value for each rat. In each rat, the mean white matter optical density values for each antibody were subtracted.
Figure 1.
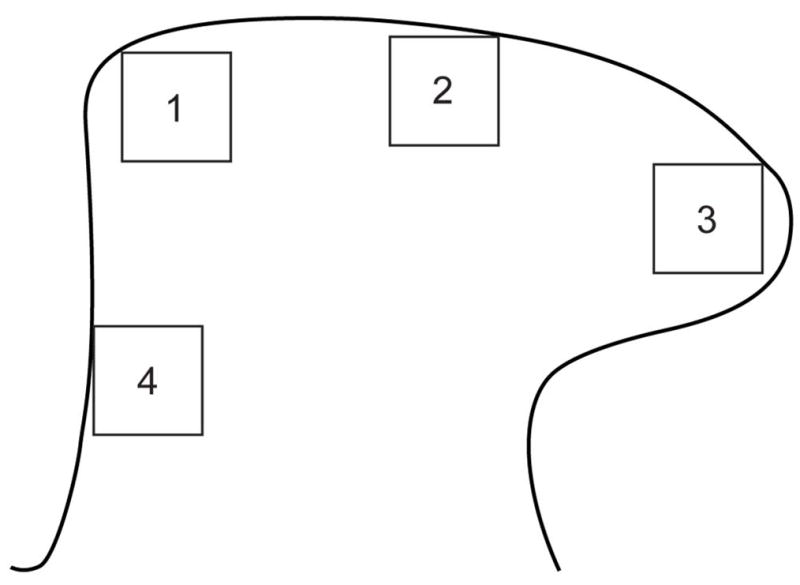
Areas of interest (AOIs) used for image analysis of L5 spinal cord dorsal horn. The diagram is oriented with the dorsal surface at the top and medial to the left. Three AOIs (boxes 1–3) were assessed for GFRα1, GFRα2, GFRα3 and CGRP. An additional AOI (box 4) in the deep medial aspect of the dorsal horn was assessed for GFRα1. Dimensions of each AOI were 130 μm × 130 μm. CGRP, calcitonin gene-related peptide; GFR, GDNF family receptor.
DRGs: neuronal counts and intensity anlaysis
DRG sections were viewed under an Olympus BX51 fluorescence microscope. For analysis of neuronal phenotype (e.g., coexpression patterns), neuronal counts were obtained manually from 5 sections per ganglion, under a 40× objective. For each antibody, approximately 150 somata were counted from a total of five sections of each ganglion. Only neurons sectioned through the nucleus were counted. For analysis of immunofluorescence intensity, 8-bit unsaturated monochrome images (1600 × 1200 pixels) were obtained under a 40× objective using an RT Spot camera (Diagnostic Instruments, Sterling Heights, MI) and digitised using Image Pro Plus. For a given antibody, images were captured using the same settings and exposure times for both left and right ganglia taken from the same animal. Using Image Pro Plus, intensity (pixel grey level) was measured in a circular AOI (6 μm diameter) located in the cytoplasm of each positively stained neuron, measuring at least 70 neurons per DRG.
Statistics and image production
Statistical analyses were performed using SPSS v16 for PC (Chicago, IL) or GraphPad Prism 5.0a (Graphpad Software Inc., La Jolla, CA). A repeated measures analysis of variance (ANOVA) and Tukey’s multiple comparison post hoc tests were to detect changes in paw withdrawal threshold in CCI rats 4 and 7 days after injury, compared with baseline prior to injury. To detect an effect of injury on the mean intensity of GFR and CGRP immunostaining in each AOI (boxes 1–3), we performed a 2-way ANOVA with repeated measures, with the side (injured or not injured) and AOI as within subject factors and correcting for violations of sphericity using the Greenhouse-Geisser method. Differences between sides of GFRα1 immunostaining intensity in the deep medial dorsal horn (box 4) were analysed using a paired t-test. The percentages of DRG neurons that expressed each of the GFRs, CGRP or NeuN was estimated in L5 DRG from each side of each rat and the arcsine transformed data compared using a paired t-test. Differences between sides of GFR immunostaining intensity in DRG neurons were analysed using paired t-tests. The proportions of neurons expressing different markers were plotted as untransformed data. All results were expressed as the mean ± SEM and P < 0.05 was regarded as statistically significant.
For image production, some minor adjustments were made to brightness and contrast to most closely demonstrate the staining as it appeared under the microscope (Adobe Photoshop CS2, San Jose, CA). Coloured monochrome or merged images were produced by pasting monochrome images into the appropriate colour channel of a 24-bit RGB file created in Photoshop. Red-green pairs were converted into magenta-green pairs to make images more accessible to readers with impaired colour discrimination.
Results
For each GFR, the distribution of immunoreactivity in lumbar spinal cord (L1-L6) and the impact of spinal cord injury on this distribution are described in detail below. Table 1 provides a summary of the primary effects of injury.
Table 1.
Summary of changes in GDNF-family receptor (GFR) immunoreactivity in L5 spinal cord and dorsal root ganglia (DRG) after sciatic nerve injury
| Tissue | GFRα1 | GFRα2 | GFRα3 |
|---|---|---|---|
| Spinal cord | |||
| Control | |||
| Location | Lamina II (outer) | Lamina II (inner) | Laminae I and II (outer) |
| Injured | |||
| Location | Laminae II–V (except lateral lamina V) | Lamina II (inner) but loss in medial and central regions | Laminae I – II (inner) |
| Intensity | Increase | Decrease | Increase |
| DRG | |||
| Control | |||
| Phenotype | Mainly small-medium non-peptidergic and large myelinated neurons | Small-medium non-peptidergic neurons | Small-medium peptidergic neurons |
| Injured | |||
| Phenotype | Increased proportion of myelinated neurons | No change | Increased proportion of myelinated and small-medium non-peptidergic neurons |
| Prevalence | Increase | Decrease | Increase |
| Intensity | Increase in small-medium and large neurons | No change | No change |
Sciatic nerve injury caused a widespread increase in GFRα1-IR in the dorsal horn of lumbar spinal cord
Contralateral to either type of sciatic nerve injury, GFRα1-IR nerve fibers were distributed primarily in lamina II (outer) in the dorsal horn of the lumbar spinal cord. In L1-L3 these terminals extended in a uniformly dense band across the entire dorsal horn (Fig. 2a–c, m–o) but in L4-5 the terminals were less dense in the medial third to half of this region (Fig. 2d, e, p, q). In L2-L5, the most medial aspect of lamina IV also showed some weak, diffuse GFRα1-IR, but individual nerve fibers were difficult to distinguish (Fig. 2a–e, m–q). In L6, the GFRα1-IR fibers extended uniformly across lamina II (outer) of the dorsal horn (Fig. 2f, r) and in some sections extended in a band of fibres along the lateral edge of the dorsal horn, terminating dorsal to the sacral preganglionic nucleus, as described previously (Fig. 2f and/or 2r) (Forrest and Keast, 2008). Weakly stained GFRα1-IR fibres were present in the dorsal commissure at this spinal level. GFRα1-IR motoneurons were present in the ventral horn of L1-L6 (not shown).
Figure 2.
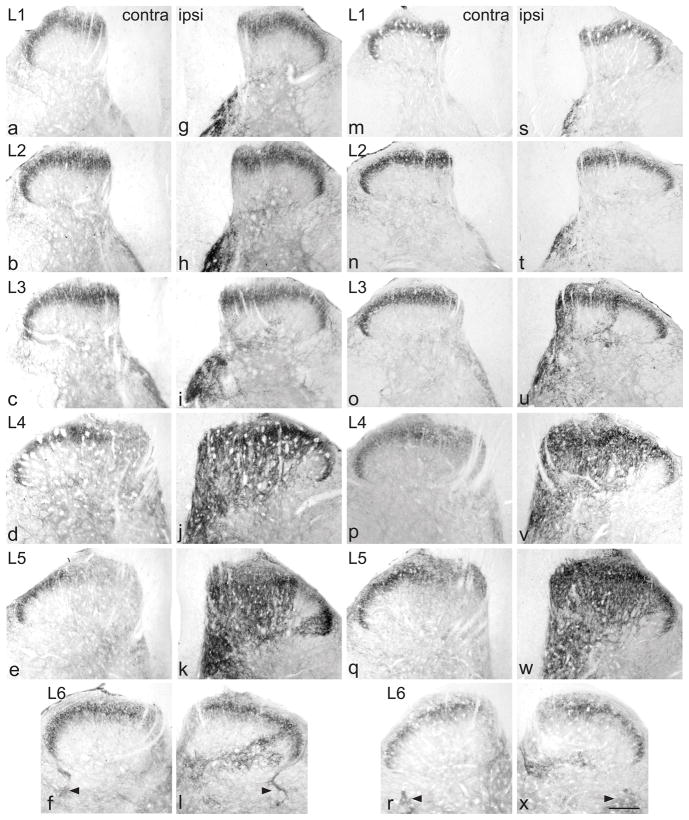
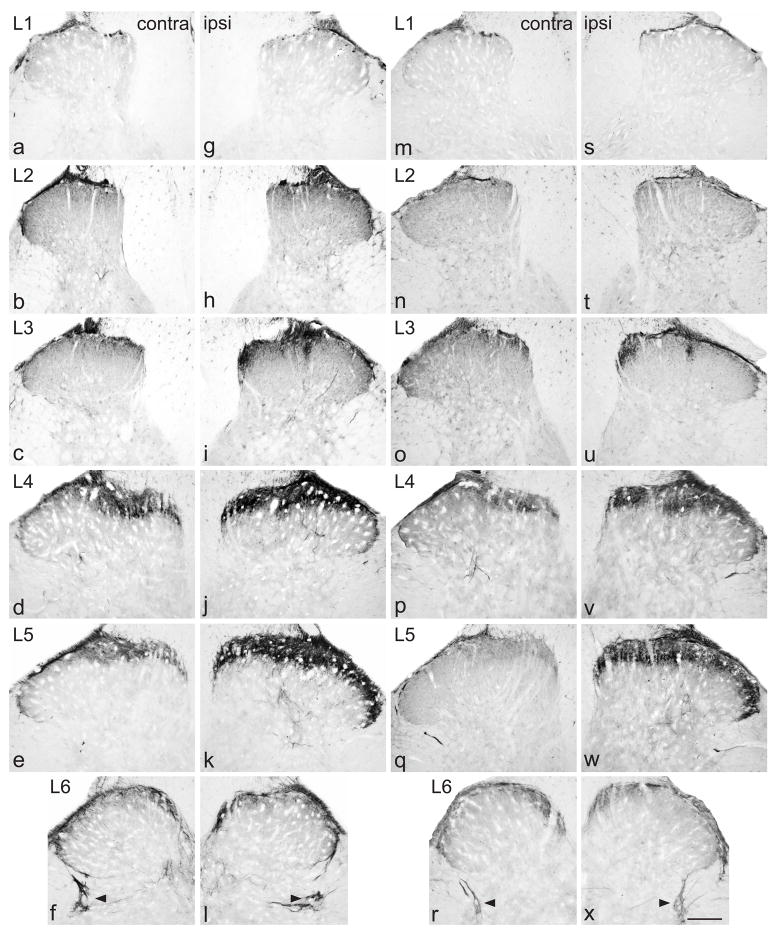
Up-regulation of GFRα1-immunoreactivity (IR) in the dorsal horn from lumbar spinal cord, 7 days after chronic constriction injury (a–l) or transection (m–x) of the sciatic nerve. For each type of injury, images are taken from left and right sides of the same section, and segments of each spinal level from the same animal. GFRα1-IR was present primarily in lamina II (outer) on the uninjured side (a–f, m–r). After chronic constriction injury (g–l), these superficial fibres remained, but a patch of GFRα1-IR fibers appeared on the medial aspect of the deep dorsal horn, extending beyond the central canal. Progressing caudally, these fibers extended to cover most of the dorsal horn (j, k), with the exception of a small area on the most lateral aspect of the grey matter. A smaller increase in GFRα1-IR innervation of the deep dorsal horn was evident in the deep dorsal horn of L6 (l). After transection injury (m–x), similar changes were seen in the ipsilateral dorsal horn (s–x). Sacral preganglionic nucleus (L6) is indicated with arrowheads (f, l, r, x). Scale bar applies to all panels and represents 200 μm. GFR, GDNF family receptor.
All rats in the CCI group developed mechanical allodynia ipsilateral to the injury (baseline 15 g; post-surgery: 4d, 12.43 ± 1.49 g, range: 6.91–15 g; 7d, 8.19 ±1.24 g, range: 4.79–11.981 g; mean ± SE, n = 6; 7d measurements significantly less than baseline [P < 0.005] and 4d [P < 0.05]). Ipsilateral to CCI, there was an increase in the area of dorsal horn innervated by GFRα1-IR fibers and in the intensity of GFRα1 immunostaining throughout L1-6, both aspects being maximal at L5 (Fig. 2g–k). The location of this upregulation varied between spinal segments. In L1, a patch of intensely stained GFRα1-IR fibers was present along the medial aspect of lamina IV (Fig. 2g). This patch increased in width in L2 (Fig. 2h) and by L3 was even larger, extending laterally towards the middle of the dorsal horn and ventrally towards the central canal (Fig. 2i). At this spinal level, in most sections there was a band of GFRα1-IR fibers spanning lamina III, approximately midway between the medial and lateral aspects. In L4, GFRα1-IR fibers extended throughout most of the depth and width of the dorsal horn (Fig. 2j), and in L5 only a lateral region of lamina V did not show dense, intensely-stained GFRα1-IR fibers (Fig. 2k). In L4 and L5, GFRα1-IR fibers in the deep laminae of the dorsal horn commonly extended laterally to the level of the central canal, and some to the ventral horn, just dorsal to motoneurons. Many GFRα1-IR motoneurons showed more intense immunostaining on the injured side of L4 and L5 and, rarely, in L6 (not shown), as previously described (Hammarberg et al., 2000). In some sections of L6, a delicate plexus of GFRα1-IR fibers was present in the deep dorsal horn, ipsilateral to the injury (Fig. 2l). The intensity of immunostaining in fibers in lamina II, travelling along the lateral edge of the dorsal horn and dorsal to the sacral preganglionic nucleus was also slightly increased in the ipsilateral side, in some sections. Very similar changes occurred after transection injury of the sciatic nerve (Fig. 2s–x).
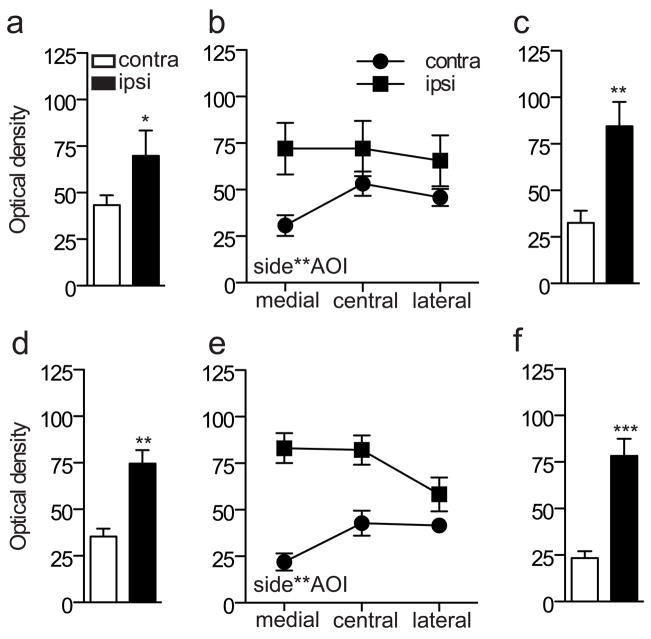
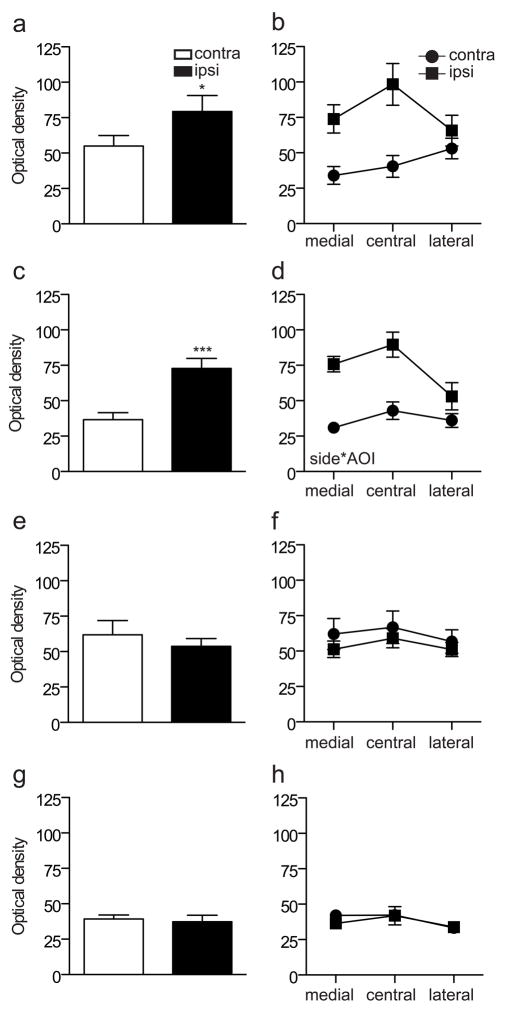
To quantify and compare the effects of each type of injury, we focused on L5 spinal cord, where the largest changes were evident. Optical density measurements of the superficial dorsal horn (3 AOI’s) were compared between sides and showed a main effect of injury to increase GFRα1-IR after both CCI (Fig. 3a; P = 0.039) and transection (Fig. 3d; P = 0.002). Considering the data from each region of the superficial dorsal horn separately, we found a significant interaction between AOI and injury (CCI: P = 0.007; Tx: P = 0.001) (Fig. 3b, e). These data reflect our qualitative observations of the contralateral side, where lower levels of GFRα1-IR occurred in the medial than either the central or lateral aspects of the superficial dorsal horn. We also quantified optical density in an AOI within the medial aspect of the deep dorsal horn and found a significant increase on the ipsilateral side after each type of injury (Fig. 3c, f; CCI: P = 0.002; Tx: P <0.001; P = 0.001).
Figure 3.
Quantitation of immunostaining intensity for GFRα1 in the L5 dorsal horn following sciatic nerve injury. Optical density measurements in spinal cord sections from chronic constriction injury (CCI) animals are shown in a–c, whereas data from transection injury are shown in d–f. a, Optical density measurements pooled from all superficial areas of interest (AOI 1–3) on each side showed a main effect of CCI (*P = 0.039, F1, 5 = 7.688), with an increase in GFRα1-IR ipsilateral to injury. b, Optical density measurements from each AOI in the superficial dorsal horn showed a significant interaction between side and AOI (P = 0.007, F1.184, 5.922 = 15.041). c, After CCI, there was an increase in GFRα1-IR ipsilateral to injury in the deep dorsal horn (** P = 0.002). d, Optical density measurements pooled from all superficial AOI (1–3) on each side showed a main effect of transection (**P = 0.002, F1, 5 = 32.702), with an increase in GFRα1-IR ipsilateral to injury. e, Optical density measurements from each AOI in the superficial dorsal horn showed a significant interaction between side and AOI (P = 0.001, F12.63, 6.317 = 31.368). f, After transection, there was an increase in GFRα1-IR ipsilateral to injury in the deep dorsal horn (*** P = 0.001). Data represent mean ± SEM (n= 6 rats per group), analysed by ANOVA. GFR, GDNF family receptor; IR, immunoreactivity.
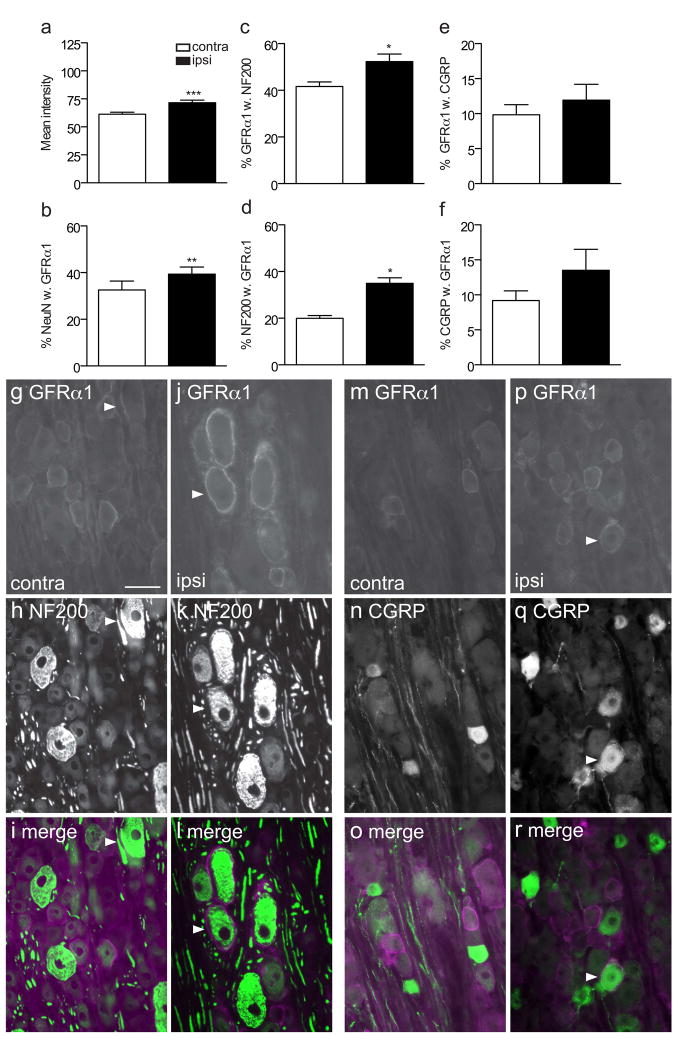
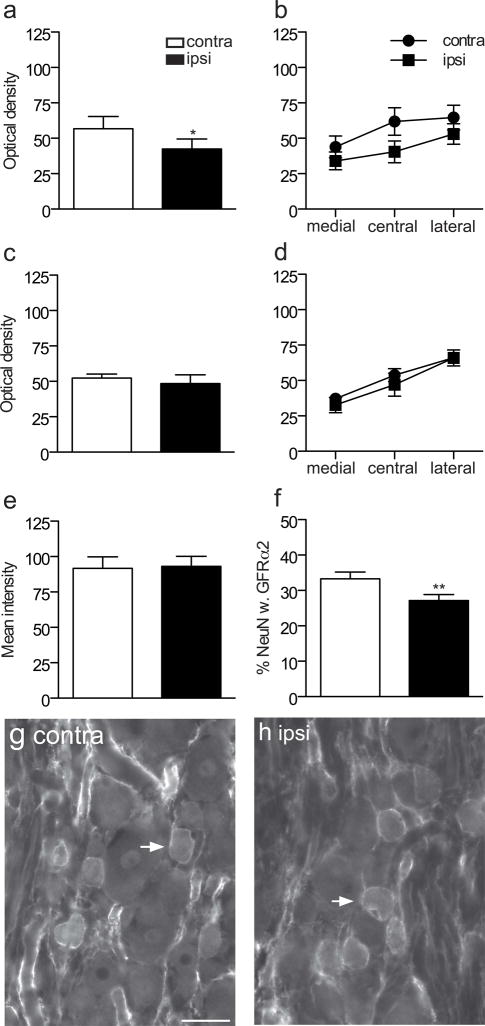
We next investigated a number of mechanisms that could underlie the widespread increase in GFRα1-IR fibers in the dorsal horn in L5 DRG following sciatic nerve transection (n = 6 rats). As injury can upregulate GFRα1 mRNA in DRG neurons (Bennett et al., 2000), we determined if a corresponding upregulation of protein could be detected by measuring the intensity of GFRα1-immunofluorescence in DRG sections. GFRα1-IR was observed in numerous DRG somata of all sizes, where it was present in the cytoplasm and the plasma membrane. We found a small but statistically significant increase in fluorescence intensity ipsilateral to injury (Fig. 4a; P = 0.0005). Qualitative assessment of the sections showed that the GFRα1 immunostaining was brighter in both small and large neurons (Fig. 4g–j). We then determined the expression of GFRα1 in neurons labelled for the neuronal nuclear marker, NeuN. The results showed that about one-third of DRG neurons were immunostained for GFRα1 and this proportion increased on the ipsilateral side (Fig. 4b; P = 0.0086). Together, these results suggest injury increased the basal expression of GFRα1 with an associated increase in GFRα1-IR neurons.
Figure 4.
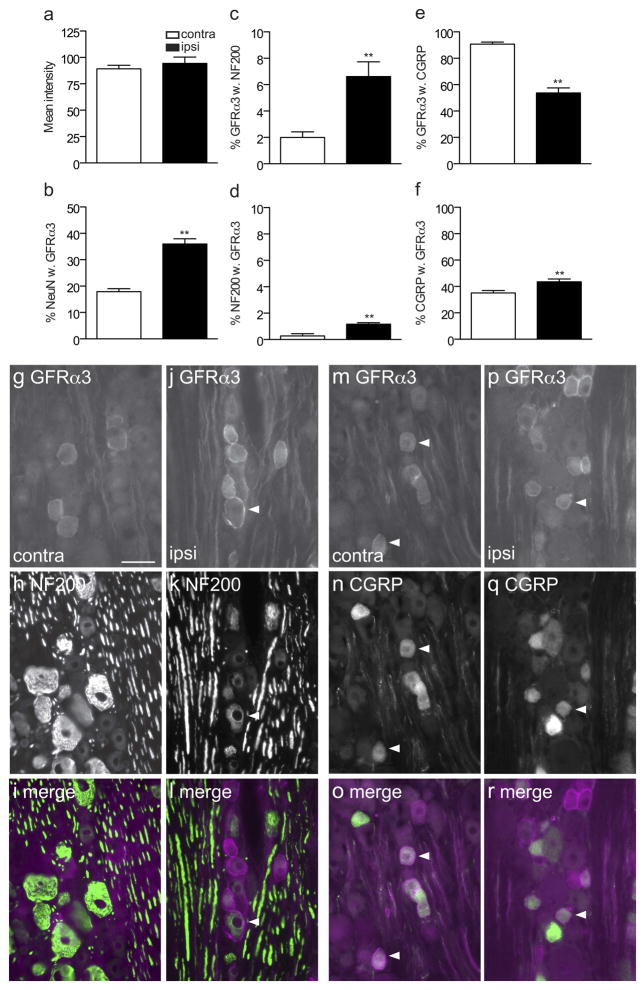
Co-localisation studies of GFRα1-immunoreactive (IR) somata in L5 dorsal root ganglia (DRG), ipsilateral and contralateral to transection of the sciatic nerve. a, Mean fluorescence intensity of GFRα1-IR neurons showed a small but significant increase ipsilateral to injury (*** P = 0.0005). b, The proportion of NeuN somata exhibiting GFRα1-IR increased ipsilateral to injury (** P = 0.0086). c, d, The proportion of GFRα1-IR neurons expressing NF200 (* P = 0.0198) and the proportion of NF200 neurons showing GFRα1-IR (*P = 0.0109) were also increased after sciatic transection. e, f, No difference was detected in the proportion of GFRα1-IR neurons expressing CGRP or the proportion of CGRP neurons showing GFRα1-IR following injury. Comparisons of ipsi- and contralateral ganglia were made by performing a paired t-test on arcsine transformed data. Data have been expressed as the mean percentage ± SEM (n = 5 rats per group), where each measurement from each ganglion represented the mean of at least 150 neuronal sections, including only those neurons sectioned through the nucleus. g–r show colocalisation of GFRα1-IR with NF200 (g–l) or calcitonin gene-related peptide (CGRP, m–r) in L5 dorsal root ganglion sections, contra- and ipsilateral to the sciatic nerve transection, shown as image pairs and corresponding merged images. Contralateral to injury (g–i), GFRα1-IR was found in small, NF200-negative and large, NF200-positive neurons. Ipsilateral to injury (j–l), GFRα1-IR was brighter in both of these groups of neurons. Colocalisation of GFRα1-IR and CGRP-IR was infrequent both contralateral (m–o) and ipsilateral (p–r) to injury. Arrowheads show examples of double-labeled neurons. Scale bar = 50 μm applies to g–r. CGRP, calcitonin gene-related peptide; GFR, GDNF family receptor.
To determine if the chemical phenotype of neurons expressing GFRα1-IR was changed after injury, we conducted quantitative double-labelling studies. GFRα1 is normally expressed by small-medium diameter unmyelinated DRG neurons (including many non-peptidergic but not peptidergic nociceptors), as well as larger diameter myelinated DRG neurons (Bennett et al., 2000). This is consistent with our data from DRG contralateral to the injury as ~40% of GFRα1-IR neurons expressed the marker of myelinated neurons, NF200, and these comprised ~20% of all NF200 neurons (Fig. 4c, d, g–i). Moreover, only ~10% of GFRα1-IR neurons coexpressed CGRP, which comprised ~10% of all CGRP neurons (Fig. 4e, f, m–o). The proportion of presumptive myelinated GFRα1-IR neurons were increased in DRG ipsilateral to the injury (Fig. 4j–l), as a larger proportion of GFRα1-IR neurons were immunoreactive for NF200 (Fig. 4c; P = 0.0198) and a larger proportion of NF200-positive neurons showed GFRα1-IR (Fig. 4d; P = 0.0109). We also investigated the possibility that injured peptidergic DRG neurons may begin to express GFRα1. Neurons showing immunoreactivity for both GFRα1 and CGRP were seen infrequently in DRG ipsilateral or contralateral to injury (Fig. 4m–r). Quantitative analyses showed no effect of injury on the proportion of GFRα1-IR neurons stained for CGRP (Fig. 4e) or the proportion of CGRP-positive neurons immunostained for GFRα1 (Fig. 4f, p–r). Therefore, the increase in number of GFRα1-IR neurons after injury is likely to comprise primarily myelinated rather than unmyelinated peptidergic neurons.
Sciatic nerve injury caused a localised decrease in GFRα2-IR in the dorsal horn of L3-L5 spinal cord and a loss of GFRα2-IR neurons in DRG
Contralateral to either type of sciatic nerve injury, GFRα2-IR fibers were distributed primarily in lamina II (inner) of the dorsal horn (Fig. 5a–f, m–r). In L1-3 and L6, these fibers showed a similar density across the dorsal horn, but in L4-5 there were slightly less GFRα2-IR fibers in the most medial region. In L6, a few pale fibers were also found in the dorsal commissure but none were associated with the sacral preganglionic nucleus (Fig. 5f, r). GFRα2-IR was not observed in motoneurons.
Figure 5.
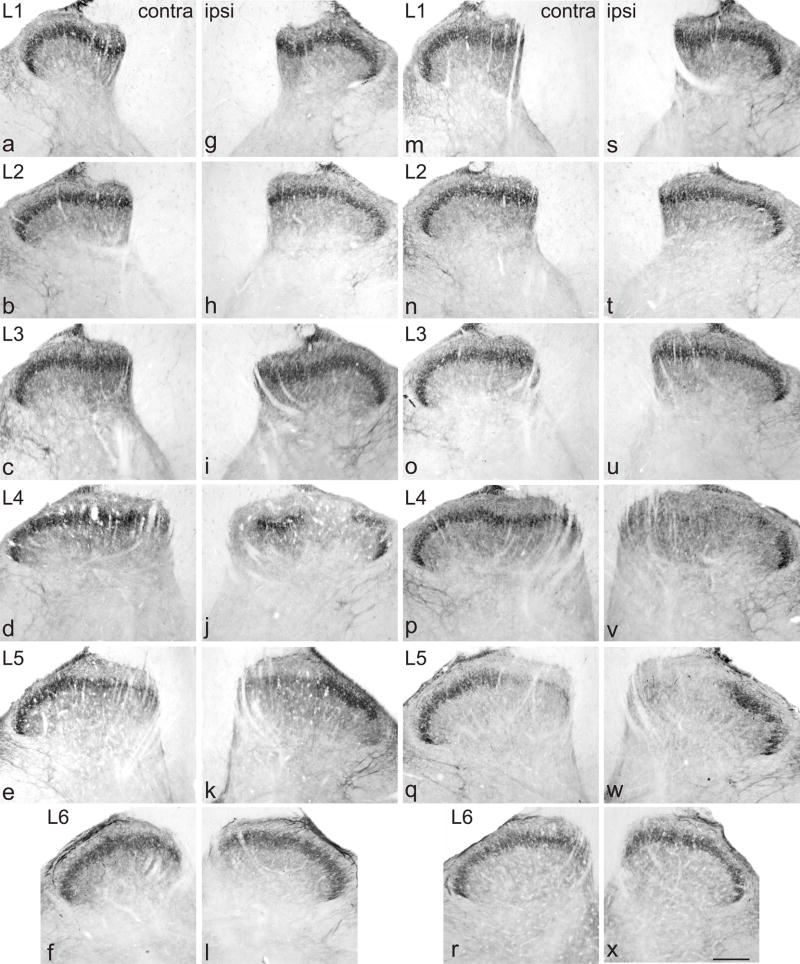
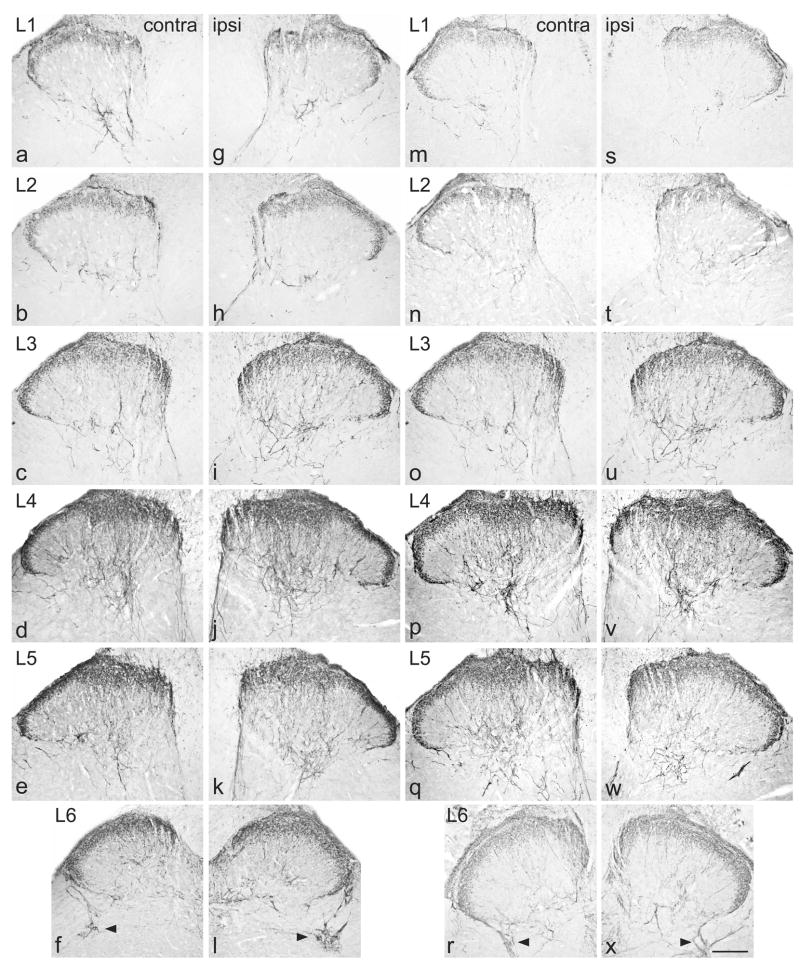
Decrease in GFRα2-immunoreactivity (IR) in the dorsal horn from lumbar spinal cord, 7 days after chronic constriction injury (a–l) or transection (m–x) of the sciatic nerve. For each type of injury, images are taken from left and right sides of the same section, and segments of each spinal level from the same animal. a–f, Distribution of GFRα2-IR in lamina II (inner) contralateral to a chronic constriction injury. g–l, Ipsilateral to injury, GFRα2-IR was decreased only in L4 and L5, showing areas with lower levels or absent immunostaining in the most medial and the central aspects of the dorsal horn. m–r, Distribution of GFRα2-IR in lamina II (inner) contralateral to a transection injury. s–x, Ipsilateral to injury, GFRα2-IR was decreased only in L4 and L5, showing substantive areas with absent immunostaining in the most medial and the central aspects of the dorsal horn. Scale bar applies to all panels and represents 200 μm. GFR, GDNF family receptor.
Following CCI, there was no effect on GFRα2 immunostaining in lamina II of the dorsal horn of L1-L3 (Fig. 5g, h) but in L4-L5 many sections showed a loss of staining from selected regions of superficial dorsal horn (Fig. 5i–k). In L4-5, some sections showed that GFRα2-IR fibers were lost from the region approximately in the middle of this lamina, but in many others this area of loss was broader and included loss from the most medial aspect. In L6 dorsal horn there was no obvious effect of injury on GFRα2-IR intensity or location (Fig. 5l). GFRα2-IR was not observed in motoneurons. Following transection injury, in L4-5 there were broadly similar changes to those observed after CCI but the loss of fibres was more extensive after transection. In some sections of L5 almost all of the GFRα2-IR was lost, except for the most lateral aspect of lamina II (Fig. 5s–x). Quantification of immunostaining intensity in L5 spinal cord after each type of injury revealed a main effect of CCI (Fig. 6a; P = 0.039) but not transection (Fig. 6c). The lack of statistically significant effect of transection may reflect the slightly differing locations of fiber loss between different sections, which may not always have coincided with the AOI used for quantitative analysis. There was no interaction between AOI and injury (Fig 6b, d).
Figure 6.
Quantitation of immunostaining intensity for GFRα2 in the L5 dorsal horn and characterisation of GFRα2-IR dorsal root ganglion neurons following sciatic nerve injury. a–d show data from the dorsal horn and e–h from dorsal root ganglia. Optical density measurements in spinal cord sections from chronic constriction injury (CCI) animals are shown in a and b, whereas data from transection injury are shown in c and d. a, Optical density measurements pooled from all superficial areas of interest (AOI 1–3) on each side showed a main effect of CCI (*P = 0.039, F1, 5 = 7.777), with an decrease in GFRα2-IR ipsilateral to injury. b, Optical density measurements from each AOI in the superficial dorsal horn showed no interaction between side and AOI. c, Optical density measurements pooled from all superficial AOIs (1–3) on each side did not detect a significant effect of transection. e, Optical density measurements from each AOI in the superficial dorsal horn showed no significant interaction between side and AOI. Data represent mean ± SEM (n= 6 rats per group), analysed by ANOVA. e, The mean fluorescence intensity of GFRα2-IR neurons did not change following transection injury. f, The proportion of NeuN-positive neurons showing GFRα2-IR was significantly decreased in ganglia ipsilateral to transection (P = 0.004). Comparisons of ipsi- and contralateral ganglia were made by performing a paired t-test on arcsine transformed data. Data have been expressed as the mean percentage ± SEM (n = 5 rats per group), where each measurement from each ganglion represented the mean of at least 150 neuronal sections, including only those neurons sectioned through the nucleus. g and h show examples of GFRα2-IR neurons contralateral and ipsilateral to injury, demonstrating the similar intensity of immunofluorescence. Scale bar = 50 μm and applies to both g and h. GFR, GDNF family receptor; IR, immunoreactivity.
To address the possibility that these changes in spinal cord labelling are due to downregulation of GFRα2 protein in DRG neurons (as described previously for GFRα2 mRNA (Bennett et al., 2000), we assessed the intensity of GFRα2-immunofluorescence in L5 DRG sections after sciatic nerve transection. In ganglia of both sides, GFRα2-IR was observed in small-medium size DRG somata (Bennett et al., 2000), where immunoreactivity was particularly pronounced in the plasma membrane (Fig. 6g). Immunofluorescence intensity was comparable between contralateral and ipsilateral sides (Fig. 6c, g, h). Quantification of the proportion of NeuN-labelled neurons labelled for GFRα2-IR revealed a small but significant decrease ipsilateral to the injury (P = 0.004; Fig. 6f).
Sciatic nerve injury caused a localised increase in GFRα3-IR but no change in CGRP fibers in the dorsal horn of lumbar spinal cord
Contralateral to injury, the distribution of GFRα3-IR fibers varied considerably between lumbar segments (Fig. 7a–f, m–r). In all lumbar levels, a small band of intensely stained fibers was present across lamina I. In occasional sections, bundles of GFRα3-IR axons could be seen in the dorsal root entry zone. In lamina II (outer), no GFRα3-IR fibers were present in L1, but some weak-moderate intensity fibers were present across the entire dorsal horn of L2-3 and more intensely stained GFRα3-IR fibers in the medial half in L4-5. In L6, GFRα3-IR fibers of moderate intensity were present in lamina II (outer) across the entire dorsal horn and a dense band of fibers extended along the lateral edge of the dorsal horn to terminate in a plexus just dorsal to the sacral preganglionic nucleus (Fig. 7f, l), as described previously (Forrest and Keast, 2008). At this spinal level, GFRα3-IR fibers were also found in the dorsal commissure. In all lumbar levels, a small number of single GFRα3-IR fibers were present in the deep dorsal horn. No GFRα3-IR was present in motoneurons.
Figure 7.
Up-regulation of GFRα3-immunoreactivity (IR) in the dorsal horn from lumbar spinal cord, 7 days after chronic constriction injury (a–l) or transection (m–x) of the sciatic nerve. For each type of injury, images are taken from left and right sides of the same section, and segments of each spinal level from the same animal. a–f, Distribution of GFRα3-IR in laminae I and II (outer), contralateral to a chronic constriction injury (CCI). In L6 (f), GFRα3-IR fibers also travel towards the sacral preganglionic nucleus (asterisks). g–l, Ipsilateral to CCI injury, GFRα3-IR is increased in the superficial dorsal of L3-L5, especially the most medial and the central aspects of the dorsal horn. m–r, Distribution of GFRα3-IR in laminae I and II (outer), contralateral to a transection injury. In L6 (r), GFRα3-IR fibers also travel towards the sacral preganglionic nucleus (arrowheads). s–x, Ipsilateral to transection injury, GFRα3-IR was increased in the superficial dorsal of L3-L5, especially the most medial and the central aspects of the dorsal horn. Scale bar applies to all panels and represents 200 μm. GFR, GDNF family receptor.
Following CCI, GFRα3-IR nerve fibers in the L3-5 dorsal horn ipsilateral to injury appeared more intensely stained and occupied a larger area than in the contralateral side (Fig. 7i–k). In L3, this increase was restricted to the medial half of the dorsal horn, but extended into lamina II (inner); in some sections a small but intensely stained patch of dense GFRα3-IR fibers was present in lamina II, near the middle of the dorsal horn. In L4 and L5, this increased intensity and extension into lamina II (inner) extended across the width of the dorsal horn. There was no obvious effect of injury on L1, L2 or L6 innervation by GFRα3-IR nerve fibers. Similar changes in GFRα3-IR innervation were observed following transection injury (Fig. 7s–x). Quantification of immunostaining in the superficial laminae showed a main effect of CCI (Fig. 8a; P = 0.013) and transection (Fig. 8c; P < 0.0001) on GFRα3-IR in the superficial dorsal horn), as well as a significant interaction between AOI and injury following transection (Fig. 8b, d; P <0.0001 for transection). No GFRα3-IR motoneurons were observed after either type of injury.
Figure 8.
Quantitation of immunostaining intensity for GFRα3 and CGRP in the L5 dorsal horn following sciatic nerve injury. Optical density measurements in spinal cord sections immunostained for GFRα3 are shown in a–d, whereas data from sections stained for CGRP are shown in e–h. a, Optical density measurements pooled from all superficial areas of interest (AOI 1–3) on each side showed a main effect of CCI (*P = 0.013, F1, 5 = 14.031), with an increase in GFRα3-IR ipsilateral to injury. b, Optical density measurements from each AOI in the superficial dorsal horn did not detect a significant interaction between side and AOI after CCI. c, Optical density measurements pooled from all superficial AOI (1–3) on each side showed a main effect of transection (***P <0.0001, F1, 5 = 85.214), with an increase in GFRα3-IR ipsilateral to injury. d, Optical density measurements from each AOI in the superficial dorsal horn detected a significant interaction between side and AOI (P = 0.014, F1.359, 6.793 = 9.697). e, There was no effect of CCI on optical density measurements of CGRP-IR when all AOIs were pooled and no interaction was detected between side and AOI (f). g, There was no effect of transection on optical density measurements of CGRP-IR when all AOIs were pooled and no interaction was detected between side and AOI (h). Data represent mean ± SEM (n= 6 rats per group), analysed by ANOVA. CGRP, calcitonin gene-related peptide; GFR, GDNF family receptor; IR, immunoreactivity.
In the absence of injury, GFRα3 is expressed almost exclusively by a sub-population of peptidergic C-fiber afferents (Bennett et al., 2006; Kalous et al., 2009). Therefore we compared the effects of sciatic nerve injury on CGRP-IR fibers in the lumbar spinal cord of the same animals from which GFR analysis had been performed. CGRP-IR fibers were distributed in the lumbar spinal cord as described previously in many mammalian species (Gibson et al., 1984) and in subsequent studies in rodents by many other research groups. Contralateral to either type of injury, numerous fibers were present through laminae I and II and in deeper laminae of the dorsal horn (Fig. 9a–e). In L6, many immunostained fibers were also present in the dorsal commissure and associated with the sacral preganglionic nucleus, and bundles of axons travelled along the lateral aspect of the dorsal horn (Fig. 9f, r). Some motoneurons were immunostained with weak to moderate intensity in all lumbar levels. Ipsilateral to CCI or transection, there was no obvious effect on CGRP-immunostaining (intensity or distribution) in any spinal level (Fig. 9g–l, s–x). Quantification of immunostaining in the superficial laminae showed no significant effect of either injury type on CGRP in the superficial dorsal horn (Fig. 8e–g).
Figure 9.
Calcitonin gene-related peptide (CGRP)-immunoreactivity (IR) in the dorsal horn from lumbar spinal cord, 7 days after chronic constriction injury (a–l) or transection (m–x) of the sciatic nerve. For each type of injury, images are taken from left and right sides of the same section, and segments of each spinal level from the same animal. Distribution of CGRP-IR in superficial laminae contralateral to a chronic constriction (a–f) and transection (m–r) injury. There was no obvious change in the area or intensity of CGRP-IR ipsilateral to chronic constriction (g–l) or transection (s–x) injury. In all images of L6, CGRP-IR fibers travel towards the sacral preganglionic nucleus (arrowheads). Scale bar applies to all panels and represents 200 μm.
To address the possibility that injury triggered upregulation of GFRα3 protein in DRG neurons, as reported previously for GFRα3 mRNA (Bennett et al., 2000), we assessed the intensity of GFRα3-immunofluorescence in L5 DRG following sciatic nerve transection. GFRα3-IR was observed in numerous somata, primarily of small size (Bennett et al., 2000), where strong immunoreactivity occurred in the plasma membrane and cytoplasm. We found no change in fluorescence intensity ipsilateral to injury (Fig. 10a, g, j, m, p). We then quantified the presence of GFRα3-IR in neurons labelled for NeuN and found an increased proportion of GFRα3-IR neurons on the ipsilateral side (P = 0.006; Fig. 10b). Together, these results suggest that neurons expressing GFRα3 do not express higher levels of this receptor in their somata after injury but additional neurons begin to express GFRα3 after injury.
Figure 10.
Co-localisation studies of GFRα3-immunoreactive (IR) somata in L5 dorsal root ganglia (DRG), ipsilateral and contralateral to transection of the sciatic nerve. a, Mean fluorescence intensity of GFRα3-IR neurons showed no difference between ganglia ipsilateral and contralateral to injury (n = 5). b, The proportion of NeuN somata exhibiting GFRα3-IR increased ipsilateral to injury (** P = 0.006). c, d, The proportion of GFRα3-IR neurons expressing NF200 (** P = 0.007) and the proportion of NF200 neurons showing GFRα1-IR (*P = 0.002) was also increased after sciatic transection. e, Following injury there was a decrease in the proportion of GFRα3-IR neurons expressing CGRP (P = 0.002). f, There was a very small but significant increase in the proportion of CGRP neurons showing GFRα3-IR following injury (P = 0.004). Comparisons of ipsi- and contralateral ganglia were made by performing a paired t-test on arcsine transformed data. Data have been expressed as the mean percentage ± SEM (n = 5 rats per group), where each measurement from each ganglion represented the mean of at least 150 neuronal sections, including only those neurons sectioned through the nucleus. g–r show colocalisation of GFRα3-IR with NF200 (g–l) or calcitonin gene-related peptide (CGRP, m–r) in L5 dorsal root ganglion sections, contra- and ipsilateral to the sciatic nerve transection, shown as image pairs and corresponding merged images. Both contralateral (g–i) and ipsilateral (j–l) to injury, GFRα3-IR neurons were rarely NF200-positive (example indicated by arrowheads in j–l). However on both sides, many neurons showing colocalisation of GFRα3-IR and CGRP-IR. Arrowheads show examples of double-labeled neurons. Scale bar = 50 μm applies to g–r. CGRP, calcitonin gene-related peptide; GFR, GDNF family receptor; IR, immunoreactivity.
We next investigated the effects of sciatic nerve transection on the chemical phenotype of GFRα3-IR DRG neurons. Previous reports of strong localisation of GFRα3 to unmyelinated, peptidergic afferents (see above), compared strongly with our data from DRG contralateral to the injury. Here, only ~10% of the GFRα3-IR neurons expressed the marker of myelinated neurons, NF200, and these comprised <2% of all NF200 neurons (Fig. 10c, d, g–i); ~90% GFRα3-IR neurons stained for CGRP, while about one-third of CGRP neurons showed GFRα3-IR (Fig. 10e, f, m–o). After injury there was a higher proportion of NF200-positive (presumptive myelinated) GFRα3-IR neurons ipsilateral to the injury, demonstrated by a higher proportion of GFRα3-IR neurons immunoreactive for NF200 (Fig. 10c; P = 0.007) and more NF200-positive neurons showing GFRα3-IR (Fig. 10d; P = 0.002). However, this increase represents only a numerically small population of neurons (Fig. 10j–l), so we also investigated the possibility that injury increased the proportion of peptidergic neurons expressing GFRα3. The results showed that this occurred in a small but statistically significant proportion of CGRP neurons (Fig. 10f, p–r; P = 0.004), again unlikely to account for the overall increase in number of GFRα3-IR NeuN-labelled neurons (Fig. 10b). This suggested GFRα3 was upregulated in non-peptidergic, NF200-negative (unmyelinated) neurons, which was consistent the proportion of GFRα3-IR DRG neurons expressing CGRP being also decreased by injury (Fig. 10e; P = 0.002).
Discussion
This study revealed distinct effects of peripheral nerve injury on three different populations of primary afferent fibres immunolabelled for receptors of the GDNF family ligands (Table 1). Sciatic nerve injury caused a widespread increase in nerve fibre labelling for GFRα1, a localised increase in GFRα3, and a loss of GFRα2 in the lumbar dorsal horn. Complete and incomplete injury models showed similar outcomes, although the loss of GFRα2 was more pronounced after complete injury. Together these results suggest that the functional impact of nerve injury may differ widely between these three groups of neurons, in the nature of their communication within the spinal cord and in their responses to neurotrophic factors.
The changes in GFR expression we observed are unlikely to be causally related to the development of neuropathic pain, an outcome of incomplete injury model (Bennett and Xie, 1988). However, they may impact on the site and degree of sensitisation induced by neurotrophic factors or related pro-regenerative therapies administered during this state. GDNF, neurturin and artemin have all been shown to promote growth of sensory neurons and to influence nociceptive signalling and pain behaviours (Hoke et al., 2000; Hoke et al., 2002; Stucky et al., 2002; Gardell et al., 2003; Mills et al., 2007; Wang et al., 2008; Widenfalk et al., 2009). From the results of our study, we predict the effects of experimental regenerative therapies based on GDNF or artemin could be broader than anticipated, due to the enhanced receptor expression in the spinal cord. Whilst this could impact favourably on regeneration, it could also promote less desirable sensitising effects on nociceptors. Conversely, treatments based on neurturin could have less impact on regeneration than predicted from uninjured systems, due to loss of receptors from many of the sensory neurons after injury. We did not examine the expression of GFRs in peripheral processes of injured DRGs that would normally be exposed to tissue-derived ligands, but if these receptors are regulated in parallel with the central processes, this would further enhance the effects of GDNF and artemin, while reducing the effects of neurturin in an injured system.
The changes in GFR-IR we observed in dorsal root ganglia broadly agree with previously reported changes in GFR mRNA after injury, with upregulation of GFRα1 and GFRα3 but downregulation or no change in GFRα2 (Bennett and Xie, 1988; Kashiba et al., 1998; Mills et al., 2007). Therefore, it is likely that the changes in GFRα1 and GFRα3 we observed in spinal cord immunostaining at least in part represent increased levels of receptor protein in central terminals, although there may also be increased GFR trafficking to these central processes. We also found evidence of GFR expression in neuronal classes that did not appear to express these GFRs prior to injury. Whether this is truly a phenotypic change or simply a limitation of the sensitivity of our immunodetection method (e.g., if we underestimated the population of neurons expressing a particular GFR in tissues from naïve animals or contralateral to injury) cannot be determined in this study. We also cannot discount the possibility that some GFRα1 and GFRα3 neurons undergo central sprouting after injury. However, the pattern of distribution of the increased immunoreactivity for these two GFRs matches very closely the projection patterns of different branches of the sciatic nerve to the hindlimb (see below), which does not support the innervation of novel territories after injury but does allow the possibility of sprouting within its normal territory. It is very difficult to resolve this issue without ultrastructural studies or the availability of alternative markers that are not modulated by injury.
The distinct patterns of central termination of each GFR-IR nerve population before and after injury raise a number of important issues. In the absence of injury, these unique patterns may indicate that each neuronal population has different spinal (and therefore supraspinal) connectivity, pointing to a much more diverse and complex range of sensory and nociceptive circuitry than previously indicated by visualising CGRP or IB4. A similar type of complexity has recently been demonstrated in the L6-S1 spinal cord (Forrest and Keast, 2008), a region that has a major role in pelvic visceral function. These patterns of termination are of particular interest when compared with the topography of projections from different nerve supplies to the hindlimb, especially different branches of the sciatic nerve. The major spinal targets of sensory neurons projecting in the sciatic nerve are lumbar levels 4 and 5, where each branch of the nerve terminates in a different territory across the mediolateral aspect of the dorsal horn (Swett and Woolf, 1985; Woolf and Fitzgerald, 1986; LaMotte et al., 1991; Rivero-Melian, 1996; Shehab, 2009) and in different regions of the hindlimb (Decosterd and Woolf, 2000). Therefore, if each class of GFR-IR DRG neuron projected similarly in each sciatic nerve branch, one would predict that fibers of each type innervate the mediolateral extent of the dorsal horn, albeit with some diversity in whether they terminate in deep or superficial laminae or both (as indicated by their expression in myelinated and/or unmyelinated fibers). However, in naive animals and contralateral to injury, each GFR-IR class of fibers showed uneven distribution, suggesting that there is either heterogeneous expression or trafficking in each sciatic nerve projection field.
Prior to injury, GFRα1-IR was found in small unmyelinated and larger myelinated neurons, yet fibers were present only in lamina II, where the most medial aspect of this lamina (a target of the tibial nerve) showed weaker innnervation. One interpretation of the uneven distribution in lamina II might be that few GFRα1-IR sensory neurons project in the tibial nerve or that the receptor is not normally transported to the central projections of this nerve. Low levels of transport to central terminals may also explain the absence of GFRα1-IR fibers from deeper laminae, even though many GFRα1-IR DRG neurons were large and immunoreactive for NF200. After injury, GFRα1-IR fibers were visible throughout the dorsal horn – across the entire mediolateral aspect (except a small region on the most lateral aspect) and in both deep and superficial laminae – consistent with increased expression within both myelinated and unmyelinated fibers that project in each branch of the sciatic nerve. The upregulation of GFRα1 was most pronounced in L4 and L5, but it also occurred from L1 to L6, consistent with the known broad projection territory of the sciatic nerve (Swett and Woolf, 1985; Woolf and Fitzgerald, 1986; LaMotte et al., 1991). The small region on the most lateral aspect of the dorsal horn that did not show an increase in GFRα1-IR after injury may be the territory supplied by the uninjured posterior cutaneous nerve (Swett and Woolf, 1985).
The increase in GFRα1-immunostaining intensity observed in the superficial laminae indicated an upregulation in smaller diameter and unmyelinated neurons. However, we were unable to identify an injury-induced expression of GFRα1 in peptidergic DRG neurons, suggesting that GFRα1 could have been upregulated in non-peptidergic, unmyelinated neurons that normally express GFRα2. We could not test this possibility directly because commercially available GFRα and GFRα2 antisera are raised in the same host species. Moreover, effects of injury on GFRα2-IR (see below) are likely to confound this analysis. It is also possible that changes in GFR distribution in the central terminals of DRG neurons do not occur in parallel with the levels in their somata. We observed an increase in the proportion of large diameter, NF200-IR (myelinated) neurons immunostained for GFRα1 after injury. Consistent with this, the labelling in deeper laminae resembled the pattern of immunoreactivity for the vesicular glutamate transporter, vGlut1 (Persson et al., 2006), after sciatic nerve transection and fibers visualised by retrograde labelling using the tracer cholera toxin (CTb) (Shehab, 2009), which preferentially labels large diameter myelinated axons (Robertson and Grant, 1985). Together, these observations are consistent with increased activation of deep dorsal horn circuits after injury, as suggested by an increase in Fos activation in this region after CCI (Ro et al., 2004). The function of the centrally transported GFRα1 is not known but the receptor is predicted to be physiologically active because a pre-conditioning injury of the sciatic nerve leads to an enhanced growth response of DRG neurons to GDNF in vitro (Mills et al., 2007). Moreover, intraspinal administration of GDNF reverses a number of changes caused by sciatic nerve injury, including the downregulation of P2x3 purinoceptors, reduction in IB4-binding and decreased conduction velocity (Munson and McMahon, 1997; Bennett et al., 1998; Bradbury et al., 1998).
The source of endogenous ligand for GFRα in the spinal cord is unclear, although many DRG neurons contain GDNF protein (Holstege et al., 1998; Jongen et al., 1999) and could potentially release it from their central terminals. Moreover, intraspinal administration of GDNF causes rapid upregulation of c-Fos in the superficial dorsal horn (Jongen et al., 2005). There have been a number of reports that sciatic nerve injury triggers GDNF synthesis within the DRG and Schwann cells proximal to injury (Hammarberg et al., 1996; Cameron et al., 1997; Chao et al., 2008), although Nagano et al (2003) showed a decrease within DRG after CCI or spared nerve injury; this is supported by a report of decreased levels of GDNF in dorsal horn (Jongen et al., 1999), suggested to be due to reduced transport from the periphery. In the absence of more detailed studies, it is very difficult to reconcile this data in order or to interpret our own results, or propose specific contexts for physiological activation of GFRα within the spinal cord after peripheral nerve injury.
The potential targets of GFRα3-IR nerve fibers also varied between lumbar spinal segments and following injury, when immunostaining became more pronounced in the superficial laminae of L3-L5. In naïve animals and contralateral to injury, GFRα3-IR was restricted to lamina I and lamina II (outer), with lamina II staining more pronounced in L4 and L5. Ipsilateral to injury, in these spinal segments and in both laminae, GFRα3-IR nerve fibers increased in staining intensity and in area, extending deeper into the dorsal horn; there was a smaller change apparent in the most central part of the superficial dorsal horn in L3. In L4 and L5, this pattern of staining did not indicate preferential upregulation of GFRα3 in particular branches of the sciatic nerve. However, the small patch of increased immunostaining in the central region of L3 may coincide with territory supplied by the superficial peroneal nerve.
The upregulation of GFRα3-IR we observed in the superficial laminae was due at least in part to increased synthesis in a minority of myelinated (NF200-IR) and peptidergic fibers. We cannot discount an increased expression by GFRα1- or GFRα2-IR neurons but could not test this directly because all GFR antisera were raised in the same species. Unexpectedly, we failed to see an effect of injury on the intensity of GFRα3-immunofluorescence within the DRG which, given the previous reports of increased GFRα3 mRNA after injury (Bennett et al., 2000), may reflect a limitation with the immunofluorescence quantitative method or that the additional message is not translated into protein. Alternatively, more GFRα3 protein may be synthesised after injury but trafficked to the central terminals rather than stored in the soma. The role of endogenous artemin in the normal spinal cord or following peripheral nerve injury is not known.
Different issues were raised by our observations that GFRα2–IR was lost from discrete zones in the dorsal horn in L4 and L5. The areas where GFRα2-IR was lost within the dorsal horn after sciatic nerve injury closely match the topography of projections from the sciatic nerve. In particular, the residual GFRα2–IR in the most lateral aspect of the dorsal horn may indicate a territory not strongly supplied by branches of the sciatic nerve, but instead innervated by the uninjured posterior cutaneous nerve (Swett and Woolf, 1985). This was also identified as a region where GFRα-IR did not increase after injury (see above).
The reduction in GFRα2–IR could be due to degeneration of GFRα2-IR axons, downregulation of GFRα2 protein, or a mixture of both events. We consider that overt degeneration is the primary response, for the following reasons. First, there is a greater extent of loss after sciatic nerve transection compared with incomplete injury (CCI). Second, our studies on DRG found no change in intensity of GFRα2-IR soma immunofluorescence, but did identify fewer GFRα2-IR somata following injury. While this could be due to lower levels of receptor expression, it could also indicate death of a minority of GFRα2-IR DRG neurons— although previous evidence suggest only limited neuronal cell death occurs within a week of injury (Hu and McLachlan, 2003). Moreover, following peripheral nerve injury, IB4 labelling is also transiently decreased in the dorsal horn (Hammond et al., 2004), and this comprises loss of terminals, not just loss of IB4 binding (Bailey and Ribeiro-da-Silva, 2006). If the loss of GFRα2–IR was due to degeneration of central projections from surviving DRG neurons, this raises the question of why only this particular population of neurons changed in this way, whereas CGRP-, GFRα1– and GFRα2–IR increased or did not change after injury. Evidence obtained with a different injury model— spinal cord injury— also suggests central projections GFRα2–IR DRG neurons are especially susceptible to loss or receptor down-regulation (Kalous et al., 2007; Kalous et al., 2009). Evidence that IB4-binding DRG neurons are less likely than peptidergic neurons to compensate for loss of adjacent terminals fields in the dorsal horn by sprouting (Belyantseva and Lewin, 1999; Runyan et al., 2007) cannot explain our result, as the IB4-binding population includes both GFRα1– and GFRα2–IR neurons. Further studies of how GFRα2–IR DRG neurons respond to injury are needed to resolve this issue.
Signalling by GDNF family members utilises a specific GFR and, in most cases described to date, the common receptor tyrosine kinase, RET (rearranged during transfection) (Airaksinen and Saarma, 2002). Therefore interpretation of our data should take into account the effect of injury on ret expression. In a recent study employing an epitope unmasking method (Jongen et al., 2007), RET-IR was found in deep and superficial laminae, abolished by dorsal rhizotomy. After sciatic nerve transection, RET-IR was reduced in lamina II but showed a hint of an increase in lamina III (although this did not reach statistical significance). Interestingly, neither the control nor injury groups showed RET expression that resembled the sum of the three types of GFR expression in our study, suggesting either that the RET-immunolabelling underestimated the distribution of RET protein or that many potential sites of RET-independent signalling (Paratcha and Ledda, 2008) exist in the cord. The effects of injury on RET expression in DRG have been studied but have yielded equivocal results (Naveilhan et al., 1997; Bennett et al., 2000; Nagano et al., 2003; Mills et al., 2007), so require further study.
The marked changes we observed in GFR levels within the spinal cord contrasted with the absence of effect of injury on CGRP fibres. While there are some reports of a decrease in CGRP fibre density or CGRP levels following peripheral injury (Cameron et al., 1991; Kajander and Xu, 1995; Sommer and Myers, 1995; Casals-Diaz et al., 2009), a number of groups have found no change (Cameron et al., 1997; Bailey and Ribeiro-da-Silva, 2006). It is possible that changes in peptide level did occur in our experiments but were not detectable with our method of analysis. Moreover, we did not determine the effect of injury on number or fluorescence intensity of CGRP+ DRG neurons, as described previously by other groups who identified a decrease in peptide level (Bennett et al., 1998; Shi et al., 2001). Irrespective, given that almost all of the GFRα3-IR DRG neurons express CGRP and substance P and, in turn, about half of the CGRP neurons show GFRα3-IR (Bennett et al., 2006; Kalous et al., 2009), it is interesting that there was so little change in the spinal distribution of CGRP fibers, yet a noticeable increase in GFRα3-IR (albeit in discrete regions of the dorsal horn). Our observations in spinal cord from naïve uninjured rats and contralateral to injury suggest that the two populations of CGRP neurons (GFRα3-positive and –negative) have different spinal projection sites and respond quite differently to injury.
In conclusion, the profound and distinctive effects of sciatic nerve injury on different populations of sensory neurons identified by GFR expression raises interesting questions about the magnitude and specificity of neurotrophic factor signalling in sensory neurons after injury. This may impact on sensitisation of nociceptor neurons and regenerative responses.
Acknowledgments
Acknowledgements for support: This work was supported by the NSW Office for Science and Medical Research (Spinal Cord Injury and Other Neurological Conditions Program), National Institutes of Health (DK-069351), University of Sydney Medical Foundation and the National Health and Medical Research Council of Australia (Senior Research Fellowship #358709 to JRK)
Other acknowledgements:
We are grateful to Ms Kathryn Evans for conducting pilot experiments on spinal cord tissues.
Literature Cited
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AL, Ribeiro-da-Silva A. Transient loss of terminals from non-peptidergic nociceptive fibers in the substantia gelatinosa of spinal cord following chronic constriction injury of the sciatic nerve. Neuroscience. 2006;138:675–690. doi: 10.1016/j.neuroscience.2005.11.051. [DOI] [PubMed] [Google Scholar]
- Belyantseva IA, Lewin GR. Stability and plasticity of primary afferent projections following nerve regeneration and central degeneration. Eur J Neurosci. 1999;11:457–468. doi: 10.1046/j.1460-9568.1999.00458.x. [DOI] [PubMed] [Google Scholar]
- Bennett DLH, Boucher TJ, Armanini MP, Poulsen KT, Michael GJ, Priestley JV, Phillips HS, McMahon SB, Shelton DL. The glial cell line-derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J Neurosci. 2000;20:427–437. doi: 10.1523/JNEUROSCI.20-01-00427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DLH, Boucher TJ, Michael GJ, Popat RJ, Malcangio M, Averill SA, Poulsen KT, Priestley JV, Shelton DL, McMahon SB. Artemin has potent neurotrophic actions on injured C-fibres. J Peripher Nerv Syst. 2006;11:330–345. doi: 10.1111/j.1529-8027.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Bennett DLH, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bespalov MM, Saarma M. GDNF family receptor complexes are emerging drug targets. Trends Pharmacol Sci. 2007;28:68–74. doi: 10.1016/j.tips.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Cliffer KD, Dougherty PM, Garrison CJ, Willis WD, Carlton SM. Time course of degenerative and regenerative changes in the dorsal horn in a rat model of peripheral neuropathy. J Comp Neurol. 1997;379:428–442. doi: 10.1002/(sici)1096-9861(19970317)379:3<428::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Cliffer KD, Dougherty PM, Willis WD, Carlton SM. Changes in lectin, GAP-43 and neuropeptide staining in the rat superficial dorsal horn following experimental peripheral neuropathy. Neurosci Lett. 1991;131:249–252. doi: 10.1016/0304-3940(91)90625-4. [DOI] [PubMed] [Google Scholar]
- Casals-Diaz L, Vivo M, Navarro X. Nociceptive responses and spinal plastic changes of afferent C-fibers in three neuropathic pain models induced by sciatic nerve injury in the rat. Exp Neurol. 2009;217:84–95. doi: 10.1016/j.expneurol.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Chao T, Pham K, Steward O, Gupta R. Chronic nerve compression injury induces a phenotypic switch of neurons within the dorsal root ganglia. J Comp Neurol. 2008;506:180–193. doi: 10.1002/cne.21537. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cho J, Yarygina O, Oo TF, Kholodilov NG, Burke RE. Glial cell line-derived neurotrophic factor receptor GFRalpha1 is expressed in the rat striatum during postnatal development. Brain Res Mol Brain Res. 2004;127:96–104. doi: 10.1016/j.molbrainres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Ernsberger U. The role of GDNF family ligand signalling in the differentiation of sympathetic and dorsal root ganglion neurons. Cell Tissue Res. 2008;333:353–371. doi: 10.1007/s00441-008-0634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest SL, Keast JR. Expression of receptors for glial cell line-derived neurotrophic factor family ligands in sacral spinal cord reveals separate targets of pelvic afferent fibers. J Comp Neurol. 2008;506:989–1002. doi: 10.1002/cne.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, Wang R, Ehrenfels C, Ossipov MH, Rossomando AJ, Miller S, Buckley C, Cai AK, Tse A, Foley SF, Gong B, Walus L, Carmillo P, Worley D, Huang C, Engber T, Pepinsky B, Cate RL, Vanderah TW, Lai J, Sah DW, Porreca F. Multiple actions of systemic artemin in experimental neuropathy. Nat Med. 2003;9:1383–1389. doi: 10.1038/nm944. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Polak JM, Bloom SR, Sabate IM, Mulderry PM, Ghatei MA, McGregor GP, Morrison JF, Kelly JS, Evans RM, et al. Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species. J Neurosci. 1984;4:3101–3111. doi: 10.1523/JNEUROSCI.04-12-03101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hammarberg H, Piehl F, Cullheim S, Fjell J, Hokfelt T, Fried K. GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury. Neuroreport. 1996;7:857–860. doi: 10.1097/00001756-199603220-00004. [DOI] [PubMed] [Google Scholar]
- Hammarberg H, Piehl F, Risling M, Cullheim S. Differential regulation of trophic factor receptor mRNAs in spinal motoneurons after sciatic nerve transection and ventral root avulsion in the rat. J Comp Neurol. 2000;426:587–601. doi: 10.1002/1096-9861(20001030)426:4<587::aid-cne7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hammond DL, Ackerman L, Holdsworth R, Elzey B. Effects of spinal nerve ligation on immunohistochemically identified neurons in the L4 and L5 dorsal root ganglia of the rat. J Comp Neurol. 2004;475:575–589. doi: 10.1002/cne.20209. [DOI] [PubMed] [Google Scholar]
- Hoke A, Cheng C, Zochodne DW. Expression of glial cell line-derived neurotrophic factor family of growth factors in peripheral nerve injury in rats. Neuroreport. 2000;11:1651–1654. doi: 10.1097/00001756-200006050-00011. [DOI] [PubMed] [Google Scholar]
- Hoke A, Gordon T, Zochodne DW, Sulaiman OA. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol. 2002;173:77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- Holstege JC, Jongen JL, Kennis JH, van Rooyen-Boot AA, Vecht CJ. Immunocytochemical localization of GDNF in primary afferents of the lumbar dorsal horn. Neuroreport. 1998;9:2893–2897. doi: 10.1097/00001756-199808240-00039. [DOI] [PubMed] [Google Scholar]
- Hu P, McLachlan EM. Selective reactions of cutaneous and muscle afferent neurons to peripheral nerve transection in rats. J Neurosci. 2003;23:10559–10567. doi: 10.1523/JNEUROSCI.23-33-10559.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis JC, Hu S, Altrock BW, Fox GM. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- Jongen JL, Dalm E, Vecht CJ, Holstege JC. Depletion of GDNF from primary afferents in adult rat dorsal horn following peripheral axotomy. Neuroreport. 1999;10:867–871. doi: 10.1097/00001756-199903170-00036. [DOI] [PubMed] [Google Scholar]
- Jongen JL, Haasdijk ED, Sabel-Goedknegt H, van der Burg J, Vecht Ch J, Holstege JC. Intrathecal injection of GDNF and BDNF induces immediate early gene expression in rat spinal dorsal horn. Exp Neurol. 2005;194:255–266. doi: 10.1016/j.expneurol.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Jongen JLM, Jaarsma D, Hossaini M, Natarajan D, Haasdijk ED, Holstege JC. Distribution of RET immunoreactivity in the rodent spinal cord and changes after nerve injury. J Comp Neurol. 2007;500:1136–1153. doi: 10.1002/cne.21234. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Xu J. Quantitative evaluation of calcitonin gene-related peptide and substance P levels in rat spinal cord following peripheral nerve injury. Neurosci Lett. 1995;186:184–188. doi: 10.1016/0304-3940(95)11294-7. [DOI] [PubMed] [Google Scholar]
- Kalous A, Osborne PB, Keast JR. Acute and chronic changes in dorsal horn innervation by primary afferents and descending supraspinal pathways after spinal cord injury. J Comp Neurol. 2007;504:238–253. doi: 10.1002/cne.21412. [DOI] [PubMed] [Google Scholar]
- Kalous A, Osborne PB, Keast JR. Spinal cord compression injury in adult rats initiates changes in dorsal horn remodeling that may correlate with development of neuropathic pain. J Comp Neurol. 2009;513:668–684. doi: 10.1002/cne.21986. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Hyon B, Senba E. Glial cell line-derived neurotrophic factor and nerve growth factor receptor mRNAs are expressed in distinct subgroups of dorsal root ganglion neurons and are differentially regulated by peripheral axotomy in the rat. Neurosci Lett. 1998;252:107–110. doi: 10.1016/s0304-3940(98)00558-8. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Uchida Y, Senba E. Distribution and colocalization of NGF and GDNF family ligand receptor mRNAs in dorsal root and nodose ganglion neurons of adult rats. Brain Res Mol Brain Res. 2003;110:52–62. doi: 10.1016/s0169-328x(02)00584-3. [DOI] [PubMed] [Google Scholar]
- Kitchener PD, Wilson P, Snow PJ. Selective labelling of primary sensory afferent terminals in lamina II of the dorsal horn by injection of Bandeiraea simplicifolia isolectin B4 into peripheral nerves. Neuroscience. 1993;54:545–551. doi: 10.1016/0306-4522(93)90274-j. [DOI] [PubMed] [Google Scholar]
- LaMotte CC, Kapadia SE, Shapiro CM. Central projections of the sciatic, saphenous, median, and ulnar nerves of the rat demonstrated by transganglionic transport of choleragenoid-HRP (B-HRP) and wheat germ agglutinin-HRP (WGA-HRP) J Comp Neurol. 1991;311:546–562. doi: 10.1002/cne.903110409. [DOI] [PubMed] [Google Scholar]
- Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp Physiol. 2002;87:239–244. [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Martin CL, Fenwick NM, Dicarlo SE, Lujan HL, Schreihofer AM. VGLUT1 and VGLUT2 innervation in autonomic regions of intact and transected rat spinal cord. J Comp Neurol. 2007;503:741–767. doi: 10.1002/cne.21414. [DOI] [PubMed] [Google Scholar]
- Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26:8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Allchorne AJ, Griffin RS, Woolf CJ, Costigan M. GDNF selectively promotes regeneration of injury-primed sensory neurons in the lesioned spinal cord. Mol Cell Neurosci. 2007;36:185–194. doi: 10.1016/j.mcn.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver DC, Radeke MJ, Feinstein SC, Snider WD. Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J Comp Neurol. 1995;361:404–416. doi: 10.1002/cne.903610305. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Munson JB, McMahon SB. Effects of GDNF on axotomized sensory and motor neurons in adult rats. Eur J Neurosci. 1997;9:1126–1129. doi: 10.1111/j.1460-9568.1997.tb01465.x. [DOI] [PubMed] [Google Scholar]
- Nagano M, Sakai A, Takahashi N, Umino M, Yoshioka K, Suzuki H. Decreased expression of glial cell line-derived neurotrophic factor signaling in rat models of neuropathic pain. Br J Pharmacol. 2003;140:1252–1260. doi: 10.1038/sj.bjp.0705550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci. 1997;9:1450–1460. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Orozco OE, Walus L, Sah DW, Pepinsky RB, Sanicola M. GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci. 2001;13:2177–2182. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F. GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–391. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Persson S, Boulland JL, Aspling M, Larsson M, Fremeau RT, Jr, Edwards RH, Storm-Mathisen J, Chaudhry FA, Broman J. Distribution of vesicular glutamate transporters 1 and 2 in the rat spinal cord, with a note on the spinocervical tract. J Comp Neurol. 2006;497:683–701. doi: 10.1002/cne.20987. [DOI] [PubMed] [Google Scholar]
- Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- Pierchala BA, Milbrandt J, Johnson EM., Jr Glial cell line-derived neurotrophic factor-dependent recruitment of Ret into lipid rafts enhances signaling by partitioning Ret from proteasome-dependent degradation. J Neurosci. 2006;26:2777–2787. doi: 10.1523/JNEUROSCI.3420-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowicz WP, Staples CS, Milbrandt J, Brunstrom JE, Johnson EM., Jr Glial cell line-derived neurotrophic factor promotes the survival of early postnatal spinal motor neurons in the lateral and medial motor columns in slice culture. J Neurosci. 2002;22:3953–3962. doi: 10.1523/JNEUROSCI.22-10-03953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero-Melian C. Organization of hindlimb nerve projections to the rat spinal cord: a choleragenoid horseradish peroxidase study. J Comp Neurol. 1996;364:651–663. doi: 10.1002/(SICI)1096-9861(19960122)364:4<651::AID-CNE4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ro LS, Li HY, Huang KF, Chen ST. Territorial and extra-territorial distribution of Fos protein in the lumbar spinal dorsal horn neurons in rats with chronic constriction nerve injuries. Brain Res. 2004;1004:177–187. doi: 10.1016/j.brainres.2003.12.047. [DOI] [PubMed] [Google Scholar]
- Robertson B, Grant G. A comparison between wheat germ agglutinin-and choleragenoid-horseradish peroxidase as anterogradely transported markers in central branches of primary sensory neurones in the rat with some observations in the cat. Neuroscience. 1985;14:895–905. doi: 10.1016/0306-4522(85)90152-6. [DOI] [PubMed] [Google Scholar]
- Runyan SA, Roy RR, Zhong H, Phelps PE. L1 cell adhesion molecule is not required for small-diameter primary afferent sprouting after deafferentation. Neuroscience. 2007;150:959–969. doi: 10.1016/j.neuroscience.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah DW, Ossipov MH, Rossomando A, Silvian L, Porreca F. New approaches for the treatment of pain: the GDNF family of neurotrophic growth factors. Curr Top Med Chem. 2005;5:577–583. doi: 10.2174/1568026054367593. [DOI] [PubMed] [Google Scholar]
- Schumacher PA, Eubanks JH, Fehlings MG. Increased calpain I-mediated proteolysis, and preferential loss of dephosphorylated NF200, following traumatic spinal cord injury. Neuroscience. 1999;91:733–744. doi: 10.1016/s0306-4522(98)00552-1. [DOI] [PubMed] [Google Scholar]
- Shehab SA. Acute and chronic sectioning of fifth lumbar spinal nerve has equivalent effects on the primary afferents of sciatic nerve in rat spinal cord. J Comp Neurol. 2009;517:481–492. doi: 10.1002/cne.22163. [DOI] [PubMed] [Google Scholar]
- Shi TJ, Tandrup T, Bergman E, Xu ZQ, Ulfhake B, Hokfelt T. Effect of peripheral nerve injury on dorsal root ganglion neurons in the C57 BL/6J mouse: marked changes both in cell numbers and neuropeptide expression. Neuroscience. 2001;105:249–263. doi: 10.1016/s0306-4522(01)00148-8. [DOI] [PubMed] [Google Scholar]
- Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol. 1990;19:789–801. doi: 10.1007/BF01188046. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Sommer C, Myers RR. Neurotransmitters in the spinal cord dorsal horn in a model of painful neuropathy and in nerve crush. Acta Neuropathol. 1995;90:478–485. doi: 10.1007/BF00294809. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Rossi J, Airaksinen MS, Lewin GR. GFR alpha2/neurturin signalling regulates noxious heat transduction in isolectin B4-binding mouse sensory neurons. J Physiol (Lond) 2002;545:43–50. doi: 10.1113/jphysiol.2002.027656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swett JE, Woolf CJ. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neurol. 1985;231:66–77. doi: 10.1002/cne.902310106. [DOI] [PubMed] [Google Scholar]
- Voikar V, Rossi J, Rauvala H, Airaksinen MS. Impaired behavioural flexibility and memory in mice lacking GDNF family receptor alpha2. Eur J Neurosci. 2004;20:308–312. doi: 10.1111/j.1460-9568.2004.03475.x. [DOI] [PubMed] [Google Scholar]
- Wang R, King T, Ossipov MH, Rossomando AJ, Vanderah TW, Harvey P, Cariani P, Frank E, Sah DW, Porreca F. Persistent restoration of sensory function by immediate or delayed systemic artemin after dorsal root injury. Nat Neurosci. 2008;11:488–496. doi: 10.1038/nn2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanigasekara Y, Airaksinen MS, Heuckeroth RO, Milbrandt J, Keast JR. Neurturin signalling via GFRalpha2 is essential for innervation of glandular but not muscle targets of sacral parasympathetic ganglion neurons. Mol Cell Neurosci. 2004;25:288–300. doi: 10.1016/j.mcn.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Lundstromer K, Jubran M, Brene S, Olson L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci. 2001;21:3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenfalk J, Wu W, Hao J, Person JK, Wiesenfeldt-Hallin Z, Risling M. Treatment of transected peripheral nerves with artemin improved motor neuron regeneration, but did not reduce nerve injury-induced pain behaviour. Scand J Plast Reconstr Surg Hand Surg. 2009;43:245–250. doi: 10.3109/02844310903259082. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Fitzgerald M. Somatotopic organization of cutaneous afferent terminals and dorsal horn neuronal receptive fields in the superficial and deep laminae of the rat lumbar spinal cord. J Comp Neurol. 1986;251:517–531. doi: 10.1002/cne.902510407. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Yang Z, You Y, Levison SW. Neonatal hypoxic/ischemic brain injury induces production of calretinin-expressing interneurons in the striatum. J Comp Neurol. 2008;511:19–33. doi: 10.1002/cne.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]